Abstract
Prokaryotes and lower eukaryotes, such as yeasts, utilize two-component signal transduction pathways to adapt cells to environmental stress and to regulate the expression of genes associated with virulence. One of the central proteins in this type of signaling mechanism is the phosphohistidine intermediate protein Ypd1. Ypd1 is reported to be essential for viability in the model yeast Saccharomyces cerevisiae. We present data here showing that this is not the case for Candida albicans. Disruption of YPD1 causes cells to flocculate and filament constitutively under conditions that favor growth in yeast form. To determine the function of Ypd1 in the Hog1 mitogen-activated protein kinase (MAPK) pathway, we measured phosphorylation of Hog1 MAPK in ypd1Δ/Δ and wild-type strains of C. albicans. Constitutive phosphorylation of Hog1 was observed in the ypd1Δ/Δ strain compared to the wild-type strain. Furthermore, fluorescence microscopy revealed that green fluorescent protein (GFP)-tagged Ypd1 is localized to both the nucleus and the cytoplasm. The subcellular segregation of GFP-tagged Ypd1 hints at an important role(s) of Ypd1 in regulation of Ssk1 (cytosolic) and Skn7 (nuclear) response regulator proteins via phosphorylation in C. albicans. Overall, our findings have profound implications for a mechanistic understanding of two-component signaling pathways in C. albicans, and perhaps in other pathogenic fungi.
INTRODUCTION
Two-component signaling systems are composed of a membrane-bound sensor histidine kinase (HK) protein, a cytoplasmic response regulator (RR) protein, and an intermediate histidine phosphotransfer (HPt) protein. The proteins which participate in this pathway are unique in regard to the amino acids that accept phosphoryl groups, which include either aspartate or histidine residues. The term “two-component” signaling was first described for bacterial systems, which are usually less complex in regard to the number of participating proteins. In bacteria, generally, a membrane-associated HK is autophosphorylated from ATP on a conserved histidine residue in response to an environmental signal. This autophosphorylation event is followed by transfer of the phosphoryl group to a cognate RR protein on a conserved aspartate residue. The phosphorylated RR then usually acts directly as a transcription factor to activate genes associated with chemotaxis, stress responses, virulence factor expression, and antibiotic resistance (1).
Fungal two-component phosphorelays are a bit more intricate in two respects. First, a third protein is required for phosphotransfer and is positioned in the pathway between the HK and RR proteins. This protein is referred to as the phosphohistidine intermediate protein Ypd1. Ypd1 has transferase activity, and the major function of this protein is to shuttle phosphate from histidine kinase to response regulator proteins (2, 3). The phosphorelay typically consists of a total of four phosphorylation events on three proteins. First, the HK is autophosphorylated on a histidine residue within the histidine kinase domain, followed by an intramolecular transfer of the phosphate group to its receiver domain aspartate (His → Asp). This protein then participates in a third phosphotransfer to the histidine residue present in the HPt domain on Ypd1 (His → Asp → His). Finally, the histidine phosphotransfer relays phosphoryl groups to the RR protein in an ATP-independent manner (3). Thus, a total of four phosphotransfer reactions occur (His → Asp → His → Asp), as described above. The outcome of this series of reactions is a phosphorylated RR protein, which terminates any further response, because the phosphorylated protein is unable to activate the downstream mitogen-activated protein kinase (MAPK) pathway (4–6). The reactions described above occur in unstressed cells, but under conditions of stress, the RR protein is dephosphorylated, by a poorly understood mechanism, and is able to activate a downstream MAPK pathway, the result of which is a functional response. The best-studied MAPK pathway that includes upstream two-component proteins is the Hog1 (hyperosmotic glycerol) MAPK pathway in Saccharomyces cerevisiae (7, 8). In Candida albicans, Hog1 MAPK regulates glycerol accumulation and adaptation to high osmolarity, as well as oxidative stress, morphogenesis, and cell wall biosynthesis (9–11).
The current paradigm that Ypd1 is indispensable for viability in eukaryotic microorganisms was established mainly by studies done with S. cerevisiae and, more recently, with Neurospora crassa, Aspergillus nidulans, and Cryptococcus neoformans (2, 12–14). S. cerevisiae Ypd1 is essential for viability and has been shown to interact with both Ssk1 and Skn7 response regulators (15, 16). Ssk1 is a response regulator upstream of the Hog1 MAPK pathway, and its major function in S. cerevisiae is in the osmotic stress response, while Skn7 provides antioxidant functions and cell wall biosynthesis regulation (8). In C. albicans, Ssk1 is a response regulator that is not functionally related to the S. cerevisiae ortholog. Previous studies with Ssk1 have shown that it is required for pathogenesis of C. albicans in a mouse model of hematogenously disseminated candidiasis, survival in human polymorphonuclear leukocytes (PMNs) in vitro, adaptation to oxidants, and adherence to human esophageal tissue (17–20). This suggests a more expansive role for two-component proteins in C. albicans than in S. cerevisiae. The genome of C. albicans has been shown to encode three HKs, three RRs, and a single HPt protein, Ypd1 (5, 6, 21, 22). The function of the phosphohistidine intermediate protein Ypd1 in C. albicans is not well understood, in part because, thus far, there have been no reports of a YPD1 disruption mutant. We report here that Ypd1 is not essential for viability in C. albicans. Gene deletion strains lacking YPD1 filament constitutively under noninducing growth conditions, as evidenced by extensive flocculation of ypd1Δ/Δ null mutant strains compared to control strains in yeast extract-peptone-dextrose (YPD) broth at 30°C. We also observed constitutive phosphorylation of Hog1 MAP kinase in our ypd1Δ/Δ mutant, suggesting constitutive activation of the Ssk1-dependent Hog1 pathway in the mutant. Taking both the data presented here and our earlier findings indicating the presence of a two-component response regulator in the mitochondria (23), we propose that the functional circuitry of two-component signal transduction pathways in C. albicans may be more divergent than previously thought.
MATERIALS AND METHODS
C. albicans strains, plasmids, primers, and growth conditions.
The C. albicans strains used in the present study are listed in Table 1. Lists of all the plasmids and primers used in the present study are given in Tables S1 and S2 in the supplemental material. All Candida strains were maintained as frozen stocks and grown on YPD agar (1% yeast extract, 2% peptone, 2% dextrose, and 2% agar). The C. albicans strains were grown routinely in liquid YPD medium at 30°C in an incubator shaker overnight prior to use in the experiments. For drop plate assays, overnight cultures of C. albicans cells were harvested by centrifugation, washed with phosphate-buffered saline (PBS), and enumerated with a hemacytometer prior to use.
TABLE 1.
C. albicans strains used in the present study
| Strain | Genotype | Reference |
|---|---|---|
| SN152 (YPD1/YPD1) | arg4Δ/arg4Δ leu2Δ/leu2Δ his1Δ/his1Δ URA3/ura3Δ::imm434 IRO1/iro1::imm434 | 24 |
| SN425 (YPD1/YPD1) | leu2Δ::C.d.HIS1/leu2Δ::C.m.LEU2 arg4Δ/arg4Δ::C.d.ARG4 his1Δ/his1Δ ura3Δ/URA3 iro1Δ/IRO1 | 25 |
| NC6 (ypd1/YPD1) | ypd1Δ::C.m.LEU2/YPD1 ura3Δ-iro1Δ::imm434/ura3Δ-iro1Δ::imm434 his1Δ/his1Δ arg4Δ/arg4Δ leu2Δ/leu2Δ | This study |
| NC7 (ypd1Δ/Δ) | ypd1Δ::C.m.LEU2/ypd1Δ::C.d.ARG4 URA3/ura3Δ::imm434 IRO1/iro1::imm434 his1Δ/his1Δ arg4Δ/arg4Δ leu2Δ/leu2Δ | This study |
| NC8 (ypd1Δ/Δ::YPD1) | RPS10-YPD1::C.d.HIS1::ypd1Δ::C.m.LEU2/ypd1Δ::C.d.ARG4 ura3Δ-iro1Δ::imm434/ura3Δ-iro1Δ::imm434 his1Δ/his1Δ arg4Δ/arg4Δ leu2Δ/leu2Δ | This study |
| NC9 (SN148::YPD1-GFP) | RPS10-ACT1-YPD1-GFP::C.a.URA3 ura3Δ-iro1Δ::imm434/ura3Δ-iro1Δ::imm434 his1Δ/his1Δ arg4Δ/arg4Δ leu2Δ/leu2Δ | This study |
| NC10 (ypd1Δ/Δ::YPD1-GFP) | RPS10-ACT1-YPD1-GFP::C.a.URA3::ypd1Δ::C.m.LEU2/ypd1Δ::C.d.ARG4 URA3/ura3Δ::imm434 IRO1/iro1::imm434 his1Δ/his1Δ arg4Δ/A4Δ leu2Δ/leu2Δ | This study |
Construction of YPD1 deletion mutant and reconstituted strains.
The YPD1 deletion mutant strain was constructed by following the method of Noble and Johnson (24). To generate deletion strains, we first constructed a plasmid carrying a disruption cassette for each allele to be deleted. Briefly, the 5′- and 3′-untranslated regions (5′- and 3′-UTRs, respectively) of YPD1 (orf19.4443) were PCR amplified and cloned into the pGEM-T Easy vector (Promega). Each pair of fragments was then subcloned into pSN40, flanking the LEU2 expression cassette, and pSN69, flanking the ARG4 expression cassette (plasmids pSN40 and pSN69, used to generate knockout mutant strains, were a generous gift from Suzanne Noble of the University of California, San Francisco [UCSF]). The plasmids generated in this way were used to generate allelic replacements of the YPD1 gene to be deleted by homologous recombination in a two-step gene replacement procedure. Briefly, each disruption cassette was digested with appropriate restriction enzymes that cut at both ends of the cloned 5′- and 3′-flanking fragments, generating ends homologous to each specific YPD1 allele to be deleted in the C. albicans genome. These released disruption cassettes were used to transform wild-type C. albicans strain SN152 (Leu− His− Arg−) by standard methods (19) to create heterozygous and homozygous null mutants. A gene-reconstituted strain was created by PCR amplifying the full-length YPD1 open reading frame (ORF), including its promoter, and subcloning it into the pSN75 plasmid vector (24). This plasmid was linearized with XhoI and transformed into a previously generated ypd1Δ/Δ homozygous null mutant strain to reintegrate the YPD1 ORF at the RPS10 (ribosomal protein 10) locus in the C. albicans genome, creating the gene-reconstituted strain. To generate strains with matched auxotrophic requirements, the HIS1 auxotrophic marker was restored by integration of empty pSN75 (which contains the HIS1 auxotrophic marker) vector into the ypd1Δ/Δ null mutant at the RPS10 locus. The C. albicans strains (Table 1) generated in this way were auxotrophically identical (His+ Leu+ Arg+). All the experiments reported in the current study were performed by using the SN425 (YPD1/YPD1), ypd1Δ/Δ, and ypd1Δ/Δ::YPD1 strains. The C. albicans strain SN425 (His+ Leu+ Arg+) was used as the wild-type strain in all experiments and is described in detail elsewhere (25). All the strains were confirmed by PCR (see Fig. S1 in the supplemental material).
Determination of generation time.
Overnight cultures of each strain were prepared in YPD medium at 30°C. These overnight cultures were diluted to an initial optical density at 600 nm (OD600) of 0.1 in YPD broth (50 ml) and then grown at 30°C. The OD600 was measured every hour until the stationary phase of the growth curve was reached. Each C. albicans strain was vortexed vigorously prior to every OD measurement to ensure that the absorbance readings obtained were consistent and not affected by flocculating ypd1Δ/Δ cells. The generation time during the log phase (exponential growth) was determined as described previously (21). The generation times calculated for each strain are the averages for two independent experiments.
Phenotypic assays.
The sensitivities of the C. albicans SN425 (YPD/YPD1), ypd1Δ/Δ, and ypd1Δ/Δ::YPD1 strains to different stressors were assayed by spotting dilutions of 5 × 101 to 5 × 105 cells (each in a total volume of 5 μl) from an overnight culture of yeast cells grown in YPD broth at 30°C onto YPD agar plates containing sodium dodecyl sulfate (SDS), calcofluor white, Congo red, and caffeine. The growth of each strain was examined after 24 h of incubation at 30°C. The sensitivities of the wild-type strain SN425 (YPD1/YPD1), the null mutant ypd1Δ/Δ strain, and the reintegrant ypd1Δ/Δ::YPD1 strain to other stressors, such as sodium chloride (NaCl), hydrogen peroxide (H2O2), menadione, and potassium superoxide (KO2), were also evaluated.
MAPK phosphorylation assay.
Western blot analysis for the detection of phosphorylation of Hog1 MAP kinase was performed as described previously (20). Briefly, overnight cultures of the parental strain and the ypd1Δ/Δ null mutant were grown to log phase in YPD broth at 30°C. At this time, the cells were exposed to oxidative stress by supplementing the medium with 5 mM H2O2. At designated times following incubation (0 to 30 min), cells were collected, proteins extracted, and equal amounts separated by SDS-PAGE. The electrophoresed proteins were then transferred to nitrocellulose membranes and first probed with a phospho-p38 MAPK (Thr180/Tyr182) monoclonal antibody (MAb) (D3F9; Cell Signaling Technology Inc.). Subsequently, the blots were stripped and incubated with an α-tubulin polyclonal antibody (a generous gift from Katsunori Sugimoto, Rutgers University). Blots were developed as recommended by the manufacturer (GE Healthcare).
Construction of Ypd1-GFP strains.
To determine the subcellular localization of Ypd1, the coding sequence of YPD1 was fused in frame with the yeast enhanced green fluorescent protein gene (yEGFP) (26). The C. albicans YPD1-GFP fusion construct pACT1-YPD1-GFP was generated by PCR amplifying the YPD1 ORF by using high-fidelity Phusion DNA polymerase (NEB). The PCR-amplified product was cloned into the HindIII site of plasmid pACT1-GFP (27) to generate pACT1-YPD1-GFP. This construct utilizes the ACT1 promoter for expression of the YPD1-GFP gene fusion. All constructs were verified by sequencing to exclude any point mutations due to PCR amplification reactions prior to transformation into C. albicans. The recombinant plasmid containing the YPD1-GFP fusion was linearized by using StuI and transformed into C. albicans SN148 (Ura−), and the transformants were selected by uracil prototrophy.
Fluorescence microscopy.
To determine the subcellular localization of Ypd1, we performed fluorescence microscopy of green fluorescent protein (GFP)-tagged Ypd1. Briefly, a C. albicans strain expressing YPD1-GFP was grown at 30°C overnight and then diluted to a starting OD600 of 0.1 in 50 ml of YPD broth. Cells were grown at 30°C and, upon reaching log phase, were stained with 250 nM MitoTracker Red (Molecular Probes) for 45 min. To investigate the effect of stress on localization of GFP-tagged Ypd1, C. albicans cells expressing YPD1-GFP were stressed with either 1.5 M NaCl (osmotic stress) or 5 mM H2O2 (oxidative stress) for 15 min and then imaged by using a fluorescence microscope. For DAPI (4′,6-diamidino-2-phenylindole) staining, log-phase cells were fixed with 4% formaldehyde for 10 min, washed twice with PBS, and resuspended in PBS. One microliter of Prolong Gold-DAPI (Invitrogen) was added to the cells on a slide and covered with a coverslip, and the samples were imaged as described above.
RESULTS
YPD1 (orf19.4443) encodes a putative histidine phosphotransfer protein.
The C. albicans YPD1 gene has an open reading frame of 555 nucleotides which encodes a 184-amino-acid protein with an estimated molecular mass of 20.57 kDa. The histidine-containing phosphotransfer (HPt) domain (amino acids 26 to 125) of this protein has the characteristics of a prototypical histidine phosphotransfer protein, including the conserved histidine residue (putative site of phosphorylation). According to Clustalw2, a multiple-sequence alignment program (http://www.ebi.ac.uk/Tools/msa/clustalw2), the percent identity between C. albicans Ypd1 and various fungal Ypd1 orthologs was highest with the N. crassa histidine phosphotransfer protein Hpt-1, at ∼44%. The percent identities with its orthologs in S. cerevisiae, A. nidulans, Aspergillus fumigatus, C. neoformans, and Dictyostelium discoideum were approximately 32, 34, 33, 14, and 19%, respectively (data not shown). The identity of each protein was generally restricted to the HPt domain, which contains the conserved histidine residue that is putatively phosphorylated during multistep phosphorelay from the histidine kinase to the response regulator. A sequence alignment (Fig. 1) of the HPt domains of C. albicans Ypd1 and its orthologs described above revealed the conserved histidine residue as the putative site of phosphorylation. Indeed, in C. albicans, the histidine (H69) residue was shown to be the site of phosphorylation by Calera et al. (22). This was accomplished by in vivo complementation of the loss of YPD1 in a conditional S. cerevisiae strain by the wild-type YPD1 allele of C. albicans but not the YPD1H69Q point mutant, indicating that H69 is the site of phosphorylation (22). Previous in vitro work also established that Ypd1 participates in the multistep Sln-Ypd1-Ssk1 phosphorelay (28). Although there is a high degree of conservation of Ypd1 proteins among eukaryotic microorganisms harboring two-component signaling pathways, there is a marked degree of difference between gain or loss of essential function by Ypd1 in these organisms. For instance, YPD1 is essential in S. cerevisiae, N. crassa, A. nidulans, and C. neoformans, while its orthologs in Schizosaccharomyces pombe (29), D. discoideum (30), and C. albicans (as shown below) are dispensable for viability. The exact mechanism and underlying reasons for acquisition of essential function by Ypd1 in the few two-component signaling cascades studied are not well understood. The generation of a C. albicans ypd1Δ/Δ deletion mutant strain and its characterization, described herein, provide further fundamental insights into the function of this very important protein, as well as the two-component signal transduction pathway circuitry in general.
FIG 1.
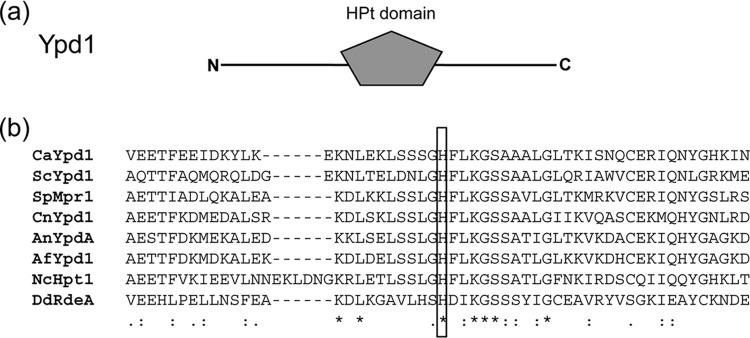
(a) Schematic representation (size not to scale) of Ypd1 (282 amino acids) depicting the conserved histidine phosphotransfer (HPt) domain. The sequence prediction was done by using SMART (http://smart.embl-heidelberg.de/). (b) Sequence alignment of the HPt domains of C. albicans Ypd1 and S. cerevisiae, S. pombe, C. neoformans, A. nidulans, A. fumigatus, N. crassa, and D. discoideum histidine phosphotransfer proteins. The conserved histidine residue within the HPt domain is boxed. The boxed histidine amino acid is the putative site of phosphorylation.
Verification of ypd1Δ/Δ mutant and reconstituted strains.
To determine the function(s) of YPD1 in C. albicans, we constructed a ypd1Δ/Δ mutant by following the method of Noble and Johnson (24), using the strain SN152 background. The first YPD1 allele was replaced by homologous recombination with the 5′-UTR-LEU2-3′-UTR cassette; likewise, the second YPD1 allele was replaced by the 5′-UTR-ARG4-3′-UTR cassette, as described in Materials and Methods. Furthermore, the complete YPD1 ORF, along with its native promoter, was restored at the RPS10 locus in the ypd1Δ/Δ mutant by using the pSN75 integrating plasmid. Homologous recombination and YPD1 allele replacement of each locus, as well as reconstitution at RPS10, were verified by PCR using a primer that anneals in the sequences external to the cloned fragments and a primer annealing within the LEU2, ARG4, and RPS10 cassettes (see Fig. S1 in the supplemental material). We also verified the absence of both alleles of YPD1 by the inability to PCR amplify an internal fragment from each deleted gene. All strains were verified by PCR. For PCR analyses (see Fig. S1 in the supplemental material), genomic DNAs from wild-type strain SN425 (YPD1/YPD1), heterozygous strain NC6 (ypd1Δ/YPD1), homozygous strain NC7 (ypd1Δ/Δ), and reconstituted strain NC8 (ypd1Δ/Δ::YPD1) were used as templates to confirm YPD1 allele replacement and reconstitution of YPD1 at the RPS10 locus.
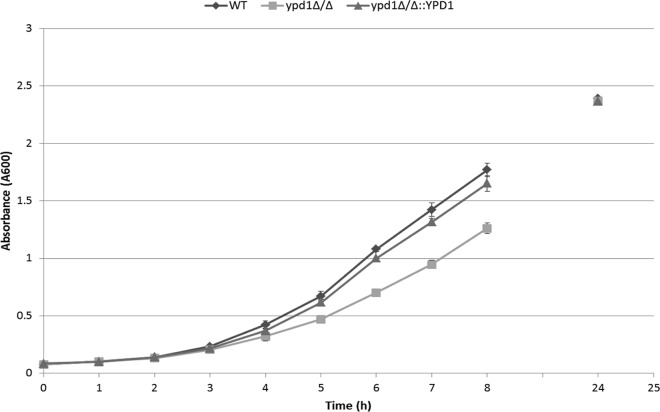
Growth rates of C. albicans strains.
To determine the effect of YPD1 disruption on growth rates, we compared the generation times of the ypd1Δ/Δ deletion strain, wild-type strain SN425 (YPD1/YPD1), and the gene-reconstituted ypd1Δ/Δ::YPD1 strain (Fig. 2). When incubated in YPD broth at 30°C, the ypd1Δ/Δ strain grew slower than the wild-type strain, whereas the growth rate of the gene-reconstituted strain was similar to that of the wild-type strain. The calculated generation time for the ypd1Δ/Δ null strain (1.93 h) was higher than the 1.76-h and 1.77-h generation times for the wild-type and gene-reconstituted strains, respectively. However, all strains reached similar cell densities after 24 h of growth in YPD broth (Fig. 2).
FIG 2.
Growth curve of C. albicans wild-type (WT), ypd1Δ/Δ, and ypd1Δ/Δ::YPD1 strains. Overnight cultures were transferred to fresh YPD medium to a starting OD of 0.1, and growth was measured every hour until stationary growth phase was reached.
Deletion of YPD1 results in increased flocculation and constitutive filamentation in C. albicans.
To determine the effect of deletion of YPD1 in C. albicans, the ypd1Δ/Δ null mutant strain and control strains were grown in YPD broth overnight at 30°C. Interestingly, we noticed that the ypd1Δ/Δ null mutant strain flocculated in YPD broth at 30°C (Fig. 3). Flocculation of the ypd1Δ/Δ null mutant strain was associated with constitutive filamentation, in contrast to the case for the wild-type and reconstituted strains (Fig. 3). The flocculation phenotype of the ypd1Δ/Δ null mutant strain was more dramatic in RPMI medium (pH 7.0) at 37°C (Fig. 4). The flocculation was observed at both 30 and 37°C, in contrast to the case for the wild-type and gene-reconstituted strains, indicating that temperature had very little role in this phenotype, except to accelerate the flocculation, perhaps due to faster growth of C. albicans at 37°C than at 30°C. Microscopic observation of flocculating cells revealed a mesh of hyphal growth of the ypd1Δ/Δ null mutant in both YPD broth and RPMI medium, in contrast to the case for the control strains (Fig. 4).
FIG 3.
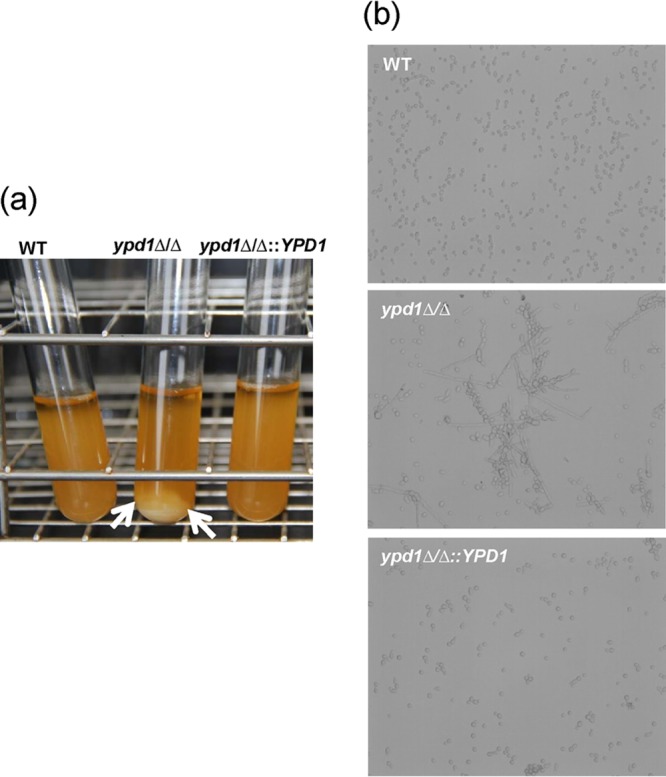
Observation of flocculation of ypd1Δ/Δ mutant cells. (a) Log-phase cultures of WT (YPD1/YPD1), ypd1Δ/Δ, and ypd1Δ/Δ::YPD1 strains of C. albicans at 30°C. Flocculation of ypd1Δ/Δ cells is indicated by the formation of clumps of cells settled at the bottom of the tube (arrows). (b) Microscopic observation indicates a mix of hyphae and yeast cells clumped together in the ypd1Δ/Δ mutant compared to the WT and gene-reconstituted strains.
FIG 4.

Growth of C. albicans WT (YPD1/YPD1), ypd1Δ/Δ, and ypd1Δ/Δ::YPD1 strains in RPMI growth medium at 37°C for 3 h. (a) Extensive flocculation is observed in ypd1Δ/Δ mutant cells. (b) Microscopic observation of ypd1Δ/Δ cells indicates a thick mesh of hyphae clumped together, while WT and gene-reconstituted cells form small germ tubes.
Disruption of YPD1 results in reduced resistance to SDS.
To determine the role of Ypd1 in stress adaptation, the wild-type strain SN425 (YPD1/YPD1), the ypd1Δ/Δ null mutant strain, and the reconstituted ypd1Δ/Δ::YPD1 strain were incubated at 30°C for 24 h on YPD agar containing hydrogen peroxide (H2O2), menadione, potassium superoxide, sodium chloride, calcofluor white, Congo red, or SDS. No major differences between the sensitivities of the wild type, the ypd1Δ/Δ null mutant strain, and the reconstituted ypd1Δ/Δ::YPD1 strain were observed with hydrogen peroxide (H2O2), potassium superoxide, menadione, calcofluor white, Congo red, and caffeine (data not shown). However, we found that the ypd1Δ/Δ null mutant strain was hypersensitive to 0.025% SDS compared to the wild type (YPD1/YPD1). Reintroduction of a wild-type copy of YPD1 in the gene-reconstituted strain (ypd1Δ/Δ::YPD1) restored the sensitivity to SDS to levels similar to wild-type levels (Fig. 5). The sensitivity of the ypd1Δ/Δ null mutant strain to SDS is indicative of cell surface defects (31). This hypothesis correlates well with the constitutive filamentation and flocculation phenotype observed with the ypd1Δ/Δ mutant. Our previous studies have shown that the Ssk1 response regulator, which is downstream of Ypd1, regulates expression of various cell wall biosynthesis genes (20). Taken together, these data emphasize the importance of the Ypd1-mediated two-component signal transduction pathway in C. albicans for mediating resistance to cell wall stress caused by SDS.
FIG 5.
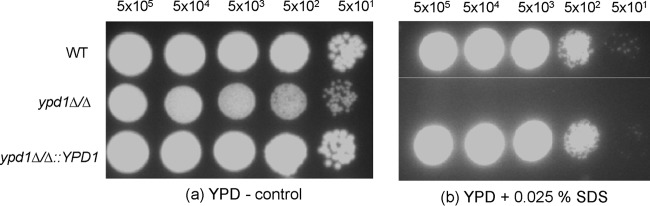
Growth of WT (YPD1/YPD1), ypd1Δ/Δ, and ypd1Δ/Δ::YPD1 strains of C. albicans at 30°C for 48 h on YPD agar (control plate) (a) and YPD agar containing 0.025% SDS (b). Five-microliter cell dilutions (5 × 105 to 5 × 101 cells) were spotted on each plate.
C. albicans Ypd1 is located in both the cytoplasm and the nucleus.
Ypd1 is critical for transfer of a phosphoryl group from the phosphorylated histidine kinase to the response regulator. The three C. albicans response regulator proteins, Ssk1, Skn7, and Srr1, are reported to be located in three distinct cellular compartments—cytosol, nucleus, and mitochondria, respectively (23, 32, 33). To determine the subcellular localization of Ypd1, we performed fluorescence microscopy of GFP-tagged Ypd1 (Fig. 6). The plasmid expressing the GFP-tagged Ypd1 fusion was tested to determine whether the GFP fusion was functional. It was found to retain function by complementation of the phenotypes of the ypd1Δ/Δ null mutant (see Fig. S2 in the supplemental material). The C. albicans strain expressing YPD1-GFP was grown to log phase and, in two separate experiments, stained with either DAPI (Invitrogen) or MitoTracker Red (Molecular Probes). We observed that GFP-tagged Ypd1 was located in both the cytosol and the nucleus (Fig. 6a). This was evident by diffuse fluorescence throughout the cell, along with bright spots of high fluorescence. These spots match perfectly with the DAPI staining, indicating a nuclear localization of GFP-tagged Ypd1 (Fig. 6a). In this regard, C. albicans Ypd1 appears to be similar to S. cerevisiae Ypd1, which is also reported to be localized to the cytosol and the nucleus (34). These data suggest that the subcellular segregation of GFP-tagged Ypd1 is probably due to an important role(s) of Ypd1 in regulation of the Ssk1 (cytosolic) and Skn7 (nuclear) response regulator proteins via phosphorylation in C. albicans. Because C. albicans contains a response regulator protein located in the mitochondria (23), we wanted to investigate whether Ypd1 also translocates to mitochondria to phosphorylate Srr1. For these experiments, log-phase C. albicans cells expressing YPD1-GFP were stained with MitoTracker Red (Molecular Probes). Mitochondria were visible by the presence of an intricate network of tube-like structures in cells stained with MitoTracker Red (Fig. 6b). However, the fluorescence signal from GFP-tagged Ypd1 did not match the MitoTracker Red signal, suggesting that Ypd1 does not localizes to mitochondria (Fig. 6b). These results also raise important questions, perhaps for future studies, about the role of Ypd1 in phosphorylation of the Srr1 response regulator. Fluorescence microscopy experiments were also performed to determine the effects of stress (oxidative and osmotic) on subcellular localization of Ypd1. No changes were observed in the localization pattern of Ypd1-GFP in the presence of stress compared to unstressed growth conditions (data not shown).
FIG 6.

Subcellular localization of Ypd1-GFP. C. albicans strains expressing GFP-tagged Ypd1 were grown to log phase and prepared for microscopy as described in Materials and Methods. The Ypd1-GFP cells were stained with DAPI and MitoTracker Red. (a) Ypd1-GFP with DAPI. Merged DAPI and GFP fluorescence images indicate nuclear and cytosolic localization of Ypd1. (b) Ypd1-GFP and MitoTracker Red. BF, bright field. All the images have similar levels of contrast.
Deletion of Ypd1 results in constitutive phosphorylation of Hog1 MAPK in C. albicans.
Activation of the Hog1 MAPK via phosphorylation is critical for adaptation to oxidative and osmotic stress in C. albicans and S. cerevisiae (5, 6, 8). Previous studies have shown that Hog1 is downstream of the Sln1-Ypd1-Ssk1 two-component pathway in C. albicans (28). We have also shown that the Ssk1 response regulator is required for phosphorylation of Hog1 under oxidative stress (20). Thus, it was of interest to determine the effect of deletion of YPD1 on phosphorylation of the Hog1 MAPK. We used a monoclonal antibody (Cell Signaling Technology) that recognizes phosphorylation of Thr180/Tyr182 within the TGY motif of the Hog1 MAPK (Fig. 7). The C. albicans wild-type and ypd1Δ/Δ strains were grown in YPD broth at 30°C, and upon reaching the logarithmic growth phase, cells were exposed to oxidative stress. At different time points (0 to 30 min), samples were taken and protein extracts were electrophoresed, transferred to a nitrocellulose membrane, and probed with a phospho-p38 MAPK antibody. A reactive band of approximately 40 kDa was observed in protein extracts from both the wild-type and ypd1Δ/Δ strains. Interestingly, phosphorylation of Hog1 was observed in the ypd1Δ/Δ null mutant strain in the absence of stress, indicating constitutive phosphorylation of Hog1 in the ypd1Δ/Δ mutant compared to the wild-type strain. However, temporal phosphorylation of Hog1 remained strong in both wild-type and ypd1Δ/Δ cells, even 30 min after treatment with H2O2. Protein extracts from the hog1Δ/Δ null mutant were included as a negative control in the same blot (Fig. 7). Subsequently, to confirm equal protein loading, the same blot was stripped and reprobed with α-tubulin antibody. Thus, the results of these experiments indicate an increased phosphorylation of Hog1 in the ypd1Δ/Δ mutant. We hypothesize that the constitutive activation of Hog1 is perhaps due to disruption of Ypd1-mediated phosphorelay, resulting in an unphosphorylated Ssk1 response regulator, which leads to activation of the downstream Hog1 MAPK pathway. We also hypothesize that the phenotypes observed with the ypd1Δ/Δ strain, such as constitutive filamentation and sensitivity to SDS, may also be due to constitutive activation of the Hog1 pathway.
FIG 7.

Detection of phosphorylation of Hog1 MAPK by Western blot analysis. C. albicans strain SN425 (YPD1/YPD1) and the ypd1Δ/Δ mutant were grown to log phase in YPD broth and treated with H2O2. Samples were taken at the indicated times (minutes; given above the lanes). Western blots were performed using either a phospho-p38 MAPK monoclonal antibody or an α-tubulin antibody. The phospho-p38 MAPK antibody detects endogenous levels of Hog1 MAP kinase only when the kinase is phosphorylated. The α-tubulin antibody was used to detect equal loading of protein samples.
DISCUSSION
Two-component signal transduction pathways are used extensively for signal transduction by bacteria, eukaryotic microorganisms, and plants (35). These signaling cascades originated in prokaryotes and are thought to have entered the eukaryotic domain of life through endosymbiotic, lateral gene transfer from their cyanobacterial ancestors (36). The genome of C. albicans has been reported to include genes for three histidine kinases (HKs), three response regulators (RRs), and one histidine phosphotransfer (HPt) protein (Ypd1). The three histidine kinases (encoded by SLN1, CHK1, and NIK1) and the response regulators (encoded by SSK1, SKN7, and SRR1) have all been characterized extensively (37–41). The biological function of the histidine phosphotransfer protein Ypd1, which is a vital constituent of the multistep phosphorelay, is poorly understood for the human-pathogenic fungus C. albicans. As noted earlier, YPD1 is essential in S. cerevisiae, N. crassa, A. nidulans, and C. neoformans. However, YPD1 orthologs in S. pombe, D. discoideum, and C. albicans are dispensable for viability. By sequence analysis, the histidine phosphotransfer protein Ypd1 appears to be highly conserved across species, but the apparent difference in essentiality could be due to partial duplication of ancestral genes. Other factors may also contribute to the nonessential function of Ypd1 in C. albicans, such as the presence of a redundant, Ypd1-like protein in C. albicans, or this organism may have evolved special mechanisms by which the phosphotransfer can bypass Ypd1 from the upstream histidine kinase to the response regulator.
Of the two-component phosphorelay proteins that regulate the downstream Hog1 MAPK pathway, Sln1 (HK) and Ssk1 (RR) have been studied in great detail in both S. cerevisiae and C. albicans (5–8). Skn7 (RR) is a transcription factor and is independent of the Hog1 MAPK pathway (33).There are functional and regulatory differences between the C. albicans and S. cerevisiae Hog1 MAPK pathways. For example, deletion of SLN1 or YPD1 in S. cerevisiae is lethal (15). This lethality is reported to be a consequence of overproduction of glycerol (an osmolyte required to maintain internal cellular turgor pressure) due to constitutive activation of the downstream Hog1 MAPK pathway (8). In C. albicans, deletion of SLN1 results in a mild to moderate sensitivity to osmotic stress, but the mutation is not lethal (41). We present data here showing that deletion of YPD1 is not a lethal event in C. albicans. Data obtained from our comprehensive analysis of the ypd1Δ/Δ mutant and from earlier studies with the Chk1 histidine kinase (37, 42, 43) and the Ssk1 and Skn7 response regulators (20, 27, 33) support the idea of substantial rewiring of two-component signaling pathways in C. albicans compared to S. cerevisiae. At present, the molecular genetic basis for this difference in regulation is not understood, but it may be explained by the presence of a robust Sho1-mediated or alternate pathway that bypasses Ssk1 and Sln1 in C. albicans.
The phosphohistidine intermediate protein Ypd1 of S. cerevisiae is reported to shuttle between the nucleus and the cytoplasm for SLN1-dependent phosphorylation of Ssk1 and Skn7 (34). We demonstrated a similar subcellular localization pattern with C. albicans Ypd1. In addition to S. cerevisiae and C. albicans, the cellular compartmentalization of two-component phosphorelay proteins is also reported for higher eukaryotes, such as Arabidopsis thaliana (44). The Arabidopsis histidine phosphotransfer proteins (AHPs) are reported to be localized to both the cytosol and the nucleus (44). The subcellular separation of GFP-tagged Ypd1 may provide important clues to the role(s) of Ypd1 in regulation of the Ssk1 (cytosolic) and Skn7 (nuclear) response regulator proteins via phosphorylation in C. albicans. However, C. albicans contains another response regulator protein, i.e., Srr1 (27). Srr1 is reported to be located in the mitochondria, and there is no evidence of mitochondrial localization of C. albicans Ypd1. These observations raise two intriguing possibilities: either interaction of Ypd1 with Srr1 is transient, or Srr1 follows a prokaryotic, single-step His → Asp phosphotransfer mechanism instead of the multistep phosphorelay reactions more prevalent in eukaryotes.
The phenotypes (flocculation, constitutive filamentation, and hypersensitivity to SDS) observed with the ypd1Δ/Δ mutant may be due to constitutive phosphorylation of the Hog1 MAPK. Cellular responses of C. albicans and the model yeast S. cerevisiae to a wide variety of environmental signals, such as oxidative stress, cell wall defects, morphogenesis, etc., are reported to be processed through the Hog1 MAPK pathway (5, 8). The requirement of an upstream two-component signaling pathway for activation of the Hog1 pathway in the oxidative stress response was demonstrated in an earlier study, in which it was shown that the Ssk1 response regulator protein is indispensable for phosphorylation of the Hog1 MAP kinase (8).
In summary, we show here that Ypd1, a histidine phosphotransfer protein, is not essential for viability in C. albicans. Ypd1 is localized to both the nucleus and the cytoplasm. Based upon data presented in this paper, we hypothesize that C. albicans utilizes a Ypd1-mediated two-component signal transduction pathway to adapt cells to various environmental conditions important for maintaining cellular homeostasis. Our results also provide new fundamental insights and lay the foundation for future work to explore in detail the mechanisms of two-component signaling pathways in C. albicans.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported in part by PHRI startup funds to N.C.
We are thankful to Suzanne Noble, University of California, San Francisco, for the generous gift of C. albicans strains and plasmids for this work. We thank Al Brown, University of Aberdeen, United Kingdom, for the pACT-GFP plasmid and Katsunori Sugimoto for the α-tubulin antibody.
Footnotes
Published ahead of print 31 January 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/EC.00243-13.
REFERENCES
- 1.Mascher T, Helmann JD, Unden G. 2006. Stimulus perception in bacterial signal-transducing histidine kinases. Microbiol. Mol. Biol. Rev. 70:910–938. 10.1128/MMBR.00020-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fassler JS, West AH. 2013. Histidine phosphotransfer proteins in fungal two-component signal transduction pathways. Eukaryot. Cell 12:1052–1060. 10.1128/EC.00083-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Janiak-Spens F, Cook PF, West AH. 2005. Kinetic analysis of YPD1-dependent phosphotransfer reactions in the yeast osmoregulatory phosphorelay system. Biochemistry 44:377–386. 10.1021/bi048433s [DOI] [PubMed] [Google Scholar]
- 4.Posas F, Saito H. 1998. Activation of the yeast SSK2 MAP kinase kinase kinase by the SSK1 two-component response regulator. EMBO J. 17:1385–1394. 10.1093/emboj/17.5.1385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chauhan N, Latge JP, Calderone R. 2006. Signalling and oxidant adaptation in Candida albicans and Aspergillus fumigatus. Nat. Rev. Microbiol. 4:435–444. 10.1038/nrmicro1426 [DOI] [PubMed] [Google Scholar]
- 6.Chauhan N, Calderone R. 2008. Two-component signal transduction proteins as potential drug targets in medically important fungi. Infect. Immun. 76:4795–4803. 10.1128/IAI.00834-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Posas F, Wurgler-Murphy SM, Maeda T, Witten EA, Thai TC, Saito H. 1996. Yeast HOG1 MAP kinase cascade is regulated by a multistep phosphorelay mechanism in the SLN1-YPD1-SSK1 “two-component” osmosensor. Cell 86:865–875. 10.1016/S0092-8674(00)80162-2 [DOI] [PubMed] [Google Scholar]
- 8.Hohmann S. 2002. Osmotic stress signaling and osmoadaptation in yeasts. Microbiol. Mol. Biol. Rev. 66:300–372. 10.1128/MMBR.66.2.300-372.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.San Jose C, Monge RA, Perez-Diaz R, Pla J, Nombela C. 1996. The mitogen-activated protein kinase homolog HOG1 gene controls glycerol accumulation in the pathogenic fungus, Candida albicans. J. Bacteriol. 178:5850–5852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alonso-Monge R, Navarro-García F, Roman E, Negredo AI, Eisman B, Nombela C, Pla J. 2003. The Hog1 MAP kinase is essential in the oxidative stress response and chlamydospore formation in Candida albicans. Eukaryot. Cell 2:351–361. 10.1128/EC.2.2.351-361.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alonso-Monge R, Navarro-García F, Molero G, Diez-Orejas R, Gustin M, Pla J, Sánchez M, Nombela C. 1999. Role of mitogen-activated protein kinase Hog1p in morphogenesis and virulence of Candida albicans. J. Bacteriol. 181:3058–3068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Banno S, Noguchi R, Yamashita K, Fukumori F, Kimura M, Yamaguchi I, Fujimura M. 2007. Roles of putative His-to-Asp signaling modules HPT-1 and RRG-2, on viability and sensitivity to osmotic and oxidative stresses in Neurospora crassa. Curr. Genet. 51:197–208. 10.1007/s00294-006-0116-8 [DOI] [PubMed] [Google Scholar]
- 13.Vargas-Pérez I, Sánchez O, Kawasaki L, Georgellis D, Aguirre J. 2007. Response regulators SrrA and SskA are central components of a phosphorelay system involved in stress signal transduction and asexual sporulation in Aspergillus nidulans. Eukaryot. Cell 6:1570–1583. 10.1128/EC.00085-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee JW, Ko YJ, Kim SY, Bahn YS. 2011. Multiple roles of Ypd1 phosphotransfer protein in viability, stress response, and virulence factor regulation in Cryptococcus neoformans. Eukaryot. Cell 10:998–1002. 10.1128/EC.05124-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giaever G, Chu AM, Ni L, Connelly C, Riles L, Véronneau S, Dow S, Lucau-Danila A, Anderson K, André B, Arkin AP, Astromoff A, El-Bakkoury M, Bangham R, Benito R, Brachat S, Campanaro S, Curtiss M, Davis K, Deutschbauer A, Entian KD, Flaherty P, Foury F, Garfinkel DJ, Gerstein M, Gotte D, Güldener U, Hegemann JH, Hempel S, Herman Z, Jaramillo DF, Kelly DE, Kelly SL, Kötter P, LaBonte D, Lamb DC, Lan N, Liang H, Liao H, Liu L, Luo C, Lussier M, Mao R, Menard P, Ooi SL, Revuelta JL, Roberts CJ, Rose M, Ross-Macdonald P, Scherens B, Schimmack G, Shafer B, Shoemaker DD, Sookhai-Mahadeo S, Storms RK, Strathern JN, Valle G, Voet M, Volckaert G, Wang CY, Ward TR, Wilhelmy J, Winzeler EA, Yang Y, Yen G, Youngman E, Yu K, Bussey H, Boeke JD, Snyder M, Philippsen P, Davis RW, Johnston M. 2002. Functional profiling of the Saccharomyces cerevisiae genome. Nature 418:387–391. 10.1038/nature00935 [DOI] [PubMed] [Google Scholar]
- 16.Porter SW, West AH. 2005. A common docking site for response regulators on the yeast phosphorelay protein YPD1. Biochim. Biophys. Acta 1748:138–145. 10.1016/j.bbapap.2004.12.009 [DOI] [PubMed] [Google Scholar]
- 17.Calera JA, Zhao XJ, Calderone R. 2000. Defective hyphal development and avirulence caused by a deletion of the SSK1 response regulator gene in Candida albicans. Infect. Immun. 68:518–525. 10.1128/IAI.68.2.518-525.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Du C, Calderone R, Richert J, Li D. 2005. Deletion of the SSK1 response regulator gene in Candida albicans contributes to enhanced killing by human polymorphonuclear neutrophils. Infect. Immun. 73:865–871. 10.1128/IAI.73.2.865-871.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li D, Bernhardt J, Calderone R. 2002. Temporal expression of the Candida albicans genes CHK1 and CSSK1, adherence, and morphogenesis in a model of reconstituted human esophageal epithelial candidiasis. Infect. Immun. 70:1558–1565. 10.1128/IAI.70.3.1558-1565.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chauhan N, Inglis D, Roman E, Pla J, Li D, Calera JA, Calderone R. 2003. Candida albicans response regulator gene SSK1 regulates a subset of genes whose functions are associated with cell wall biosynthesis and adaptation to oxidative stress. Eukaryot. Cell 2:1018–1024. 10.1128/EC.2.5.1018-1024.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Desai C, Mavrianos J, Chauhan N. 2011. Candida albicans SRR1, a putative two-component response regulator gene, is required for stress adaptation, morphogenesis, and virulence. Eukaryot. Cell 10:1370–1374. 10.1128/EC.05188-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Calera JA, Herman D, Calderone R. 2000. Identification of YPD1, a gene of Candida albicans which encodes a two-component phosphohistidine intermediate protein. Yeast 16:1053–1059. [DOI] [PubMed] [Google Scholar]
- 23.Mavrianos J, Berkow EL, Desai C, Pandey A, Batish M, Rabadi MJ, Barker KS, Pain D, Rogers PD, Eugenin EA, Chauhan N. 2013. Mitochondrial two-component signaling systems in Candida albicans. Eukaryot. Cell 12:913–922. 10.1128/EC.00048-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Noble SM, Johnson AD. 2005. Strains and strategies for large-scale gene deletion studies of the diploid human fungal pathogen Candida albicans. Eukaryot. Cell 4:298–309. 10.1128/EC.4.2.298-309.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Noble SM, French S, Kohn LA, Chen V, Johnson AD. 2010. Systematic screens of a Candida albicans homozygous deletion library decouple morphogenetic switching and pathogenicity. Nat. Genet. 42:590–598. 10.1038/ng.605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cormack BP, Bertram G, Egerton M, Gow NA, Falkow S, Brown AJ. 1997. Yeast-enhanced green fluorescent protein (yEGFP): a reporter of gene expression in Candida albicans. Microbiology 143:303–311. 10.1099/00221287-143-2-303 [DOI] [PubMed] [Google Scholar]
- 27.Barelle CJ, Manson CL, MacCallum DM, Odds FC, Gow NA, Brown AJ. 2004. GFP as a quantitative reporter of gene regulation in Candida albicans. Yeast 21:333–340. 10.1002/yea.1099 [DOI] [PubMed] [Google Scholar]
- 28.Menon V, Li D, Chauhan N, Rajnarayanan R, Dubrovska A, West AH, Calderone R. 2006. Functional studies of the Ssk1p response regulator protein of Candida albicans as determined by phenotypic analysis of receiver domain point mutants. Mol. Microbiol. 62:997–1013. 10.1111/j.1365-2958.2006.05438.x [DOI] [PubMed] [Google Scholar]
- 29.Aoyama K, Mitsubayashi Y, Aiba H, Mizuno T. 2000. Spy1, a histidine-containing phosphotransfer signaling protein, regulates the fission yeast cell cycle through the Mcs4 response regulator. J. Bacteriol. 182:4868–4874. 10.1128/JB.182.17.4868-4874.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang WT, Thomason PA, Gross JD, Neweil PC. 1998. Evidence that the RdeA protein is a component of a multistep phosphorelay modulating rate of development in Dictyostelium. EMBO J. 17:2809–2816. 10.1093/emboj/17.10.2809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pagé N, Gérard-Vincent M, Ménard P, Beaulieu M, Azuma M, Dijkgraaf GJ, Li H, Marcoux J, Nguyen T, Dowse T, Sdicu AM, Bussey H. 2003. A Saccharomyces cerevisiae genome-wide mutant screen for altered sensitivity to K1 killer toxin. Genetics 163:875–894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Calera JA, Calderone RA. 1999. Identification of a putative response regulator two-component phosphorelay gene (CaSSK1) from Candida albicans. Yeast 15:1243–1254. [DOI] [PubMed] [Google Scholar]
- 33.Singh P, Chauhan N, Ghosh A, Dixon F, Calderone R. 2004. SKN7 of Candida albicans: mutant construction and phenotype analysis. Infect. Immun. 72:2390–2394. 10.1128/IAI.72.4.2390-2394.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu JM, Deschenes RJ, Fassler JS. 2003. Saccharomyces cerevisiae histidine phosphotransferase Ypd1p shuttles between the nucleus and cytoplasm for SLN1-dependent phosphorylation of Ssk1p and Skn7p. Eukaryot. Cell 2:1304–1314. 10.1128/EC.2.6.1304-1314.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stock AM, Robinson VL, Goudreau PN. 2000. Two-component signal transduction. Annu. Rev. Biochem. 69:183–215. 10.1146/annurev.biochem.69.1.183 [DOI] [PubMed] [Google Scholar]
- 36.Koretke KK, Lupas AN, Warren PV, Rosenberg M, Brown JR. 2000. Evolution of two-component signal transduction. Mol. Biol. Evol. 17:1956–1970. 10.1093/oxfordjournals.molbev.a026297 [DOI] [PubMed] [Google Scholar]
- 37.Calera JA, Zhao XJ, De Bernardis F, Sheridan M, Calderone R. 1999. Avirulence of Candida albicans CaHK1 mutants in a murine model of hematogenously disseminated candidiasis. Infect. Immun. 67:4280–4284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kruppa M, Krom BP, Chauhan N, Bambach AV, Cihlar RL, Calderone RA. 2004. The two-component signal transduction protein Chk1p regulates quorum sensing in Candida albicans. Eukaryot. Cell 3:1062–1065. 10.1128/EC.3.4.1062-1065.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Torosantucci A, Chiani P, De Bernardis F, Cassone A, Calera JA, Calderone R. 2002. Deletion of the two-component histidine kinase gene (CHK1) of Candida albicans contributes to enhanced growth inhibition and killing by human neutrophils in vitro. Infect. Immun. 70:985–987. 10.1128/IAI.70.2.985-987.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamada-Okabe T, Mio T, Ono N, Kashima Y, Matsui M, Arisawa M, Yamada-Okabe H. 1999. Roles of three histidine kinase genes in hyphal development and virulence of the pathogenic fungus Candida albicans. J. Bacteriol. 181:7243–7247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nagahashi S, Mio T, Ono N, Yamada-Okabe T, Arisawa M, Bussey H, Yamada-Okabe H. 1998. Isolation of CaSLN1 and CaNIK1, the genes for osmosensing histidine kinase homologues, from the pathogenic fungus Candida albicans. Microbiology 144:425–432. 10.1099/00221287-144-2-425 [DOI] [PubMed] [Google Scholar]
- 42.Li D, Gurkovska V, Sheridan M, Calderone R, Chauhan N. 2004. Studies on the regulation of the two-component histidine kinase gene CHK1 in Candida albicans using the heterologous lacZ reporter gene. Microbiology 150:3305–3313. 10.1099/mic.0.27237-0 [DOI] [PubMed] [Google Scholar]
- 43.Li D, Williams D, Lowman D, Monteiro MA, Tan X, Kruppa M, Fonzi W, Roman E, Pla J, Calderone R. 2009. The Candida albicans histidine kinase Chk1p: signaling and cell wall mannan. Fungal Genet. Biol. 46:731–741. 10.1016/j.fgb.2009.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Punwani JA, Hutchison CE, Schaller GE, Kieber JJ. 2010. The subcellular distribution of the Arabidopsis histidine phosphotransfer proteins is independent of cytokinin signaling. Plant J. 62:473–482. 10.1111/j.1365-313X.2010.04165.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.