Abstract
Many systemic treatment options are available for advanced breast cancer, including endocrine therapy, chemotherapy, anti-human epidermal growth factor receptor 2 (HER2) therapy, and other targeted agents. Recently, everolimus, a mammalian target of rapamycin (mTOR) inhibitor, combined with exemestane, an aromatase inhibitor, has been approved in Europe and the USA for patients suffering from estrogen receptor-positive, HER2-negative advanced breast cancer previously treated by a nonsteroidal aromatase inhibitor, based on the results of BOLERO-2 (Breast cancer trials of OraL EveROlimus). This study showed a statistically significant and clinically meaningful improvement in median progression-free survival. Results concerning the impact on overall survival are expected in the near future. This clinically oriented review focuses on the use of mTOR inhibitors in breast cancer. Results reported with first-generation mTOR inhibitors (ridaforolimus, temsirolimus, everolimus) are discussed. The current and potential role of mTOR inhibitors is reported according to breast cancer subtype (estrogen receptor-positive HER2-negative, triple-negative, and HER2-positive ER-positive/negative disease). Everolimus is currently being evaluated in the adjuvant setting in high-risk estrogen receptor-positive, HER2-negative early breast cancer. Continuing mTOR inhibition or alternatively administering other drugs targeting the phosphatidylinositol-3-kinase/protein kinase B-mTOR pathway after progression on treatments including an mTOR inhibitor is under evaluation. Potential biomarkers to select patients showing a more pronounced benefit are reviewed, but we are not currently using these biomarkers in routine practice. Subgroup analysis of BOLERO 2 has shown that the benefit is consistent in all subgroups and that it is impossible to select patients not benefiting from addition of everolimus to exemestane. Side effects and impact on quality of life are other important issues discussed in this review. Second-generation mTOR inhibitors and dual mTOR-phosphatidylinositol-3-kinase inhibitors are currently being evaluated in clinical trials.
Keywords: breast cancer, treatment, everolimus, mTOR inhibitors, biomarkers, phosphatidylinositol-3-kinase/protein kinase B-mTOR pathway
Introduction
Dysregulation of the phosphatidylinositol-3-kinase/protein kinase B/mammalian target of rapamycin (PI3K-AKT-mTOR) pathway is frequently observed in tumors, including breast cancer. This pathway plays an important role in the regulation of cell proliferation, metabolism, motility, angiogenesis, and survival. Pathway hyperactivation has been linked to cancer pathogenesis, progression, and treatment resistance.1 mTOR is a serine-threonine kinase which plays a key role in cell regulation, including responses to multiple stimuli such as amino acid availability, energy and oxygen stresses, and growth factor receptor signaling.2–5
Alterations in receptor tyrosine kinases can constitutively activate the PI3K-AKT pathway upstream in breast cancer, leading to increased activity of the mTOR pathway. Aberrant activation of insulin-like growth factor-1, the fibroblast growth factor receptor family, and the epidermal growth factor/HER family, in particular human epidermal growth factor receptor 2 (HER2) have all been observed in breast cancer.6 Abnormalities in the PI3K-AKT pathway itself including loss of phosphatase and tensin homolog (PTEN) function, PI3K mutations, and aberrant activation of AKT are other possibilities of mTOR pathway activation. The liver kinase B1 (LKB1)/adenosine monophosphate-activated protein kinase (AMPK) pathway is the other major pathway regulating mTOR. Hyperactivation of this pathway, known as the metabolic pathway, can also be responsible for mTOR pathway activation.
A close interaction between the mTOR pathway and estrogen receptor (ER) signaling has been reported. A substrate of the mTOR complex 1, S6 kinase 1 phosphorylates the activation function domain 1 of the ER, leading to ligand-independent receptor activation.7,8
Many drugs in development target the PI3K-AKT-mTOR pathway, but only the mTOR inhibitor, everolimus, is currently approved for use in breast cancer in combination with the aromatase inhibitor exemestane in patients with ER-positive, HER2-negative advanced breast cancer who have previously failed a nonsteroidal aromatase inhibitor. This review focuses on first-generation mTOR inhibitors, their clinical results (Table 1), their common side effects (Table 2) including the impact on quality of life in Phase III trials, and perspectives of mTOR inhibitors in the treatment of advanced or early-stage breast cancer.
Table 1.
Results of key clinical trials
| Study | Study design | Patients | Treatments | Primary endpoint | Secondary endpoints |
|---|---|---|---|---|---|
| ER-positive, HER2-negative advanced BC | |||||
| Wolff et al14 (HORIZON) | Placebo-controlled, randomized, Phase III | Postmenopausal AI-naïve, ER-positive, advanced BC, first-line (n=1,112) | Letrozole 2.5 mg daily, temsirolimus 30 mg daily, 5 days every 2 weeks or letrozole 2.5 mg daily + placebo | Median PFS 9 versus 8.9 months (comparable) | Median OS not reached (comparable) ORR 27% versus 27% SD at least 24 weeks 17% versus 19% |
| Bachelot et al16 (TAMRAD) | Randomized, open, Phase II | Postmenopausal AI-resistant, ER-positive, HER2-negative, metastatic BC (n=111) | Tamoxifen 20 mg daily + everolimus 10 mg daily or tamoxifen 20 mg daily | 6 month CBR 61% versus 42% (exploratory analysis) |
TTP 8.6 versus 4.5 months Risk of death HR 0.45 |
| Yardley et al19 (BOLERO-2) | Placebo-controlled, randomized Phase III | Postmenopausal NSAI-resistant, ER-positive, HER2-negative, advanced BC (n=724) | Exemestane 25 mg daily + everolimus 10 mg daily or exemestane 25 mg daily + placebo | Median PFS 7.8 versus 3.2 months investigator review difference statistically significant |
Median PFS 11 versus 4.1 months central review OS events 25.4% versus 32.2% ORR 12.6% versus 2.1% CBR 49.9% versus 22.2% |
| HER2-positive advanced BC | |||||
| O’Regan et al28 BOLERO-3 |
Placebo-controlled, randomized Phase III | HER2-positive advanced BC, prior taxane required, trastuzumab-resistant (n=569) | Vinorelbine 25 mg/m2 weekly + trastuzumab 2 mg/kg/week + everolimus 5 mg daily or vinorelbine 25 mg/m2 weekly + trastuzumab 2 mg/kg/week + placebo | Median PFS 7 versus 5.78 months (difference statistically significant) |
ORR 40.8% versus 37.2% CBR 59.2% versus 53.3% OS events 36.3% versus 41.1% |
| ER-positive BC : neoadjuvant therapy | |||||
| Baselga et al15 | Placebo-controlled, randomized Phase II | Postmenopausal operable ER-positive (n=270) | Letrozole 2.5 mg daily + everolimus 10 mg daily or letrozole 2.5 mg daily + placebo (4 months) | Clinical RR 68.1% versus 59.1% (difference statistically significant) | Antiproliferative response day 15 (downregulation of Ki67 expression) 57% versus 30% RR 58% versus 47% (ultrasound) RR 36.2% versus 39.4% (mammography) |
Abbreviations: ER, estrogen receptor; BC, breast cancer; RR, response rate; HER2, human epidermal growth factor 2 receptor; AI, aromatase inhibitor; PFS, progression-free survival; OS, overall survival; CBR, clinical benefit rate; TTP, time to progression; HR, hazard ratio; NSAI, non-steroidal aromatase inhibitor; ORR, overall response rate; SD, stable disease.
Table 2.
Side effects observed in key trials involving everolimus and temsirolimus
| Neoadjuvant letrozole ± everolimus 10 mg daily (Baselga et al 15)
|
TAMRAD tamoxifen ± everolimus 10 mg daily (Bachelot et al 16)
|
BOLERO-2 exemestane ± everolimus 10 mg daily (Yardley et al 19)
|
BOLERO-3 trastuzumab ± vinorelbine ± everolimus 5 mg daily (O’Regan et al 28)
|
HORIZON Letrozole ± temsirolimus 5/14 days (Wolff et al 14)
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| EVE | Placebo | EVE | Placebo | EVE | Placebo | EVE | Placebo | EVE | Placebo | |
| Stomatitis/mucositis | ||||||||||
| All grade | 36.5 | 6.1 | 56 | 7 | 59 | 12 | 63 | 28 | 26 | 4 |
| Grade 3–4 | 2.2 | 0 | 11 | 0 | 8 | 0 | 13 | 2 | 1 | <1 |
| Rash | ||||||||||
| All grade | 20.4 | 7.6 | 44 | 7 | 39 | 7 | 25 | 18 | 15 | 4 |
| Grade 3–4 | 0.7 | 0 | 4 | 0 | 1 | 0 | 0 | 1 | 1 | <1 |
| Fatigue/asthenia | ||||||||||
| All grade | 29.9 | 13.3 | 72 | 53 | 37 | 27 | 43 | 42 | 27 | 21 |
| Grade 3–4 | 1.5 | 0.8 | 6 | 11 | 4 | 1 | 12 | 4 | 3 | 2 |
| Diarrhea | ||||||||||
| All grade | NR | 39 | 11 | 34 | 19 | 38 | 31 | 21 | 9 | |
| Grade 3–4 | 2 | 0 | 2 | <1 | 4 | 1 | 2 | 1 | ||
| Nausea | ||||||||||
| All grade | NR | 35 | 35 | 31 | 29 | 35 | 37 | 16 | 16 | |
| Grade 3–4 | 4 | 0 | <1 | 1 | 3 | 1 | 1 | 1 | ||
| Decreased appetite/anorexia | ||||||||||
| All grade | 12.4 | 3.8 | 43 | 18 | 31 | 13 | 33 | 17 | 15 | 7 |
| Grade 3–4 | 0 | 0 | 7 | 4 | 1 | <1 | 1 | 1 | 1 | 1 |
| Pyrexia | ||||||||||
| All grade | NR | NR | 16 | 7 | 39 | 23 | 14 | 7 | ||
| Grade 3–4 | 3 | 1 | 1 | 1 | ||||||
| Weight decreased | ||||||||||
| All grade | NR | NR | 28 | 7 | NR | NR | ||||
| Grade 3–4 | 1 | 0 | ||||||||
| Cough | ||||||||||
| All grade | NR | NR | 26 | 12 | NR | 15 | 10 | |||
| Grade 3–4 | <1 | 0 | <1 | <1 | ||||||
| Dysgeusia | ||||||||||
| All grade | NR | NR | 22 | 6 | NR | NR | ||||
| Grade 3–4 | 0 | 0 | ||||||||
| Dyspnea | ||||||||||
| All grade | 7.3 | 1.5 | NR | 22 | 11 | NR | 13 | 10 | ||
| Grade 3–4 | 0.7 | 0 | 5 | <1 | 3 | 3 | ||||
| Peripheral edema | ||||||||||
| All grade | NR | NR | 21 | 6 | NR | 15 | 6 | |||
| Grade 3–4 | 1 | <1 | 0 | 0 | ||||||
| Epistaxis | ||||||||||
| All grade | NR | NR | 17 | 1 | NR | NR | ||||
| Grade 3–4 | 0 | 0 | ||||||||
| Hyperglycemia | ||||||||||
| All grade | 13.1 | 3 | NR | 14 | 2 | 9 | 5 | 13 | 5 | |
| Grade 3–4 | 5.1 | 0 | 5 | <1 | 6 | 3 | 4 | 1 | ||
| Pneumonitis | ||||||||||
| All grade | 2.9 | 0 | 17 | 4 | 16 | 0 | 6 | 3 | NR | |
| Grade 3–4 | 2.2 | 0 | 2 | 4 | 3 | 0 | <1 | 1 | ||
Abbreviations: EVE, everolimus; NR, not reported.
Clinical use of mTOR inhibitors
Dysregulation of the mTOR pathway leading to hyperactivation of the pathway is associated with a poor outcome in breast cancer. Cancer cells develop resistance to endocrine, cytotoxic, and HER2-targeted therapy through activation of the PI3K-AKT-mTOR pathway. This is the rationale for addition of mTOR inhibitors to endocrine therapy, chemotherapy, and antiHER2 therapy with the aim of enhancing efficacy and/or delaying resistance.
Sirolimus was the first rapalog used as an immunosuppressant agent to prevent rejection in organ transplantation. Rapalogs also have proven clinical benefit in eluting stents to prevent coronary artery reocclusion. Three first-generation rapalogs, ie, temsirolimus, everolimus, and ridaforolimus, have been evaluated in inhibition of cancer growth. They differ from the structure of the parent drug, sirolimus, at position C-42 and have more favorable pharmacokinetics. They all bind to FK506-binding protein 12 (FKBP12) and all preferentially inhibit the functions of mTOR complex 1 (mTORC-1).9 These drugs seem to have similar clinical activity and toxic effects, but with some differences in metabolism, formulation, and schedule of administration. The class-specific side effects are well known, and include stomatitis, noninfectious pneumonitis, infection, hyperglycemia, and dyslipidemia. Everolimus has been approved to overcome resistance to endocrine therapy, but there are a lot of clinical trials in progress combining mTOR inhibitors with various endocrine, targeted, or cytotoxic drugs trying to overcome resistance to therapy.
Clinical studies evaluating mTOR inhibitors in breast cancer
Ridaforolimus
Ridaforolimus is an analog of sirolimus and has been administered orally in breast cancer trials at a dose of 40 mg/day for 5 days per week. This drug is still investigational. In sarcoma, the drug offers moderate benefit as maintenance therapy after chemotherapy,10 but it has not yet been approved by the US Food and Drug Administration or European Medicines Agency in this indication. No randomized Phase II or III data are available in breast cancer. Two nonrandomized Phase II trials have finished recruitment, but the results are not yet available (oral deforolimus with trastuzumab for patients with HER2-positive, trastuzumab-refractory metastatic breast cancer, NCT00736970; and a study of ridaforolimus in combination with dalotuzumab compared with standard of care treatment in ER-positive breast cancer patients, NCT01234857).11
Temsirolimus
Temsirolimus is currently approved for advanced renal cell carcinoma. The primary active metabolite of this prodrug is rapamycin. Temsirolimus is administered either orally or intravenously.
Temsirolimus weekly as a 30-minute intravenous infusion at a dose of 75 mg or 250 mg was evaluated in a randomized, open-label trial in 109 patients presenting with locally advanced or metastatic breast cancer.12 An objective response rate of 9.2% was reported in these heavily pretreated patients. The median time to progression was 12 weeks. Similar efficacy was observed independent of the dose, but side effects were more frequent at the higher 250 mg dose.
A three-arm randomized Phase II trial evaluated the safety and activity of oral temsirolimus in combination with letrozole, a nonsteroidal aromatase inhibitor.13 The analysis was restricted to 92 patients included after amendment implementing a lower dose at treatment initiation (letrozole alone, 29 patients; letrozole + temsirolimus daily 10 mg, 33 patients; letrozole + intermittent temsirolimus 30 mg daily during 5 days every 14 days, 30 patients) and suggested that both combined treatment arms had better median progression-free survival (not reached at time of reporting in the abstract) compared with the median progression-free survival of 9.2 months in the letrozole alone arm. Based on these encouraging results, a Phase III trial evaluating intermittent temsirolimus combined with letrozole has been initiated.
An ongoing Phase I–II trial is evaluating temsirolimus in combined treatment for metastatic HER2-positive or triple-negative breast cancer (Temsirolimus plus neratinib for patients with metastatic HER2-amplified or triple-negative breast cancer, NCT01111825).11
Phase III trial
A randomized placebo-controlled Phase III study (HORIZON) tested the efficacy and safety of first-line oral letrozole 2.5 mg daily + temsirolimus 30 mg intermittent daily (5 days every 2 weeks) compared with letrozole + placebo in 1,112 patients with aromatase inhibitor-naive advanced breast cancer.14 The study was prematurely closed for futility at the preplanned second interim analysis performed after 382 median progression-free survival events. The median progression-free survival (9 and 8.9 months), objective response rate (27% both arms), and clinical benefit rate (17% and 19%) were all similar in both arms of this trial. More grade 3 or 4 toxicities were seen in the letrozole + temsirolimus arm (37% versus 24%). The median progression-free survival was also not increased by adding temsirolimus in the 40% of patients who had previously received endocrine therapy in the adjuvant setting. An exploratory analysis suggested improved median progression-free survival favoring letrozole + temsirolimus in postmenopausal patients aged 65 years or younger. Persisting ovarian function in these younger patients and its detrimental effect in the letrozole alone arm have been given as a potential explanation for this finding by the authors. Unfortunately, blood samples are not available to prove this hypothesis. These disappointing results are in contrast with those of BOLERO-217 (Breast cancer trials of OraL EveROlimus, see below). The major difference between the two trials is that, in the temsirolimus trial, no patient had previously received an endocrine therapy for advanced breast cancer. Only 40% of the patients received previous endocrine therapy in the adjuvant setting. The authors can only assume as the data were not prospectively collected that the patients received tamoxifen, taking into account that the median duration of adjuvant endocrine therapy was 34 months, that the median time since last endocrine therapy was 5 months, and that no patient had received an adjuvant aromatase inhibitor within 12 months of study entry. The high number of HER2-positive tumors (23% and an additional 36% of tumors with unknown status in the temsirolimus arm) has probably also contributed, because these patients need an anti-HER2 agent in the treatment regimen according to our current knowledge. Also, intermittent administration may not be the best schedule for optimal mTOR inhibition. Nevertheless, mTOR inhibitor-related side effects such as stomatitis have been observed in many patients, suggesting that the target had been inhibited even with this intermittent schedule. Consequently, the difference in patient characteristics is probably the most important factor explaining these negative results.
Everolimus
Proof-of-concept Phase II trials in ER-positive, HER2-negative breast cancer
Two proof-of-concept studies have shown that everolimus combined with an endocrine treatment is associated with a better outcome compared with the same endocrine therapies used alone. Baselga et al evaluated the combination of letrozole 2.5 mg/day and everolimus 10 mg/day in the neoadjuvant setting in a randomized, placebo-controlled Phase II trial which enrolled 270 postmenopausal women.15 Neoadjuvant treatment was planned to be given over 4 months. The primary endpoint, response rate by clinical examination, was significantly improved from 59.1% to 68.1% in the combined treatment arm. The patients had mandatory biopsies at baseline and at day 15, allowing key biomarker analyses as a secondary endpoint. Patients in both treatment arms showed a marked reduction in progesterone receptors and cyclin D1. A major reduction in phospo-S6 was only seen in the everolimus arm. Further, an antiproliferative response based on a reduction in Ki67 expression was more frequently seen in the everolimus arm than in the placebo arm (57% versus 30%, respectively). The relationship between specific phosphatidylinositol-4,5-bisphosphate 3-kinase, catalytic subunit alpha (PI3KCA) mutations and Ki67 was also studied, but the small sample size has to be pointed out. With this important limitation in mind, it is interesting to note that exon 9 mutants, but not exon 20 mutants, had a rather poor antiproliferative response based on Ki67 proliferation marker expression in the placebo arm but a good response similar to all the other patients, including also patients with exon 20 mutations in the everolimus arm. TAMRAD (tamoxifen plus everolimus) is the other important proof-of-concept study.16 This is a small, open-label, randomized Phase II study in the metastatic setting. One hundred and eleven postmenopausal patients have been randomized to receive either the antiestrogen agent tamoxifen 20 mg/day + everolimus 10 mg/day or tamoxifen alone. All patients had previously received an aromatase inhibitor either in the adjuvant or metastatic setting. Randomization was stratified according to primary or secondary resistance to aromatase inhibitor therapy. Primary resistance was defined as relapsing during or within 6 months of stopping adjuvant aromatase inhibitor treatment or progressing within 6 months of starting aromatase inhibitor treatment in the metastatic setting. The primary endpoint was clinical benefit rate, ie, objective response or stable disease for at least 6 months according to RECIST17 (Response Evaluation Criteria In Solid Tumors) version 1.0. The clinical benefit rate was higher in the combined treatment arm than in the tamoxifen alone arm (61% versus 42%). Concerning secondary endpoints, the median time to progression (4.5 months versus 8.6 months) and overall survival (hazards ratio [HR] 0.45) were also longer in the combined treatment arm. Interestingly, in an exploratory subgroup analysis, it has been shown that patients with secondary endocrine resistance had a more pronounced benefit than patients with primary resistance when everolimus was added to tamoxifen.
Phase III trial: BOLERO-2
Although these proof-of-concept trials are very important, the practice of oncology can only be changed based on randomized Phase III trials. Everolimus is now approved for the treatment of ER-positive, HER2-negative advanced breast cancer in combination with exemestane in patients resistant to nonsteroidal aromatase inhibitors by health authorities in Europe and the USA based on the BOLERO-2 trial (Table 3).18,19
Table 3.
Key messages concerning the BOLERO-2 trial
| Compared with exemestane alone, everolimus + exemestane improves median progression-free survival (3.2 months versus 7.8 months) in the treatment of estrogen receptor-positive, HER2-negative advanced breast cancer resistant to nonsteroidal aromatase inhibitor therapy |
| The benefit is consistent among all prespecified clinical subgroups |
| Side effects are manageable. Patient education and appropriate dose modification according to existing guidelines are indicated |
| The most frequent clinically significant side effect is stomatitis. The most medically important side effect is noninfectious pneumonitis |
Abbreviations: BOLERO, Breast cancer trials of OraL EveROlimus; HER2, human epidermal growth factor receptor 2.
This was a randomized, placebo-controlled Phase III trial comparing exemestane 25 mg/day + everolimus 10 mg/day with exemestane and placebo in 724 patients previously treated with a nonsteroidal aromatase inhibitor in the adjuvant or advanced setting. All patients suffered from recurrence of breast cancer during or within 12 months after the end of adjuvant treatment or progression during or within 1 month after the end of treatment for advanced disease. It is important to point out that patients who fail treatment with a nonsteroidal aromatase inhibitor have a very poor prognosis with standard endocrine therapy alone. This has been shown in the EFECT (Evaluation of Faslodex versus Exemestane Clinical Trial) and SoFEA (Study Of Faslodex with or without concomitant arimidex vs Exemestane following progression on non-steroidal Aromatase inhibitors) trials.20,21 The median progression-free survival was less than 5 months with fulvestrant (250 mg every 4 weeks) or exemestane in both trials. Combined endocrine therapy of anastrozole + fulvestrant (at the 250 mg dose) does not perform better compared with exemestane or fulvestrant monotherapy.21 These disappointing results illustrate well the unmet medical need in this patient population. The BOLERO-2 trial met its primary endpoint of median progression-free survival according to local assessment performed every 6 weeks. At the interim analysis, median progression-free survival was 6.9 months in the everolimus + exemestane arm compared with only 2.8 months in the placebo + exemestane arm. These results were confirmed when the final progression-free survival results were presented (median progression-free survival 7.8 and 3.2 months, respectively).19
Randomization was stratified according to the presence of visceral metastasis and previous sensitivity to endocrine therapy. Endocrine-sensitive patients were defined as having received at least 24 months of endocrine therapy before recurrence in the adjuvant setting or having presented a response or stabilization for at least 24 weeks of endocrine therapy for advanced disease. Visceral involvement was present in 56% of all patients and hormone-sensitive disease in 84%. Importantly, the improvement in median progression-free survival was consistent among all predefined subgroups including (but not limited to) age, race, baseline performance status, progesterone receptor status, prior chemotherapy for advanced disease, prior endocrine therapy other than a nonsteroidal aromatase inhibitor, presence of visceral disease, bone only disease, and sensitivity to prior endocrine therapy.19
Of course, the absolute benefit was less pronounced in poor prognostic subgroups but the relative benefit was very similar in all subgroups. For example, median progression-free survival according to local assessment increases from 2.76 months to 6.83 months in patients with visceral disease, from 4.21 months to 9.86 months in patients without visceral disease, and from 5.29 months to 12.88 months in patients with bone only disease. Very interestingly, the median progression-free survival increases from 4.17 months to 11.7 months in patients having received nonsteroidal aromatase inhibitor therapy in the adjuvant setting but having not yet received any treatment for advanced disease. This important absolute benefit is observed in patients who just failed one line of endocrine therapy. Patients do not need to be heavily pretreated before benefiting from the combined treatment approach. BOLERO-2 has shown that everolimus + exemestane offers an important alternative to chemotherapy for the large subset of patients who do not have life-threatening visceral metastatic disease.22 The use of chemotherapy can be postponed in most patients with visceral metastases.
Concerning the secondary endpoint, analysis of progression-free survival based on central radiologic assessment, a 6.9-month prolongation in median progression-free survival from 4.1 months in the placebo + exemestane arm to 11 months in the everolimus + exemestane arm was observed.19 We are eagerly awaiting the results for overall survival. Mature data are expected to be presented in 2014. The absolute difference in deaths was increasing progressively since the first interim analysis of median progression-free survival, and was 6.8% (25.4% versus 32.2%) at last update at time of final progression-free survival analysis, indicating a better outcome in the everolimus + exemestane arm, but this difference is not yet statistically significant.19
Future clinical trials in the adjuvant setting
Two investigator-initiated studies will evaluate the role of everolimus in the adjuvant setting (Safety study of adding everolimus to adjuvant hormone therapy in women with poor prognosis, ER-positive and HER2-negative primary breast cancer, free of disease after receiving 3 years of adjuvant hormone therapy, NCT01805271; S1207 hormone therapy with or without everolimus in treating patients with breast cancer, NCT01674140).11 In Europe, the UNICANCER group in France has started a large placebo-controlled Phase III trial expected to recruit 2,010 premenopausal or postmenopausal women suffering from ER-positive, HER2-negative breast cancer presenting at least four lymph nodes involved at diagnosis. Patients initially receive routine standard adjuvant therapy for 2–3 years. Only patients still relapse-free after 2–3 years of adjuvant therapy can enter the trial. Patients then receive everolimus or placebo for 2 years in addition to tamoxifen or aromatase inhibitor therapy. The primary endpoint is disease-free survival at 2 years post-randomization, and secondary endpoints include overall survival, biomarker assessments, and safety. The investigators have chosen this design with everolimus treatment starting only after some years of endocrine therapy because their previous TAMRAD study has shown in an exploratory analysis that patients with secondary endocrine resistance had a much more pronounced benefit compared with patients with primary resistance when everolimus is added to tamoxifen (see above).16
The other large placebo-controlled Phase III trial is organized by the Southwest Oncology Group and National Surgical Adjuvant Breast and Bowel Project.11 In this trial, everolimus or placebo is given in combination with standard endocrine therapy as soon as the patient starts endocrine therapy for a total duration of 1 year. A total of 3,500 high-risk patients are expected to be recruited into this trial. High risk is defined as an Oncotype DX® recurrence score over 25 or at least four lymph nodes involved.23 Use of chemotherapy in the adjuvant or neoadjuvant setting is mandatory in this study in contrast with the European study. The primary endpoint is invasive disease-free survival. Secondary endpoints include overall survival, distant recurrence-free survival, biomarker assessments, and safety. Compliance with the experimental treatment arm in both studies may be an issue because combined treatment with everolimus is more toxic than endocrine therapy alone. Everolimus has never been evaluated in the adjuvant setting in any tumor type. Consequently, no data concerning the acceptance rate in the adjuvant setting are available. In the neoadjuvant setting, twice as many patients discontinued everolimus + letrozole compared with placebo + letrozole (18.8% versus 9.1%).15 If a significant number of patients, for personal reasons, stop all adjuvant treatment including standard endocrine therapy in the case of poor tolerance of everolimus this may have a significant impact on the results in the experimental arm, in particular in the trial performed in the USA where everolimus or placebo is given as soon as endocrine therapy starts.
What is the efficacy of everolimus in the first-line setting in patients who have never been exposed to endocrine therapy? Should we change endocrine therapy and continue the mTOR inhibitor at time of progression?
BOLERO-4 (Open-label, Phase II, study of everolimus plus letrozole in postmenopausal women with ER-positive, HER2-negative metastatic or locally advanced breast cancer, NCT01698918)11 is a nonrandomized trial evaluating the first-line effectiveness of everolimus 10 mg/day in combination with letrozole 2.5 mg/day in endocrine therapy-naïve patients presenting advanced breast cancer. The first-line use of everolimus in endocrine-naïve patients is still the subject of major debate after the negative first-line temsirolimus trial.14 At the time of disease progression, patients have the option to continue everolimus at the same dose combined with exemestane 25 mg/day instead of letrozole. Given that this is a nonrandomized trial, the results can only be hypothesis-generating, but the usefulness of continuing mTOR inhibition after progression during mTOR therapy in particular is a very relevant clinical question. In HER2-positive disease, we give several lines of trastuzumab-based treatment. It is worthwhile to evaluate if this kind of approach is also of interest for agents blocking the PI3K-AKT-mTOR pathway. This concept has also been validated in colorectal cancer, where antiangiogenic drugs are continued beyond progression although a different antiangiogenic drug is used after progression. The administration of subsequent lines of endocrine therapy in metastatic ER-positive breast cancer is also standard of care. Another important question is whether, in the event of progressive disease, the optimal treatment should be continuation of the same mTOR inhibitor or if another drug targeting the PI3K-AKT-mTOR pathway should be administered in addition to a modified endocrine therapy. The latter strategy is being evaluated for example in the BELLE-3 trial (A Phase III study of BKM120 with fulvestrant in patients with hormone receptor-positive, HER2-negative, aromatase inhibitor treated locally advanced or metastatic breast cancer who progressed on or after mTOR inhibitor, NCT01633060)11 where patients who have failed everolimus + exemestane are randomized to receive fulvestrant ± BKM120, a PI3K inhibitor.
Another question is whether administration of everolimus should be considered again in a later line of therapy after use of other drugs targeting the PI3K-AKT-mTOR pathway rather than at the time of progressive disease during treatment with everolimus.
Is everolimus + exemestane at least as effective as capecitabine after failure of a nonsteroidal aromatase inhibitor? Is everolimus alone as effective as combined therapy with exemestane?
Another Phase II open-label but randomized trial is BOLERO-6 (A Phase II study of everolimus in combination with exemestane versus everolimus alone versus capecitabine in advanced breast cancer, NCT01783444).11 A total of 300 postmenopausal patients with progressive disease during or soon after nonsteroidal aromatase inhibitor therapy will receive either everolimus + exemestane, everolimus alone, or capecitabine. The primary endpoint is progression-free survival for the comparison of everolimus + exemestane versus exemestane monotherapy based on local radiologic assessment performed every 6 weeks. Comparison of everolimus + exemestane versus capecitabine is the key secondary endpoint. Overall survival, objective response rate, clinical benefit rate, safety, quality of life, patient treatment satisfaction, and potential biomarkers that may predict sensitivity to everolimus will also be evaluated. Although everolimus has some single-agent activity, our current understanding is that we should target both the estrogen signaling pathway and the PI3K-AKT-mTOR pathway. This trial will give additional information concerning the single-agent activity of everolimus. It is reasonable to expect that the combined approach is more effective and, as exemestane is in general well tolerated in patients who have already received a nonsteroidal aromatase inhibitor, we do not expect that everolimus monotherapy will be used in breast cancer in the future, although this is the case for example in renal cell carcinoma. The comparison of everolimus + exemestane with capecitabine is much more interesting from a clinical point of view, although of course this is just a secondary endpoint in a Phase II trial. Nevertheless, it will be very interesting to see a head-to-head comparison between a standard oral chemotherapy and a combined treatment approach between an endocrine agent and everolimus, particularly in patients suffering from visceral metastases. Randomization is stratified according to visceral involvement in this trial.
BOLERO-3: Phase III trial in HER2-positive disease
Nonrandomized Phase I/II trials have shown everolimus to have promising activity when combined with other agents, ie, either trastuzumab alone or combined with trastuzumab and chemotherapy (paclitaxel or vinorelbine) in HER2-positive disease.24–27
O’Regan et al presented the final progression-free survival analysis of the BOLERO-3 study28 at the 2013 American Society of Clinical Oncology meeting. This randomized, double-blind, placebo-controlled, multicenter Phase III trial compared weekly trastuzumab + vinorelbine and everolimus at the dose of 5 mg with weekly trastuzumab + vinorelbine and placebo. A total of 569 patients with advanced breast cancer who had previously failed trastuzumab and a taxane were recruited into this trial. Progressive disease was either observed during treatment with trastuzumab in the adjuvant or metastatic setting or during the 12 months following completion of trastuzumab therapy in the adjuvant setting (Table 4). The trial met its primary endpoint progression-free survival. Indeed, the median progression-free survival increases from 5.78 months to 7 months in the experimental arm. More stomatitis and neutropenic fever were observed in the experimental arm. Stomatitis was also the reason why in the Phase I/II trial the everolimus dose had not been increased to the standard 10 mg dose which allows optimal inhibition of the mTOR pathway.27 Subgroup analysis revealed a more pronounced benefit in patients who previously received trastuzumab in the adjuvant or neoadjuvant setting (HR 0.65), in those without visceral involvement (HR 0.48), and in those with ER-negative disease (HR 0.65). Neither the objective response rate nor the clinical benefit rate have been improved by addition of everolimus. However, very interestingly, only 36.3% in the everolimus arm compared with 41.1% in the placebo arm had died at the time of the interim overall survival analysis. We are now waiting for the mature overall survival data in order to determine if this trend will result in a statistically significant survival benefit.
Table 4.
Key messages concerning the BOLERO-3 trial
| The trial met its primary endpoint: median progression-free survival is increased when everolimus is added to trastuzumab and vinorelbine compared with placebo, trastuzumab, and vinorelbine |
| The average benefit in median progression-free survival is only 5 weeks, indicating the need to identify patients who benefit most |
| The benefit is more pronounced in estrogen receptor-negative tumors (hazards ratio 0.65) |
| New treatment approaches are needed for HER2-positive, estrogen receptor-positive tumors. In particular, a treatment strategy including an endocrine agent should also be evaluated |
Abbreviations: BOLERO, Breast cancer trials of OraL EveROlimus; HER2, human epidermal growth factor receptor 2.
Expected additional study results in the near future
In 2014 we expect the results of the BOLERO-1 study (Everolimus in combination with trastuzumab and paclitaxel in the treatment of HER2-positive locally advanced or metastatic breast cancer, NCT00876395)11 evaluating everolimus in less heavily pretreated HER2-positive breast cancer patients. This trial is comparing everolimus at a dose of 10 mg daily combined with weekly trastuzumab and paclitaxel with placebo and weekly trastuzumab and paclitaxel in the first-line advanced disease setting.
Triple-negative disease
Triple-negative breast cancers are very heterogeneous and represent a very aggressive subtype of breast cancer that lacks expression of ER and HER2. It is a real challenge to find specific systemic therapies according to targets present in the tumor with the aim of improving the prognosis of triple-negative breast cancer. Immunohistochemistry analyses have shown that activated mTOR is more frequently observed in triple-negative breast cancer (36%) than in other subtypes of breast cancer and is associated with poor outcome.29,30 Loss of PTEN or PI3KCA mutations have also been reported in triple-negative breast cancer, but less frequently compared with other breast cancer subtypes.31 Many clinical trials have evaluated mTOR inhibitors in triple-negative breast cancer (Table 5), but few results have been reported.11 Preliminary results of a study in the neoadjuvant setting were presented at the 2013 San Antonio Breast Cancer Symposium.32 The pathologic complete response rate was not improved by adding everolimus to the standard neoadjuvant regimen in the control arm.
Table 5.
Ongoing trials with everolimus in triple-negative breast cancer
| Study title | ClinicalTrials.gov identifier |
|---|---|
| Phase Ib/II trials of RAD001 in triple-negative metastatic breast cancer | NCT01939418 |
| A study of lapatinib in combination with everolimus in patients with advanced, triple-negative breast cancer | NCT01272141 |
| NECTAR: everolimus plus cisplatin in triple-negative breast cancer | NCT01931163 |
| Temsirolimus plus neratinib for patients with metastatic HER2-amplified or triple-negative breast cancer |
NCT01111825 |
| Study of temsirolimus, erlotinib, and cisplatin in solid tumors | NCT00998036 |
| Cisplatin and paclitaxel with or without everolimus in treating patients with stage II or stage III breast cancer | NCT00930930 |
| RAD001 plus carboplatin in breast cancer patients | NCT01127763 |
| Cisplatin, paclitaxel, and everolimus in treating patients with metastatic breast cancer | NCT01031446 |
| Trial of RAD001 in triple-negative metastatic breast cancer | NCT00827567 |
| Phase I/II study of weekly nab-paclitaxel and RAD001 in women with locally advanced or metastatic breast cancer | NCT00934895 |
| Study to compare vinorelbine in combination with the mTOR inhibitor everolimus versus vinorelbine monotherapy for second-line treatment in advanced breast cancer | NCT01520103 |
| Trial of paclitaxel/bevacizumab ± everolimus for patients with HER2-negative metastatic breast cancer | NCT00915603 |
| Efficacy of RAD001 in breast cancer patients with bone metastases | NCT00466102 |
| Paclitaxel followed by FEC versus paclitaxel and RAD001 followed by FEC in women with breast cancer | NCT00499603 |
Abbreviations: FEC, fluorouracil, epirubicin, and cyclophosphamide; HER2, human epidermal growth factor receptor 2.
Nonresponsive HER2-negative tumors treated by neoadjuvant chemotherapy
Huober et al33 reported a study evaluating the role of everolimus in addition to paclitaxel as a drug resistance-modulating agent in patients with HER2-negative tumors not responding to initial neoadjuvant cytotoxic therapy (epirubicin + cyclo-phosphamide) combined with or without antiangiogenic (bevacizumab) therapy. Patients without a clinical response were randomized to receive weekly paclitaxel (80 mg/m2) with or without everolimus (5 mg daily after a stepwise dose escalation starting from 2.5 mg every other day; daily full dose starting day 13) for 12 weeks. A total of 403 patients were randomized. The number of patients randomized in this substudy was lower than expected, and consequently, this substudy is unfortunately underpowered. The hypothesis was that adding everolimus to paclitaxel improves the pathologic complete response, defined as no invasive and no noninvasive residuals in breast and nodes from 5% to 12.1%; 566 patients had to be recruited to confirm this hypothesis. A total of 18 (4.6%) patients, ie, seven (3.6%) treated with paclitaxel and everolimus and eleven (5.6%) treated with paclitaxel alone had a pathologic complete response. This trial indicates that neoadjuvant therapy with everolimus and paclitaxel for patients with HER2-negative disease unresponsive to epirubicin-based and cyclophosphamide-based chemotherapy, with or without bevacizumab, did not improve the pathologic complete response rate. However, longer followup is needed to test the impact on other endpoints, such as progression-free survival and overall survival. Impact on the pathologic complete response rate is not necessarily the best endpoint for evaluating the potential role of everolimus in this specific patient population. Breast-conserving treatment was performed in 54.4% of patients with the combination treatment and in 61.9% of those receiving paclitaxel alone (difference not statistically different). As expected, side effects (including mucosal inflammation, thrombocytopenia, neutropenia, infection, diarrhea, allergic reactions, and skin rash) were more frequent when everolimus was added. Chemotherapy was discontinued in 11.6% of patients treated with paclitaxel alone and in 21.8% of patients treated with paclitaxel and everolimus.
Side effects of mTOR inhibitors in key Phase III studies and impact on quality of life
Temsirolimus
In the HORIZON trial (letrozole + intermittent temsirolimus versus letrozole + placebo), treatment-emergent adverse events were more frequently seen in the temsirolimus arm (91% versus 79%).14 All grade side effects included asthenia (27% versus 21%), mucositis/stomatitis (26% versus 4%), diarrhea (21% versus 9%), headache (19% versus 12%), anorexia (15% versus 7%), and rash (15% versus 4%). Grade 3 or 4 toxicities were also more frequent in the temsirolimus arm (37% versus 24%), and included hyperglycemia (4% versus 1%), diarrhea (2% versus 1%), mucositis/stomatitis (2% versus 1%), and hyperlipidemia (2% versus 1%). Permanent dose reduction (4% versus 1%) and therapy discontinuation were also more frequent in the experimental arm. No increase in noninfectious pneumonitis was reported in the experimental arm. The authors suggested that the overall much lower frequency of toxicities in HORIZON compared with BOLERO-2 can be related to a less heavily pretreated patient population. However, less effective inhibition of the mTOR pathway, in particular with intermittent administration, should be considered as an alternative explanation. The mean relative dose intensity of temsirolimus was very high (0.96). The impact of side effects on quality of life was not reported in this trial.
Everolimus
In the BOLERO-2 (exemestane + everolimus versus exemestane + placebo), serious adverse events related to study treatment were much more frequent in the everolimus arm (12% versus 1%).17 Also, a higher percentage of patients discontinued everolimus compared with placebo in the control group because of adverse events (19% versus 4%) or withdrawal of consent (5% versus 2%). Seven deaths were attributed to treatment in the everolimus arm. The most common grade 3 or 4 adverse events were stomatitis (8% versus 1%), anemia (6% versus <1%), dyspnea (4% versus 1%), hyperglycemia (4% versus <1%), fatigue (4% versus 1%), and pneumonitis (3% versus 0%). All grade adverse events independent of relationship to study treatment were also in general more frequent in the everolimus arm, ie, stomatitis (56% versus 11%), rash (36% versus 6%), fatigue (36% versus 29%), diarrhea (30% versus 16%), decreased appetite (29% versus 10%), cough (22% versus 11%), and dyspnea (18% versus 9%).
Quality of life was an important secondary endpoint in BOLERO-2 and was assessed by the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire-Core 30 (EORTC QLQ-C30) done at baseline and thereafter every 6 weeks until disease progression and/or discontinuation of treatment.34 Time to definitive deterioration analysis at a 5% decrease in the quality of life score versus baseline, with no subsequent increase above this threshold, was evaluated prospectively. The median time to definitive deterioration in quality of life was 8.3 months in the combined treatment arm versus 5.8 months in the control arm (HR 0.74; P=0.0084). The authors reported an additional sensitivity analysis using a 10-point minimal important difference decrease in the global health status score versus baseline. The median time to definitive deterioration in the combined treatment arm was 11.7 months versus 8.4 months in the control arm (HR 0.80; P=0.1017). These results show that quality of life was at least maintained in the experimental arm and that side effects were largely compensated by a better antitumoral effect in patients receiving everolimus in addition to exemestane.
In the BOLERO-3 trial (trastuzumab + vinorelbine + everolimus versus trastuzumab + vinorelbine + placebo), more patients discontinued treatment because of adverse events (10% versus 5%) and because of withdrawal of consent (6% versus 5%) in the everolimus arm.28 The median relative dose intensity was 0.77 for everolimus and 0.96 for placebo. The median relative dose intensity for vinorelbine was lower in the experimental arm (0.64 versus 0.73). More frequent all grade adverse events in the everolimus arm included stomatitis (63% versus 28%), pyrexia (39% versus 23%), decreased appetite (33% versus 17%), and febrile neutrope-nia (17% versus 4%). Several grade 3 toxicities were also more frequent in the everolimus arm, and included stomatitis (13% versus 1%), fatigue (12% versus 4%), diarrhea (4% versus 1%), hyperglycemia (grade 3, 4% versus 2%; grade 4, 2% versus 1%), and nausea (3% versus 1%), but not noninfectious pneumonitis (<1% versus 1%). The incremental toxicity observed in the everolimus arm compared with the placebo arm did not impact quality of life. The time to definitive deterioration of global health status score based on the EORTC QLQ-C30 questionnaire and defined as at least a 10% change from baseline was 8.31 months in the everolimus arm and 7.29 months in the placebo arm.
Management of side effects
Dose interruption and/or modification are key elements for the management of side effects. In general, the dose interruption and/or modification guidelines used in the key clinical trials discussed above should be used in routine care. Patient education is also very important in our opinion. When we start everolimus in our center, we inform our patients concerning early symptoms and optimal management, in particular for stomatitis and noninfectious pneumonitis. The patients are also invited to contact us as soon as any significant problem appears. We have the advantage that most of our patients are living close to our hospital (most often within 30 km). Concerning stomatitis, all our patients receive a prescription for a corticosteroid and analgesic-containing mouth bath to be started as soon as even mild stomatitis appears. As discussed above, some trials are evaluating even prophylactic mouth baths and some colleagues have reported very good results with this approach (unpublished data), but we do not use mouth baths prophylactically in our center. Others recommend a rapid progressive increase in the everolimus dose before reaching the recommended 10 mg daily. However, neither the efficacy nor the impact on the incidence of stomatitis has been evaluated prospectively within a clinical trial and consequently we do not recommend this approach. In patients with more severe pain we consider daily local laser therapy because we have observed major benefit on pain, rapidly after therapy. We use this approach routinely, although we have to admit that this approach has also not been prospectively evaluated within a clinical trial. In the event of grade 2 stomatitis (symptomatic patient, but can eat and follow a modified diet) we do a transient dose interruption. After recovery to grade 1, we restart at the 10 mg dose. It is not unusual to see that the patients are now able to tolerate the 10 mg dose, although some will present a relapse of grade 2 stomatitis, and after a second dose interruption we restart everolimus in these patients at a reduced dose of 5 mg. Patients are educated in our center about good oral hygiene. We suggest that they avoid spicy food. We ask that they come back immediately to the clinic if they develop more extensive stomatitis (more than three lesions), if the lesions are lasting over 3 days, and if they interfere with eating and drinking. In our experience, grade 3 stomatitis (a symptomatic patient who is unable to adequately aliment or hydrate orally) can be avoided by patient education. We also explain to our patients that, based on the experience in BOLERO-2, stomatitis generally starts within the first month, and ask them to come back for a routine visit in the office at day 15, and during this visit we check in particular if the patient has any sign of early onset of stomatitis.
All our patients are aware that cough and dyspnea can be the first symptoms related to noninfectious pneumonitis. They are asked to come back immediately to our office if they develop any significant cough and/or dyspnea in order to perform further work-up. They are also informed that, based on the BOLERO-2 trial experience, noninfectious pneumonitis can occur at any time point during treatment with everolimus. We perform a chest computed tomography (CT) scan routinely before starting everolimus in all patients in order to have a baseline CT that can be used for comparison if the patient presents later with any abnormality on chest CT. As recommended in the guidelines, we do not interrupt treatment or adapt the dose in patients with grade 1 noninfectious pneumonitis (asymptomatic patient, radiographic finding only), but immediately interrupt everolimus in a symptomatic patient suffering from noninfectious pneumonitis. After recovery to grade 1 noninfectious pneumonitis, we try to restart at a reduced dose of 5 mg. In symptomatic patients, we consider high-dose corticosteroid therapy (at least 1 mg/kg/day methylprednisolone) because this approach generally allows very rapid improvement of the symptoms. We recommend continuing high-dose corticosteroids for at least 4 weeks because we have seen rapid recurrence of severe noninfectious pneumonitis in a patient after a short course of corticosteroid therapy.
We have no specific recommendations for the management of the other common everolimus-related side effects, and simply follow the available guidelines at our center.
What is the optimal dose: 5 mg or 10 mg daily?
Ravaud et al35 performed a meta-analysis of clinical trials in oncology in order to evaluate the potential relationship between everolimus exposure and safety and efficacy. Previous studies have shown that maximum everolimus concentrations are reached 1–2 hours after administering 5–70 mg oral doses,36 maximum everolimus concentrations increase in a dose-proportional manner between 5 mg and 10 mg,36 continuous 5–10 mg once-daily dosing enables steady state to be achieved within 1 week,36 and the minimum concentration demonstrates a linear dose-through relationship.37 Individual patient data from five Phase II or Phase III studies, in which steady-state, predose pharmacokinetic samples were taken from patients with solid tumors receiving 10 mg daily, were pooled in the meta-analysis.35 No patients with breast cancer were included. Efficacy and safety were evaluable for 945 and 938 patients, respectively. A twofold increase in the minimum concentration of everolimus increased the probability of tumor size reduction (odds ratio 1.4), was associated with a trend for reduced risk of progression-free survival events (risk ratio [RR] 0.9), and increased the risk of at least grade 3 pulmonary toxicity (RR 1.93), stomatitis (RR 1.49), and metabolic toxicity (RR 1.3). A very important remaining question is whether experiencing an adverse event is associated with improved efficacy. The data reported in the meta-analysis suggest that this is indeed the case, because increased exposure to everolimus is associated with both increased efficacy and a higher rate of high-grade toxicities. Future studies should consider assessing the everolimus concentration at the time of occurrence of adverse events.
This meta-analysis, although not in the field of breast cancer, clearly suggests a dose-dependent antitumor effect of everolimus. Some clinicians suggest starting at 5 mg instead of the recommended 10 mg daily dose. The data available are definitely against this approach. The optimal dose can only be further explored within a randomized prospective clinical trial. For the routine care of our patients, we have to respect the approved dose and regimens. Dose reductions should only be performed according to management guidelines in patients presenting some specific high-grade toxicities. All patients should receive everolimus 10 mg in combination with exemestane based on the BOLERO-2 trial.
Biomarker data in key Phase II and III studies
Everolimus
TAMRAD
Sixty-six formalin-fixed paraffin-embedded primary breast tumors were retrospectively collected in the TAMRAD study.38 Data for 55 patients (50% of the intention-to-treat patient population) were finally available, and biomarkers in the canonical and metabolic pathways as well as downstream effectors evaluating pathway activation were presented at the 2013 American Society of Clinical Oncology meeting. The results suggest that high phosphorylated 4E-binding protein 1 (p4EBP-1), low liver kinase B1 (LKB1), and low PI3K are associated with higher efficacy of mTOR inhibition in these tumors. A prospective validation of these hypothesis-generating results is needed in an independent patient cohort.
BOLERO-2
At the same American Society of Clinical Oncology meeting, Hortobagyi et al39 reported biomarker data from BOLERO-2.40 A large panel of oncogenes and tumor suppressor genes were sequenced using next-generation sequencing of 309 formalin-fixed paraffin-embedded archival tissue samples. Successful analysis was obtained in 227 samples, representing 32% of the intention-to-treat patient population. No major baseline clinical or demographic differences were observed between the intention-to-treat and the next-generation sequencing populations. Clinical efficacy was also comparable between these populations. There were 1,476 sequence alterations, including 1,222 missense mutations. There were 548 copy number alterations, including 522 amplifications (at least six copies). Genetic alterations were most frequently seen in PI3KCA (47.6%, mostly missense mutations), cyclin D1 (CCND1) (31.3%, gene amplifications), TP53 (23.3%, missense and other sequence alterations), and FGFR1 (18.1%, mostly gene amplifications). Many other less frequent genetic alterations were also reported in other genes, some in the same pathways as the pathways which contain the most frequently altered genes. This retrospective exploratory analysis was unable to define any predictive biomarker identifying subgroups of patients, defined by each of the four most frequently altered genes/pathways assessed individually, who do not benefit from the addition of everolimus. A greater benefit from everolimus treatment was derived in patients (representing 76% of the biomarker population) with minimal genetic alterations in PI3KCA/PTEN/CCND1 or FGFR1/2 genes in a combined analysis (HR 0.24 for wild-type patients and 0.26 for patients with a single gene alteration favoring everolimus-based therapy). Patients with tumors having multiple gene alterations experienced less benefit, but still did better than those on exemestane alone (HR 0.78). It is not fully understood why patients with multiple gene abnormalities in these particular pathways benefit less than patients with no, or a single alteration.
BOLERO-3
Preliminary biomarker data from BOLERO-3 were presented at the European Cancer Congress meeting in Amsterdam in 2013.41 Archival tumor samples were available for biomarker analysis from 283 of 569 patients. Candidate biomarkers reflecting activation of the PI3K-mTOR pathway evaluated in this exploratory analysis were high phosphorylated S6 (pS6), low PTEN, and PI3KCA mutations. Results for at least one of these candidate biomarkers were available for 46% of the intention-to-treat patient population, and suggest that addition of everolimus may be most beneficial for patients with low PTEN or high pS6 levels. Addition of everolimus in these patient subgroups was respectively associated with an absolute benefit of 4 and 3 months in median progression-free survival compared with patients receiving only trastuzumab, vinorelbine, and placebo. No correlation with PI3KCA mutational status has been observed, but sample size may be a critical issue. These observations need of course to be confirmed in an independent cohort.
Future directions
Second-generation mTOR inhibitors and dual mTOR-PI3K inhibitors
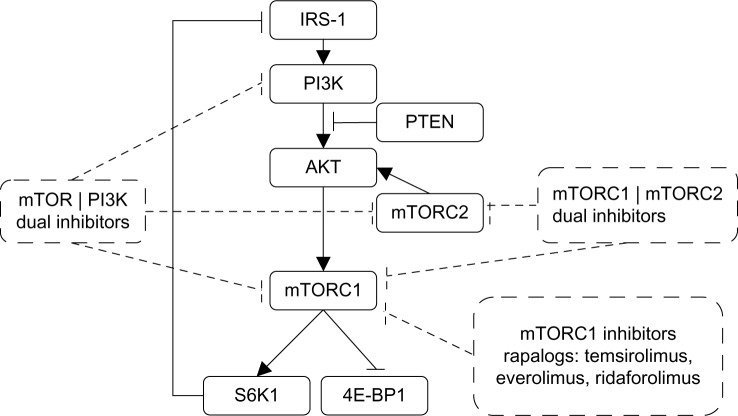
The objective response rates achieved with everolimus and other first-generation mTOR inhibitors are modest.42 mTOR forms at least two functional multiprotein complexes, ie, mTORC1 and mTORC2.43 First-generation mTOR inhibitors inhibit mTORC1 but not mTORC2, which also plays an important role in cancer growth and survival (Figure 1). Furthermore, treatment with first-generation mTOR inhibitors may also cause activation of AKT via a negative feedback loop, resulting in increased cancer cell survival.44,45 Consequently, PI3K/mTOR or mTORC1/2 dual inhibitors are currently under evaluation because these drugs can prevent elevated AKT activity and provide more complete inhibition of mTOR activity.
Figure 1.
Mechanism of action of different classes of mTOR inhibitors (rapalogs) already approved or under development.
Abbreviations: IRS-1, insulin receptor substrate 1; PI3K, phosphatidylinositol-3-kinase; AKT, protein kinase B; mTOR, mammalian target of rapamycin; PTEN, phosphatase and tensin homologue; S6K1, S6 kinase 1; 4E-BP1, eIF4E-binding protein 1.
Immunosuppression is a concern for mTOR kinase inhibitors because more potent suppression of mTOR signaling will potentially lead to impaired immune surveillance and potentially an increased risk of new cancer and metastatic progression. Increased toxicity can also become a problem with more potent pan-kinase blockade. Ongoing studies will evaluate if inhibiting multiple points of the PI3K-AKT-mTOR signaling cascade is more effective than blockade at a single node, and if catalytic mTOR kinase inhibitors can really achieve a more favorable balance of efficacy and tolerability.
Conclusion and perspectives
We are living in an exciting time for breast cancer specialists (Table 6). In particular, in HER2-negative, ER-positive breast cancer, many targeted drugs (including PI3K-AKT-mTOR, cyclin-dependent-kinase, histone deacetylase, and Src inhibitors) in combination with endocrine therapy are under development. The mTORC1 inhibitor everolimus is the first targeted therapy approved in HER2-negative, ER-positive advanced breast cancer resistant to nonsteroidal aromatase inhibitors based on the results of the BOLERO-2 study. The magnitude of benefit has never been seen in this breast cancer subtype since the introduction of tamoxifen. The median progression-free survival has more than doubled when everolimus is added to exemestane compared with exemestane alone.17 Ongoing research is evaluating if more potent inhibition of the PI3K-AKT-mTOR pathway will lead to improved outcomes. The BOLERO-3 trial evaluating everolimus in heavily pretreated patients presenting HER2-positive advanced breast cancer also met its primary endpoint. Median progression-free survival is significantly improved when everolimus is added to trastuzumab and vinorelbine compared with placebo, trastuzumab, and vinorelbine. However, the benefit was only observed in HER2-positive, ER-negative tumors. This and other trials in the neoadjuvant and metastatic setting clearly indicate that new approaches are needed for HER2-positive, ER-positive breast cancer. The addition of an endocrine therapy to a drug combination targeting the PI3K-AKT-mTOR pathway and the HER2 receptor should be evaluated. The role of mTOR inhibitors in triple-negative breast cancer also warrants further evaluation.
Table 6.
Key questions in ongoing clinical trials
| Will the BOLERO-2 trial show an overall survival benefit favoring patients receiving everolimus combined with exemestane? |
| Is there a role for everolimus in the first-line treatment of estrogen receptor-positive HER2-negative advanced breast cancer not previously exposed to any endocrine therapy? |
| Should everolimus or a drug targeting the PI3K-AKT-mTOR pathway be part of the next or later line of treatment for tumors progressing while receiving a regimen containing an mTOR inhibitor? |
| Is there any role for everolimus in the adjuvant treatment of high-risk, early-stage estrogen receptor-positive, HER2-negative breast cancer? |
| Is there any overall survival benefit in the BOLERO-3 trial comparing trastuzumab, vinorelbine, and everolimus with trastuzumab, vinorelbine, and placebo in heavily pretreated HER2-positive advanced breast cancer? |
| Do we see a more pronounced absolute benefit in HER2-positive advanced breast cancer when everolimus is added to standard therapy in less heavily pretreated patients (BOLERO-1) compared with the outcome observed in the BOLERO-3 trial? |
| Will we see improved outcome compared with everolimus-based therapy using second-generation mTOR inhibitors? |
Abbreviations: BOLERO, Breast cancer trials of OraL EveROlimus; HER2, human epidermal growth factor receptor 2; PI3K-AKT-mTOR, phosphatidylinositol-3-kinase/protein kinase B/mammalian target of rapamycin.
Footnotes
Disclosure
GJ has received consultancy fees and research funding from Novartis Pharmaceuticals Corporation. The authors report no other conflicts of interest in this work.
References
- 1.Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature. 2006;441:424–430. doi: 10.1038/nature04869. [DOI] [PubMed] [Google Scholar]
- 2.Hara K, Yonezawa K, Weng QP, et al. Amino acid sufficiency and mTOR regulate p70 S6 kinase and eIF-4E BP1 through a common effector mechanism. J Biol Chem. 1998;273:14484–14494. doi: 10.1074/jbc.273.23.14484. [DOI] [PubMed] [Google Scholar]
- 3.Brugarolas J, Lei K, Hurley RL, et al. Regulation of mTOR function in response to hypoxia by REDD1 and the TSC1/TSC2 tumor suppressor complex. Genes Dev. 2004;18:2893–2904. doi: 10.1101/gad.1256804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shaw RJ, Bardeesy N, Manning BD, et al. The LKB1 tumor suppressor negatively regulates mTOR signaling. Cancer Cell. 2004;6:91–99. doi: 10.1016/j.ccr.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 5.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 6.Ghayad SE, Cohen PA. Inhibitors of the PI3K/Akt/mTOR pathway: new hope for breast cancer patients. Recent Pat Anticancer Drug Discov. 2010;5:29–57. doi: 10.2174/157489210789702208. [DOI] [PubMed] [Google Scholar]
- 7.Yamnik RL, Holz MK. mTOR/S6K1 and MAPK/RSK signaling pathways coordinately regulate estrogen receptor alpha serine 167 phosphorylation. FEBS Lett. 2010;584:124–128. doi: 10.1016/j.febslet.2009.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamnik RL, Digilova A, Davis DC, et al. S6 kinase 1 regulates estrogen receptor alpha in control of breast cancer cell proliferation. J Biol Chem. 2009;284:6361–6369. doi: 10.1074/jbc.M807532200. [DOI] [PubMed] [Google Scholar]
- 9.Dancey J. mTOR signaling and drug development in cancer. Nat Rev Clin Oncol. 2010;7:209–219. doi: 10.1038/nrclinonc.2010.21. [DOI] [PubMed] [Google Scholar]
- 10.Demetri GD, Chawla SP, Ray-Coquard I, et al. Results of an international randomized Phase III trial of the mammalian target of rapamycin inhibitor ridaforolimus versus placebo to control metastatic sarcomas in patients after benefit from prior chemotherapy. J Clin Oncol. 2013;31:2485–2492. doi: 10.1200/JCO.2012.45.5766. [DOI] [PubMed] [Google Scholar]
- 11.US National Institutes of Health [Accessed December 12, 2013]. Available from: http://Clinicaltrials.gov.
- 12.Chan S, Scheulen ME, Johnston S, et al. Phase II study of temsirolimus (CCI-779), a novel inhibitor of mTOR, in heavily pretreated patients with locally advanced or metastatic breast cancer. J Clin Oncol. 2005;23:5314–5322. doi: 10.1200/JCO.2005.66.130. [DOI] [PubMed] [Google Scholar]
- 13.Carpenter JT, Roché H, Campone M, et al. Randomized 3-arm, Phase 2 study of temsirolimus (CCI-779) in combination with letrozole in postmenopausal women with locally advanced or metastatic breast cancer. J Clin Oncol. 2005;23:564. [Google Scholar]
- 14.Wolff AC, Lazar AA, Bondarenko I, et al. Randomized Phase III placebo-controlled trial of letrozole plus oral temsirolimus as first-line endocrine therapy in postmenopausal women with locally advanced or metastatic breast cancer. J Clin Oncol. 2013;31:195–202. doi: 10.1200/JCO.2011.38.3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baselga J, Semiglazov V, van Dam P, et al. Phase II randomized study of neoadjuvant everolimus plus letrozole compared with placebo plus letrozole in patients with estrogen receptor-positive breast cancer. J Clin Oncol. 2009;27:2630–2637. doi: 10.1200/JCO.2008.18.8391. [DOI] [PubMed] [Google Scholar]
- 16.Bachelot T, Bourgier C, Cropet C, et al. Randomized Phase II trial of everolimus in combination with tamoxifen in patients with hormone receptor-positive, human epidermal growth factor receptor 2-negative metastatic breast cancer with prior exposure to aromatase inhibitors: a GINECO study. J Clin Oncol. 2012;30:2718–2724. doi: 10.1200/JCO.2011.39.0708. [DOI] [PubMed] [Google Scholar]
- 17.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 18.Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366:520–529. doi: 10.1056/NEJMoa1109653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yardley DA, Noguchi S, Pritchard KI, et al. Everolimus plus exemes-tane in postmenopausal patients with HR(+) breast cancer: BOLERO-2 final progression-free survival analysis. Adv Ther. 2013;30:870–884. doi: 10.1007/s12325-013-0060-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chia S, Gradishar W, Mauriac L, et al. Double-blind, randomized placebo controlled trial of fulvestrant compared with exemestane after prior nonsteroidal aromatase inhibitor therapy in postmenopausal women with hormone receptor-positive, advanced breast cancer: results from EFECT. J Clin Oncol. 2008;26:1664–1670. doi: 10.1200/JCO.2007.13.5822. [DOI] [PubMed] [Google Scholar]
- 21.Johnston SR, Kilburn LS, Ellis P, et al. Fulvestrant plus anastrozole or placebo versus exemestane alone after progression on non-steroidal aromatase inhibitors in postmenopausal patients with hormone-receptor-positive locally advanced or metastatic breast cancer (SoFEA): a composite, multicentre, Phase 3 randomised trial. Lancet Oncol. 2013;14:989–998. doi: 10.1016/S1470-2045(13)70322-X. [DOI] [PubMed] [Google Scholar]
- 22.Campone M, Bachelot T, Gnant M, et al. Effect of visceral metastases on the efficacy and safety of everolimus in postmenopausal women with advanced breast cancer: subgroup analysis from the BOLERO-2 study. Eur J Cancer. 2013;49:2621–2632. doi: 10.1016/j.ejca.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 23.Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351:2817–2826. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 24.Morrow PK, Wulf GM, Ensor J, et al. Phase I/II study of trastuzumab in combination with everolimus (RAD001) in patients with HER2-overexpressing metastatic breast cancer who progressed on trastuzumab-based therapy. J Clin Oncol. 2011;29:3126–3132. doi: 10.1200/JCO.2010.32.2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andre F, Campone M, O’Regan R, et al. Phase I study of everolimus plus weekly paclitaxel and trastuzumab in patients with metastatic breast cancer pretreated with trastuzumab. J Clin Oncol. 2010;28:5110–5115. doi: 10.1200/JCO.2009.27.8549. [DOI] [PubMed] [Google Scholar]
- 26.Hurvitz SA, Dalenc F, Campone M, et al. A Phase 2 study of everolimus combined with trastuzumab and paclitaxel in patients with HER2-overex-pressing advanced breast cancer that progressed during prior trastuzumab and taxane therapy. Breast Cancer Res Treat. 2013;141:437–446. doi: 10.1007/s10549-013-2689-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jerusalem G, Fasolo A, Dieras V, et al. Phase I trial of oral mTOR inhibitor everolimus in combination with trastuzumab and vinorelbine in pre-treated patients with HER2-overexpressing metastatic breast cancer. Breast Cancer Res Treat. 2011;125:447–455. doi: 10.1007/s10549-010-1260-x. [DOI] [PubMed] [Google Scholar]
- 28.O’Regan R, Ozguroglu M, Andre F, et al. Phase III, randomized, double-blind, placebo-controlled multicenter trial of daily everolimus plus weekly trastuzumab and vinorelbine in trastuzumab-resistant, advanced breast cancer (BOLERO-3) J Clin Oncol. 2013;31(Suppl) Abstr 505. [Google Scholar]
- 29.Walsh S, Flanagan L, Quinn C, et al. mTOR in breast cancer: differential expression in triple-negative and non-triple-negative tumors. Breast. 2012;21:178–182. doi: 10.1016/j.breast.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 30.Ueng SH, Chen SC, Chang YS, et al. Phosphorylated mTOR expression correlates with poor outcome in early-stage triple negative breast carcinomas. Int J Clin Exp Pathol. 2012;5:806–813. [PMC free article] [PubMed] [Google Scholar]
- 31.Cancer Genome Atlas Network Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mayer IA, Jovanovic B, Abramson VG, et al. A randomized Phase II neoadjuvant study of cisplatin, paclitaxel with or without everolimus (an mTOR inhibitor) in patients with stage II/III triple-negative breast cancer (TNCB); Poster PD1-6 presented at the San Antonio Breast Cancer Symposium; December 10–14, 2013; San Antonio, TX, USA. [Google Scholar]
- 33.Huober J, Fasching PA, Hanusch C, et al. Neoadjuvant chemotherapy with paclitaxel and everolimus in breast cancer patients with non-responsive tumours to epirubicin/cyclophosphamide (EC) +/− bevacizumab – results of the randomised GeparQuinto study (GBG 44) Eur J Cancer. 2013;49:2284–2293. doi: 10.1016/j.ejca.2013.02.027. [DOI] [PubMed] [Google Scholar]
- 34.Burris HA, III, Lebrun F, Rugo HS, et al. Health-related quality of life of patients with advanced breast cancer treated with everolimus plus exemestane versus placebo plus exemestane in the Phase 3, randomized, controlled, BOLERO-2 trial. Cancer. 2013;119:1908–1915. doi: 10.1002/cncr.28010. [DOI] [PubMed] [Google Scholar]
- 35.Ravaud A, Urva SR, Grosch K, et al. Relationship between everolimus exposure and safety and efficacy: meta-analysis of clinical trials in oncology. Eur J Cancer. 2014;50:486–495. doi: 10.1016/j.ejca.2013.11.022. [DOI] [PubMed] [Google Scholar]
- 36.O’Donnell A, Faivre S, Burris HA, III, et al. Phase I pharmacokinetic and pharmacodynamic study of the oral mammalian target of rapamycin inhibitor everolimus in patients with advanced solid tumors. J Clin Oncol. 2008;26:1588–1595. doi: 10.1200/JCO.2007.14.0988. [DOI] [PubMed] [Google Scholar]
- 37.Tabernero J, Rojo F, Calvo E, et al. Dose- and schedule-dependent inhibition of the mammalian target of rapamycin pathway with everolimus: a Phase I tumor pharmacodynamic study in patients with advanced solid tumors. J Clin Oncol. 2008;26:1603–1610. doi: 10.1200/JCO.2007.14.5482. [DOI] [PubMed] [Google Scholar]
- 38.Treilleux I, Arnedos M, Cropet C, et al. Predictive markers of everolimus efficacy in hormone receptor positive (HR+) metastatic breast cancer (MBC): final results of the TAMRAD trial translational study. J Clin Oncol. 2013;31(Suppl) Abstr 510. [Google Scholar]
- 39.Hortobagyi G, Piccart-Gebhart M, Rugo H, et al. Correlation of molecular alterations with efficacy of everolimus in hormone receptor–positive, HER2-negative advanced breast cancer: Results from BOLERO-2. J Clin Oncol. 2013;31 abstract LBA509. [Google Scholar]
- 40.Rugo H, Campone M, Gnant M, et al. BOLERO-2: Efficacy and safety of first-line everolimus plus exemestane in advanced breast cancer. J Clin Oncol. 2013;31(Suppl) Abstr 152. [Google Scholar]
- 41.Jerusalem G, Andre F, Chen D, et al. Evaluation of everolimus (EVE) in HER2+ advanced breast cancer (BC) with activated PI3K/mTOR pathway: exploratory biomarker observations from the BOLERO-3 trial. Eur J Cancer. 2013;49(Suppl 3):PS8. [Google Scholar]
- 42.Don AS, Zheng XF. Recent clinical trials of mTOR-targeted cancer therapies. Rev Recent Clin Trials. 2011;6:24–35. doi: 10.2174/157488711793980147. [DOI] [PubMed] [Google Scholar]
- 43.Cybulski N, Hall MN. TOR complex 2: a signaling pathway of its own. Trends Biochem Sci. 2009;34:620–627. doi: 10.1016/j.tibs.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 44.Sun SY, Rosenberg LM, Wang X, et al. Activation of Akt and eIF4E survival pathways by rapamycin-mediated mammalian target of rapamycin inhibition. Cancer Res. 2005;65:7052–7058. doi: 10.1158/0008-5472.CAN-05-0917. [DOI] [PubMed] [Google Scholar]
- 45.Wan X, Harkavy B, Shen N, Grohar P, Helman LJ. Rapamycin induces feedback activation of Akt signaling through an IGF-1R-dependent mechanism. Oncogene. 2007;26:1932–1940. doi: 10.1038/sj.onc.1209990. [DOI] [PubMed] [Google Scholar]