Summary
1. Flying foxes Pteropus spp. play a key role in forest regeneration as seed dispersers and are also the reservoir of many viruses, including Nipah virus in Bangladesh. Little is known about their habitat requirements, particularly in South Asia. Identifying Pteropus habitat preferences could assist in understanding the risk of zoonotic disease transmission broadly, and in Bangladesh, could help explain the spatial distribution of human Nipah virus cases.
2. We analysed characteristics of Pteropus giganteus roosts and constructed an ecological niche model to identify suitable habitat in Bangladesh. We also assessed the distribution of suitable habitat in relation to the location of human Nipah virus cases.
3. Compared to non-roost trees, P. giganteus roost trees are taller with larger diameters, and are more frequently canopy trees. Colony size was larger in densely forested regions and smaller in flood-affected areas. Roosts were located in areas with lower annual precipitation and higher human population density than non-roost sites.
4. We predicted that 2–17% of Bangladesh's land area is suitable roosting habitat. Nipah virus outbreak villages were 2.6 times more likely to be located in areas predicted as highly suitable habitat for P. giganteus compared to non-outbreak villages.
5. Synthesis and applications. Habitat suitability modelling may help identify previously undocumented Nipah outbreak locations and improve our understanding of Nipah virus ecology by highlighting regions where there is suitable bat habitat but no reported human Nipah virus. Conservation and public health education is a key component of P. giganteus management in Bangladesh due to the general misunderstanding and fear of bats that are a reservoir of Nipah virus. Affiliation between Old World fruit bats (Pteropodidae) and people is common throughout their range, and in order to conserve these keystone bat species and prevent emergence of zoonotic viruses, it is imperative that we continue to improve our understanding of Pteropus resource requirements and routes of virus transmission from bats to people. Results presented here can be utilized to develop land management strategies and conservation policies that simultaneously protect fruit bats and public health.
Keywords: Bangladesh, conservation medicine, ecological niche model, habitat selection, Indian flying fox, Pteropus giganteus, Maxent, Nipah virus, OneHealth, zoonotic disease
Introduction
Flying foxes (genus Pteropus) are declining worldwide (Mildenstein et al. 2005; Stier & Mildenstein 2005) due to growing human populations and consequent demands for food and housing that cause destruction of bat habitat (Fujita 1991; Mickleburgh et al. 2002). Nearly 300 plant species rely on flying foxes for seed dispersal, and in turn, these plants produce almost 500 different products such as food, medicine, and timber (Fujita 1991). Additionally, flying foxes play a key role in forest regeneration because of their ability to retain viable seeds in their gut for several hours (Shilton et al. 1999), their long-distance foraging movements (Tidemann & Nelson 2004; Epstein et al. 2009), and their flight paths over forest clearings that are generally avoided by other forest animals (Fujita 1991). Bats are also increasingly recognized as reservoir hosts for viruses that can cause serious human and animal disease (Calisher et al. 2006; Halpin et al. 2007). In Bangladesh, Pteropus giganteus fruit bats have been implicated as the primary reservoir of Nipah virus (Luby et al. 2009a), a disease that was recognized in the country in 2001 and has caused human outbreaks almost every year since (Luby et al. 2009b).
Despite the ecological, economic, and public health significance of flying foxes, little is known about their habitat requirements, particularly in South Asia (Mildenstein et al. 2005). Understanding their habitat selection can provide information for the design of forest management strategies that preserve roosting and foraging landscapes (Crampton & Barclay 1998; Mildenstein et al. 2005). Furthermore, preventing viral spillover from bats to humans requires an understanding of the ecological narrative linking bat habitat with human and livestock activity to explain when, where, and why a virus emerges (Halpin et al. 2007).
In this study, we describe the characteristics and landscape context of P. giganteus roost sites across Bangladesh. Our study objectives were: (1) to understand P. giganteus roost habitat preferences at the tree-level and in relation to human settlements and the broader landscape, (2) to assess P. giganteus roosting behaviour across environmental gradients, and (3) to evaluate the use of maximum entropy modelling to identify suitable roosting habitat in unstudied areas throughout Bangladesh and relate these findings to our understanding of Nipah virus ecology.
Materials and Methods
Study area
Bangladesh is located in the world's largest delta (the Ganges) and is home to some of the most fertile agricultural land in the world; however, the low-lying plains that make up 80% of the country's landmass are subject to frequent flooding, particularly during monsoon season. Within Bangladesh, remnant tracts of native forest are rapidly being replaced by cropland to meet the needs of one of the densest populations in the world (FAO 2000; Lepers et al. 2005). Forest cover has declined from 14% of Bangladesh's land area in 1989 (Giri & Shrestha 1996) to just over 7% in 2006 (SPARRSO 2007).
Sample selection and locating roosts
This was a countrywide study conducted in Bangladesh from December 2011 to February 2012. Study sites were randomly selected among villages that have experienced a Nipah virus spillover event (where the virus was apparently introduced from a non-human source), known as “spillover villages” and among those that had not (control sites). Control sites were selected by creating a geographically random sample of points throughout Bangladesh (excluding areas within 5 km of spillover villages) that were linked to the nearest village by the field teams in situ using Garmin eTrex GPS devices and GoogleEarth (Fig. 1).
Fig. 1.
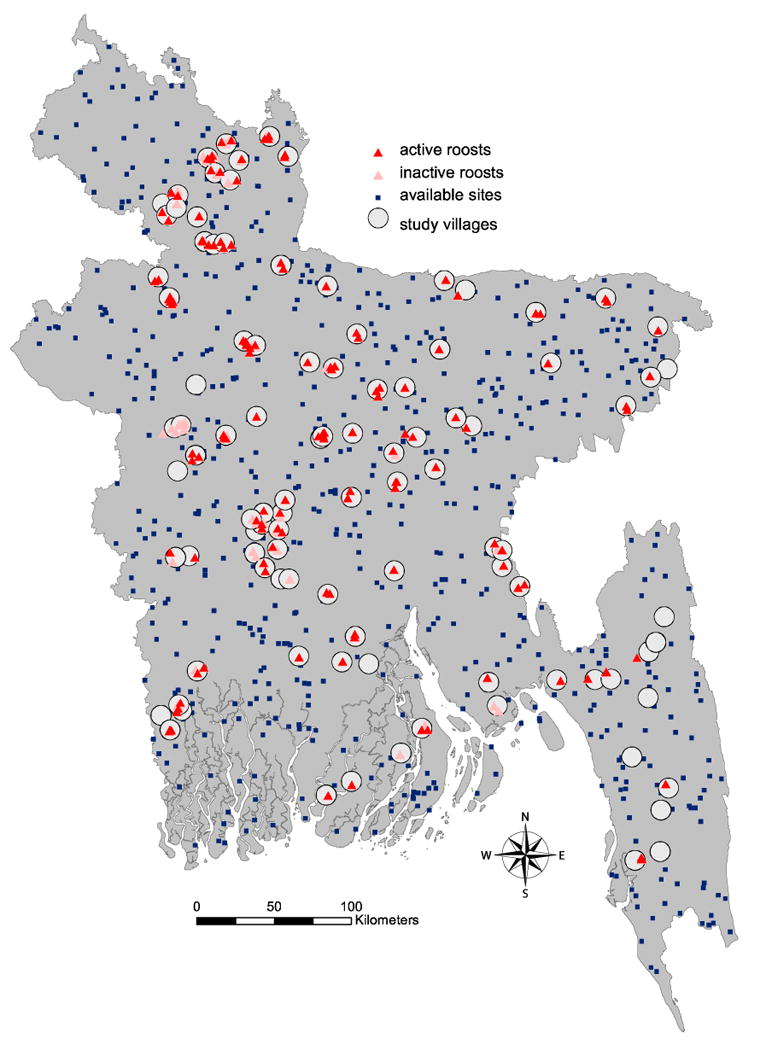
Location of study villages (circles), roosts (triangles) with active roosts denoted in dark red and inactive roosts in pink, and available sites (squares). Available sites are locations randomly selected from within the Bangladesh country boundary to characterize the habitat available for P. giganteus within the study area. Three available sites were selected for each observed roost site.
Field teams identified the location of P. giganteus roosts (arboreal sites where P. giganteus sleep, mate, or otherwise remain during the day) in a village and within 5 km of the village boundary (defined by collecting coordinates along the boundary with direction from community members) though interviews with community members. Data were collected for roost sites occupied for any amount of time within the past five years. The field teams took GPS coordinates at each roost and noted whether the roost was active (currently inhabited by bats) or inactive, as well as the number of bats if present.
Measuring roosting behaviour and structural characteristics of roost sites
Interviews were conducted with residents in the household nearest each roost about the duration and seasonality of bat activity at the roost (Hahn 2013, Appendix B). An environmental assessment was conducted at the two largest, active roosts closest to the village centre or at all roosts in the village if only 1–2 roosts were located (Hahn 2013, Appendix C). Field teams used transect lines to delineate a 20×20 m plot around the central roost tree, which was visually selected as the tree with the largest number of bats. Within each plot, they recorded tree species, diameter at breast height (DBH), tree height (using a clinometer), and canopy versus sub-canopy designation for each tree with DBH >4 cm. Trees with bats present were marked as “roost” trees and empty trees as “non-roosts.” Canopy cover was measured using densitometer readings every meter along the transect lines. Field teams recorded information about human activities within 50 m of the roost plot boundary.
Derivation of remotely sensed roost site characteristics
Three random locations for each observed roost were selected from within the Bangladesh country boundary but excluding the 20×20 m area around an identified roost using Geospatial Modeling Environment (Beyer 2012) to characterize the habitat available to P. giganteus within the study area (henceforth called “available sites,” Fig. 1).
We used ArcGIS 9.3, FRAGSTATS (McGarigal et al. 2012), and Geospatial Modeling Environment (Beyer 2012) to calculate the distance to the nearest river/perennial water body and to derive measures of land cover and forest fragmentation, climate patterns, and human disturbance within 1 km of each roost and available site (Table S1). Information on these datasets can be found in Appendix S1 in Supporting Information.
Statistical analysis
Roost tree selection
We compared the attributes of roost trees with non-roost trees within the roost plots where we conducted environmental assessments to evaluate P. giganteus roost tree selection. We used independent sample t-tests to compare DBH and tree height, and a χ2 a test to compare the percentage of roost and non-roost trees that were identified as canopy trees. We used a binomial exact test to compare the abundance of tree species that comprise roost trees and non-roost trees to identify “selected” and “avoided” species (Sedgeley & Donnell 2004).
Assessing roosting behaviour in relation to environmental characteristics of roosts
We used independent sample t-tests to test for significant differences in environmental characteristics of roosts grouped by roosting duration ( <10 years versus longer-term roosts) and by seasonal occupancy (seasonally occupied versus year-round), as reported by key informants. We used Spearman rank correlation coefficients to test for associations between the number of bats in an active roost and roost characteristics. We considered P ≤ 0.05 significant.
We used non-parametric ordination methods and PC-ORD software (McCune & Mefford 2010) to group roosts into clusters based on the basal area of the tree species present, calculated using our DBH measurements (see Appendix S2). We used χ2 and Fisher's Exact tests to examine associations between the cluster to which a roost was assigned and seasonality and years of roost activity, and analysis of variance (ANOVA) to test for associations with size of roost colony.
Roost site selection: maximum entropy modelling
We used independent sample t-tests to test for significant differences in environmental characteristics of roosts versus available sites. Then we used maximum entropy modelling to identify areas of suitable P. giganteus habitat across Bangladesh based on locations of roost sites from our field study.
We used Maxent software, version 3.3.3 (Phillips et al. 2006) to build our ecological niche model. Maximum entropy modelling is a machine learning method (Phillips et al. 2006) that only requires occurrence data (Phillips et al. 2006), is not very sensitive to small sample sizes (Wisz et al. 2008), and has been shown to consistently out-perform more traditional approaches in terms of predictive power and ability to handle noisy data (Elith et al. 2006). To prevent collinearity in our model, which can affect the interpretation of variable influence from the MaxEnt output (Phillips et al. 2006), we selected 10,000 random points across Bangladesh and calculated the pairwise Pearson's correlation coefficient for all potential predictors. We only included variables where r<0.75 in the same model (Dormann et al. 2012). To validate our model, we withheld 25% of our roost locations for cross-validation. See Appendix S3 for additional modelling methodology.
In order to assess possible bias in the model results related to sampling effort (i.e. different sample selection bias in the occurrence records compared to the background sample used by Maxent) (Elith et al. 2011), we ran the Maxent model three more times, restricting the study area first to 10 km around study villages, then to 25 and 50 km. We compared these results to identify areas where the model output was not consistent and to improve our predictions of suitable P. giganteus roosting habitat throughout Bangladesh.
Results
We located 215 roosts (Fig. 1) and completed key informant interviews at each of these sites. Of these, 68% (n=147) were active roosts where at least one bat was present at the time of the assessment. We conducted an environmental assessment at 143 of identified roosts (see above for selection methodology), and of these, 81% (n=115) were located outside the village boundary but within the 5-km search buffer.
Roost tree selection
Within the roost sites, 3782 trees were surveyed representing 78 tree species and 34 families. Roost trees were taller (19.9 m ± 7.4 v 12.2 m ± 6.5), had larger diameters (53.1 cm ± 56.6 v 22.3 cm ± 38.3), and were more frequently canopy trees than non-roost trees (88.6 v 20.8%) (Table 1). Roost tree selection was non-random with respect to tree species (χ2= 672.12, d.f.=34, P<0.0001, Table 2). For example, bamboo, Albzia spp., eucalyptus, and Shorea robusta accounted for only 23% of all trees (and grasses, in the case of bamboo) surveyed but 53% of roost trees.
Table 1. Characteristics of Pteropus giganteus roost trees versus non-roost trees and roost sites versus random comparison sites.
| Variable* | Roost features | Comparison features | P-value† |
|---|---|---|---|
| ROOST TREE CHARACTERISTICS | Roost Trees | Non-Roost Trees | |
| Diameter at breast height (cm) | 53.1 ± 56.6 | 22.3 ± 38.3 | <0.001 |
| Height (m) | 19.9 ± 7.4 | 12.2 ± 6.5 | <0.001 |
| Percentage canopy trees | 88.6 | 20.8 | <0.001 |
|
| |||
| ROOST PLOT CHARACTERISTICS‡ | Roost Plots | ||
| Species richness | 6.4 ± 3.5 | ||
| Percentage canopy cover | 61.0 ± 18.6 | ||
| Percentage roosts with <50% canopy cover | 29.3 | ||
| Mean stand basal area (m-2 / ha) | 140.2 ± 186.7 | ||
|
| |||
| ROOST SITE CHARACTERISTICS (1-km buffer) | Roost Sites (n= 215) | Random Comparison Sites (n = 645) | P-value † |
| Land Cover | |||
| Distance to river (km) | 1.9 ± 1.8 | 2.2 ± 2.4 | 0.06 |
| Percent forest/rural settlement cover | 30.5 ± 16.0 | 31.3 ± 29.2 | 0.60 |
| Forest patch density (patches km-2) | 0.85 ± 0.42 | 0.73 ± 0.49 | 0.001 |
| Forest edge density edge length (m km-2) | 21.5 ± 7.1 | 16.2 ± 10.0 | <0.0001 |
| Largest patch index (%) | 22.4 ± 17.9 | 24.9 ± 28.6 | 0.15 |
| Percent flooded area | 37.8 ± 23.2 | 53.0 ± 30.8 | <0.0001 |
| Average vegetation condition index (%) | 73.0 ± 22.2 | 67.5 ± 24.3 | 0.004 |
| Percent drought area | 14.5 ± 20.7 | 18.5 ± 25.1 | 0.02 |
| Climate | |||
| Annual mean temperature (°C) | 25.5 ± 0.4 | 25.5 ± 0.5 | 0.62 |
| Mean diurnal range (°C) | 9.8 ± 0.6 | 9.6 ± 0.8 | 0.0002 |
| Mean temperature of warmest quarter (°C) | 28.7 ± 0.4 | 28.6 ± 0.6 | 0.001 |
| Mean temperature of coldest quarter (°C) | 19.6 ± 0.7 | 19.9 ± 0.8 | <0.0001 |
| Temperature annual range (°C) | 22.5 ± 1.6 | 21.8 ± 2.1 | <0.0001 |
| Annual precipitation (mm) | 2085.5 ± 432.1 | 2268.4 ± 581.1 | <0.0001 |
| Precipitation of warmest quarter (mm) | 895.0 ± 277.2 | 936.5 ± 300.5 | 0.07 |
| Precipitation of coldest quarter (mm) | 31.9 ± 6.4 | 34.4 ± 7.2 | <0.0001 |
| Elevation (m) | 17.4 ± 9.7 | 26.0 ± 42.4 | <0.0001 |
| Latitude | 24.3 ± 1.1 | 23.8 ± 1.2 | <0.0001 |
| Human disturbance | |||
| Human population density (people km-2) | 1746 ± 3334 | 959 ± 992 | 0.001 |
| Road density (km km-2) | 9.0 ± 3.6 | 6.5 ± 4.3 | <0.0001 |
Data presented as means ± 1 SD unless otherwise noted
Based on two-tailed, independent groups t-test or χ2 test results
Roost plots were defined as the 20×20 m area around the central roost tree; No comparison plots were evaluated in the field for this study
Table 2. Pteropus giganteus roost tree selection (preferred and avoided species) based on use of a species as a roost tree versus prevalence of the species in surveyed roosts.
| Common name | Scientific name | Bangla name | Family | Proportion all trees | Proportion roost trees | P-value* | Preference† |
|---|---|---|---|---|---|---|---|
| Bamboo | Bambusoideae‡ | bash | Poaceae | 0.099 | 0.201 | <0.0001 | selected |
| Raintree/Koroi | Albizia‡ | raintree/koroi/“acacia” | Fabaceae | 0.054 | 0.145 | <0.0001 | selected |
| Eucalyptus | Myrtaceae§ | eucalyptus/akashi | Myrtaceae | 0.038 | 0.101 | <0.0001 | selected |
| Gajari | Shorea robusta | gajari/shal | Dipterocarpaceae | 0.038 | 0.087 | <0.0001 | selected |
| Mehogani | Swietenia mahagoni | mehogani | Meliaceae | 0.134 | 0.071 | <0.0001 | avoided |
| Indian Mast Tree | Polyalthia longifoliaठ| debdaru | Annonaceae | 0.055 | 0.049 | 0.52 | random |
| Teak | Tectona grandis‡ | shegun | Lamiaceae | 0.030 | 0.042 | 0.06 | random |
| Mango | Mangifera indicaठ| amm | Anacardiaceae | 0.066 | 0.035 | 0.001 | avoided |
| Banyan | Ficus bengalensisठ| bot/pakore | Moraceae | 0.009 | 0.033 | <0.0001 | selected |
| Kadam | Neolamarckia cadamba | kadam | Rubiaceae | 0.010 | 0.028 | <0.0001 | selected |
| Jackfruit | Artocarpus heterophyllus | kathal/chambol | Moraceae | 0.040 | 0.023 | 0.02 | avoided |
| Cotton Silk | Ceiba pentandraठ| shimul | Malvaceae | 0.011 | 0.023 | 0.001 | selected |
| Mabolo/Ebony | Diospyros peregrina§ | gaab | Ebenaceae | 0.041 | 0.020 | 0.01 | avoided |
| Beechwood | Gmelina arborea | gamari/pitagora | Lamiaceae | 0.006 | 0.015 | 0.005 | selected |
| Mulberry | Morus | jam | Moraceae | 0.014 | 0.010 | 0.42 | random |
| Pithraj | Aphanamixis polystachya | pit raj/royna | Meliaceae | 0.036 | 0.006 | <0.0001 | avoided |
| Rough Bush | Streblus asper | sheora | Moraceae | 0.014 | 0.006 | 0.07 | random |
| Tamarind | Tamarindus indicaठ| tatul | Fabaceae | 0.004 | 0.006 | 0.46 | random |
| Dumur | Ficus carica§ | dumur | Moraceae | 0.011 | 0.004 | 0.09 | random |
| Blackboard Tree | Alstonia scholaris‡ | chatim | Apocynaceae | 0.004 | 0.004 | 0.96 | random |
| Pitali | Trewia nudiflora‡ | pitali/pithkhuli | Euphorbiaceae | 0.004 | 0.004 | 0.78 | random |
| Indian Ash Tree | Lannea coromandelica§ | jigha | Anacardiaceae | 0.009 | 0.003 | 0.10 | random |
| Ipil Ipil | Intsia bijuga | ipil ipil | Fabaceae | 0.007 | 0.003 | 0.17 | random |
| Indian Rosewood | Dalbergia sissoo‡ | shishu | Fabaceae | 0.005 | 0.003 | 0.47 | random |
| Betel Nut | Areca catechu§ | shupari | Arecaceae | 0.104 | 0.001 | <0.0001 | avoided |
| Neem | Azadirachta indica§ | neem | Meliaceae | 0.009 | 0.001 | 0.04 | avoided |
| Palmyra Palm | Borassus flabelliferठ| tal | Arecaceae | 0.006 | 0.001 | 0.12 | random |
| Bishop Wood | Bischofia javanica | uriam/puia | Phyllanthaceae | 0.004 | 0.001 | 0.26 | random |
| Monkey Jack | Artocarpus lacucha | dewa | Moraceae | 0.003 | 0.001 | 0.38 | random |
| Hijol | Barringtonia Acutangula | hijol | Lecythidaceae | 0.003 | 0.001 | 0.48 | random |
| Date Palm | Phoenix sylvestris | khejur | Arecaceae | 0.003 | 0.001 | 0.48 | random |
| Banana | Musa paradisiaca§ | kala | Musaceae | 0.021 | 0.000 | - | not used |
| Coconut Palm | Cocos nucifera‡ | narikel | Arecaceae | 0.012 | 0.000 | - | not used |
| Sacred Fig | Ficus religiosaठ| peepal | Moraceae | 0.005 | 0.000 | - | not used |
| Custard Apple | Annona reticulata§ | ata | Annonaceae | 0.004 | 0.000 | - | not used |
Based on binomial exact test
If the abundance of a tree species within the roost trees was significantly greater than expected based on its abundance within the plots, we considered it a preferred, or “selected” species. Alternatively, if use of the tree species as a roost tree was less than expected based on its general abundance, then we labelled it as an “avoided” species. If there was no significant difference between use as a roost and availability in the plots, then the species was considered “used at random.”
Tree species has been documented in the literature as a Pteropus giganteus roosting site
Tree species has been documented in the literature as a Pteropus giganteus food source
Roost plot characteristics
Of the 143 roosts where we completed an environmental assessment, 87% of roost sites were located within 50 m of homestead activities which include the primary residence or buildings associated with the home (kitchen, bathroom, and animal house were generally separate structures) or the household's water pump. Fifty-nine percent of roosts were located near a standing water source such as a large pond, and 55% were within 50 m of agricultural land. Although the majority of roosts were located outside the village boundary, there were no roosts without human activities within 50 m including the homestead activities listed above, other buildings such as schools or mosques, agriculture, a man-made water source, or a road.
The mean stand basal area of the roost plots was 140.2 m2 ha-1 ± 186.7 (Table 1). The average canopy cover was 61% ± 18.6, with sites ranging from 21 to 100% canopy cover. Roost plots contained as few as one tree species and as many as 17, and the mean species richness was 6.4 ± 3.5.
Roosting behaviour
Key informants reported that 65% of the roosts had been occupied intermittently for 10 or more years. The mean DBH of trees in roosts that had been occupied for less than 10 years was smaller than that of trees in long-standing roosts (30.8 cm ± 15.2 vs. 43.1cm ± 35.4, P=0.01).
Respondents described over 87% of the roosts as “year-round,” meaning that bats were present throughout the year rather than seasonally. Of the 28 roosts that were identified as “seasonal” by respondents, 18 roosts (64%) were occupied in only one of the four seasons (rainy, post-monsoon, winter, and summer). The mean diurnal temperature range was lower in seasonal roosts compared to year-round roosts (9.3°C ± 0.7 vs 9.7°C ± 0.6, P=0.002). Also, the elevation above sea level in seasonal roosts was higher than year-round roosts (21.6 m ± 13.6 vs. 14.1 m ± 12.8, P=0.03).
The mean number of bats in the active roosts was 387 ± 543, and the largest roost had over 2,700 bats. The number of bats in a roost increased with the percentage canopy cover (ρ=0.24, P=0.01), the percent of the 1-km buffer area around a roost covered by forest (ρ=0.31, P=0.00), the amount of contiguous forest around the roost (ρ=0.37, P<0.0001), and distance to the nearest river (ρ=0.19, P=0.03). The size of the roosting colony decreased with the amount of flood-affected area around a roost (ρ=-0.23, P=0.01) and forest patch density (ρ=-0.24, P=0.01).
The ordination analysis grouped the roosts into 5 significant clusters, containing between 2 and 74 roosts (Table S2, Appendix S4). There was no significant association between roost tree species cluster and duration or seasonality of roost activity. The mean number of bats in a roost colony was related to tree species composition (ANOVA = 2.54, P=0.04). Raintree/mahagony roosts were more likely to support larger roosting colonies than bamboo roosts (623 bats ± 708 vs. 312 bats ± 440).
Roost site selection: maximum entropy modelling
Pteropus giganteus tended to roost in areas of the country with less annual precipitation (2085.5 mm ± 432.1 vs. 2268.4 mm ± 581.1, Table 1) and that experience a greater range in annual temperatures (22.5°C ± 1.6 vs. 21.8°C ± 2.1) when compared with non-roost locations. P. giganteus also tended to roost in areas with less flooding (37.8% ± 23.2 vs. 53.0% ± 30.8) and drought (14.5% ± 20.7 vs. 18.5% ± 25.1). Human population and road density estimates were higher in roost sites compared to available sites (1746 people km-2 ± 3334 vs. 959 people km-2 ± 992; 9.0 km of road km-2 ± 3.6 vs 6.5 km of road km-2 ± 4.3). Roosts were found in more fragmented forests than were the available sites (patch density = 0.85 patches km-2 ± 0.42 vs. 0.73 patches km-2 ± 0.49).
A total of 135 unique roost sites were used to construct the ecological niche model and 45 were withheld for testing. Our initial Maxent model that utilized all land cover, climate, and human disturbance variables had a high predictive value (AUC=0.884). After dropping the correlated variables, the AUC was 0.882. Models with and without “annual mean temperature” were similar, so our final model which included 10 predictor variables (Table S3) had an AUC=0.882.
The model output produced an estimated probability of occurrence of a P. giganteus roosting site for each pixel across Bangladesh. Our final model predicted the most suitable P. giganteus roosting habitat (probability >0.6) in a north-south band running from central Rangpur Division to the Faridpur region, south of where the Padma and Jamuna rivers join and extending southwest towards the Sundarbans (Fig. 2). There was also a small patch of suitable habitat northeast of the city of Sylhet. Relatively suitable habitat (probability = xf0.4-0.6) was predicted primarily in the central part of the country with small suitable areas on the western coast of Chittagong Division and distributed throughout Sylhet Division. Areas shown as unpredictable are outside the regions where we searched for bat roosts, and therefore, the habitat suitability predictions are uncertain in these areas based on the results of this study.
Fig. 2.
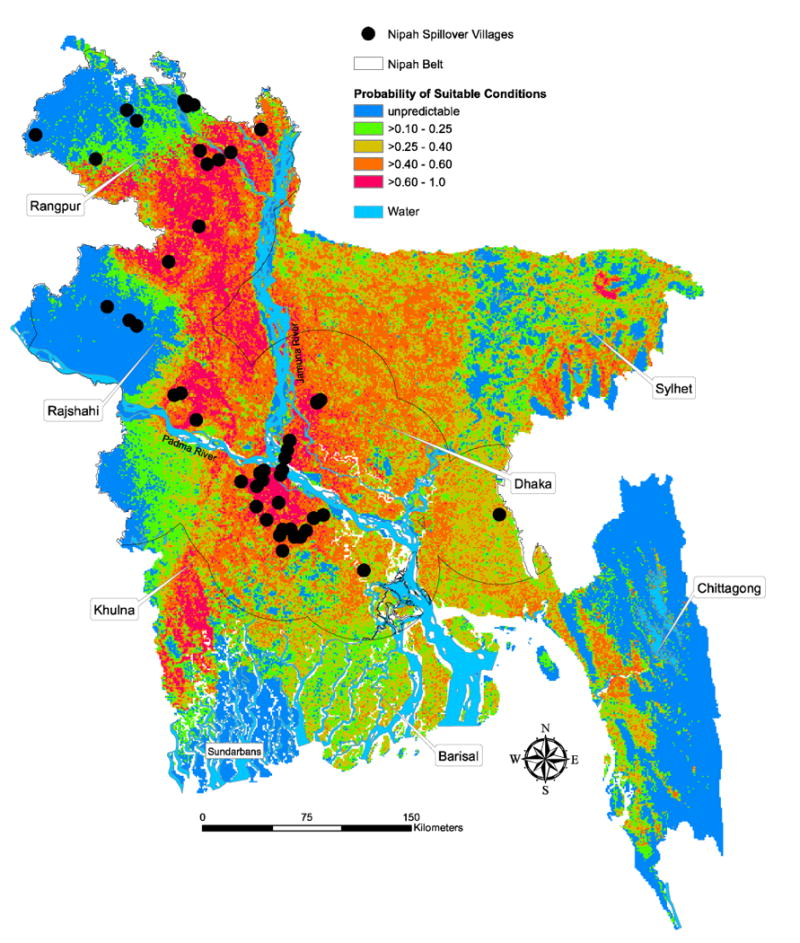
Predicted probability of suitable conditions for Pteropus giganteus roosts in Bangladesh with location of human Nipah virus spillover cases from 2001–2011 and the Nipah Belt. The probability of suitable habitat in blue regions is unpredictable based on the results of this study. See Fig. 4 for an indication of model certainty across the country and areas where future research is needed to refine the model. Bangladesh's administrative divisions are labelled for geographic reference.
Our final model predicted approximately 21,500 km2 of suitable P. giganteus roosting habitat, about 17% of Bangladesh's land area (probability >0.5, Fig. 3; “unpredictable area” is included in the land area denominator). The amount of suitable habitat was cut by more than half (10,000 km2, ∼8% total area of Bangladesh) when the probability threshold was increased to 0.6 and dropped to just over 3,000 km2, ∼2% of Bangladesh at a threshold of 0.7.
Fig. 3.
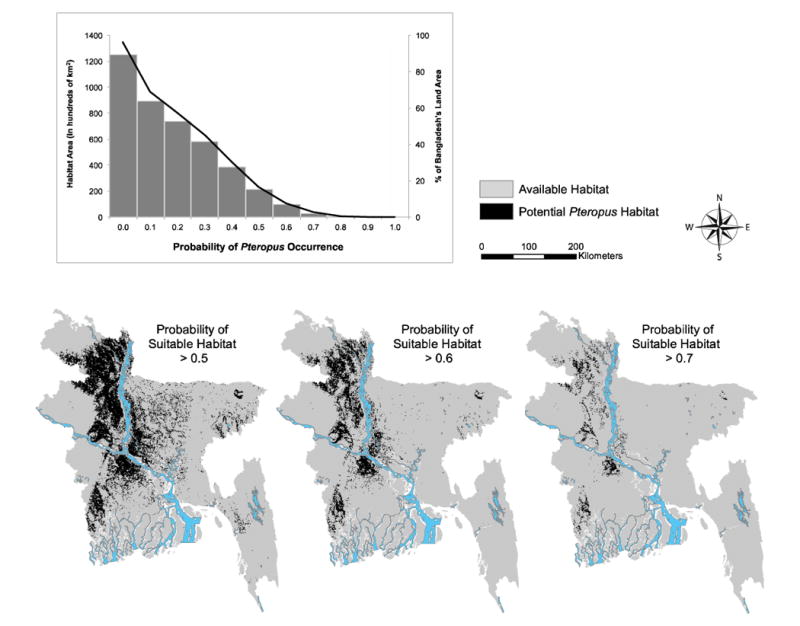
The graph shows the amount of habitat predicted as suitable at probability thresholds from 0–1.0 (bar graph) and the percent of Bangladesh's land area represented by these habitat areas (right vertical axis). The maps show areas of suitable habitat conditions for Pteropus giganteus in Bangladesh based on maximum entropy modelling results at increasingly strict probability thresholds. The most suitable conditions are shown in the threshold > 0.7 map (far right).
Our results from modelling habitat suitability within restricted geographic areas around study villages demonstrate overall consistency with the model produced using the Bangladesh country boundary (Fig. 4). Compared to the full country model, the model within 10 km of study villages shows higher P. giganteus suitability in villages in the lower third of the country near the eastern and western coasts. The models within 25 and 50 km of study villages show the areas of suitable habitat in a more focused area in the western part of the country compared to the full country model that shows suitable areas dispersed throughout eastern Bangladesh. These restricted models also extend the area of suitable habitat along the northwest and western boundaries.
Fig. 4.
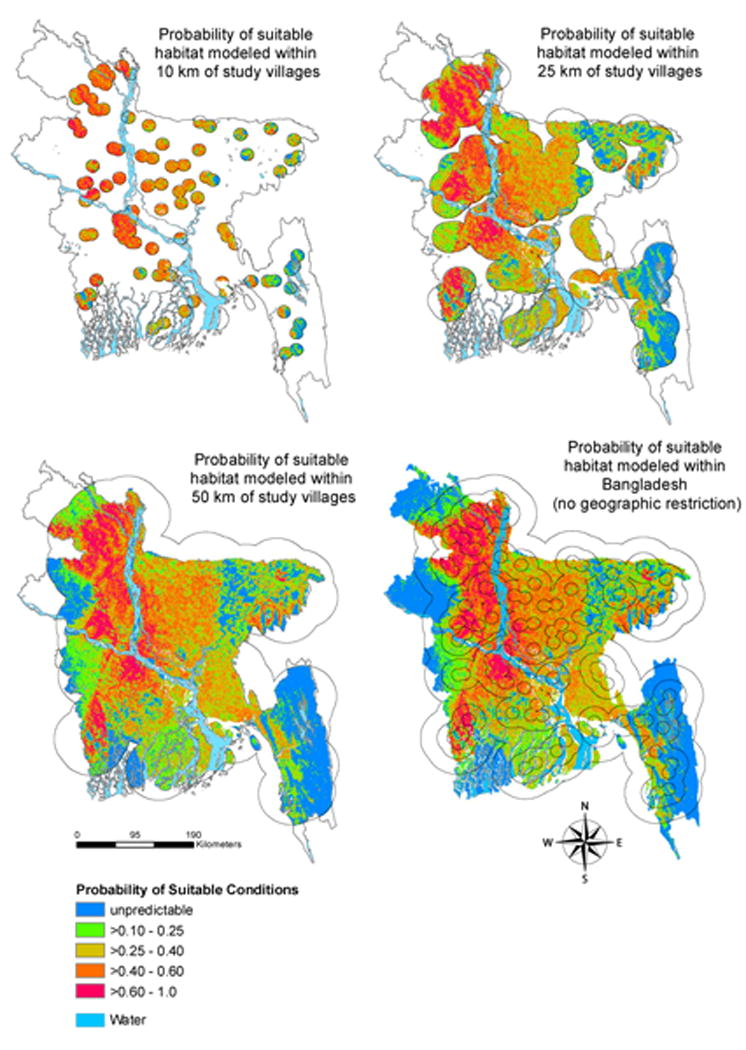
Predicted probability of suitable conditions for Pteropus giganteus roosts in Bangladesh using variable buffer sizes around study villages. Comparison of the maximum entropy model output across these restricted geographies can help identify areas where sample selection bias likely affects the results and predict what P. giganteus suitability would look like if the search for roosts was extended into the outlying regions of Bangladesh where model predictions are uncertain (shown in blue).
The variables that contributed the most information to the models were human population density, distance to roads, annual precipitation, and elevation, together accounting for 65–81% of the information in the geographically restricted and full country models (Table S3). Villages where there were Nipah virus spillovers between 2001–2011 were more likely (OR=2.6, 95% 1.2-5.8) to be located in areas identified as most suitable for P. giganteus compared to villages where there have been no reported outbreaks (Fig. 2).
Discussion
Pteropus roost selection is likely to be strongly influenced by food availability and food proximity (Palmer & Woinarski 1999). One explanation for P. giganteus roosting preference in forests near areas of high human density is that homestead gardens provide a diversity of food resources that may not be present in natural forests. More than 20 million households in Bangladesh maintain a home garden, and a survey of over 400 homesteads in southwestern Bangladesh found an average of 34 plant species per garden (Kabir & Webb 2008). We also found that roosts were located in highly fragmented forests. Gorresen and Willig (2004) observed that the abundance of generalist frugivorous bats was positively associated with fragmentation of the landscape and proposed that their ability to feed on a variety of plant species allowed them to utilize heterogeneous landscapes. In a review of the genus Pteropus, Pierson & Rainey (1992), found that P. giganteus was a species that has been documented in forest remnants in populated areas as opposed to only in undisturbed natural forests. We also found that roosts were often near large ponds, which may be used as a drinking source and are common in Bangladeshi villages.
Pteropid species are unusual within the Yinpterochiroptera in their propensity to roost within trees in large aggregations that range from tens to thousands (Marshall 1983; Pierson & Rainey 1992). Our finding that taller, larger, canopy trees were preferred as roosting sites may be because they provide more space for these large colonies (Gumal 2004). We found that the size of the bat colony was associated with tree species composition in the roost site, likely due to architectural differences between raintrees and bamboo that allow a larger number of bats to congregate in the numerous branches of the former. Others have suggested that these large bats roost in tall trees because they need room to free-fall during take-off (Pierson & Rainey 1992). We found that P. giganteus tended to roost in bamboo, Albzia spp., Eucalyptus spp., and Shorea robusta, among others. Other studies have also found that Pteropus tend to roost in only a subset of the available tree species (Pierson & Rainey 1992; Vardon et al. 2001; Gumal 2004). The reason for this is unknown, but it has been suggested that Pteropus prefer to roost in thick foliage for sun or rain protection (Vardon et al. 2001). Several of the tree species that were found within 20 m of the primary roost trees but were not frequently used for roosting have been documented elsewhere as food resources for Pteropus (Chakravarthy & Girish 2003; Stier & Mildenstein 2005). One possibility is that in addition to selecting roost sites based on the roost tree, P. giganteus also choose sites near food resources but keep their roosting and feeding sites separate, a behaviour that has been noted for other Pteropus bats (Pierson & Rainey 1992).
The majority of roosts identified in our study had been occupied for more than 10 years. This finding is consistent with observations that colonial megabats tend to have high roost fidelity (Marshall 1983; Pierson & Rainey 1992), although some have observed Pteropus fidelity to a home range rather than a single roost (Gumal 2004). The only environmental characteristic of roost sites that was associated with duration of roost occupancy was the mean diameter at breast height (DBH) of trees in the roost plot. The trees in more recently occupied roosts were smaller, which could be because they are simply younger trees that only became viable roosts within the previous 10 years. We also found that bats inhabited most roosts year-round according to key informants. The elevation was higher and there was less variation in the day to night temperatures (diurnal range) in the small percentage of seasonal roosts we identified. Lower-lying areas tend to be more humid and experience less daily variation in temperatures, which may affect the consistency of food resources for P. giganteus. Most seasonal movements of pteropid bats tend to be related to birthing season (Pierson & Rainey 1992) or changes in food abundance (Nelson 1965).
Pteropid bats are threatened throughout their range primarily due to hunting and habitat loss (Fujita 1991; Epstein et al. 2009). Rapid human population growth (Streatfield & Karar 2008) and deforestation (SPARRSO 2007) in Bangladesh will continue to threaten P. giganteus unless comprehensive protection policies and land management practices are established. Additionally, the reputation of P. giganteus as an agricultural pest and reservoir of a deadly virus highlights the perceived conflict between public health and conservation (Breed et al. 2006). While our findings highlight the affiliation between pteropid bats and villages that have experienced Nipah virus outbreaks, it is important to note that the presence of bats in and of itself is not considered a risk factor for Nipah virus infection. Rather, epidemiological studies have consistently identified the consumption of date palm sap as a significant route of transmission (Luby et al. 2005; Luby et al. 2009a). Infrared camera studies have documented P. giganteus licking the flow of date palm sap from the tree to the collection pot as well as urinating and defecating in proximity of the pot, allowing for Nipah virus to be shed into the sap hours prior to human consumption (Khan et al. 2010). Therefore, it is primarily a human agricultural practice that facilitates spillover, rather than direct exposure to bat excreta at the roost site. This was also the case in Malaysia, where Nipah virus first emerged on a large-scale pig farms that had fruit orchards planted next to animal enclosures (Pulliam et al. 2012). The human hand in promoting spillover of viruses from bats and other wildlfie via environmental change, and the often simple solutions that can reduce risk (e.g. bamboo skirts over sap pots), is a message which must be conveyed to avoid attempts at displacement or extermination of bats. In fact, conservation education directed towards local and national government agencies and the public has been a key component of P. giganteus conservation (Morton 1992). Organizations like the Group for Conservation and Research on Bats (GCRB) in Bangladesh are working to develop educational materials to raise awareness of practices that harm bats such as the use of fishing nets to protect orchards that bats get tangled in as well the importance of P. giganteus for pollination and seed dispersal (Islam 2013). Our group in collaboration with the Government of Bangladesh has incorporated both public health and conservation messages into efforts to control Nipah virus spillover at the village level. Working with communities to understand transmission routes of Nipah virus and steps they can take to prevent spillover of the virus such as the use of bamboo skirts to protect their date palm sap containers (Nahar et al. 2010) can dispel fear of flying foxes that might otherwise lead to hunting bats or cutting down roost sites.
In addition to garnering information for conservation of this ecologically important species, a dual purpose of these habitat-modelling efforts is to improve our understanding of Nipah virus ecology in Bangladesh. The location of suitable habitat for the viral reservoir will likely influence the geographic distribution of risk of viral spillover from bats to humans (Halpin et al. 2007). An overlay of Nipah virus spillover locations on our habitat suitability modelling results shows that the majority of spillover events have occurred in regions predicted as highly suitable for P. giganteus roosting. The overlap is more pronounced in the models within restricted geographic areas around the study villages (discussed below). Interestingly, our model also identified highly suitable roosting habitat in areas where human Nipah virus cases have not been reported, such as the area northeast of Sylhet, near Chittagong, and southwest of Khulna. Our current understanding of the “high risk” Nipah virus spillover region is based on the location of previous spillover events; this region is known colloquially as the Nipah Belt (Fig. 2). Our habitat suitability map raises the question as to why have there not been human Nipah cases in the areas identified as highly suitable roosting habitat outside of the Nipah Belt. A possible explanation is surveillance bias. Citizens and medical staff inside the Nipah Belt are more familiar with the disease, symptoms, and testing protocol than people living outside the Nipah Belt, so perhaps there are undocumented human Nipah cases in these roosting hotspots. The lack of human Nipah virus cases in these areas may also be attributed to differences in the intensity or method of harvesting date palm sap, a hypothesis that is currently being tested in the larger Nipah virus disease ecology study underway in Bangladesh by icddr,b and EcoHealth Alliance. Finally, a recent study pointed to differences in tree species composition and configuration of the forest inside and outside the Nipah Belt that could affect the likelihood of interactions between P. giganteus, humans, and shared food resources (Hahn et al. 2013). Further investigation of these roosting hotspots outside the Nipah Belt could be the key to preventing future Nipah virus spillovers if we can identify characteristics of these ecosystems where P. giganteus and humans co-exist without disease transmission. Landscape management or date palm sap collection practices that buffer disease spillover in these regions could be implemented throughout Bangladesh and other regions with high risk of zoonotic disease spread. For example, preserving or replanting tree species preferred by P. giganteus for roosting outside village areas could be one strategy for simultaneously protecting bats and public health.
Although we searched for roosts up to 5 km away from village boundaries, not one identified roost site was located more than 50 m from areas of human activity. This finding underscores the overlap in P. giganteus habitat and human settlements in the densely populated country. Refining and improving our knowledge of P. giganteus roosting and foraging habitat may provide further insight into the conditions that lead to spillover of Nipah virus into humans in Bangladesh. Building on the findings of this study, future assessments could utilize dynamic land cover maps to predict P. giganteus roosting, foraging, and migration that could aid in prediction of annual human Nipah virus outbreaks as well as help define suitable habitat not just in Bangladesh, but throughout its range. Our findings on the current distribution and habitat preferences of P. giganteus could also be used to aid large-scale predictions of Pteropus habitat under future climate change scenarios, which would alter the risk of disease emergence from these bats (Daszak et al. 2012). All of these techniques could be applied to other pteropid species through Asia and Australia.
Our field work was focused in and around villages in Bangladesh while our available comparison sites (and Maxent background sites) were random locations across the country. Consequently, it is possible that the identified roost sites were more likely to be located near human populations than the available sites. Similarly, there are potential biases in occurrence-only data including spatial autocorrelation and correlation with roads, which make occurrence locations easier to find (Phillips et al. 2006; Elith et al. 2011). We looked for roosts up to 5 km from the village boundary, which ensured that there was opportunity to identify roosts away from human populations. And although human population and road density were significant predictors in the ecological niche model, it is clear that these variables were not independently driving the habitat suitability map because there are densely populated areas that were predicted as low suitability such as near the large cities of Dhaka and Chittagong. Sample selection bias can also manifest if the background locations used by Maxent are sampled from a different area than the presence locations (Elith et al. 2011). Our results from the Maxent models in restricted geographic areas around study villages demonstrate the effect of using presence records from in and around study villages to predict suitable habitat for a much larger area in Bangladesh. Although the predicted habitat suitability results are fairly consistent across these models, there are some areas of inconsistency, particularly near the edges of our field work extent. Based on these results, if we continued our search for P. giganteus roosts in northwestern Bangladesh in Rangpur and Rajshahi divisions and in the Sundarban mangrove forests, the areas of high suitability predicted by our model would be likely to extend into these regions, which are currently unpredictable based on our model, and our estimates of available P. giganteus habitat would be larger. It is, however, reassuring that all the maximum entropy models based on occurrence only data and the univariate comparisons of used and available roosts yield similar results. Future studies that extend the search for P. giganteus roosts into these unexplored regions are important for refining the habitat suitability model. In addition, this study was limited to the winter months. Investigation of roosting locations in other times of year would also add to the accuracy of our model.
In summary, we found that P. giganteus shows roost habitat selection preferences at the sub-forest level and at scales of several kilometres. These bats appear to show preferences in terms of tree species and characteristics, degree of forest fragmentation, rainfall and temperature gradients, and level of human disturbance. We predicted that 2–17% of Bangladesh's land area is currently suitable roosting habitat for P. giganteus, although this is likely to be a conservative estimate. Within these areas, humans and bats share significant natural resources. This is also the case with other Old World fruit bats throughout their range (Pierson and Rainy 1992, Mickleburgh et al. 2002). In order to conserve this keystone group of bats and prevent spillover or emergence of zoonotic viruses, it is imperative that we continue to improve our understanding of Pteropus resource requirements and characteristics of the bat–human interface.
Supplementary Material
Supporting Information: Additional Supporting Information may be found in the online version of this article:
Appendix S1. Description of land cover, climate, and human disturbance datasets.
Appendix S2. Description of ordination methods used to group roosts in clusters based on tree species composition.
Appendix S3. Ecological niche modeling methodology.
Appendix S4. Ordination results.
Table S1. Derived variables, units, year of raw data acquisition, original resolution and satellite, and source of remotely sensed data.
Table S2. Diagnostic tree species identified in indicator species analysis.
Table S3. Mean, range, and percent contribution of land cover, climate, and human disturbance variables to the habitat suitability model for Pteropus giganteus in Bangladesh.
Acknowledgments
Hossain M.S. Sazzad, Golam Dostogir Harun, A.K.M. Dawlat Khan, and Sonia Hegde provided logistical support for the field component of this research. We would like to extend our gratitude to the icddr,b field staff for their dedication to collecting accurate and complete data. We also thank Tony Goldberg, Monica Turner, and Ron Gangnon for their helpful comments that greatly improved the manuscript.
Funding from the NSF/NIH Ecology and Evolution of Infectious Diseases grant, 2R01-TW005869 (Fogarty International Center) and the NSF IGERT grant, 0549407: CHANGE-IGERT in the Nelson Institute for Environmental Studies at the University of Wisconsin-Madison.
Footnotes
Data Accessibility: The land cover, Vegetation Condition Index, and flood extent maps can be obtained through the Center for Environmental and Geographic Information Services, House 6, Road 23/C, Gulshan-1, Dhaka 1212, Bangladesh. The WorldClim database (http://www.worldclim.org/), the Shuttle Radar Topography Mission (SRTM) elevation data (http://srtm.csi.cgiar.org/), and the LandScan population data (http://www.ornl.gov/sci/landscan/) are all available online at the web addresses provided. The location of the study villages and P. giganteus roosts are sensitive information and are therefore not online publicly. To obtain these data, please contact icddr,b, GPO Box 128, Dhaka 1000, Bangladesh.
Contributor Information
Micah B. Hahn, Email: micah.hahn@gmail.com, Nelson Institute, SAGE (Center for Sustainability and the Global Environment), Department of Population Health Sciences, School of Medicine and Public Health, University of Wisconsin-Madison, Madison, WI.
Jonathan H. Epstein, Email: epstein@ecohealthalliance.org, EcoHealth Alliance, New York City, NY.
Emily S. Gurley, Email: egurley@icddrb.org, International Center for Diarrheal Disease Research, Bangladesh (icddr,b), Dhaka, Bangladesh.
Mohammad S. Islam, Email: sislam@cegisbd.com, Center for Environmental and Geographic Information Services, Dhaka, Bangladesh.
Stephen P. Luby, Email: sluby@stanford.edu, International Center for Diarrheal Disease Research, Bangladesh (icddr,b), Dhaka, Bangladesh, Centers for Disease Control and Prevention, Atlanta, Georgia, current: Stanford University, Stanford, California.
Peter Daszak, Email: daszak@ecohealthalliance.org, EcoHealth Alliance, New York City, NY.
Jonathan A. Patz, Email: patz@wisc.edu, Nelson Institute, SAGE (Center for Sustainability and the Global Environment), Department of Population Health Sciences, School of Medicine and Public Health, University of Wisconsin-Madison, Madison, WI.
References
- Beyer HL. [Last accessed 29 April 2013];Geospatial Modelling Environment v0.7.0. 2012 http://www.spatialecology.com/gme/
- Breed AC, Field HE, Epstein JH, Daszak P. Emerging henipaviruses and flying foxes - Conservation and management persepctives. Biological Conservation. 2006;131:211–220. doi: 10.1016/j.biocon.2006.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calisher CH, Childs JE, Field HE, Holmes KV, Schountz T. Bats: important reservoir hosts of emerging viruses. Clinical Microbiology Reviews. 2006;19:531–545. doi: 10.1128/CMR.00017-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarthy AK, Girish AC. Crop protection and conservation of frugivorous bats in orchards of hill and coastal regions of Karnataka. Zoos’ Print Journal. 2003;18:1169–1171. [Google Scholar]
- Crampton LH, Barclay RMR. Selection of roosting and foraging habitat by bats in different-aged aspen mixedwood stands. Conservation Biology. 1998;12:1347–1358. [Google Scholar]
- Daszak P, Zambrana-Torrelio C, Bogich TL, Fernandez M, Epstein JH, Murray Ka, et al. Interdisciplinary approaches to understanding disease emergence: The past, present, and future drivers of Nipah virus emergence. PNAS. 2012:1–8. doi: 10.1073/pnas.1201243109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dormann CF, Elith J, Bacher S, Buchmann C, Carl G, Carré G, et al. Collinearity: a review of methods to deal with it and a simulation study evaluating their performance. Ecography. 2012;35:001–020. [Google Scholar]
- Elith J, Graham CH, Anderson PR, Dudík M, Ferrier S, Guisan A, et al. Novel methods improve prediction of species’ distributions from occurrence data. Ecography. 2006;29:129–151. [Google Scholar]
- Elith J, Phillips SJ, Hastie T, Dudík M, Chee YE, Yates CJ. A statistical explanation of MaxEnt for ecologists. Diversity and Distributions. 2011;17:43–57. [Google Scholar]
- Epstein JH, Olival KJ, Pulliam JRC, Smith C, Westrum J, Hughes T, et al. Pteropus vampyrus, a hunted migratory species with a multinational home-range and a need for regional management. Journal of Applied Ecology. 2009;46:991–1002. [Google Scholar]
- FAO. Forest Resources Assessment Program Forest resources of Bangladesh - country report. 2000 [Google Scholar]
- Fujita MS. Flying foxes (Chiroptera: Pteropodidae): threatened animals of key ecological and economic importance. Conservation Biology. 1991;5:455–463. [Google Scholar]
- Giri C, Shrestha S. Land cover mapping and monitoring from NOAA AVHRR data in Bangladesh. International Journal of Remote Sensing. 1996;17:2749–2759. [Google Scholar]
- Gorresen PM, Willig MR. Landscape Responses of bats to habitat fragmentation in Atlantic Forest of Paraguay. Journal of Mammalogy. 2004;85:688–697. [Google Scholar]
- Gumal MT. Diurnal home range and roosting trees of a maternity colony of Pteropus vampyrus natunae (Chiroptera: Pteropodidae) in Sedilu, Sarawak. Journal of Tropical Ecology. 2004;20:247–258. [Google Scholar]
- Hahn MB. Land use and forest composition effects on the ecology of zoonotic and vector-borne disease, Ph D dissertation. University of Wisconsin-Madison; 2013. [Google Scholar]
- Hahn MB, Gurley ES, Epstein JH, Islam MS, Patz JA, Daszak P, Luby SP. The role of landscape composition and configuration on Pteropus giganteus roosting ecology and Nipah virus spillover risk in Bangladesh. American Journal of Tropical Medicne and Hygiene. 2013 doi: 10.4269/ajtmh.13-0256. Advanced online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpin K, Hyatt AD, Plowright RK, Epstein JH, Daszak P, Field HE, et al. Emerging viruses: coming in on a wrinkled wing and a prayer. Clinical Infectious Diseases. 2007;44:711–717. doi: 10.1086/511078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam N. BATS. Vol. 31. Bat Conservation International Magazine; 2013. A first for bats in Bangladesh; pp. 13–14. [Google Scholar]
- Kabir E, Webb EL. Can homegardens conserve biodiversity in Bangladesh? Biotropica. 2008;40:95–103. [Google Scholar]
- Khan SU, Hossain J, Gurley ES, Nahar N, Sultana R, Luby SP. Use of infrared camera to understand bats' access to date palm sap: Implications for preventing Nipah virus transmission. EcoHealth. 2010;7:517–25. doi: 10.1007/s10393-010-0366-2. [DOI] [PubMed] [Google Scholar]
- Lepers E, Lambin EF, Janetos AC, Fries RDE, Achard F, Ramankutty N, et al. A Synthesis of Information on rapid land-cover change for the period 1981–2000. BioScience. 2005;55:115–124. [Google Scholar]
- Luby SP, Gurley ES, Hossain MJ. Transmission of human infection with Nipah virus. Clinical Infectious Diseases. 2009a;49:1743–1748. doi: 10.1086/647951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby SP, Hossain MJ, Gurley ES, Ahmed BN, Banu S, Khan SU, et al. Recurrent zoonotic transmission of Nipah virus into humans, Bangladesh, 2001-2007. Emerging Infectious Diseases. 2009b;15:1229–1235. doi: 10.3201/eid1508.081237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall AG. Bats, flowers and fruit: evolutionary relationships in the Old World. Biological Journal of the Linnean Society. 1983;20:115–135. [Google Scholar]
- McCune B, Mefford M. PC-ORD: software for multivariate analysis of ecological data, Version 6 2010 [Google Scholar]
- McGarigal K, Cushman SA, Ene E. FRAGSTATS v4: Spatial pattern analysis program for categorical and continuous maps. [Last accessed 29 April 2013];2012 http://www.umass.edu/landeco/research/fragstats/fragstats.html.
- Mickleburgh SP, Hutson AM, Racey PA. A review of the global conservation status of bats. Oryx. 2002;36:18–34. [Google Scholar]
- Mildenstein TL, Stier SC, Nuevo-Diego CE, Mills LS, Nuevodiego C. Habitat selection of endangered and endemic large flying-foxes in Subic Bay, Philippines. Biological Conservation. 2005;126:93–102. [Google Scholar]
- Morton PA. Suggestions for long- and short-term education strategies to address the conservation of Pacific Island Flying Foxes. In: Rockwell ED, et al., editors. Pacific Island Flying Foxes: Proceedings of an International Conservation Conference. U.S Department of the Interior; 1992. pp. 167–171. [Google Scholar]
- Nahar N, Sultana R, Gurley ES, Hossain MJ, Luby SP. Date palm sap collection: Exploring opportunities to prevent Nipah transmission. EcoHealth. 2010;7:196–203. doi: 10.1007/s10393-010-0320-3. [DOI] [PubMed] [Google Scholar]
- Nelson J. Movements of Australian flying foxes (Pteropodidae: Megachiroptera) Australian Journal of Zoology. 1965;13:53–73. [Google Scholar]
- Palmer C, Woinarski JCZ. Seasonal roosts and foraging movements of the black flying fox (Pteropus alecto) in the Northern Territory: resource tracking in a landscape mosaic. Wildlife Research. 1999;26:823–838. [Google Scholar]
- Phillips SJ, Anderson RP, Schapire RE. Maximum entropy modeling of species geographic distributions. Ecological Modelling. 2006;190:231–259. [Google Scholar]
- Pierson ED, Rainey WE. The biology of flying foxes of the Genus Pteropus: A Review. In: Rockwell ED, et al., editors. Pacific Island Flying Foxes: Proceedings of an International Conservation Conference. U.S Department of the Interior; 1992. pp. 1–17. [Google Scholar]
- Pulliam JRC, Epstein JH, Dushoff J, Rahman SA, Bunning M, Jamaluddin AA, et al. Agricultural intesnsification, priming for persistence and the emergence of Nipah virus: a lethal bat-borne zoonosis. Journal of the Royal Society Interface. 2012;9:89–101. doi: 10.1098/rsif.2011.0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedgeley JA, Donnell CFJO. Roost use by long-tailed bats in South Canterbury: examining predictions of roost-site selection in a highly fragmented landscape. New Zealand Journal of Ecology. 2004;1:1–18. [Google Scholar]
- Shilton LA, Altringham JD, Compton SG, Whittaker RJ. Old World fruit bats can be long-distance seed dispersers through extended retention of viable seeds in the gut. Proceedings of the Royal Society B Biological Sciences. 1999;266:219. [Google Scholar]
- SPARRSO. Bangladesh Space Research and Remote Sensing Organization. Forest and other land uses of Bangladesh: a technical report on the remote sensing monitoring component of the project strengthening capacity to generate quality information on forest resources 2007 [Google Scholar]
- Stier SC, Mildenstein TL. Dietary habits of the world's largest bats: the Philippine Flying Foxes, Acerodon Jubatus and Pteropus Vampyrus Lanensis. Journal of Mammalogy. 2005;86:719–728. [Google Scholar]
- Streatfield PK, Karar ZA. Population challenges for Bangladesh in the coming decades. Journal of Health, Population, and Nutrition. 2008;26:261–272. [PMC free article] [PubMed] [Google Scholar]
- Tidemann CR, Nelson JE. Long-distance movements of the grey-headed flying fox (Pteropus poliocephalus) Society. 2004;263:141–146. [Google Scholar]
- Vardon MJ, Brocklehurst PS, Woinarski JCZ, Cunningham RB, Donnelly CF, Tidemann CR. Seasonal habitat use by flying-foxes, Pteropus alecto and P. scapulatus (Megachiroptera), in monsoonal Australia. Journal of Zoological Society of London. 2001;253:523–535. [Google Scholar]
- Wisz MS, Hijmans RJ, Li J, Peterson aT, Graham CH, Guisan A. Effects of sample size on the performance of species distribution models. Diversity and Distributions. 2008;14:763–773. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information: Additional Supporting Information may be found in the online version of this article:
Appendix S1. Description of land cover, climate, and human disturbance datasets.
Appendix S2. Description of ordination methods used to group roosts in clusters based on tree species composition.
Appendix S3. Ecological niche modeling methodology.
Appendix S4. Ordination results.
Table S1. Derived variables, units, year of raw data acquisition, original resolution and satellite, and source of remotely sensed data.
Table S2. Diagnostic tree species identified in indicator species analysis.
Table S3. Mean, range, and percent contribution of land cover, climate, and human disturbance variables to the habitat suitability model for Pteropus giganteus in Bangladesh.


