Abstract
The development of ectopic gastric, intestinal, or pancreatic tissue in the gastrointestinal tract is extremely rare in rats, although it is fairly common in humans. In this report, we describe an unusual case in which a mixture of different types of ectopic tissue was found in the forestomach of a rat. A solitary white nodular/polypoid structure, which measured 5 mm in size, was detected on the luminal surface of the greater curvature of the forestomach in an 8-week-old female Crl:CD(SD) rat. A histological examination revealed that the lesion contained ectopic glandular gastric tissue, including gastric surface mucous cells, parietal cells, and pyloric gland cells, which was confirmed by immunohistochemistry. Moreover, the lesion also contained villin-positive columnar intestinal absorptive cells and chymotrypsin-positive pancreatic exocrine tissue. To the best of our knowledge, this is the first study to detect a mixture of ectopic glandular gastric, intestinal, and exocrine pancreatic tissue in a rat.
Keywords: choristoma, ectopic, forestomach, heterotopic, rat
The development of ectopic tissue (developmental rests) in the gastrointestinal tract is fairly common in humans1, 2. For example, heterotopic pancreatic tissue is often found in the stomach, duodenum, proximal jejunum or Meckel’s diverticulum2,3,4. Such nodules are usually asymptomatic but can cause damage and/or local inflammation2. However, the development of ectopic tissue in the gastrointestinal tract is extremely rare in rats5. In fact, only one case, which involved a F344 rat, has been reported6. In the latter case, the ectopic tissue was composed of small intestinal glands, which were comprised of absorptive columnar cells, goblet cells, and Paneth cells6.
The present report describes a case in which ectopic tissue consisting of a mixture of glandular gastric, intestinal, and exocrine pancreatic tissue developed in the forestomach of a Crl:CD(SD) rat. To the best of our knowledge, this is the first report to describe the presence of such a mixture of ectopic tissue in a rat.
An eight-week-old female Crl:CD(SD) rat (Charles River Laboratories Japan, Shiga, Japan) was sacrificed at the end of a 14-day repeated-dose oral toxicity study. The rat had been housed in a plastic cage in an environmentally controlled room (room temperature, 23 ± 3°C; relative humidity, 30–60%; lighting cycle, 12 h light/ 12 h dark) and supplied with a pellet diet and tap water ad libitum. All experimental procedures were conducted after approval for the study had been obtained from the Animal Care and Use Committee of Shionogi Research Laboratories.
The stomach of the rat was routinely infused with 10% neutral buffered formalin and then subjected to an inspection of its inner surface. During the examination, a solitary white polypoid nodule, which measured 5 mm in diameter, was observed on the luminal surface of the greater curvature of the forestomach. Although the rat had been assigned to the dosing group, no test substance-related findings were observed. The forestomach lesion was considered to have developed spontaneously because no similar lesions were found in the other rats given the same compound. All the tissues including its stomach were fixed in 10% neutral buffered formalin, processed and embedded in paraffin. Then, paraffin-embedded sections were cut and stained with hematoxylin and eosin (HE) or a combination of Alcian Blue and periodic acid-Schiff (AB-PAS) stain. The HIK1083 antibody (1:50; Kanto Chemical Co., Inc., Tokyo, Japan) and antibodies against chromogranin A (1:1600; Abcam, Cambridge, UK), chymotrypsin (1:1000; AbD Serotec, Oxford, UK), cytokeratin AE1/AE3 (ready to use; Dako, Glostrup, Denmark), lysozyme (1:800; Dako), mucin 5AC (Muc5AC, 1:100; Abcam), proton pumps (ready to use; MBL, Nagoya, Japan), and villin (1:800; Novocastra, Newcastle, UK) were selected for the immunohistochemical study. Sections for lysozyme were treated with proteinase K. No antigen retrieval was carried out for HIK1083. For the other antibodies, heat-induced antigen retrieval with citrate buffer was performed. In rats, the HIK1083 antibody reacts with gastric gland cells including mucous neck, pyloric gland and Brunner’s gland cells7. The gastric Muc5AC antigen is found in the columnar mucous cells of the surface gastric epithelium but not in the normal colon, whereas villin is found in the microvilli of the digestive and urinary tracts8, 9.
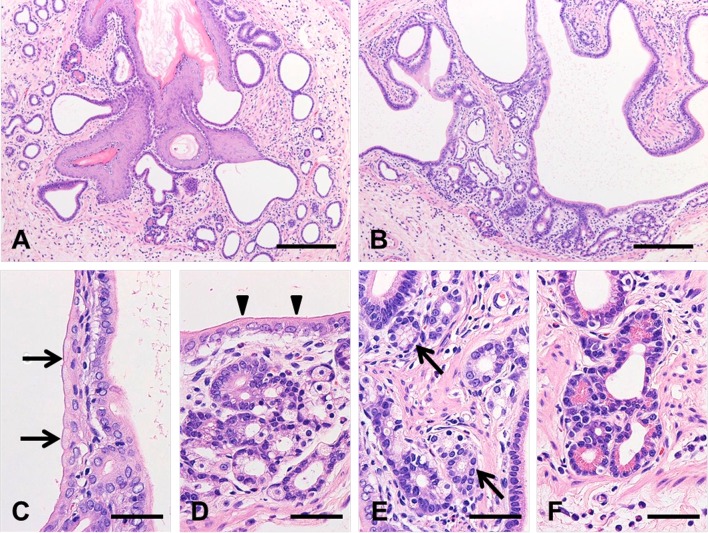
Microscopically, the nodular/polypoid structure observed in the lamina propria of the forestomach was composed of branching ducts, which were clearly contiguous with the squamous epithelia and opened into the forestomach lumen, and all of the ectopic columnar epithelia were surrounded by muscularis mucosae (Fig. 1). The lumens of the branching ducts were composed of mucous cells, which were stained a reddishpurple color by AB-PAS staining (data not shown) and exhibited a brush border, suggesting that they possessed the characteristics of gastric surface mucous cells or intestinal absorptive columnar cells (Fig. 2A–D). Around the branching ducts, a number of glandular epithelial tissues were observed. These tissues were composed of cells that resembled parietal cells (Fig. 2D) and pyloric gland cells (Fig. 2E). Cells containing eosinophilic granules, which were similar in appearance to pancreatic acinar cells or Paneth cells, were also found in the glandular structures (Fig. 2F). In addition, a small number of eosinophils and lymphocytes were observed in the laminae propria and submucosa around the ectopic tissue.
Fig. 1.
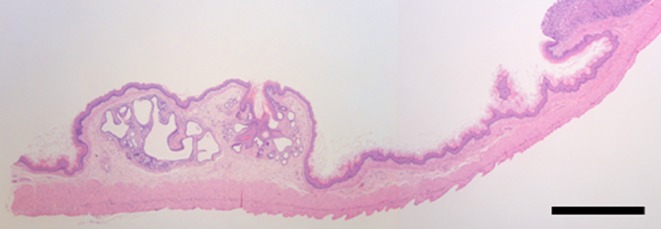
Location of the ectopic tissue in the forestomach. Hematoxylin and eosin staining. Bars: 1 mm.
Fig. 2.
Histological appearance of the ectopic tissue in the forestomach. Hematoxylin and eosin staining (A–F). The lesion occupied the lamina propria under the normal forestomach mucosa and consisted of branching epithelia (A, B), which exhibited the characteristics of gastric surface mucous cells (C, arrow) or columnar intestinal absorptive cells (D, arrowhead). The lesion contained parietal cells (D), pyloric gland-like cells (E, arrow), and pancreatic acinar cells or Paneth cells (F). Bars: 200 μm (A, B), 50 μm (C–F).
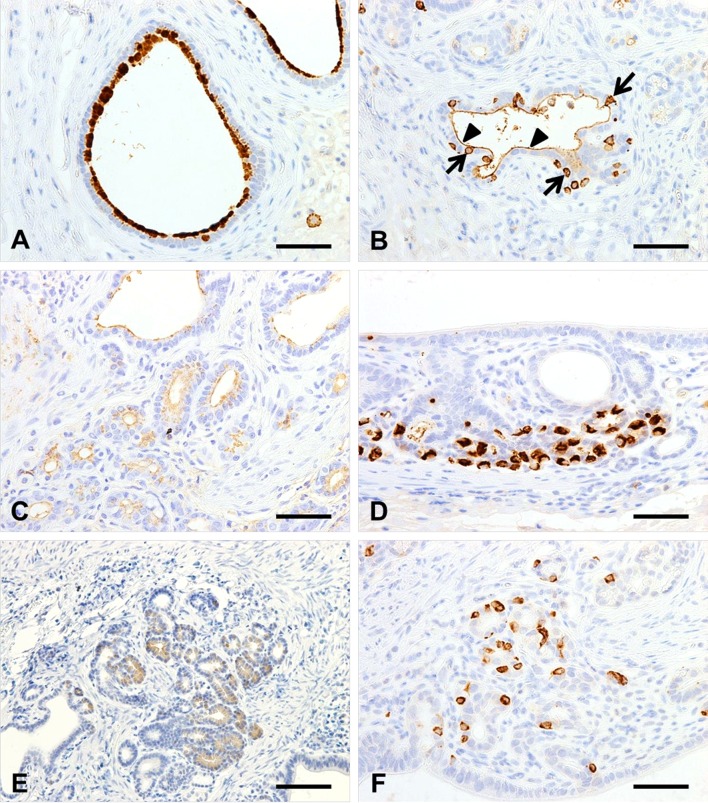
Immunohistochemistry demonstrated that all of the columnar ductal and glandular epithelia were composed of cytokeratin AE1/AE3-positive ectopic tissue (data not shown). Some of the gastric surface mucous cells in the lesion were positive for Muc5AC (Fig. 3A), and a small number of the intestinal absorptive columnar cell microvilli were positive for villin (Fig. 3B, arrowhead). On the other hand, some of the cells that were located between the columnar cells displayed positivity for villin in their cytoplasm (Fig. 3B, arrow). These reactions were considered to be nonspecific because the villin-positive cells did not co-localize with the microvilli. Moreover, the ectopic gastric gland cells and parietal cells were positively stained by the HIK1083 antibody (Fig. 3C) and the antibody used to detect proton pumps (Fig. 3D), respectively. The eosinophilic granule-containing cells were positive for chymotrypsin (Fig. 3E), but negative for lysozyme (data not shown), indicating that they had differentiated into pancreatic acinar cells but not Paneth cells. Chromogranin A-positive endocrine cells were scattered throughout the columnar ductal and glandular epithelia (Fig. 3F). AB-PAS staining did not detect any goblet cells in the lesion.
Fig. 3.
Immunostaining of Muc5AC (A), villin (B), HIK1083 (C), proton pumps (D), chymotrypsin (E) and chromogranin A (F). Some of the gastric surface mucous cells in the lesion were positive for Muc5AC (A), and a small number of the intestinal absorptive columnar cell microvilli (arrowhead) were positive for villin (B). The cytoplasmic immunoreaction to villin (arrow) was considered to be nonspecific (B). The gastric gland cells were positively stained by the HIK1083 antibody (C), and the parietal cells and pancreatic acinar cells were positive for proton pumps (D) and chymotrypsin (E), respectively. Chromogranin A-positive endocrine cells were present in the branching columnar and glandular epithelia (F). Bars: 50 μm (A–D, F), 100 μm (E).
In humans, ectopic tissue can arise at any point in the gastrointestinal tract10. Heterotopic pancreatic tissue, which is also known as ectopic, aberrant or accessory pancreatic tissue, is a type of ectopic tissue that often develops in the gastrointestinal tract11. It is found during approximately one in every 500 operations involving the upper gastrointestinal tract and in 0.6–13% of necropsies12, 13. Heterotopic pancreatic tissue is usually asymptomatic and tends to be found during endoscopic or imaging examinations or surgery but can also produce clinical symptoms depending on its size and pathological changes (acute or chronic pancreatitis, cyst formation, malignant degeneration, etc.) 2, 14, 15.
Two hypotheses have been proposed to explain the development of heterotopy. The first suggests a metaplastic origin for the condition, whereas the other proposes an embryonal origin, which has become more commonly accepted10. In embryos, the stomach and pancreas are derived from the foregut, and gastric and pancreatic heterotopia have been proposed to be caused by the abnormal differentiation of multipotent regional endoderms16.
It is worth noting that not only gastric, but also intestinal and pancreatic tissues, which originate from the foregut, were observed in the present lesion. Although no consensus has been reached regarding the origin of ectopic tissue, it was considered to have arisen during fetal development via the erroneous differentiation of pluripotent endodermal cells in the present case. The latter process might have been influenced by inflammatory conditions in the surrounding region. It is unlikely that epithelial metaplasia developed in the forestomach after embryo development because the lesion consisted of multiple differentiated epithelia.
Acknowledgments
We thank Dr. Mitsuru Kuwamura of the Laboratory of Veterinary Pathology, Osaka Prefecture University, for providing the chymotrypsin antibody.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives (by-nc-nd) License <http://creativecommons.org/licenses/by-nc-nd/3.0/>.
References
- 1.Razi MD. Ectopic pancreatic tissue of esophagus with massive upper gastrointestinal bleeding. Arch Surg. 92(1): 101–104. 1966. [DOI] [PubMed] [Google Scholar]
- 2.Vinay K, Abul KA, Nelson F, and Jon CA. Robbins and Cotran Pathologic Basis of Disease, 8th ed. Saunders, Philadelphia. 2010. [Google Scholar]
- 3.Gucer H, Bagci P, Coskunoglu EZ, and Karadag C. Heterotopic pancreatic tissue located in the gallbladder wall. A case report. JOP. 12(2): 152–154. 2011. [PubMed] [Google Scholar]
- 4.Rana SS, Bhasin DK, Nada R, Gupta R, and Singh K. Heterotopic pancreas in the jejunum presenting as a submucosal lesion on endoscopy. JOP. 10(4): 419–420. 2009. [PubMed] [Google Scholar]
- 5.Boorman GA, Montgomery CA, and MacKenzie WF. Pathology of the Fischer Rat. 1st ed., Academic Press, Inc., San Diego, CA. 1990. [Google Scholar]
- 6.Hagiwara A, Kurata Y, and Tamano S. Ectopic intestinal glands in the forestomach of a F344 rat. Vet Pathol. 27(3): 201–203. 1990. [DOI] [PubMed] [Google Scholar]
- 7.Ishihara K, Kurihara M, Goso Y, Urata T, Ota H, Katsuyama T, and Hotta K. Peripheral alpha-linked N-acetylglucosamine on the carbohydrate moiety of mucin derived from mammalian gastric gland mucous cells: epitope recognized by a newly characterized monoclonal antibody. Biochem J. 318(Pt 2): 409–416. 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kikuchi M, Nagata H, Watanabe N, Watanabe H, Tatemichi M, and Hibi T. Altered expression of a putative progenitor cell marker DCAMKL1 in the rat gastric mucosa in regeneration, metaplasia and dysplasia. BMC Gastroenterol. 10: 65 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garcia MI, Ghiani M, Lefort A, Libert F, Strollo S, and Vassart G. LGR5 deficiency deregulates Wnt signaling and leads to precocious Paneth cell differentiation in the fetal intestine. Dev Biol. 331(1): 58–67. 2009. [DOI] [PubMed] [Google Scholar]
- 10.Yiğit T, Yiğitler C, Güleç B, Atabek C, Ozcan A, Kozak O, and Oner K. Abdominal heterotopic tissues: review of 24 cases diagnosed on postoperative histological evaluation. Turk J Gastroenterol. 17(1): 20–24. 2006. [PubMed] [Google Scholar]
- 11.Christodoulidis G, Zacharoulis D, Barbanis S, Katsogridakis E, and Hatzitheofilou K. Heterotopic pancreas in the stomach: a case report and literature review. World J Gastroenterol. 13(45): 6098–6100. 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tanaka K, Tsunoda T, Eto T, Yamada M, Tajima Y, Shimogama H, Yamaguchi T, Matsuo S, and Izawa K. Diagnosis and management of heterotopic pancreas. Int Surg. 78(1): 32–35. 1993. [PubMed] [Google Scholar]
- 13.Trifan A, Târcoveanu E, Danciu M, Huţanaşu C, Cojocariu C, and Stanciu C. Gastric heterotopic pancreas: an unusual case and review of the literature. J Gastrointestin Liver Dis. 21(2): 209–212. 2012. [PubMed] [Google Scholar]
- 14.Emerson L, Layfield LJ, Rohr LR, and Dayton MT. Adenocarcinoma arising in association with gastric heterotopic pancreas: A case report and review of the literature. J Surg Oncol. 87(1): 53–57. 2004. [DOI] [PubMed] [Google Scholar]
- 15.Hsia CY, Wu CW, and Lui WY. Heterotopic pancreas: a difficult diagnosis. J Clin Gastroenterol. 28(2): 144–147. 1999. [DOI] [PubMed] [Google Scholar]
- 16.Willis RA. Some unusual developmental heterotopias. Br Med J. 3(5613): 267–272. 1968. [DOI] [PMC free article] [PubMed] [Google Scholar]