Abstract
Background
We previously showed that parenteral nutrition (PN) compared with formula feeding results in hepatic insulin resistance and steatosis in neonatal pigs. The current aim was to test whether the route of feeding (intravenous [IV] vs enteral) rather than other feeding modalities (diet, pattern) had contributed to the outcome.
Methods
Neonatal pigs were fed enterally or parenterally for 14 days with 1 of 4 feeding modalities as follows: (1) enteral polymeric formula intermittently (FORM), (2) enteral elemental diet (ED) intermittently (IEN), (3) enteral ED continuously (CEN), and (4) parenteral ED continuously (PN). Subgroups of pigs underwent IV glucose tolerance tests (IVGTT) and hyperinsulinemic-euglycemic clamps (CLAMP). Following CLAMP, pigs were euthanized and tissues collected for further analysis.
Results
Insulin secretion during IVGTT was significantly higher and glucose infusion rates during CLAMP were lower in CEN and PN than in FORM and IEN. Endogenous glucose production rate was suppressed to zero in all groups during CLAMP. In the fed state, plasma glucose-dependent insulinotropic polypeptide (GIP), glucagon-like peptide (GLP)–1, and GLP-2 were different between feeding modalities. Insulin receptor phosphorylation in liver and muscle was decreased in IEN, CEN, and PN compared with FORM. Liver weight was highest in PN. Steatosis and myeloperoxidase (MPO) activity tended to be highest in PN and CEN. Enterally fed groups had higher plasma GLP-2 and jejunum weight compared with PN.
Conclusions
PN and enteral nutrition (EN) when given continuously as an elemental diet reduces insulin sensitivity and the secretion of key gut incretins. The intermittent vs continuous pattern of EN produced the optimal effect on metabolic function.
Keywords: premature infant, PN, elemental diet, fatty liver, glucose metabolism, gut hormones, IRS-1
Clinical Relevancy Statement
Neonates who are challenged by premature birth, very low birth weight, or other medical conditions often are dependent on nutrition support because they cannot be breastfed by their own mothers. The choice of feeding modality—optimized for each patient’s condition—ranging from continuous parenteral nutrition to intermittent and/or continuous enteral feeding, including polymeric formulas vs elemental diets, is critical to avoid nutrition deficiencies and, importantly, to achieve normal maturation and growth. In an animal model for the human term and preterm neonate, we found that continuous feeding of an elemental diet—parenterally as well as enterally—decreases secretion of incretin hormones, induces insulin resistance, and leads to hepatic steatosis. Research over the past 2 decades has linked the neonatal environment to later-in-life onset of insulin resistance, a key factor in the etiology of type 2 diabetes. Our results suggest that the mode of (life-saving) nutrition support for the neonate may predispose these individuals to later development of type 2 diabetes. The long-term goal of this research is to optimize perinatal nutrition support and to investigate how subsequent nutrition or lifestyle modification can prevent later-in-life chronic disease.
Introduction
Optimal nutrition support is vital to the clinical care and outcome of premature infants of very low birth weight (VLBW). Due to the immaturity of their gastrointestinal tract, congenital abnormalities, or other medical conditions, many of these infants experience feeding intolerance and are at risk to develop necrotizing enterocolitis. Intestinal failure, including short bowel syndrome (SBS), may be the consequence. Moreover, as survival rates of even the smallest infants are increasing, so is the number of patients susceptible to gastrointestinal complications. Thus, many of these infants receive life-saving parenteral nutrition (PN; prior to reaching full enteral feeding), lasting from a few days to weeks or months. To prevent postnatal growth failure and optimize growth and neurodevelopment,1 more aggressive nutrition regimens (such as increased rates of PN) have been implemented.2,3 Unfortunately, complications of aggressive PN are hyperglycemia, liver dysfunction, and sepsis, contributing to increased morbidity and mortality in PN-fed VLBW infants.4–7
The etiology of neonatal hyperglycemia is poorly understood but is thought to develop from a combination of persistent hepatic glucose production despite parenteral glucose infusion, insulin resistance, impaired pancreatic β-cell secretion, immaturity of the glucose transport system, and a limited number of insulin-dependent tissues.8 The most common cause of hyperglycemia is excessive parenteral glucose and lipid infusion. However, in preterm infants, stress-reactive hormones also can uncouple the action of insulin and glucagon and promote endogenous hepatic glucose production and decrease peripheral glucose utilization,9,10 leading to hyperglycemia.3 Two studies in premature infants specifically attributed hyperglycemia to continued hepatic gluconeogenesis during routine PN.11,12 It is uncertain whether hyperglycemia per se is a cause of adverse clinical outcomes or whether outcomes can be improved by preventing hyperglycemia. It was concluded that more large randomized trials are needed to determine whether lowering glucose infusion rates or insulin therapy to prevent hyperglycemia could improve clinical outcomes.13
The absence of nutrients in the gastrointestinal tract during PN produces mucosal and villus atrophy. Enzymes necessary for digestion and substrate absorption are reduced, and trophic hormones normally produced by the gut in response to enteral feeding are diminished. Hence, a critical goal for nutrition support of parenterally fed premature infants, as well as those receiving PN due to intestinal failure, is early introduction of “minimal enteral feeding.” The “trophic effect” of enteral nutrients coupled with the release of intestinal hormones accelerates the maturation of gastrointestinal function and tolerance to full enteral feeding.14 The release of gut incretin hormones— namely, glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide 1 (GLP-1)—are also critical for the maintenance of normal glucose homeostasis and insulin sensitivity.15 Accelerated tolerance to enteral feeding reduces the duration of PN and risk of adverse clinical outcomes, including hepatic cholestasis and sepsis.3,6,16 Another consideration when administering enteral nutrition (EN) to VLBW infants is whether to give feedings as continuous intragastric infusion or intermittent bolus.17,18 There is limited clinical evidence showing which method is optimal, but in some premature infants, continuous feeding seems to be superior to intermittent feeding.18,19 In contrast, bolus feeding had greater stimulatory effects on intestinal and muscle growth in neonatal pigs.20,21 The chemical form of the diet is another factor that influences effectiveness of EN in preterm infants. Human milk has been shown to decrease the incidence of necrotizing entercolitis22 and thus is preferred to polymeric preterm formula. But when human milk is unavailable and polymeric preterm formula is not tolerated due to food allergy23 or malabsorption of nutrients due to immature digestive function24 or SBS, elemental diets (EDs) are used for enteral feedings. EDs are hypoallergenic, readily assimilated, and almost completely absorbed in the upper gastrointestinal tract without the requirement of full digestive capacity.25
We recently examined the metabolic effect of PN-fed neonatal pigs and showed that continuous PN compared with intermittent, enteral polymeric formula feeding (FORM) resulted in insulin resistance, glucose intolerance, and hepatic steatosis.26 We used the neonatal pig given its relevance as an animal model of the human neonate based on comparative aspects of metabolism, body composition, organ function, and stage of development.27–30 With respect to glucose metabolism, the fasted newborn pig28 and the VLBW infant31,32 are both susceptible to glucose extremes, and the relationship between body weight and glucose turnover in the 2 species is strikingly similar. Importantly, the larger body size of the neonatal pig compared with neonatal rodents allows investigation of the effect of PN at birth. On the basis of our recent results in PN-fed piglets, we hypothesized that the route of nutrition, intravenous (IV) vs enteral, was the major determinant contributing to the adverse metabolic outcome rather than the feeding pattern, continuous vs intermittent, or the nature of the diet, elemental vs polymeric. Thus, our current aim was to test whether administration of the PN solution (ED) enterally either continuously (continuous enteral nutrition, CEN) or intermittently (intermittent enteral nutrition, IEN) would reproduce our previous findings with enteral polymeric formula fed intermittently (FORM). Our main end points were insulin sensitivity and endogenous hepatic glucose production during fasting and insulin stimulation. Furthermore, we measured the effect of feeding modalities on the release of incretin hormones, GIP and GLP-1, and intestinal growth.
Research Design and Methods
Animals and Study Design
The study protocol was approved by the Animal Care and Use Committee of Baylor College of Medicine and was conducted in accordance with the Guide for the Care and Use of Laboratory Animals (DHHS publication no. [NIH] 85-23, revised 1985, Office of Science and Health Reports, DRR/NIH, Bethesda, MD). Newborn (2-day-old), crossbred (female) pigs (n = 60), obtained from the Texas Department of Criminal Justice (Huntsville, TX), were transported to the animal facility at the Children’s Nutrition Research Center (Houston, TX). Upon arrival (day 0), piglets were weighed and placed in cages in a heated room (~30°C). Pigs were randomly assigned to 1 of the 4 feeding modalities shown in Table 1 as follows: (1) enteral polymeric formula (protein, lactose, fat) every 4 hours (FORM), (2) enteral ED (amino acid, glucose, lipid) every 4 hours (IEN), (3) enteral ED continuously (CEN), and (4) parenteral ED continuously (PN). Subgroups of pigs (n = 4–8/group) underwent IV glucose tolerance tests (IVGTT) on days 7 and 14 and hyperinsulinemic-euglycemic clamps (CLAMP) on day 14 as described below. Fed-state blood samples collected between days 9–12 were used for analysis of intestinal peptide hormones (GLP-1, GLP-2, GIP). Following CLAMP, pigs were euthanized, and tissues were quickly excised, weighed, and stored for further analysis.
Table 1.
Scheme of Feeding Modalities
| FORM | IEN | CEN | PN | |
|---|---|---|---|---|
| Route | Enteral | Enteral | Enteral | Parenteral |
| Diet | Polymeric | Elemental | Elemental | Elemental |
| Pattern | Intermittent | Intermittent | Continuous | Continuous |
CEN, continuous enteral nutrition; FORM, intermittent formula feeding; IEN, intermittent enteral nutrition; PN, parenteral nutrition.
Upon arrival, piglets were implanted with silastic catheters into the jugular vein, carotid artery, and stomach as previously described.33,34 After surgery, all piglets received PN at 50% of their requirement for 24 hours to provide nutrients and fluid during recovery. Subsequently, piglets were started on their assigned feeding modality, FORM, IEN, CEN, or PN, which was maintained until the end of the study period. Their respective dietary intakes were increased to 100% within the next 48 hours. FORM piglets were fed a polymeric cow’s milk–based formula for baby pigs (Litter Life; Merrick, Middleton, WI) at 50 g·kg−1·d−1, suspended in 240 mL water, providing, in g·kg−1·d−1, 25 lactose, 12.5 protein, 5 fat, and electrolytes, (trace) minerals, and vitamins. Pigs receiving ED (ie, IEN, CEN, PN) were fed 240 mL·kg−1·d−1 providing, in g·kg−1·d−1, 25 glucose, 13 L-amino acids, 5 lipids (Intralipid 20%; Fresenius Kabi, Bad Homburg, Germany) and electrolytes, (trace) minerals, and vitamins. Both diets, polymeric formula and ED (Table 2), were isocaloric and isonitrogenous and provided electrolytes, minerals, and vitamins according to piglets’ requirement.35 All piglets had the same daily macronutrient and fluid intake, but FORM piglets received polymeric formula, including whole protein, lactose, and fat from animal and vegetable sources, whereas ED consisted of individual amino acids, glucose, and soybean oil–based lipid emulsion (Table 2). FORM and IEN piglets received their nutrition via intragastric catheter in 4-hour intervals, whereas CEN and PN piglets were infused continuously via an intragastric and jugular catheter, respectively. Piglets were weighed daily and their intakes adjusted accordingly.
Table 2.
Daily Macronutrient Intake and Composition of Polymeric Enteral Formula (FORM) and Elemental Diet (ED)
| Intake | Unit/kg Body Weight·d |
FORMa | EDb,c,d |
|---|---|---|---|
| Fluid | mL | 240 | 240 |
| Energy | kJ | 816 | 824 |
| Protein | g | 12.5 | 13 |
| Carbohydrate | g | 25 | 25 |
| Fat | g | 5 | 5 |
| Composition, g/L | FORM | ED | |
| Alanine | 2.60 | 2.71 | |
| Arginine | 1.43 | 2.35 | |
| Aspartic acid | 4.65 | 4.18 | |
| Cysteinee | — | 0.83 | |
| Cystine | 1.31 | — | |
| Glutamic acid | 7.65 | 5.22 | |
| Glutaminee | — | 4.18 | |
| Glycine | 1.28 | 2.04 | |
| Histidine | 0.90 | 1.36 | |
| Isoleucine | 2.97 | 3.03 | |
| Leucine | 5.55 | 5.38 | |
| Lysine | 4.87 | 4.07 | |
| Methionine | 1.13 | 1.36 | |
| Phenylalanine | 1.67 | 2.77 | |
| Proline | 3.45 | 3.92 | |
| Serine | 2.59 | 2.93 | |
| Threonine | 3.31 | 3.29 | |
| Tryptophane | — | 0.63 | |
| Tyrosine | 1.36 | 0.63 | |
| Valine | 2.97 | 3.29 | |
| Palmitic acid (C16:0) | — | 2.19 | |
| Stearic acid (C18:0) | 2.13 | 0.72 | |
| Oleic acid (C18:1, n-9) | 6.40 | 5.10 | |
| Linoleic acid (C18:2, n-6) | 3.61 | 11.04 | |
| Linolenic acid (C18:3, n-3) | 0.31 | 1.56 | |
| Lactose | 106 | — | |
| Dextrose | — | 104 | |
Dashes used to show that respective item was not detected or not contained in diet. NA, not available from manufacturer.
Soweena LitterLife, Merrick (Middleton, WI). Ingredient list includes dried whey protein concentrate, dried whey product, dried whey, animal plasma, animal and vegetable fat preserved with butylated hydroxyanisole (BHA), lecithin, dicalcium phosphate, vitamin A acetate, d-activated animal sterol (source of vitamin D3), vitamin E supplement, menadione dimethylpyrimidinol bisulfite (source of vitamin K activity), choline chloride, riboflavin supplement, calcium pantothenate, niacin supplement, vitamin B12 supplement, biotin, ascorbic acid, magnesium sulfate, manganese sulfate, ferrous sulfate, zinc sulfate, cobalt sulfate, copper sulfate, calcium iodate, sodium selenite, dried lactose, and natural and artificial flavors.
ED solution includes lipid as Intralipid (20%) containing 20% soybean oil, 1.2% egg yolk phospholipids, and 2.25% glycerin; the major component fatty acids and their range of content as percentage of total lipid are linoleic (44%–62%), oleic (19%–30%), palmitic (7%–14%), linolenic (4%–11%), and stearic (1.4%–5.5%). Values in table represent mean values of these given ranges.
ED electrolyte composition includes (g/L): sodium, 0.67; chloride, 1.28; potassium, 1.02; phosphorus, 0.47; magnesium, 0.10; and calcium, 0.54.
ED vitamins and trace minerals includes (mg/L): vitamin A, 1.375; vitamin D, 0.0104; vitamin E, 0.0123; vitamin K, 0.21; thiamin vitamin B1, 20.83; riboflavin vitamin B2,1.04; niacin vitamin B3, 20.83; pantothenic acid vitamin B5, 4.17; pyridoxine vitaminB6, 2.08; biotin vitamin B7/H, 0.042; folic acid vitamin B9, 0.42; cyanocobalamin vitamin B12, 0.021; ascorbic acid vitamin C, 208.33; iron, 26.25; zinc, 26.25; copper, 1.67; manganese, 1.04; selenium, 0.083; chromium, 0.042; and iodine, 0.042.
Cysteine, glutamine, and tryptophan in FORM not detected due to loss during protein hydrolysis for analysis. Cysteine was measured as cystine and glutamine was measured as glutamic acid.
IV glucose tolerance test and plasma analysis
On days 7 and 14, after an 8-hour fast, subgroups of pigs were submitted to an IVGTT (1 g glucose · kg body weight [BW]−1) with arterial blood sampled over 60 minutes for analysis of plasma glucose and insulin as in Stoll et al.26
Hyperinsulinemic-euglycemic clamp
On day 14, after being feed-deprived for 8–10 h, 4-hour CLAMP were performed, targeting circulating insulin concentrations at the physiological peak level of the fed state (417 pmol·L−1; ie, 60 µIU·mL−1). CLAMP were performed in subgroups of FORM, IEN, CEN, and PN pigs as described in Stoll et al26 and Wray-Cahen et al.36 Briefly, 30 minutes before the clamp procedure was initiated, 5 blood samples were obtained to establish the average basal (fasting) glucose concentration. Blood glucose during the clamp protocol was determined rapidly using a glucose analyzer (YSI 2300 STAT Plus Glucose Analyzer; YSI Incorporated, Yellow Springs, OH). After a 10-minute priming infusion, porcine insulin (Sigma-Aldrich, St Louis, MO) was infused for 4 hours at 31 pmol·kg−0.66·min−1 (517 nmol·L−1, 0.9% saline, 0.1% human serum albumin) to achieve fed-state plasma insulin levels. During the infusion, arterial blood samples were obtained in 5-minute intervals and immediately analyzed for glucose. Subsequently, glucose infusion rates (20% solution) were adjusted to maintain blood glucose concentrations within ±10% of the average basal glucose concentration. Additional blood samples were acquired for later analysis of insulin and glucagon. Glucagon was analyzed according to Holst37 using antiserum 4305, which is specific for the C-terminal part of the peptide. After completing the CLAMP, pigs were euthanized, and tissue samples were quickly excised and either frozen in liquid nitrogen or fixed for histology.
Endogenous glucose production during fasting and hyperinsulinemic-euglycemic clamp
On day 14, 2 hours before submitting pigs to CLAMP, primed (300 µmol·kg−1) IV infusions of D-[13C6]glucose (300 µmol·kg−1·h−1; ISOTEC, Sigma-Aldrich, Miamisburg, OH) were initiated to determine endogenous glucose production (EGP) rates during fasting (0–2 hours) and 4-h CLAMP (2–6 hours). The rate of appearance (Ra) of total (endogenous and exogenous) glucose,
was determined from the13C6 -enrichment (tracer-to-tracee ratio) of glucose during steady state, which was achieved during the last 30–60 minutes of each period using established isotope dilution equations
where Ei is the13C6 -enrichment of the infusate; Ep is the13C6 -enrichment in plasma, and I is the infusion rate of D-[13C6] glucose (µmol·kg−1·h−1). Under steady-state conditions, glucose rate of disappearance (Rd) from plasma is assumed to be equal to total glucose Ra. Thus,
where exogenous glucose infusion during fasting included D-[13C6]glucose only and D-[13C6]glucose + unlabeled glucose to maintain euglycemia during CLAMP.
Analysis of intestinal hormones
Between days 9 and 12, 2-mL blood samples were collected 30–60 min after feeding (FORM, IEN) or during continuous feeding (CEN, PN), and EDTA-plasma was stored at −80°C until analyzed for GLP-1, GLP-2, and GIP as described in Jain et al38 and Lindgren et al.39
Tissue insulin signaling
Immunoblotting was performed on tissue extracts of frozen liver and muscle tissue to detect insulin receptor (IR), insulin receptor substrate 1 (IRS-1), and phosphatidylinositol 3 kinase (PI3K), as well as their phosphorylated forms as described in Stoll et al.26 All Western blots were run with 6 pigs from each treatment group and used for statistical analysis. Treatment means and standard errors are shown as bar graphs. The abundances of PI3K are expressed relative to that of tubulin measured after stripping and reprobing membranes. To quantify the phosphorylated forms of specific proteins, we expressed the calculated densitometric band intensities of the phosphorylated form relative to that of the total protein on the same immunoblot; the latter was determined after stripping and reprobing the membranes. These values are expressed as arbitrary units for each treatment group in the figures.
Liver Analyses and Histology
Fresh liver was fixed in O.C.T. Compound (Sakura Finetek USA, Inc, Torrance, CA) and frozen in liquid nitrogen, and then frozen sections were stained with Oil Red O. Liver triglyceride (TG) concentrations,26 liver myeloperoxidase activity,40 and tissue protein concentration33 were determined as described previously. Organ weight and composition were expressed relative to BW in kilograms.
Intestinal Morphometry
For morphometric analysis, paraffin-embedded sections (5 µm) of formalin-fixed jejunal and ileal segments were stained with eosin and hematoxylin. Villus height, crypt depth, and muscularis thickness were measured by using an Axiophot microscope (Carl Zeiss, Inc, Werk Göttingen, Germany) and National Institutes of Health (NIH) Image software, Version 1.60 (NIH, Bethesda, MD) in 15 vertically well-oriented cryptvillus units.33
Statistical Analysis
Data for the 4 treatment groups were analyzed using Minitab statistical software (Minitab, Inc, State College, PA). Data were analyzed by 1-way analysis of variance (ANOVA) with nutrition treatment as a main effect. The area under the curve (AUC) for glucose and insulin data derived from the IVGTT tests were calculated using GraphPad statistical software (GraphPad Software, Inc, La Jolla, CA). The correlation between hepatic TG content and glucose infusion rate during CLAMP was analyzed using nonlinear regression analysis, and best-fit line was generated using the Origin software program (Microcal Software, Inc, Northampton, MA). Results are expressed as mean ± SEM, and a P value of <.05 was considered statistically significant.
Results
Whole-Body Insulin Sensitivity, Glucose Tolerance, and Incretin Secretion
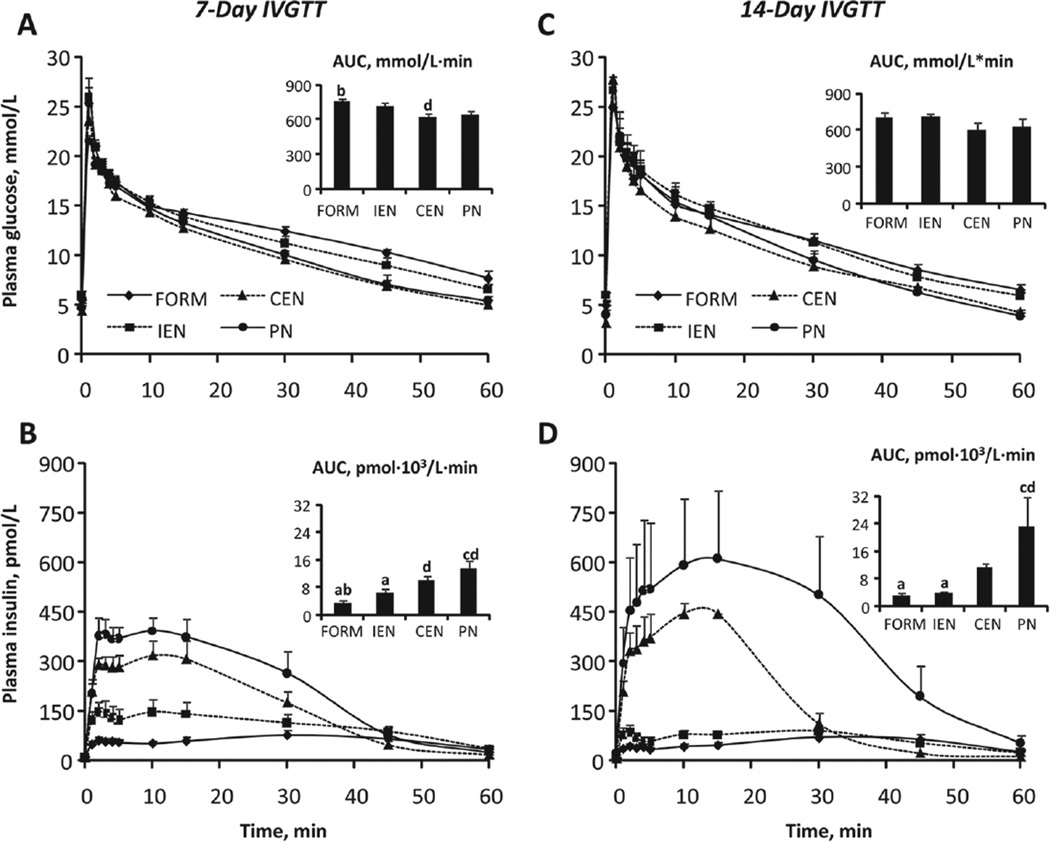
We examined the impact of feeding modality on whole-body glucose tolerance based on an IVGTT on days 7 and 14 of treatment (Figure 1). Plasma glucose responses (Figure 1A,C) were similar among most groups as evidenced by similar AUC estimates; only FORM and CEN on day 7 were different (P < .05; Figure 1A). In contrast, insulin responses were strikingly higher in continuously fed groups CEN and PN (P < .05; Figure 1B,D). This effect was even more pronounced in PN on day 14 compared with day 7.
Figure 1.
Intravenous glucose tolerance tests (IVGTT). Plasma glucose and insulin concentrations and respective AUC values during IVGTT on day 7 (A, B) and on day 14 (C, D) in FORM, IEN, CEN, and PN pigs. Results are expressed as mean ± SEM; n = 5–8 per group;abcd different from PN, CEN, IEN, and FORM, respectively; P < .05. CEN, continuous enteral nutrition; FORM, intermittent formula feeding; IEN, intermittent enteral nutrition; PN, parenteral nutrition.
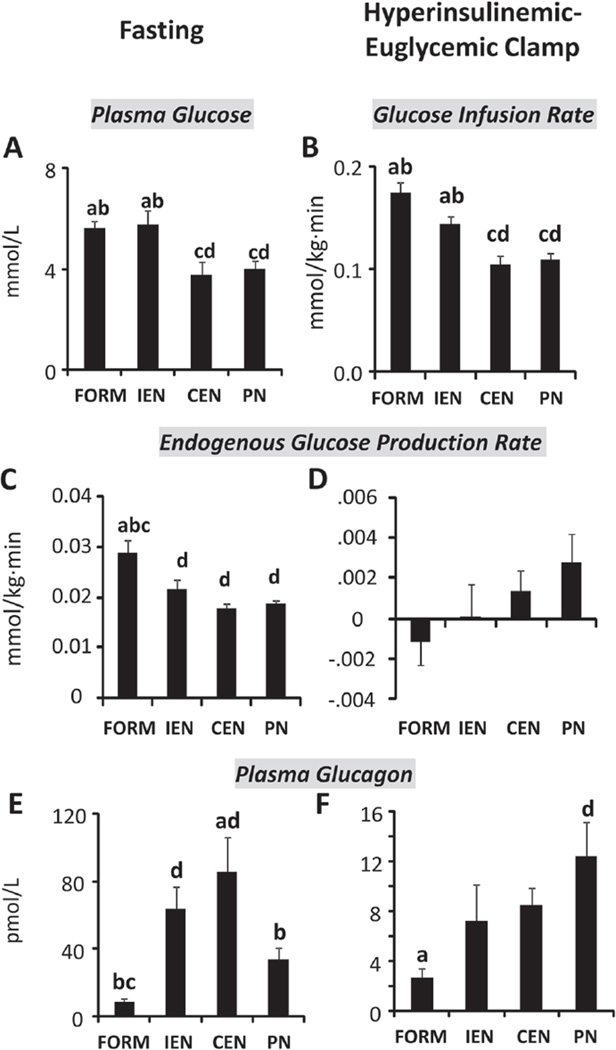
On day 14 of treatment, we assessed insulin sensitivity using the hyperinsulinemic-euglycemic clamp technique. Insulin was infused at a rate to achieve plasma insulin levels of 417 pmol·L−1 (60 µIU·mL−1). Plasma insulin concentrations during fasting before CLAMP and at steady state during CLAMP were not different between groups; average concentrations (pmol·L−1) including all 4 groups (n = 32) were 12 ± 1 and 378 ± 12, respectively. In contrast, plasma glucose concentrations (mmol·L−1) during fasting before CLAMP were lower (P < .05) in CEN and PN (3.78 ± 0.48 and 4.01 ± 0.31) compared with FORM and IEN (5.62 ± 0.28 and 5.75 ± 0.56) and were maintained at those levels in CEN and PN (3.67 ± 0.29 and 3.74 ± 0.36) compared with FORM and IEN (5.72 ± 0.55 and 5.59 ± 0.65) during insulin infusion (Figure 2A). Glucose infusion rates necessary to maintain euglycemia at the fasting glucose concentrations were significantly lower (P < .05) in continuously fed compared with intermittently fed pigs (Figure 2B). Using stable isotope technique, we determined the rate of EGP. During fasting, EGP was higher (P < .05) in FORM than in all other treatments (Figure 2C) and was suppressed in all treatments during CLAMP (Figure 2D). Plasma glucagon was highest in IEN and CEN during fasting (Figure 2E). Hyperinsulinemia during CLAMP greatly decreased glucagon secretion in all groups where differences between FORM and PN reached significance (P < .05).
Figure 2.
Hyperinsulinemic-euglycemic clamp (CLAMP). Glucose, insulin, and glucagon during 6-hour infusion of D[13C6] glucose (0.005 mmol/kg·min) during fasting (0–2 hours) and CLAMP (3–6 hours) with insulin infusion of 31 pmol/kg0.66·min. Plasma glucose during fasting (A), glucose infusion rates during CLAMP (B), endogenous glucose production and plasma glucagon during fasting (C, E), and CLAMP (D, F). Plasma insulin (pmol/L) between groups was not different during fasting (12 ± 1), n = 54, or CLAMP (378 ± 12), n = 32. Results are expressed as mean ± SEM; n = 6–20 per group; abcd different from PN, CEN, IEN, and FORM, respectively; P < .05. CEN, continuous enteral nutrition; FORM, intermittent formula feeding; IEN, intermittent enteral nutrition; PN, parenteral nutrition.
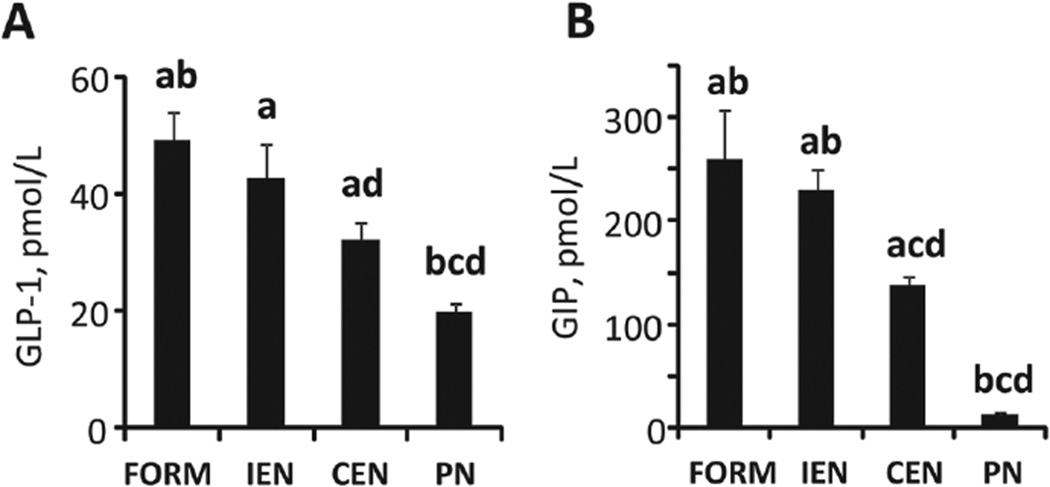
Given the effects of different feeding modalities on glucose metabolism and insulin and glucagon secretion, we measured plasma concentrations of the incretin hormones GIP and GLP-1 in the fed state (Figure 3). For both hormones, the levels were higher in FORM and IEN compared with CEN and PN. As expected, due to the lack of enteral feeding, hormone levels were significantly different in PN (P < .05) from all other treatments.
Figure 3.
Incretin hormones. Plasma GLP-1 (A) and GIP (B) concentrations in the fed state after 9–12 days of treatment. Results are expressed as mean ± SEM; n = 6–20 per group;abcd different from PN, CEN, IEN, and FORM, respectively; P < .05. GLP-1, glucagon-like peptide-1; GIP, glucose-dependent insulinotropic polypeptide; CEN, continuous enteral nutrition; FORM, intermittent formula feeding; IEN, intermittent enteral nutrition; PN, parenteral nutrition.
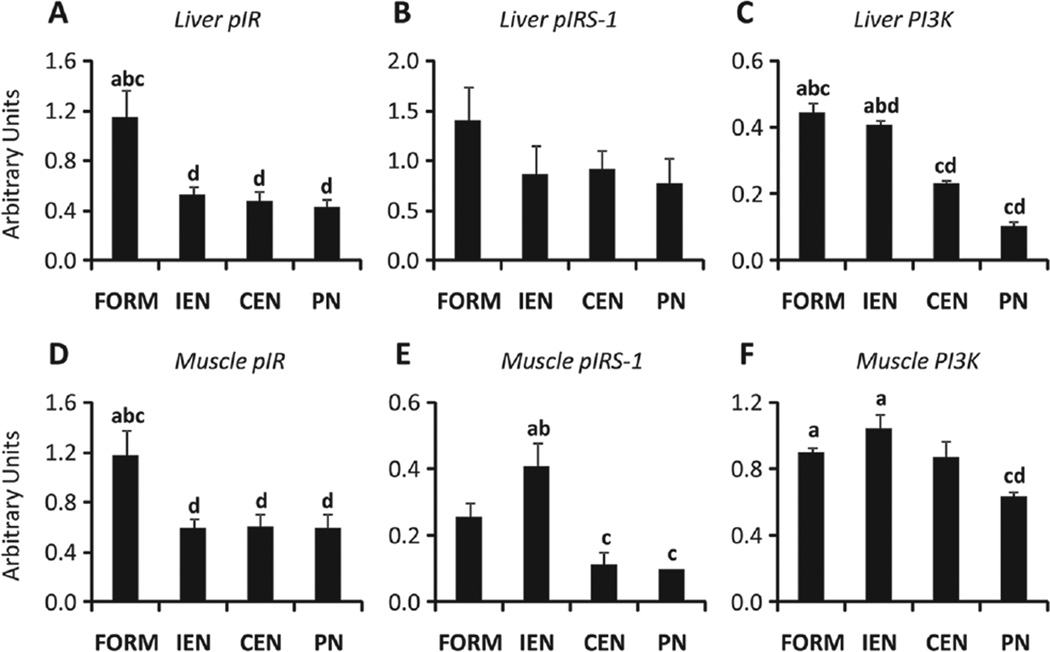
Tissue Insulin Signaling
To further assess the effects of PN on tissue-specific insulin resistance, we measured the liver and skeletal muscle tissue abundance of the IR, IRS-1, and PI3K. Our results indicate that phosphorylation of hepatic IR and IRS-1 was or tended to be reduced in all pigs receiving ED compared with FORM; the abundance of PI3K protein was decreased (P < .05) in CEN and PN compared with FORM and IEN (Figure 4A–C). Similarly, in skeletal muscle, phosphorylation of IR showed the same pattern as in liver, and abundance of PI3K protein was decreased in PN (P < .05) vs FORM and IEN (Figure 4D,F).
Figure 4.
Insulin signaling. Relative abundance and phosphorylation of proteins in liver and skeletal muscle tissue samples from neonatal pigs treated for 14 days after a 4-hour hyperinsulinemic-euglycemic clamp (CLAMP) on day 14. Liver tissue relative abundance of phospho-IR (pIR) (A), phospho-IRS-1 (pIRS-1) (B), and PI3K (C). Muscle tissue relative abundance of phospho-IR (pIR) (D), phospho-IRS-1 (pIRS-1) (E), and PI3K (F). Phosphorylated proteins expressed relative to total protein and PI3K protein expressed relative to tubulin. Results are expressed in arbitrary units as mean ± SEM; n = 6 per group;abcd different from PN, CEN, IEN, and FORM, respectively; P < .05. IR, insulin receptor; IRS-1, insulin receptor substrate 1; PI3K, phosphatidylinositol 3 kinase; CEN, continuous enteral nutrition; FORM, intermittent formula feeding; IEN, intermittent enteral nutrition; PN, parenteral nutrition.
Hepatic Steatosis, Inflammation, and Insulin Resistance
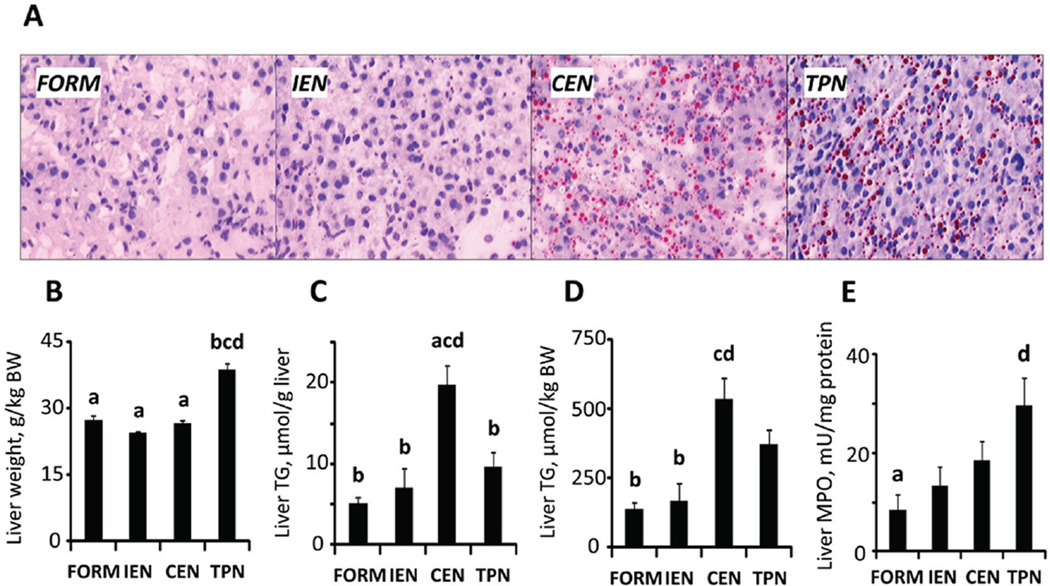
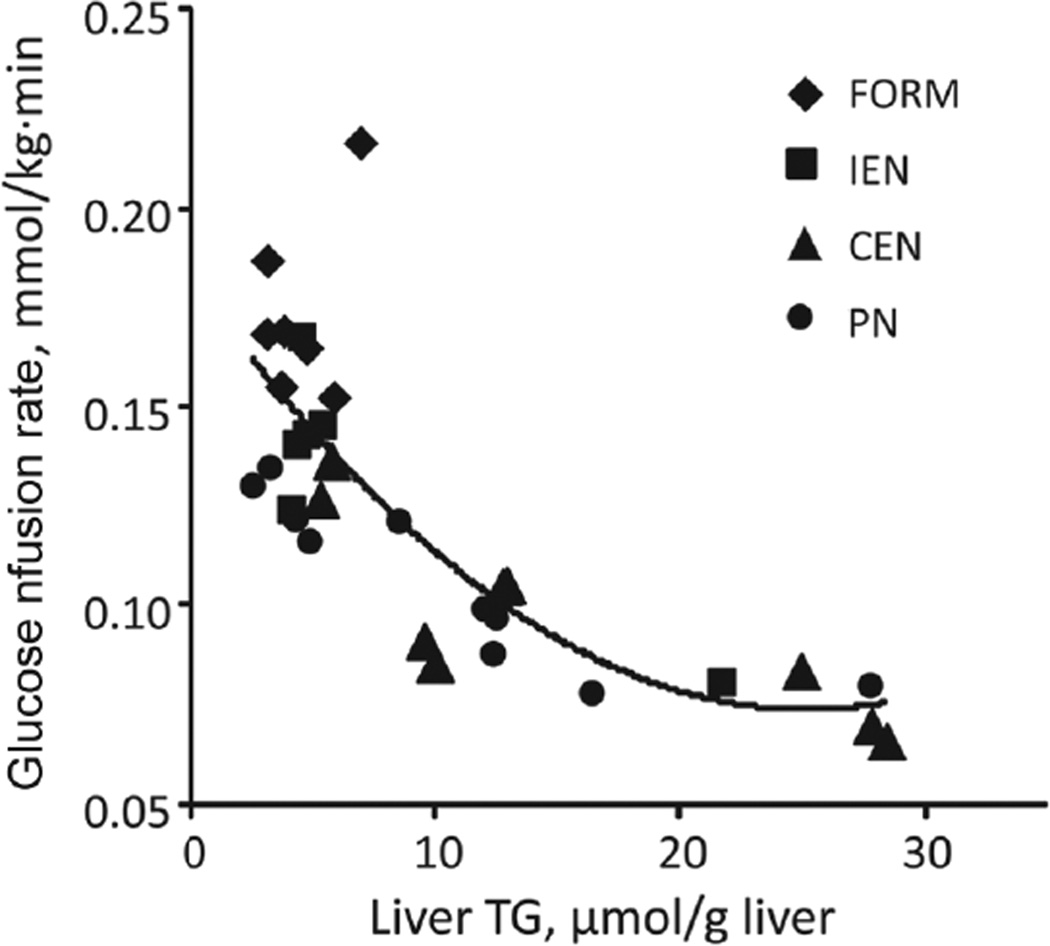
In accordance to our previous studies, liver weight was significantly higher in PN (P < .05) compared with all enterally fed groups (Figure 5B, Table 2). However, PN and CEN induced hepatic steatosis compared with FORM and IEN, as indicated by increased lipid content and microscopic lipid droplets (Figure 5A). Compared with FORM, hepatic lipid content was increased in all other treatments but only significantly for CEN (P < .05; Figure 5C,D). As a measure of hepatic inflammation, we quantified myeloperoxidase activity, which was increased in the order FORM < IEN < CEN < PN, with FORM and PN being significantly different (P < .05; Figure 5E). Furthermore, regardless of treatment, hepatic TG content was strongly correlated (R2 = 0.63, P < .0001) with insulin resistance measured by CLAMP (Figure 6).
Figure 5.
Hepatic steatosis and inflammation. Livers from neonatal pigs treated for 14 days. Representative Oil Red O–stained liver sections showing lipid droplets (red) (A); liver weight per kilogram of body weight (BW) (B), hepatic triglyceride (TG) content per gram of liver tissue (C) and per kilogram of BW (D); hepatic myeloperoxidase (MPO) activity (E). Results are expressed as mean ± SEM; n = 7–17 per group;abcd different from PN, CEN, IEN, and FORM, respectively; P < .05. CEN, continuous enteral nutrition; FORM, intermittent formula feeding; IEN, intermittent enteral nutrition; PN, parenteral nutrition.
Figure 6.
Hepatic steatosis is associated with increased insulin resistance. Nonlinear regression analysis of hepatic triglyceride (TG) content and glucose infusion rate during hyperinsulinemic euglycemic clamp (y = 1.72E−4 x2 – 8.67E−3×+ 1.82E−1; R2 = 0.637; P < .0001; n = 32). CEN, continuous enteral nutrition; FORM, intermittent formula feeding; IEN, intermittent enteral nutrition; PN, parenteral nutrition.
Body and Organ Weights and Intestinal Morphometry
Body and organ weights are presented in Table 3. Initial BWs were similar between the 4 groups, although IEN pigs tended to be slightly heavier. This resulted in higher final BW in IEN compared with CEN and PN. Daily BW gain tended to be higher in FORM than in all other groups receiving ED.
Table 3.
Body and Organ Weights in Neonatal Pigs Fed FORM, IEN, CEN, or PN for 14 Days
| FORM | IEN | CEN | PN | |
|---|---|---|---|---|
| Initial BW, g | 2191 ± 94 | 2649 ± 153 | 2270 ± 115 | 2369 ± 111 |
| Final BW, g | 4081 ± 161 | 4108 ± 160ab | 3433 ± 153 | 3450 ± 152 |
| Daily weight gain, g·kg BW−1 | 53 ± 2 | 46 ± 2 | 46 ± 2 | 47 ± 1 |
| Liver, g·kg BW−1 | 27.5 ± 1.1a | 24.5 ± 0.4a | 26.8 ± 0.7a | 38.9 ± 1.3bcd |
| Jejunum, g·kg BW−1 | 17.1 ± 0.8a | 17.1 ± 0.5a | 15.9 ± 0.5a | 9.7 ± 0.6bcd |
| Ileum, g·kg BW−1 | 25.9 ± 2.0abc | 19.1 ± 0.6abd | 16.5 ± 0.4acd | 11.0 ± 0.5bcd |
| Total SI, g·kg BW−1 | 43.0 ± 1.6abc | 36.2 ± 0.8abd | 32.4 ± 0.7acd | 20.6 ± 1.0bcd |
| Stomach, g·kg BW−1 | 5.3 ± 0.1bc | 7.9 ± 0.2abd | 6.7 ± 0.2acd | 5.8 ± 0.3bc |
| Pancreas, g·kg BW−1 | 1.5 ± 0.1 | 1.4 ± 0.1 | 1.4 ± 0.1 | 1.5 ± 0.1 |
| Spleen, g·kg BW−1 | 2.8 ± 0.2a | 3.6 ± 0.3a | 3.4 ± 0.2a | 5.9 ± 0.7bcd |
Organ weights are expressed relative to final body weight. Results are expressed as mean ± SEM; n = 8–20 per group. BW, body weight; CEN, continuous enteral nutrition; FORM, intermittent formula feeding; IEN, intermittent enteral nutrition; PN, parenteral nutrition; SI, small intestine.
different from PN, CEN, IEN, and FORM, respectively; P < .05.
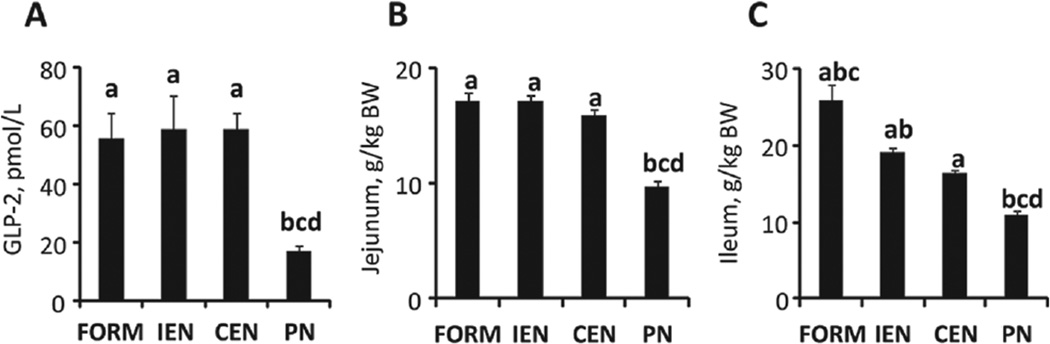
Small intestinal weights decreased in the order FORM > IEN > CEN > PN. This difference reflected the major effects found in ileal weights (Figure 7C). The significant effect of enteral feeding on the jejunum and ileum (Figure 7B,C) is in accord with higher levels of the intestinotrophic peptide GLP-2 in all enterally fed groups compared with PN (Figure 7A). Morphometric measurements of villus height in jejunal and ileal segments (Table 4) largely paralleled the effects of enteral feeding compared with PN, but jejunal villus height in IEN was significantly decreased (P < .05) compared with FORM and CEN. No differences in crypt depth and muscuaris thickness were detected. Stomach weights were highest in pigs receiving ED enterally (ie, IEN and CEN). PN pigs had significantly enlarged livers (Figure 5) and spleens (P < .05). There were no differences in pancreatic weights.
Figure 7.
Intestinal growth. Plasma GLP-2 concentrations in the fed state after 9–12 days of treatment (A). Jejunum (B) and ileum (C) weight per kilogram of body weight (BW) of neonatal pigs treated for 14 days. Results are expressed as mean ± SEM; n = 5–20 per group;abcd different from PN, CEN, IEN, and FORM, respectively; P < .05. GLP-2, glucagon-like peptide-2; CEN, continuous enteral nutrition; FORM, intermittent formula feeding; IEN, intermittent enteral nutrition; PN, parenteral nutrition.
Table 4.
Intestinal Morphometry in Neonatal Pigs Fed FORM, IEN, CEN, or PN for 14 Days
| FORM | IEN | CEN | PN | |
|---|---|---|---|---|
| Jejunum | ||||
| Villus height, µm | 655 ± 42ac | 346 ± 42bd | 586 ± 69c | 404 ± 58d |
| Crypt depth, µm | 124 ± 15 | 129 ± 7 | 113 ± 7 | 97 ± 8 |
| Muscularis thickness, µm | 128 ± 6 | 158 ± 16 | 128 ± 10 | 136 ± 14 |
| Ileum | ||||
| Villus height, µm | 820 ± 140 | 769 ± 133 | 651 ± 142 | 416 ± 71 |
| Crypt depth, µm | 110 ± 9 | 93 ± 4 | 103 ± 5 | 95 ± 8 |
| Muscularis thickness, µm | 164 ± 14 | 159 ± 7 | 150 ± 11 | 165 ± 1 |
Results are expressed as mean ± SEM; n = 6 per group. CEN, continuous enteral nutrition; FORM, intermittent formula feeding; IEN, intermittent enteral nutrition; PN, parenteral nutrition.
different from PN, CEN, IEN, and FORM, respectively; P < .05.
Discussion
Nutrition support has a critical impact on the clinical outcome, growth, and development of infants, especially those born premature. In the past few decades, considerable research has shown the importance of EN compared with PN in many clinical outcomes, especially gut function. Yet, it is becoming increasingly evident that the mode of nutrition support has implications beyond the gut and may be linked to poor growth and metabolic dysfunction. We recently found that continuous PN compared with intermittent enteral formula feeding induces hepatic inflammation, steatosis, and insulin resistance in neonatal pigs.26 Although there were fundamental differences in the nature of the diet (elemental vs polymeric) and feeding pattern (continuous vs intermittent), we hypothesized that the IV vs the enteral route of feeding was the major cause of this outcome. An important finding in this study was that switching the route of continuous feeding of elemental diet from parenteral to enteral did not improve the PN-induced insulin resistance. Unexpectedly, our results show that CEN, similar to PN, induced metabolic dysfunction marked by hepatic steatosis and inflammation and insulin resistance. Our analysis also revealed that defects in liver and muscle insulin signaling were similar in PN, CEN, and IEN animals, suggesting that the elemental form of macronutrients in the diet may have played a role. The improved results found for IEN vs CEN with respect to insulin sensitivity imply that intermittent feeding is a more beneficial modality compared with continuous enteral nutrient administration. Another aspect of the current treatment design was the chemical composition of nutrients (ie, elemental vs polymeric). However, the metabolic responses were in most cases similar in FORM and IEN groups, indicating that this factor had limited influence under intermittent feeding conditions.
Our IVGTT results showed that continuous nutrition, either parenteral or enteral, induced insulin resistance mainly evident as hypersecretion of insulin, whereas glucose excursions were similar between all treatments. This adaptive secretory response of pancreatic β-cells is a critical factor for normal glucose tolerance, and a reduction in β-cell function and mass over time eventually leads to diminished insulin secretion and hyperglycemia. Our previous study demonstrated suppressed β-cell proliferation in PN-fed neonatal pigs while still exhibiting hyperinsulinemia in response to insulin resistance.26 β-Cell failure is the hallmark of type 2 diabetes mellitus (T2DM) and may be precipitated by the development of insulin resistance, which is considered the major factor in the onset of pediatric nonalcoholic fatty liver disease (NAFLD),41,42 a potential precursor to overt T2DM. Over the past decades, it has become clear that by the time hyperglycemia develops during the pathogenesis of T2DM, reductions in insulin sensitivity and β-cell function have already occurred.43 And given the presence of a tightly regulated feedback system incorporating β-cell and insulin-sensitive tissues, it becomes apparent that reductions in both parameters are present early in the course of the disease.43 Interestingly, follow-up studies have shown that children44 and young adults45 born with VLBW have higher insulin resistance compared with subjects born at term. This issue may be significant given the current recommendations to pursue aggressive early nutrition in premature and VLBW infants because it becomes apparent that rapid catch-up growth may predispose these infants to increased adiposity and later obesity.46
Our CLAMP measurements after 2 weeks confirmed the presence of insulin resistance in PN and CEN compared with IEN and FORM and suggest that the underlying cause is due to diminished peripheral glucose disposal. We also tested the possibility that insulin resistance occurs in the liver, manifest as persistent glucose production under conditions of insulin stimulation. To do this, we measured hepatic EGP during CLAMP. Contrary to our hypothesis that EGP would persist during CLAMP, we found that EGP was completely suppressed in all treatment groups (Figure 2D). Yet, this is in agreement with normoglycemia during fasting and suggests that insulin levels during CLAMP were sufficiently high to suppress EGP. We also measured glucagon, which counteracts insulin actions and thereby maintains euglycemia by stimulating EGP in the fasted state. The primary stimulus for glucagon secretion is hypoglycemia, whereas insulin and glucose exert strong inhibitory actions on the normal α-cell; under diabetic conditions, the α-cell seems to be less responsive to these factors (for review, see Hare47). Accordingly, in FORM, glucagon levels were lowest during fasting, yet plasma glucose was higher than in continuous groups (CEN and PN). In contrast, all other elemental diet groups exhibited higher glucagon levels during fasting. Our results suggest nutrients given in the elemental form for 14 days not only affect the pancreatic β-cell insulin secretion but also the pancreatic α-cell glucagon secretion. In line with these findings are those in obese insulin-resistant adolescents with normal fasting glucose compared with healthy lean controls.48 In a longitudinal follow-up in these individuals, fasting glucagon concentration was the main determinant in the transition from insulin sensitivity to insulin resistance, marked by α-cell upregulation early in the course of deteriorating glucose tolerance.48 Because increased glucagon levels in the current study did not translate into persistence of hepatic EGP, evidence points to a defect in peripheral insulin action to explain the insulin resistance observed in CEN and PN groups.
Given the differences in EN vs PN, we predicted that this would result in differences in the release of gut incretin hormones, GIP and GLP-1, which positively affect insulin secretion and glucose disposal. These 2 hormones are responsible for the so-called incretin effect (ie, the amplification of insulin secretion in response to oral glucose ingestion to produce normoglycemic plasma glucose levels).49 As expected, both GIP and GLP-1 levels were significantly lower in PN compared with all enterally fed piglets. However, we found distinct differences between FORM, IEN, and CEN groups showing that the GIP and GLP-1 levels were positively related to the degree of insulin sensitivity. The incretin effect is also known to be dependent on the dose of glucose,50 and thus it could be argued that the difference in GIP and GLP-1 levels between intermittent and continuous (IEN vs CEN) enteral feeding is due to the dose delivered at a specific time point. On the other hand, incretin levels were similar between FORM and IEN despite lactose in FORM being a less potent secretagogue than glucose.51 This suggests that other components in FORM, such as type of protein,52 contributed to higher release of incretins. Thus, although considerable evidence shows that GLP-1 improves β-cell function and insulin release, in the current study, we postulate that feeding modality-related differences in GLP-1 level may have augmented peripheral glucose disposal, which is thought to occur via neural circuits involving the vagus nerve or brain.53,54
To further explain the differences in insulin sensitivity, we examined the most predominant insulin-responsive peripheral tissue in neonates—namely, muscle—and found that feeding modality strongly affected the initial steps of insulin signaling under conditions of insulin stimulation. Insulin signaling involves a cascade of events initiated by insulin binding to the cell surface IR, followed by receptor autophosphorylation and activation of receptor tyrosine kinases, which results in tyrosine phosphorylation of IRS-1 and IRS-2. Binding of IRSs to PI3K, a key component of the pathway, results in its activation, which ultimately leads to transport of glucose into the cell.55 Our results show that, under insulin-stimulated conditions, there was less phosphorylation and hence activation of IR and IRS-1 in ED-fed piglets compared with FORM. Moreover, the abundance of PI3K in continuously fed piglets was mostly decreased, confirming our previous results for PN.26 The dampening of insulin signaling in all ED-fed piglets may explain the insulin resistance and reduction of glucose disposal during CLAMP. Indeed, IRS-1 phosphorylation closely correlates with PI3K activation in insulin-resistant ob/ob mice,56 and insulin-stimulated PI3K activity was decreased in skeletal muscle of T2DM subjects.55 However, during insulin signaling, IR is also dephosphorylated and inactivated by protein tyrosine phosphatases (PTPs). Thus, the physiological regulation of insulin is controlled by the balance between phosphorylation and dephosphorylation,57 and it is plausible that enhanced activity of 1 or more PTPs could lead to insulin resistance by increased dephosphorylation of IR and thus IRS-1.55
An additional explanation for a peripheral defect in insulin signaling is the role of inflammation associated with PN-associated liver disease (PNALD). In accordance with our previous study,26 PN induced markers of PNALD—namely, enlarged livers, enlarged spleens, inflammation, and steatosis. Interestingly, the increase in liver mass with PN compared with enteral feeding was not due to increased lipid content but rather substantial increases in protein and DNA content.26 The latter finding is suggestive of increased hepatic acute phase protein synthesis secondary to the mild inflammatory stimulus. Surprisingly, CEN induced the greatest degree of steatosis, whereas IEN was similar to FORM. This is agreement with a study in mice where intermittent administration of parenteral lipid emulsion enterally protected mice against hepatic steatosis, whereas the intermittent administration intravenously did not.58 Yet, that study did not include continuous enteral administration of the lipid emulsion. All piglets receiving ED showed a hepatic inflammatory response based on myeloperoxidase (MPO) measurements, albeit only FORM and PN were significantly different. The coinciding features we found in PN and CEN resemble those contributing to NAFLD characterized by elevated hepatic TG content with varying degrees of inflammation. In adults and adolescents, NAFLD is associated with insulin resistance in both liver and muscle.59 A major factor in the development of insulin resistance is obesity, particularly increased visceral, muscle, and liver fat.60 Studies have shown that intrahepatic fat deposition is a contributing factor in the pathogenesis of insulin resistance in childhood and adolescence.61,62 Our current results demonstrate that insulin sensitivity is negatively correlated with increasing hepatic TG content (Figure 5). Importantly, since the introduction of noninvasive methods such as magnetic resonance imaging (MRI) and magnetic resonance spectroscopy (MRS) for measurement of ectopic fat,63 it has become clear that insulin resistance is associated with hepatic steatosis because measurements do not require a liver biopsy. Furthermore, measurements in preterm born adults showed “aberrant” adiposity and ectopic lipid deposition,64 and preterm-at-term infants had increased intrahepatocellular lipid compared with term-born infants and adults.65
Finally, we examined the effect of feeding modality on whole-body and visceral organ growth. This was in part based on results from a previous study in infant pigs where we demonstrated that continuous enteral infusion of an elemental diet compared with intermittent formula feeding for 7 days did not decrease intestinal growth.66 In line with our previous studies,26,66 ED-fed piglets tended to grow slower than FORM piglets. This may be an effect of the nature of ED, where the elemental form of its macronutrients—namely, amino acids and glucose—renders them into easily accessible fuel for intestinal tissues that are oxidized and thus not available for absorption. PN piglets presented with enlarged livers and spleens but smaller intestines due to lack of enteral nutrients and therefore low levels of GLP-2 compared with enterally fed groups. The greatest effects of feeding modality were seen in stomach mass, small intestinal mass, and morphology. While EDs are known to be primarily absorbed in the upper gastrointestinal tract, more nutrients may have reached the ileum with polymeric formula feeding. This effect translated into distinct differences in stomach mass and ileal mass, suggesting that ED enhances stomach growth and that giving ED intermittently (IEN) enhances ileal growth compared with continuous administration (CEN). Furthermore, the fact that GLP-2 secretory L-cells are predominantly located in the ileum and colon leaves the possibility of enhanced intestinotrophic local effects of the secreted hormone in response to feeding. The same trend was detected for ileal villus height, but because of higher variability, the differences were not statistically significant.
In conclusion, our results show that feeding modality significantly affects metabolic function as well as gut growth in neonatal pigs. We demonstrated that the increased insulin resistance and hepatic steatosis induced by continuous PN are not resolved when the same elemental diet is given enterally. However, intermittent feeding of either an elemental or polymeric diet significantly improved insulin sensitivity. A weakness of our current study design was that we did not include a group that was fed continuous EN with a polymeric formula, but we speculate that this treatment also would have ameliorated the metabolic effect compared with CEN, although this issue warrants further study. We also show that fasting hepatic EGP and glucagon levels in the neonatal pig were effectively suppressed by insulin in all groups. Thus, insulin resistance induced by continuous PN and EN was not due to persistent hepatic EGP but more likely peripheral muscle tissue IR signaling defects, which appeared to be induced by the elemental form of the diet. It is also possible that diminished gut incretin secretion—namely, GLP-1—observed with continuous PN and EN contributed to insulin resistance via lower peripheral glucose disposal. The trophic effects of EN vs PN were evident on gut growth, strongly linked to increased GLP-2 secretion, and optimal when given intermittently vs continuously. Our findings suggest that the positive effects of EN on metabolic function are optimal when given intermittently rather than continuously, although these positive effects may be diminished when macronutrients are given in elemental rather than polymeric form.
Acknowledgments
This work is a publication of the USDA/ARS Children’s Nutrition Research Center, Department of Pediatrics, Baylor College of Medicine and Texas Children’s Hospital, Houston, Texas. The contents of this publication do not necessarily reflect the views or policies of the U.S. Department of Agriculture, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. government.
Financial disclosure: U.S. Department of Agriculture, Agricultural Research Service under Cooperative Agreement Number 58-6250-6-001 (DB); A.S.P.E.N. Rhoads Research Foundation of the American Society for Parenteral and Enteral Nutrition; and Texas Medical Center Digestive Diseases Center (NIH Grant P30 DK-56338) (DB).
References
- 1.Ehrenkranz RA, Dusick AM, Vohr BR, Wright LL, Wrage LA, Poole WK. Growth in the neonatal intensive care unit influences neurodevelopmental and growth outcomes of extremely low birth weight infants. Pediatrics. 2006;117(4):1253–1261. doi: 10.1542/peds.2005-1368. [DOI] [PubMed] [Google Scholar]
- 2.Ziegler EE, Thureen PJ, Carlson SJ. Aggressive nutrition of the very low birthweight infant. Clin Perinatol. 2002;29(2):225–244. doi: 10.1016/s0095-5108(02)00007-6. [DOI] [PubMed] [Google Scholar]
- 3.Hay WW., Jr Strategies for feeding the preterm infant. Neonatology. 2008;94(4):245–254. doi: 10.1159/000151643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kao LS, Morris BH, Lally KP, Stewart CD, Huseby V, Kennedy KA. Hyperglycemia and morbidity and mortality in extremely low birth weight infants. J Perinatol. 2006;26:730–736. doi: 10.1038/sj.jp.7211593. [DOI] [PubMed] [Google Scholar]
- 5.Hays SP, Smith EO, Sunehag AL. Hyperglycemia is a risk factor for early death and morbidity in extremely low birth-weight infants. Pediatrics. 2006;118(5):1811–1818. doi: 10.1542/peds.2006-0628. [DOI] [PubMed] [Google Scholar]
- 6.Nehra D, Fallon EM, Puder M. The prevention and treatment of intestinal failure–associated liver disease in neonates and children. Surg Clin North Am. 2011;91(3):543–563. doi: 10.1016/j.suc.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 7.Sondheimer JM, Asturias E, Cadnapaphornchai M. Infection and cholestasis in neonates with intestinal resection and long-term parenteral nutrition. J Pediatr Gastroenterol Nutr. 1998;27(2):131–137. doi: 10.1097/00005176-199808000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Raney M, Donze A, Smith JR. Insulin infusion for the treatment of hyperglycemia in low birth weight infants: examining the evidence. Neonatal Netw. 2008;27(2):127–140. doi: 10.1891/0730-0832.27.2.127. [DOI] [PubMed] [Google Scholar]
- 9.Cowett RM, Oh W, Schwartz R. Persistent glucose production during glucose infusion in the neonate. J Clin Invest. 1983;71(3):467–475. doi: 10.1172/JCI110791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sunehag A, Gustafsson J, Ewald U. Very immature infants (< or = 30 wk) respond to glucose infusion with incomplete suppression of glucose production. Pediatr Res. 1994;36(4):550–555. doi: 10.1203/00006450-199410000-00024. [DOI] [PubMed] [Google Scholar]
- 11.Chacko SK, Ordonez J, Sauer PJ, Sunehag AL. Gluconeogenesis is not regulated by either glucose or insulin in extremely low birth weight infants receiving total parenteral nutrition. J Pediatr. 2011;158(6):891–896. doi: 10.1016/j.jpeds.2010.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chacko SK, Sunehag AL. Gluconeogenesis continues in premature infants receiving total parenteral nutrition. Arch Dis Child Fetal Neonatal Ed. 2010;95(6):F413–F418. doi: 10.1136/adc.2009.178020. [DOI] [PubMed] [Google Scholar]
- 13.Sinclair JC, Bottino M, Cowett RM. Interventions for prevention of neonatal hyperglycemia in very low birth weight infants. Cochrane Database Syst Rev. 2011;(10):CD007615. doi: 10.1002/14651858.CD007615.pub3. [DOI] [PubMed] [Google Scholar]
- 14.Lucas A, Bloom SR, Aynsley-Green A. Gut hormones and ‘minimal enteral feeding’. Acta Paediatr Scand. 1986;75(5):719–723. doi: 10.1111/j.1651-2227.1986.tb10280.x. [DOI] [PubMed] [Google Scholar]
- 15.Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;3(3):153–165. doi: 10.1016/j.cmet.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 16.Tyson JE, Kennedy KA. Trophic feedings for parenterally fed infants. Cochrane Database Syst Rev. 2005;(3):CD000504. doi: 10.1002/14651858.CD000504.pub2. [DOI] [PubMed] [Google Scholar]
- 17.Hanson C, Sundermeier J, Dugick L, Lyden E, Anderson-Berry AL. Implementation, process, and outcomes of nutrition best practices for infants <1500 g. Nutr Clin Pract. 2011;26(5):614–624. doi: 10.1177/0884533611418984. [DOI] [PubMed] [Google Scholar]
- 18.Rojahn A, Lindgren CG. Enteral feeding in infants <1250 g starting within 24 h post-partum. Eur J Pediatr. 2001;160(10):629–632. doi: 10.1007/s004310100814. [DOI] [PubMed] [Google Scholar]
- 19.Premji SS, Chessell L. Continuous nasogastric milk feeding versus intermittent bolus milk feeding for premature infants less than 1500 grams. Cochrane Database Syst Rev. 2011;(11):CD001819. doi: 10.1002/14651858.CD001819.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shulman RJ, Redel CA, Stathos TH. Bolus versus continuous feedings stimulate small-intestinal growth and development in the newborn pig. J Pediatr Gastroenterol Nutr. 1994;18(3):350–354. doi: 10.1097/00005176-199404000-00017. [DOI] [PubMed] [Google Scholar]
- 21.Gazzaneo MC, Suryawan A, Orellana RA, et al. Intermittent bolus feeding has a greater stimulatory effect on protein synthesis in skeletal muscle than continuous feeding in neonatal pigs. J Nutr. 2011;141(12):2152–2158. doi: 10.3945/jn.111.147520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neu J, Mihatsch W. Recent developments in necrotizing enterocolitis. JPEN J Parenter Enteral Nutr. 2012;36(1) suppl:30S–35S. doi: 10.1177/0148607111422068. [DOI] [PubMed] [Google Scholar]
- 23.Martinez JA, Ballew MP. Infant formulas. Pediatr Rev. 2011;32(5):179–189. doi: 10.1542/pir.32-5-179. [DOI] [PubMed] [Google Scholar]
- 24.Raimondi F, Spera AM, Sellitto M, Landolfo F, Capasso L. Amino acid based formula as a rescue strategy in feeding very low birth weight infants with intrauterine growth restriction. J Pediatr Gastroenterol Nutr. 2012 Jan 10; doi: 10.1097/MPG.0b013e3182483e8f. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 25.Russell RI. Intestinal adaptation to an elemental diet. Proc Nutr Soc. 1985;44(1):87–93. doi: 10.1079/pns19850014. [DOI] [PubMed] [Google Scholar]
- 26.Stoll B, Horst DA, Cui L, et al. Chronic parenteral nutrition induces hepatic inflammation, steatosis, and insulin resistance in neonatal pigs. J Nutr. 2010;140(12):2193–2200. doi: 10.3945/jn.110.125799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Book SA, Bustad LK. The fetal and neonatal pig in biomedical research. J Anim Sci. 1974;38(5):997–1002. doi: 10.2527/jas1974.385997x. [DOI] [PubMed] [Google Scholar]
- 28.Flecknell PA, Wootton R, John M. Total body glucose metabolism in the conscious, unrestrained piglet and its relation to body- and organ weight. Br J Nutr. 1980;44(2):193–203. doi: 10.1079/bjn19800027. [DOI] [PubMed] [Google Scholar]
- 29.Moughan PJ, Birtles MJ, Cranwell PD, Smith WC, Pedraza M. The piglet as a model animal for studying aspects of digestion and absorption in milkfed human infants. World Rev Nutr Diet. 1992;67:40–113. doi: 10.1159/000419461. [DOI] [PubMed] [Google Scholar]
- 30.Schook L, Beattie C, Beever J, et al. Swine in biomedical research: creating the building blocks of animal models. Anim Biotechnol. 2005;16(2):183–190. doi: 10.1080/10495390500265034. [DOI] [PubMed] [Google Scholar]
- 31.Cornblath M, Hawdon JM, Williams AF, et al. Controversies regarding definition of neonatal hypoglycemia: suggested operational thresholds. Pediatrics. 2000;105(5):1141–1145. doi: 10.1542/peds.105.5.1141. [DOI] [PubMed] [Google Scholar]
- 32.Sunehag AL, Haymond MW. Glucose extremes in newborn infants. Clin Perinatol. 2002;29(2):245–260. doi: 10.1016/s0095-5108(02)00006-4. [DOI] [PubMed] [Google Scholar]
- 33.Burrin DG, Stoll B, Jiang R, et al. Minimal enteral nutrient requirements for intestinal growth in neonatal piglets: how much is enough? Am J Clin Nutr. 2000;71(6):1603–1610. doi: 10.1093/ajcn/71.6.1603. [DOI] [PubMed] [Google Scholar]
- 34.Burrin DG, Stoll B, Guan X, Cui L, Chang X, Holst JJ. Glucagon-like peptide 2 dose-dependently activates intestinal cell survival and proliferation in neonatal piglets. Endocrinology. 2005;146(1):22–32. doi: 10.1210/en.2004-1119. [DOI] [PubMed] [Google Scholar]
- 35.NRC. Nutrient Requirements of Swine. 10th ed. Washington, DC: National Academies Press; 1998. [Google Scholar]
- 36.Wray-Cahen D, Beckett PR, Nguyen HV, Davis TA. Insulin-stimulated amino acid utilization during glucose and amino acid clamps decreases with development. Am J Physiol. 1997;273(2, pt 1):E305–E314. doi: 10.1152/ajpendo.1997.273.2.E305. [DOI] [PubMed] [Google Scholar]
- 37.Holst JJ. Evidence that glicentin contains the entire sequence of glucagon. Biochem J. 1980;187(2):337–343. doi: 10.1042/bj1870337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jain AK, Stoll B, Burrin DG, Holst JJ, Moore DD. Enteral bile acid treatment improves parenteral nutrition related liver disease and intestinal mucosal atrophy in neonatal pigs. Am J Physiol Gastrointest Liver Physiol. 2012;302(2):G218–G224. doi: 10.1152/ajpgi.00280.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lindgren O, Carr RD, Deacon CF, et al. Incretin hormone and insulin responses to oral versus intravenous lipid administration in humans. J Clin Endocrinol Metab. 2011;96(8):2519–2524. doi: 10.1210/jc.2011-0266. [DOI] [PubMed] [Google Scholar]
- 40.Kansagra K, Stoll B, Rognerud C, et al. Total parenteral nutrition adversely affects gut barrier function in neonatal piglets. Am J Physiol Gastrointest Liver Physiol. 2003;285(6):G1162–G1170. doi: 10.1152/ajpgi.00243.2003. [DOI] [PubMed] [Google Scholar]
- 41.Mendez-Sanchez N, Arrese M, Zamora-Valdes D, Uribe M. Current concepts in the pathogenesis of nonalcoholic fatty liver disease. Liver Int. 2007;27(4):423–433. doi: 10.1111/j.1478-3231.2007.01483.x. [DOI] [PubMed] [Google Scholar]
- 42.Alisi A, Panera N, Agostoni C, Nobili V. Intrauterine growth retardation and nonalcoholic fatty liver disease in children. Int J Endocrinol. 2011;2011:269853. doi: 10.1155/2011/269853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kahn SE. The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of type 2 diabetes. Diabetologia. 2003;46(1):3–19. doi: 10.1007/s00125-002-1009-0. [DOI] [PubMed] [Google Scholar]
- 44.Hofman PL, Regan F, Jackson WE, et al. Premature birth and later insulin resistance. N Engl J Med. 2004;351(21):2179–2186. doi: 10.1056/NEJMoa042275. [DOI] [PubMed] [Google Scholar]
- 45.Hovi P, Andersson S, Eriksson JG, et al. Glucose regulation in young adults with very low birth weight. N Engl J Med. 2007;356(20):2053–2063. doi: 10.1056/NEJMoa067187. [DOI] [PubMed] [Google Scholar]
- 46.Dunger DB, Salgin B, Ong KK. Session 7: early nutrition and later health early developmental pathways of obesity and diabetes risk. Proc Nutr Soc. 2007;66(3):451–457. doi: 10.1017/S0029665107005721. [DOI] [PubMed] [Google Scholar]
- 47.Hare KJ. Role of GLP-1 induced glucagon suppression in type 2 diabetes mellitus. Dan Med Bull. 2010;57(9):B4181. [PubMed] [Google Scholar]
- 48.Weiss R, D’Adamo E, Santoro N, Hershkop K, Caprio S. Basal alphacell up-regulation in obese insulin-resistant adolescents. J Clin Endocrinol Metab. 2011;96(1):91–97. doi: 10.1210/jc.2010-1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Holst JJ, Vilsboll T, Deacon CF. The incretin system and its role in type 2 diabetes mellitus. Mol Cell Endocrinol. 2009;297(1–2):127–136. doi: 10.1016/j.mce.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 50.Nauck MA, Homberger E, Siegel EG, et al. Incretin effects of increasing glucose loads in man calculated from venous insulin and C-peptide responses. J Clin Endocrinol Metab. 1986;63(2):492–498. doi: 10.1210/jcem-63-2-492. [DOI] [PubMed] [Google Scholar]
- 51.Shima K, Suda T, Nishimoto K, Yoshimoto S. Relationship between molecular structures of sugars and their ability to stimulate the release of glucagon-like peptide-1 from canine ileal loops. Acta Endocrinol (Copenh) 1990;123(4):464–470. doi: 10.1530/acta.0.1230464. [DOI] [PubMed] [Google Scholar]
- 52.Nilsson M, Stenberg M, Frid AH, Holst JJ, Bjorck IM. Glycemia and insulinemia in healthy subjects after lactose-equivalent meals of milk and other food proteins: the role of plasma amino acids and incretins. Am J Clin Nutr. 2004;80(5):1246–1253. doi: 10.1093/ajcn/80.5.1246. [DOI] [PubMed] [Google Scholar]
- 53.Williams DL. Minireview: finding the sweet spot: peripheral versus central glucagon-like peptide 1 action in feeding and glucose homeostasis. Endocrinology. 2009;150(7):2997–3001. doi: 10.1210/en.2009-0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Johnson KM, Edgerton DS, Rodewald T, et al. Intraportal GLP-1 infusion increases nonhepatic glucose utilization without changing pancreatic hormone levels. Am J Physiol Endocrinol Metab. 2007;293(4):E1085–E1091. doi: 10.1152/ajpendo.00275.2007. [DOI] [PubMed] [Google Scholar]
- 55.Choi K, Kim YB. Molecular mechanism of insulin resistance in obesity and type 2 diabetes. Korean J Intern Med. 2010;25(2):119–129. doi: 10.3904/kjim.2010.25.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Folli F, Saad MJ, Backer JM, Kahn CR. Regulation of phosphatidylinositol 3-kinase activity in liver and muscle of animal models of insulin-resistant and insulin-deficient diabetes mellitus. J Clin Invest. 1993;92(4):1787–1794. doi: 10.1172/JCI116768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Goldstein BJ, Ahmad F, Ding W, Li PM, Zhang WR. Regulation of the insulin signalling pathway by cellular protein-tyrosine phosphatases. Mol Cell Biochem. 1998;182(1–2):91–99. [PubMed] [Google Scholar]
- 58.Javid PJ, Greene AK, Garza J, et al. The route of lipid administration affects parenteral nutrition-induced hepatic steatosis in a mouse model. J Pediatr Surg. 2005;40(9):1446–1453. doi: 10.1016/j.jpedsurg.2005.05.045. [DOI] [PubMed] [Google Scholar]
- 59.Deivanayagam S, Mohammed BS, Vitola BE, et al. Nonalcoholic fatty liver disease is associated with hepatic and skeletal muscle insulin resistance in overweight adolescents. Am J Clin Nutr. 2008;88(2):257–262. doi: 10.1093/ajcn/88.2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stefan N, Machann J, Schick F, et al. New imaging techniques of fat muscle and liver within the context of determining insulin sensitivity. Horm Res. 2005;64(suppl 3):38–44. doi: 10.1159/000089316. [DOI] [PubMed] [Google Scholar]
- 61.D’Adamo E, Cali AM, Weiss R, et al. Central role of fatty liver in the pathogenesis of insulin resistance in obese adolescents. Diabetes Care. 2010;33(8):1817–1822. doi: 10.2337/dc10-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Springer F, Nguyen HP, Machann J, et al. Normal-weight 14-year-old girl with acanthosis nigricans and markedly increased hepatic steatosis: evidence for the important role of ectopic fat deposition in the pathogenesis of insulin resistance in childhood and adolescence. Horm Res Paediatr. 2010;74(5):376–380. doi: 10.1159/000319707. [DOI] [PubMed] [Google Scholar]
- 63.Lettner A, Roden M. Ectopic fat and insulin resistance. Curr Diabetes Rep. 2008;8(3):185–191. doi: 10.1007/s11892-008-0032-z. [DOI] [PubMed] [Google Scholar]
- 64.Thomas EL, Parkinson JR, Hyde MJ, et al. Aberrant adiposity and ectopic lipid deposition characterize the adult phenotype of the preterm infant. Pediatr Res. 2011;70(5):507–512. doi: 10.1203/PDR.0b013e31822d7860. [DOI] [PubMed] [Google Scholar]
- 65.Thomas EL, Uthaya S, Vasu V, et al. Neonatal intrahepatocellular lipid. Arch Dis Child Fetal Neonatal Ed. 2008;93(5):F382–F383. doi: 10.1136/adc.2007.127431. [DOI] [PubMed] [Google Scholar]
- 66.Stoll B, Price PT, Reeds PJ, et al. Feeding an elemental diet vs a milk-based formula does not decrease intestinal mucosal growth in infant pigs. JPEN J Parenter Enteral Nutr. 2006;30(1):32–39. doi: 10.1177/014860710603000132. [DOI] [PubMed] [Google Scholar]