Abstract
Microtubule turnover in the growing axons is required for directional axonal growth and synapse formation in the developing brain. In this issue of The EMBO Journal, Tortoriello et al (2014) show that the microtubule-binding protein SCG10/stathmin-2 is a specific molecular target for a CB1 receptor-mediated effect of Δ9-tetrahydrocannabinol (THC), the psychoactive ingredient of smoked marijuana, in the fetal brain. Considering the role of CB1 in modulating the specification and long-distance migration of neurons in the perinatal brain, this study reveals an interesting mechanism potentially accounting for connectivity deficits during cortical development following exposure to CB1 agonists or THC during pregnancy.
See also: G Tortoriello et al (April 2014)
Heavy use of marijuana during pregnancy can impair social behaviors, cognition, and motor functions in the offspring, with impact lasting well into adulthood (Fried et al, 2002; Huizink & Mulder, 2006; Passey et al, 2014; Shabani et al, 2014). Approximately 1/3 of Δ9-tetrahydrocannabinol (THC), the psychoactive constituent of cannabis, and its metabolites can pass through the placental barrier upon marijuana smoking during pregnancy (Hutchings et al, 1989). Nonetheless, over 10% of pregnancies in Western countries are associated with maternal cannabis exposure, whereas, during the past decades, selective agriculture of Cannabis has resulted in an increased THC content at the expense of the most abundant non-psychotropic component, cannabidiol (Pijlman et al, 2005). Therefore, and taking into consideration the increasing awareness of the role of CB1 receptors in embryonic neuronal differentiation, axonal pathfinding, and target selection (Berghuis et al, 2007), intrauterine THC exposure may lead to long-lasting developmental brain defects. However, it is presently unknown whether and how THC triggers CB1-driven signaling cascades to disrupt fetal cortical circuitry specification.
In this issue of The EMBO Journal, Tortoriello et al (2014) use a combination of pharmacological methods, organotypic slice preparations, quantitative and qualitative morphometric analysis, and unbiased proteomic profiling to identify SCG10/stathmin-2, a microtubule-binding protein, as the first THC-sensitive molecular effector modulating directional growth of corticofugal axons in the developing cerebrum. In the rodent perinatal brain, cannabinoid CB1 and CB2 receptors and their endogenous agonists, the “endocannabinoids” anandamide and 2-arachidonoylglycerol (2-AG), are present from early developmental stages onwards (Berrendero et al, 1999). Evidence shows that this endocannabinoid system plays a key role in determining the synaptic structure and function of the adult brain by correctly “wiring” the fetal cortical circuitries through the strict spatio-temporal dynamics of CB1 activation.
To investigate its effect on cortical network assembly, Tortoriello and co-authors were careful to administer THC to pregnant mice from embryonic day 5.5 through 17.5 at a “non-intoxicating” daily dose (3 mg/kg, intraperitoneal), that is, a dose that did not alter maternal behavior or physical measures. They then allowed offspring to mature without postnatal re-introduction of the drug. By postnatal day 10, they observed redistribution of CB1-expressing inputs in both the neocortex and hippocampus. This is reminiscent of the long-term structural and functional corticofugal alterations previously observed in CB1-“knock-out” mice (Berghuis et al, 2007). However, the authors also noted novel features. SCG10 mapping in long-range forebrain projections was enriched in growth cone-like structures and co-distributed with CB1 in both the intermediate zone of the cerebral cortex and the primordial hippocampus of vehicle-treated fetuses. While, under normal conditions, SCG10 is proximal to CB1 in corticofugal axons and could therefore be a downstream target of endocannabinoid actions in particular neurite domains, THC-treated fetuses exhibited a depletion of SCG10 from growing neurites, including the growth cones. Moreover, growth cones underwent morphological reorganization, suggesting their altered rate of motility and reduced outgrowth due to impaired microtubule dynamics. Tortoriello and colleagues also showed that THC induces CB1-mediated rapid axonal degradation of SCG10 through its phosphorylation by JNK1 (Fig 1). Consistent with this latter finding, several studies have revealed that JNK1-mediated phosphorylation of SCG10 at Ser62 and Ser73 negatively regulates the activity of SCG10 by promoting its proteasomal degradation in injured axons (Shin et al, 2012) and limiting the crucial role of this protein in regulating microtubule dynamics in the developing brain (Grenningloh et al, 2004). Finally, to demonstrate that the CB1-JNK-SCG10 pathway alone is sufficient to account for the THC-induced cytoarchitectural modifications of cortical neurons, the authors overexpressed a pseudophosphorylated SCG10 mutant refractory to phosphorylation by JNK1 and degradation, and found that this process limits neurite outgrowth in competition with endogenous (wild-type) SCG10 and occludes THC effects. Thus, while the “physiological” phosphorylation of SCG10 downstream of 2-AG-activated CB1 reduces to an essential level the cytoskeletal instability required to maintain the rate of neurite outgrowth, its “pathological” hyperphosphorylation by THC triggers ectopic axonal growth.
Figure 1. THC deregulating effect on microtubule dynamics in growth cones.
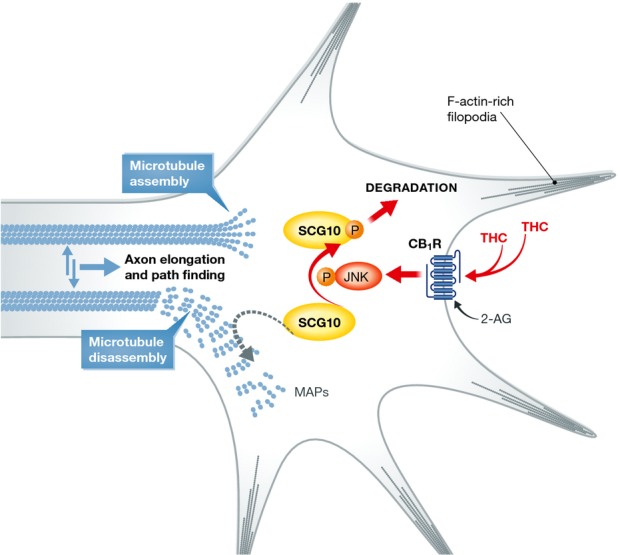
An integrated signaling transduced by CB1 via JNKs and executed by SCG10 is disrupted by THC during the corticogenesis of prenatal brains exposed to a dose of THC (3 mg/kg/day, embryonic day E5.5–17.5) non-aversive to dams. SCG10 is normally enriched near CB1 receptors in growth cone-like structures, mainly traversing the pallio-subpallial boundary, where its microtubule-destabilizing action represents an important factor for the dynamic assembly and disassembly of growth cone microtubules during axonal elongation. Phosphorylation at Ser62 and Ser73 by JNK1, the active brain-specific JNK isoform, exerted also through the tonic action of the endocannabinoid 2-AG, promotes SCG10 degradation with ensuing fine regulation of microtubule dynamics and axodendritic morphology. Chronic THC administration during cortical development disrupts this action through both reduced 2-AG biosynthesis and CB1 expression, and excessive SCG10 phosphorylation and degradation. THC also increases the formation of F-actin-rich filopodia in the distant motile axon segment. 2-AG, 2-arachidonoylglycerol; CB1R, cannabinoid receptor type 1; JNK1, c-Jun N-terminal kinase 1; MAPs, microtubule-associated proteins; THC, Δ9-tetrahydrocannabinol; SCG10, superior cervical ganglion 10.
To make their study relevant to a condition potentially occurring in marijuana smoking pregnant mothers, Tortoriello and colleagues assessed the distribution and density of SCG10 mRNA in the primordial hippocampus and parahippocampal gyrus of electively aborted second-trimester human fetuses exposed prenatally to cannabis vs. age-matched controls. They found that, also in the human developing brain, SCG10 mRNA levels were significantly reduced in cannabis-exposed subjects during the temporal window coincident with the formation of intra- and extracortical axonal trajectories (Kostovic′ & Judaš, 2010). Indeed, retrospective longitudinal studies have documented potential long-term behavioral abnormalities in marijuana-exposed offspring—including exaggerated startle response and poor habituation to novel stimuli in infants, hyperactivity, inattention, and cognitive retardation in adolescents (Fried et al, 2002; Huizink & Mulder, 2006). Experimental studies substantiate these observations and link behavioral and cognitive deficits and emotional responsiveness to pharmacological interference with endocannabinoid signals during fetal development (Passey et al, 2014; Shabani et al, 2014). Increased incidence of schizophrenia, depression, and predisposition to substance abuse in offspring prenatally exposed to cannabis (Substance Abuse and Mental Health Service Administration, 2012) might also be due, in part, to CB1-induced altered developmental synaptic organization, which, even when remaining latent for long periods, might be prone to failure if challenged by environmental cues.
In conclusion, the important findings by Tortoriello et al provide a mechanistic explanation as to why marijuana abuse should be avoided during pregnancy. Future investigations should address the effect of THC on axon targeting processes in neonates and developing pups, thus allowing us to understand whether or not these observations are relevant also to the ever-increasing use of cannabidiol-enriched, but still THC-containing, cannabis extracts for pediatric indications, such as epilepsy (Porter & Jacobson, 2013).
Conflict of interest
Vicenzo Di Marzo is a consultant for GW Pharmaceuticals, UK, and receives research funds from the same company. The other author, Luigia Cristino, declares that she has no conflict of interest.
References
- Berghuis P, Rajnicek AM, Morozov YM, Ross RA, Mulder J, Urbán GM, Monory K, Marsicano G, Matteoli M, Canty A, Irving AJ, Katona I, Yanagawa Y, Rakic P, Lutz B, Mackie K, Harkany T. Hardwiring the brain: endocannabinoids shape neuronal connectivity. Science. 2007;316:1212–6. doi: 10.1126/science.1137406. [DOI] [PubMed] [Google Scholar]
- Berrendero F, Sepe N, Ramos JA, Di Marzo V, Fernández-Ruiz JJ. Analysis of cannabinoid receptor binding and mRNA expression and endogenous cannabinoid contents in the developing rat brain during late gestation and early postnatal period. Synapse. 1999;33:181–91. doi: 10.1002/(SICI)1098-2396(19990901)33:3<181::AID-SYN3>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Fried P, Watkinson B, James D, Gray R. Current and former marijuana use: preliminary findings of a longitudinal study of effects on IQ in young adults. CMAJ. 2002;166:887–91. [PMC free article] [PubMed] [Google Scholar]
- Grenningloh G, Soehrman S, Bondallaz P, Ruchti E, Cadas H. Role of the microtubule destabilizing proteins SCG10 and stathmin in neuronal growth. J Neurobiol. 2004;58:60–9. doi: 10.1002/neu.10279. [DOI] [PubMed] [Google Scholar]
- Huizink AC, Mulder EJ. Maternal smoking, drinking or cannabis use during pregnancy and neurobehavioral and cognitive functioning in human offspring. Neurosci Biobehav Rev. 2006;30:24–41. doi: 10.1016/j.neubiorev.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Hutchings DE, Hutchings DE, Martin BR, Gamagaris Z, Miller N, Fico T. Plasma concentrations of Δ9-tetrahydrocannabinol in dams and fetuses following acute or multiple prenatal dosing in rats. Life Sci. 1989;44:697–701. doi: 10.1016/0024-3205(89)90380-9. [DOI] [PubMed] [Google Scholar]
- Kostovic′ I, Judaš M. The development of the subplate and thalamocortical connections in the human foetal brain. Acta Paediatr. 2010;99:1119–1127. doi: 10.1111/j.1651-2227.2010.01811.x. [DOI] [PubMed] [Google Scholar]
- Passey ME, Sanson-Fisher RW, D'Este CA, Stirling JM. Tobacco, alcohol and cannabis use during pregnancy: clustering of risks. Drug Alcohol Depend. 2014;134:44–50. doi: 10.1016/j.drugalcdep.2013.09.008. [DOI] [PubMed] [Google Scholar]
- Pijlman FT, Rigter SM, Hoek J, Goldschmidt HM, Niesink RJ. Strong increase in total delta-THC in cannabis preparations sold in Dutch coffee shops. Addict Biol. 2005;10:171–80. doi: 10.1080/13556210500123217. [DOI] [PubMed] [Google Scholar]
- Porter BE, Jacobson C. Report of a parent survey of cannabidiol-enriched cannabis use in pediatric treatment-resistant epilepsy. Epilepsy Behav. 2013;29:574–7. doi: 10.1016/j.yebeh.2013.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Mental Health, United States, 2010. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2012. HHS Publication No. (SMA) 12-4681. [PubMed] [Google Scholar]
- Shabani M, Mahnam A, Sheibani V, Janahmadi M. Alterations in the intrinsic burst activity of Purkinje neurons in offspring maternally exposed to the CB1 Cannabinoid agonist WIN 55212-2. J Membr Biol. 2014;247:63–72. doi: 10.1007/s00232-013-9612-1. [DOI] [PubMed] [Google Scholar]
- Shin JE, Miller BR, Babetto E, Cho Y, Sasaki Y, Qayum S, Russler EV, Cavalli V, Milbrandt J, DiAntonio A. SCG10 is a JNK target in the axonal degeneration pathway. Proc Natl Acad Sci USA. 2012;109:E3696–705. doi: 10.1073/pnas.1216204109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tortoriello G, Morris CV, Alpar A, Fuzik J, Shirran SL, Calvigioni D, Keimpema E, Botting CH, Reinecke K, Herdegen T, Courtney M, Hurd YL, Harkany T. Miswiring the brain: Δ9-tetrahydrocannabinol disrupts cortical development by inducing an SCG10/stathmin-2 degradation pathway. EMBO J. 2014 doi: 10.1002/embj.201386035. DOI 10.1002/embj.201386035. [DOI] [PMC free article] [PubMed] [Google Scholar]


