Abstract
Inhibition, the ability to suppress inappropriate cognitions or behaviors, can be measured using computer tasks and questionnaires. Inhibition depends on the frontal cortex, but the role of the underlying white matter (WM) is unclear. We assessed the specific impact of frontal WM damage on inhibition in 29 children with moderate-to-severe traumatic brain injury (15 with and 14 without frontal WM damage), 21 children with orthopedic injury, and 29 population controls. We used the Stop Signal Task to measure response inhibition, the Behavior Rating Inventory of Executive Function to assess everyday inhibition, and T2 fluid-attenuated inversion recovery magnetic resonance imaging to identify lesions. Children with frontal WM damage had impaired response inhibition compared with all other groups and poorer everyday inhibition than the orthopedic injury group. Frontal WM lesions most often affected the superior frontal gyrus. These results provide evidence for the critical role of frontal WM in inhibition.
Keywords: Traumatic brain injury, Response inhibition, Impulsiveness, Brain lesions, White matter, Frontal lobe
Introduction
Inhibition can broadly be defined as the ability to inhibit cognitions or behaviors that are inappropriate. Response inhibition is a cognitive process that is important to development and to the flexible execution and alteration of action and behavior (Miller & Cohen, 2001; Shallice & Burgess, 1996). Cancellation inhibition, a form of response inhibition (Schachar et al., 2007), requires withdrawal of an ongoing motor response. Yet, cognitive function does not map directly to functioning in everyday life (i.e., everyday inhibition; McAuley, Chen, Goos, Schachar, & Crosbie, 2010), which is typically assessed by questionnaire. Both types of inhibition are impaired in neuropathologies such as traumatic brain injury (TBI; Gioia, Isquith, Kenworthy, & Barton, 2002; Konrad, Gauggel, Manz, & Scholl, 2000; Ornstein et al., 2009; Schachar, Levin, Max, Purvis, & Chen, 2004; Stewart & Tannock, 1999) and in psychopathologies, such as attention-deficit hyperactivity disorder (ADHD; Gioia et al., 2002; Lipszyc & Schachar, 2010). Consequently, studying the neural basis of inhibition is of importance to the understanding of both rehabilitation following TBI and psychopathology. Diffuse axonal injury is a common finding after TBI that involves widespread injury to white matter (WM) tracts (Levine, Katz, Dade, & Black, 2002). Studies using magnetic resonance imaging (MRI) have found that frontal WM is most susceptible to diffuse axonal injury in TBI (Gentry, Godersky, & Thompson, 1988). Whether damage to these connections impairs inhibition is unclear.
Children often exhibit cognitive and behavioral impairments following TBI (Levin & Hanten, 2005). Frontal WM damage in children with TBI has been found to impair various cognitive processes, such as restraint inhibition (Levin et al., 2008), planning (Wozniak et al., 2007), and processing speed (Levin et al., 2008). Restraint is the ability to withhold a response that has yet to be initiated and is usually measured using the Go/No-Go Task. Cancellation, which is assessed by the Stop Signal Task (SST; Logan, 1994), activates different neural regions (Chevrier, Noseworthy, & Schachar, 2007) and is regulated by different neurotransmitter systems than restraint (Eagle, Bari, & Robbins, 2008). In terms of behavior, frontal WM damage has been associated with emotional control problems in children with TBI (Wozniak et al., 2007). None of these studies, though, considered the role of frontal WM in cancellation and everyday inhibition.
The neural basis of cancellation has been studied using various neuroimaging techniques. Functional MRI (fMRI) indicates that a fronto-basal ganglia network is active during cancellation (Aron & Poldrack, 2005; Chevrier et al., 2007). However, fMRI cannot distinguish between regions that are necessary for cancellation and those that are activated along with the necessary regions (Noppeney, Friston, & Price, 2004). Damage to a region that is necessary should result in a deficit unless there are alternative brain regions that can support the function. To date, lesion studies of cancellation, the vast majority of which have been conducted in adults, suggest that lesions to the frontal lobe have an impairing effect (Aron, Fletcher, Bullmore, Sahakian, & Robbins, 2003; Chambers et al., 2006; Chen, Muggleton, Tzeng, Hung, & Juan, 2009; Clark et al., 2007; Floden & Stuss, 2006; Muggleton, Chen, Tzeng, Hung, & Juan, 2010; Rieger & Gauggel, 2002; Rieger, Gauggel, & Burmeister, 2003; for an exception, see Rieger & Gauggel, 2002). An important caveat is that these studies either focused on frontal gray matter (GM) or did not distinguish between frontal GM and WM.
There is a paucity of research on the neural basis of everyday inhibition. In children, everyday inhibition is usually assessed by parental report measures, such as the Behavior Rating Inventory of Executive Function (BRIEF). The parent form of the BRIEF includes several scales, one of which is the Inhibit scale (Gioia, Isquith, Guy, & Kenworthy, 2000). Frontal damage has been found to result in children being reported by their parents as having poorer everyday inhibition than normal controls (Anderson, Anderson, Northam, Jacobs, & Mikiewicz, 2002). However, the children in this study had damage limited to the frontal cortex.
In order to determine the impact of frontal WM damage on cancellation and everyday inhibition, we examined children with TBI, children with orthopedic injury, and population controls. We divided the children with TBI into two groups: children with frontal WM damage and children without frontal WM damage as assessed by T2 fluid-attenuated inversion recovery (FLAIR) MRI. The orthopedic injury control group was included to control for pre-injury factors, such as behavioral problems (Stancin et al., 1998), that could impact cancellation (Lipszyc & Schachar, 2010). We administered the SST to obtain a measure of cancellation. Children with TBI and orthopedic injury performed the task and underwent MRI at 3 months after injury. If frontal WM is necessary for cancellation, then children with frontal WM damage should show impairment in this ability. Similarly, if frontal WM is necessary for everyday inhibition, then this behavior should be reported by parents as being poorer for children in the frontal WM group.
Method
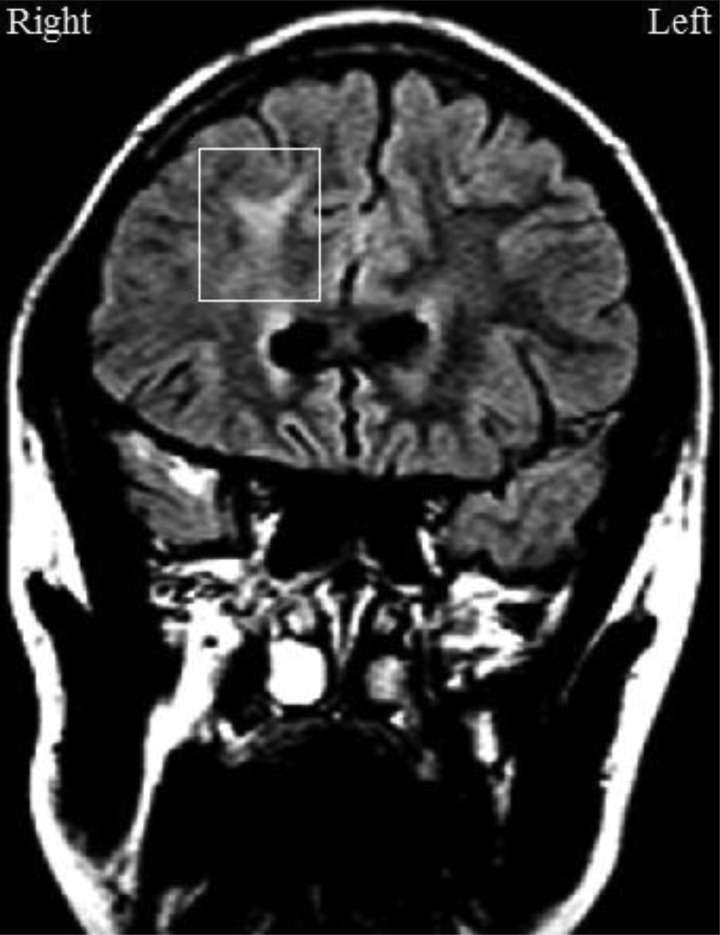
In order to isolate the impact of frontal WM damage, four groups of children were examined: a TBI group with frontal WM damage (n = 15), a TBI group without frontal WM damage (n = 14), an orthopedic injury group (n = 21), and a population control group (n = 29). All children in the frontal WM group had damage in, but not limited to, the frontal WM (see Fig. 1 for an example). Damage was also present in other frontal tissue (GM, GM–WM junction) and/or in any non-frontal tissue (GM, WM, GM–WM junction). The TBI group without frontal WM damage had damage in any frontal and/or non-frontal tissue with the exception of the frontal WM. The proportion of patients with frontal GM damage was markedly, but not significantly, higher in the frontal WM group than in the TBI group without frontal WM damage (see “Results” section for further details). As a check on the effect of frontal GM damage on our findings, we compared children with frontal GM in the frontal WM group to those with frontal GM damage in the TBI group without frontal WM damage. We recruited children in the TBI groups and the orthopedic injury group from consecutive admissions to hospitals in Houston, Dallas, and Miami. Those in the population control group were recruited from visitors to the Ontario Science Centre in Toronto and matched for age and sex to the children with TBI. Study procedures were approved by the Internal Review Board at the Baylor College of Medicine and the Research Ethics Board at The Hospital for Sick Children. Informed consent from parents and assent from children were obtained.
Fig. 1.
Coronal T2-weighted FLAIR image of a patient with right frontal WM damage due to TBI at 3 months post-injury.
Inclusion/Exclusion Criteria
All participants were between 8 and 17 years of age. Children in the TBI groups had moderate-to-severe closed-head injury as assessed by the lowest post-resuscitation score on the Glasgow Coma Scale (GCS; Teasdale & Jennett, 1974), which takes into account eye opening, verbal, and motor responses. The possible range of scores on the GCS is 3 (worst) to 15 (best). Severe TBI was ascribed to children with a GCS score of 3–8. Children were considered to have moderate TBI if they presented with a GCS score of 9–12 or 13–15 accompanied by a brain lesion. The orthopedic injury group consisted of children who underwent hospitalization for a traumatic bone fracture (upper/lower extremities or pelvis). Children in the TBI groups and the orthopedic injury group were screened for exclusion criteria using an interview with parents. Reasons for exclusion included: pre-injury diagnosis of ADHD or other psychiatric disorders such as pervasive developmental disorder or schizophrenia, prior hospitalization for brain injury, penetrating head injury, hypoxia or hypotension lasting for 30 min or more after resuscitation, history of child abuse, and pre-existing neurological disorders (e.g., cerebral palsy, epilepsy, mental retardation). A diagnosis of ADHD prior to injury was based on the Schedule for Affective Disorders and Schizophrenia for School Aged Children (Kaufman et al., 1997). Children with orthopedic injury who had brain lesions were excluded. In the population control group, children were excluded if they had vision, hearing, or motor impairments.
MRI Acquisition
Children with TBI and orthopedic injury group were imaged without sedation in Philips Intera 1.5 T MRI scanners (Philips, Best, The Netherlands) at 3 months post-injury. This time interval was selected based on previous TBI research, indicating that cognitive outcome is more strongly associated with MRI at follow-up than at baseline (Wilson et al., 1988).
Lesion Analysis
T2-weighted FLAIR images were inspected by an expert neuroradiologist (J.H.) for lesions. Picture Archiving and Communication System software was used for this purpose. Some studies have reported an association between the extent of brain damage and cognitive functioning in TBI (Levin et al., 1997; Slomine et al., 2002). In order to quantify the damage, the total number and volume (in cm3) of lesions were calculated.
The template method (Damasio, 1995; Damasio & Damasio, 1989) was employed to localize lesions. Frontal WM lesions were subjacent to the superior frontal gyrus (SFG; Brodmann Area [BA] 6, 8, 9), middle frontal gyrus (MFG; BA 6, 8, 9, 46), inferior frontal gyrus (IFG; BA 44, 45), precentral gyrus (BA 4, 6), orbital frontal gyrus (OFG; BA 11, 47), and other frontal regions.
Cancellation
The SST is rooted in the race model of response inhibition (Logan, 1994). According to this model, inhibitory success is decided by a race between independent go and stop processes. If the go process completes the race first, the response will be committed, whereas if the stop process completes the race first, the response will be inhibited. The SST was comprised of go and stop trials (75% and 25%, respectively). Go trials required children to press one button in response to the letter X and another button in response to the letter O. They were asked to respond as quickly and accurately as possible. On stop trials, which were infrequent, a tone (the stop signal) was presented after the go stimulus. Upon hearing the tone, children were to withdraw their response. The delay separating the go stimulus from the stop signal was dynamically altered via a tracking algorithm. At first, the length of the delay was 250 ms. Successful inhibition resulted in a 50-ms lengthening of the delay. Conversely, failed inhibition resulted in a 50-ms shortening of the delay. The purpose of the algorithm was to establish an inhibition rate of around 50%. The latency of the inhibitory process, referred to as the stop signal reaction time (SSRT), was computed by deducting the mean delay from the mean go RT.
All trials commenced with a 500-ms fixation point. Subsequently, a go stimulus was displayed in the middle of the computer screen for 1000 ms. A blank screen was then presented for 2000 ms. The stop signal tone was 1000 Hz (for a more detailed description of the SST, see Schachar, Mota, Logan, Tannock, & Klim, 2000). The following dependent variables were examined: SSRT, go RT, the within-subject standard deviation of RT (SDRT), probability of inhibition, and go accuracy. We excluded blocks in which the probability of inhibition was 0% or 100% and/or go accuracy was less than 66%. Scores exceeding 3 standard deviations from the mean of the TBI groups and the orthopedic injury group were not included in the analysis. This resulted in the omission of three go RT scores (two from the frontal WM group and one from the orthopedic injury group), one go accuracy score (from the orthopedic injury group), and one probability of inhibition score (from the frontal WM group). Studies have indicated that SSRT, go RT, and SDRT vary with age (Johnstone et al., 2007; Williams, Ponesse, Schachar, Logan, & Tannock, 1999). Therefore, these data were residualized for age prior to analysis (for the procedure, see Leblanc et al., 2005). For descriptive purposes, SST data are presented in Table 1 in raw form.
Table 1.
SST performance of the TBI groups and the control groups
| Variable | TBI with frontal WM damage (n = 15) |
TBI without frontal WM damage (n = 14) |
Orthopedic injury (n = 21) |
Population control (n = 29) |
F-value | p-value | Contrasts | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | M | SD | ||||
| SSRT | 305.79 | 99.73 | 214.89 | 63.24 | 253.51 | 70.14 | 201.97 | 59.70 | 8.80 | <.001 | 1 > 2,3,4 |
| Go RT | 553.93 | 87.83 | 566.86 | 137.46 | 547.41 | 125.41 | 441.63 | 93.65 | 8.95 | <.001 | 1,2 > 4 |
| SDRT | 140.68 | 52.78 | 128.32 | 55.54 | 119.54 | 39.72 | 113.85 | 32.74 | 2.34 | .08 | – |
| % inhibition | 53.63 | 7.47 | 52.86 | 5.08 | 50.80 | 6.40 | 49.83 | 2.27 | 2.45 | .09 | – |
| Go accuracy | 96.61 | 2.98 | 96.21 | 3.87 | 96.86 | 3.17 | 97.48 | 2.19 | 0.68 | .57 | – |
Notes: WM = white matter; SSRT = stop signal reaction time; go RT = go reaction time; SDRT = standard deviation of reaction time; TBI = traumatic brain injury.
Everyday Inhibition
The parent form of the BRIEF was administered to caregivers of children in the TBI groups and the orthopedic injury group. It consists of 86 items that assess cognitive functioning as reflected in everyday life and is designed for use in children aged 5–18 years (Gioia et al., 2000). The BRIEF is valid for use in normal children (Gioia et al., 2000) and in those with TBI (Mangeot, Armstrong, Colvin, Yeates, & Taylor, 2002). It is comprised of eight clinical scales, two indices, and a Global Executive Composite. Given that the focus of this study is on inhibition, we considered only the Inhibit scale, which has a test–retest reliability correlation coefficient of .84 (Gioia et al., 2000). Raw scores on the Inhibit scale were transformed to T-scores (M = 50, SD = 10). Scores of 65 or greater are clinically significant, whereas those between 60 and 64 are borderline clinically significant. The BRIEF was not administered to children in the population control group. BRIEF data were unavailable for four children in the TBI group without frontal WM damage, three in the frontal WM group, and three in the orthopedic injury group.
Socioeconomic Status
Socioeconomic status (SES) was determined using the Socioeconomic Composite Index (SCI; Yeates et al., 1997), which considers annual family income, maternal education, and occupational prestige. These three variables were z-transformed. The SCI was the average of these z-scores. SES data were not collected for children in the population control group, who likely had a higher SES than children in the TBI groups and the orthopedic injury group. This was not expected to influence the cancellation result, however, because previous research has found no significant correlation between SSRT and SES (e.g., Leblanc et al., 2005).
Analysis
Group differences were compared using one-way analysis of variance (ANOVA) for continuous variables and Fisher's exact test for categorical variables. When ANOVA was significant, post hoc comparisons were performed using Tukey's test. If variances were not homogeneous, the Games–Howell test was used. Linear regression was performed where appropriate. Analyses were conducted using Statistical Package for the Social Sciences version 17.0 for Windows. Significance for analyses was set at p < .05. We calculated effect sizes (ESs) using Cohen's d (Cohen, 1992). ESs can be classified as small (0.2), medium (0.5), or large (0.8).
Results
Participant Characteristics
Table 2 presents the characteristics of the frontal WM group, the TBI group without frontal WM damage, the orthopedic injury group, and the population control group. The four groups did not differ significantly in age (F = 1.23, p = .31) or in the proportion of male participants (p = .45). SES was available only for children in the TBI groups and the orthopedic injury group. There was no significant difference between the three groups in SES (F = 0.20, p = .82). The TBI groups did not differ significantly in the proportion of children with a severe GCS score (p = .69).
Table 2.
Characteristics of the TBI groups and the control groups
| Measure | TBI with frontal WM damage (n = 15) |
TBI without frontal WM damage (n = 14) |
Orthopedic injury (n = 21) |
Population control (n = 29) |
F-value | p-value | Contrasts | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | M | SD | ||||
| Age at testing | 14.09 | 2.97 | 13.42 | 2.60 | 12.54 | 2.29 | 13.77 | 2.84 | 1.23 | .31 | |
| Percent male | 73.33 | 57.14 | 61.90 | 65.52 | .82 | ||||||
| SES | 0.09 | 0.86 | −0.11 | 0.79 | 0.02 | 0.89 | 0.20 | .82 | |||
| GCS | 6.92 | 3.52 | 8.54 | 4.75 | 0.97 | .34 | |||||
| BRIEF Inhibit | 56.83 | 11.84 | 52.50 | 15.17 | 43.89 | 4.00 | 7.56 | .006 | 1 > 3 | ||
Notes: WM = white matter; SES = socioeconomic status; GCS = Glasgow Coma Scale; TBI = traumatic brain injury.
Lesion Characteristics
Table 3 presents the distribution of lesions across all children with TBI. Each child with TBI had at least one brain lesion. The mean total number and volume of lesions in the TBI groups is provided in Table 4. The TBI groups did not differ significantly in the total number of lesions (F = 1.99, p = .17). The frontal WM group had a significantly greater total lesion volume than the TBI group without frontal WM damage (F = 5.79, p = .03). Later, we examine whether the total volume of lesions influences cancellation.
Table 3.
Distribution of lesions across all children with TBI
| Lesion location | Overall |
WM |
GM |
GM–WM junction |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T | R | L | B | T | R | L | B | T | R | L | B | T | R | L | B | |
| Frontal | 25 | 4 | 2 | 19 | 15 | 7 | 2 | 6 | 21 | 5 | 2 | 14 | 11 | 2 | 4 | 5 |
| Superior frontal gyrus | 15 | 3 | 2 | 10 | 10 | 5 | 2 | 3 | 11 | 3 | 8 | 8 | 2 | 3 | 3 | |
| Middle frontal gyrus | 13 | 8 | 2 | 3 | 7 | 6 | 1 | 8 | 5 | 1 | 2 | 3 | 2 | 1 | ||
| Inferior frontal gyrus | 13 | 7 | 1 | 5 | 3 | 1 | 1 | 1 | 12 | 6 | 1 | 5 | 2 | 1 | 1 | |
| Precentral gyrus | 1 | 1 | 1 | 1 | ||||||||||||
| Cingulate gyrus | ||||||||||||||||
| Anterior cingulate gyrus | ||||||||||||||||
| Posterior cingulate gyrus | ||||||||||||||||
| Medial frontal gyrus | ||||||||||||||||
| Orbital gyrus | 13 | 2 | 3 | 8 | 1 | 1 | 12 | 2 | 2 | 8 | 2 | 2 | ||||
| Gyrus rectus | 6 | 1 | 1 | 4 | 5 | 1 | 1 | 3 | 1 | 1 | ||||||
| Operculum | ||||||||||||||||
| Other frontal | 4 | 2 | 2 | 4 | 2 | 2 | ||||||||||
| Non-frontal | 28 | 3 | 5 | 19 | 17 | 7 | 3 | 5 | 23 | 2 | 5 | 16 | 11 | 5 | 4 | 2 |
Notes: Values reflect number of children. Groups are not mutually exclusive. Overall = across all of the tissue types; WM = white matter; GM = gray matter; T = total number of children with a lesion; R = number of children with a lesion in the right hemisphere; L = number of children with a lesion in the left hemisphere; B = number of children with a lesion in both hemispheres.
Table 4.
Mean total number and volume of lesions in each TBI group
| Measure | TBI with frontal WM damage (n = 15) |
TBI without frontal WM damage (n = 14) |
F-value | p-value | ||
|---|---|---|---|---|---|---|
| M | SD | M | SD | |||
| Total number of lesions | 12.33 | 6.61 | 8.57 | 7.74 | 1.99 | .17 |
| Total volume of lesions | 21.58 | 29.32 | 3.12 | 4.65 | 5.79* | .03 |
Notes: Volumes are in cm3. WM = white matter.
*p < .05.
In the frontal WM group, children had frontal WM damage subjacent to the SFG (67%), MFG (47%), IFG (20%), precentral gyrus (7%), OFG (7%), and other frontal regions (27%). Of the children in this group, 47% had right-sided, 13% had left-sided, and 40% had bilateral frontal WM damage. Eighty-seven percent had frontal GM damage. In the TBI group without frontal WM damage, 57% of children had frontal GM damage. There was no significant difference in the proportion of patients with frontal GM damage between the frontal WM group and the TBI group without frontal WM damage (p = .11), but this was likely due to the small sample size. All children in the frontal WM group and 93% of children in the TBI group without frontal WM damage had non-frontal damage.
Everyday Inhibition
Table 2 provides the mean BRIEF T-scores of the frontal WM group (range = 37–76), the TBI group without frontal WM damage (range = 41–81), and the orthopedic injury group (range = 37–55). Mean T-scores on the Inhibit scale fell within the normal range (<60) for all groups, but significant group differences emerged (F = 7.56, p = .006). Post hoc tests revealed significantly higher scores (indicating poorer everyday inhibition) in the frontal WM group compared with the orthopedic injury group (d = 1.46). Conversely, there was no significant difference in scores between the frontal WM group and the TBI group without frontal WM damage (d = 0.32).
The proportion of children with T-scores of ≥60 (i.e., in the borderline or clinically significant range) on the Inhibit scale was 50%, 20%, and 0% in the frontal WM group, the TBI group without frontal WM damage, and the orthopedic injury group, respectively. A significantly larger proportion of children in the frontal WM group had T-scores of ≥60 relative to the orthopedic injury group (p = .002). The proportion of children with T-scores in this range did not differ between the TBI group without frontal WM damage and the orthopedic injury group (p = .12). We later assess the association between scores on the Inhibit scale and SSRT.
Cancellation
Table 1 presents SST performance according to the group. Results showed a significant difference between groups in SSRT (F = 8.80, p < .001). Post hoc analysis revealed that the frontal WM group had significantly longer SSRTs than the TBI group without frontal WM damage (d = 1.26), the orthopedic injury group (d = 0.77), and the population control group (d = 1.45). There was also a significant difference in go RT between the four groups (F = 8.95, p < .001). Post hoc analysis indicated that children with and without frontal WM damage had significantly longer go RTs than population controls (d = 1.52 and d = 1.12, respectively). There was a go RT ES of d = 0.79 for the orthopedic injury group relative to the population control group. Later, the association between SSRT and go RT is considered. No significant difference in SDRT was found between groups (F = 2.34, p = .08). Comparing the frontal WM group to the TBI group without frontal WM damage, the orthopedic injury group, and the population control group yielded SDRT ESs of d = 0.35, d = 0.73, and d = 0.79, respectively. The groups did not differ significantly in probability of inhibition or go accuracy.
Effect of Frontal GM Damage
We compared children with GM damage with and without frontal WM damage. Children with frontal GM damage in the frontal WM group had significantly longer SSRTs than children with frontal GM damage in the TBI group without frontal WM damage (F = 11.58, p = .003; d = 1.64). No significant differences emerged between these two groups in MRT (F = 0.01, p = .92; d = −0.05), SDRT (F = 2.50; p = .13; d = 0.66), or in scores on the Inhibit scale of the BRIEF (F = 1.58, p = .23, d = 0.60). These results suggest that the presence of children with frontal GM damage in the frontal WM group did not significantly impact our findings.
Regression Analysis
Regression analyses across all children with TBI revealed no significant relationships between SSRT and the total volume of lesions (r = .26, r2 = .07, p = .17), ratings on the Inhibit scale of the BRIEF (r = .06, r2 = .003, p = .81), or go RT (r = .17, r2 = .03, p = .41). There was also no significant association between ratings on the Inhibit scale of the BRIEF and the total volume of lesions (r = .13, r2 = .02, p = .58).
Discussion
We examined children with and without frontal WM lesions, children with orthopedic injury, and population controls in order to determine the influence of frontal WM damage on cancellation and everyday inhibition. Frontal WM emerged as a common location of lesions in our TBI sample, with over 50% of children showing evidence of such damage. Our results showed that children with frontal WM damage had significantly poorer cancellation than all of the other groups, agreeing with research reporting that cancellation depends on a frontal-subcortical network (Aron, Behrens, Smith, Frank, & Poldrack, 2007). This result held even after taking into account the presence of children with frontal GM damage. We also found that children with frontal WM damage showed poorer everyday inhibition than children with orthopedic injury. These results indicate that frontal WM is necessary for cancellation and everyday inhibition. According to previous studies, children with TBI are impaired in cancellation and everyday inhibition (Konrad et al., 2000; Mangeot et al., 2002). Our results suggest that frontal WM damage may be responsible, at least in part, for these deficits.
This study compliments lesion studies in adults suggesting that the frontal lobe is necessary for cancellation. Aron and colleagues (2003) found a cancellation deficit in adults with right frontal lesions stemming from aneurysm, hemorrhage, or excisions of meningioma and noted a significant relationship between SSRT and volume of right IFG damage. Clark and colleagues (2007) confirmed the findings of Aron and colleagues with a larger sample. Rieger and colleagues (2003) found a similar deficit in adults with frontal lobe damage resulting from cerebrovascular disorders or brain tumor resections. In addition, Floden and Stuss (2006) reported that adults with damage to the right SFG due to stroke, tumor/epilepsy resections, or traumatic focal contusions showed impairment in SSRT. However, these studies did not specifically investigate the impact of frontal WM damage on cancellation.
Virtual lesion studies using transcranial magnetic stimulation (TMS) have also found that cancellation depends critically on the frontal lobe. Cancellation has been reported to be impaired by TMS over the right IFG (Chambers et al., 2006), the left SFG (Chen et al., 2009), and the right frontal eye fields (Muggleton et al., 2010) in healthy adults. TMS is useful because its effects are transient and do not result in neural reorganization. On the other hand, it penetrates only ∼1–2 cm into the brain (Schutter, 2009), and as such, cannot adequately test the involvement of frontal WM.
Rodent models have also been used to study the neural basis of cancellation. Eagle and colleagues (2008) found that rats with fiber-sparing lesions of the orbitofrontal cortex had a deficit. According to Bari and colleagues (2011), inactivation of the dorsomedial prefrontal cortex (homologous to both the dorsolateral prefrontal cortex and the anterior cingulate cortex in primates; Seamans, Lapish, & Durstewitz, 2008) impaired SSRT in rats. Collectively, these studies highlight the necessity of frontal GM in cancellation, but do not speak to the role of frontal WM.
Impaired cancellation in children with frontal WM damage may be a consequence of disrupted communication between brain regions. A fronto-basal-ganglia network is thought to underlie cancellation (Aron et al., 2007), but the mechanism by which frontal WM damage impairs cancellation is unclear. Cognitive dysfunction has been previously related to frontal WM abnormalities in disorders such as sickle cell disease vasculopathy (Hogan, Vargha-Khadem, Saunders, Kirkham, & Baldeweg, 2006) and multiple sclerosis (Arnett et al., 1994) as well as in a community sample of adults (Bunce et al., 2010). Conversely, our results showed that children in the TBI group without frontal WM damage had intact cancellation, perhaps because they compensated by more effectively recruiting alternative regions to support the function.
Most children in the frontal WM group had right-sided or bilateral frontal WM damage. Prior studies do point to a right hemispheric network subserving cancellation (Aron & Poldrack, 2005). We also found that frontal WM lesions often occurred subjacent to the SFG. FMRI research has reported that the SFG is activated by various types of response inhibition (Swick, Ashley, & Turken, 2008). Moreover, Easdon, Levine, O'Connor, Tisserand, and Hevenor (2004) noted that, during successful inhibition, adults with TBI showed significantly less activation than controls in the dorsolateral prefrontal cortex, a region that encompasses part of the SFG.
Diffusion tensor imaging (DTI) is an MRI technique designed to assess the integrity of WM tracts. It is more sensitive than conventional MRI in detecting WM damage (Ghajar & Ivry, 2008). Madsen and colleagues (2010) found that better cancellation was significantly associated with higher fractional anisotropy (greater WM integrity) in the right IFG and pre-supplementary motor area in healthy children. The present study complements the work of Madsen and colleagues by implicating SFG WM. Levin and colleagues (2008) reported that higher fractional anisotropy in the left dorsolateral frontal region was significantly associated with fewer errors in the no-go condition of a Flanker Task (a measure of restraint) in children with moderate-to-severe TBI. Therefore, various forms of response inhibition may depend on the integrity of frontal WM connections.
We found no evidence that the cancellation deficit in the frontal WM group could be explained by a generalized slowing of response speed. Previous research has indicated that stop and go processes are independent of one another (Logan, 1994). Our results showed no significant relationship between SSRT and go RT across all children with TBI. Indeed, children in the TBI group without frontal WM damage had impaired go RTs but intact SSRTs. Children with orthopedic injury had moderately longer go RTs compared with population controls, but this finding failed to reach statistical significance. The latter finding hints to the possibility that slowed RT speed may be related to hospitalization or risk factors that predispose children to injury.
In terms of everyday inhibition, no significant relationship emerged between SSRT and scores on the Inhibit scale in children with TBI, consistent with studies reporting that questionnaires tap different aspects of inhibition than cognitive tasks (McAuley et al., 2010; but see Young et al., 2009 for an exception). We found that significantly more children with TBI who showed evidence of frontal WM damage had scores in the clinically significant/borderline range on the Inhibit scale of the BRIEF than children with orthopedic injury, indicating that frontal WM may be necessary for everyday inhibition. In contrast, there was no significant difference in the proportion of patients with scores in this range between the TBI group without frontal WM damage and the orthopedic injury group, but this may have been an issue of statistical power. There may be mechanisms other than frontal WM damage by which TBI can produce impairment in everyday inhibition.
We assessed the extent of brain damage by measuring the total number and volume of lesions. The TBI groups did not differ significantly in the total number of lesions. The frontal WM group did have a significantly greater total volume of lesions than the TBI group without frontal WM damage. Nevertheless, we did not find a significant relationship between SSRT or ratings on the Inhibit scale of the BRIEF and the total volume of lesions, indicating that cancellation and everyday inhibition are not predicted by the total volume of brain damage.
There are some limitations to our study to take into consideration. This study had a small sample size, which may have reduced our ability to detect subtle differences between groups. Another limitation is that conventional MRI is not as sensitive as more cutting edge techniques for detecting WM damage, such as multidirection DTI, raising the possibility that some children in the TBI group without frontal WM damage may have had frontal WM lesions that went undetected. It should also be emphasized that most patients in the frontal WM group also had frontal GM damage, making it difficult to determine whether frontal WM damage alone can impair inhibition. There is also the issue that some children in the frontal WM group had intact inhibition. In these patients, it is possible that pre-injury inhibition may have been superior or frontal WM critical for inhibition could have been spared. Furthermore, the present data were from a single point in time, which is important to mention because brain and behavior change with time since injury (Wu et al., 2010).
A number of recommendations for future research can be made based on the findings of this study. First, this study should be replicated with a larger sample of children. Second, it may be useful to investigate whether our findings are generalizable to adults with TBI as well as other patient populations. Third, DTI should be used to confirm the relationship between frontal WM damage and cancellation in children with TBI. Finally, it would be informative to use fMRI to establish whether or not activity elicited by cancellation is reorganized after various lesions to the brain.
In summary, this study assessed the influence of lesions arising from TBI on cancellation and everyday inhibition. We found impaired cancellation in children with TBI who had frontal WM damage, but not in those without such damage, suggesting that this cognitive process depends critically on intact frontal WM connections. The most common location of frontal WM damage was the SFG, suggesting an important role for this region in cancellation. Our finding of intact cancellation in children with TBI who did not have frontal WM damage suggests that neural reorganization may have occurred. We also found that a significantly larger proportion of children in the frontal WM group had poorer everyday inhibition relative to the orthopedic injury group. Yet, there was no evidence of a significant relationship between cancellation and everyday inhibition. Overall, our results suggest that frontal WM damage can lead to both cognitive and behavioral impairment in children with TBI.
Funding
This work was supported by the Canadian Institutes of Health Research (Frederick Banting and Charles Best Canada Graduate Scholarship to J.L. and MOP-64277, MOP-44070, and MOP-74699 to R.S.); the Hospital for Sick Children (Restracomp Studentship to J.L.); and the National Institutes of Health (NS-21889 to H.L.).
Acknowledgements
This work was part of the first author's Master's thesis in the Institute of Medical Science at the University of Toronto.
References
- Anderson V. A., Anderson P., Northam E., Jacobs R., Mikiewicz O. Relationships between cognitive and behavioral measures of executive function in children with brain disease. Child Neuropsychology. 2002;8(4):231–240. doi: 10.1076/chin.8.4.231.13509. doi:10.1076/chin.8.4.231.13509. [DOI] [PubMed] [Google Scholar]
- Arnett P. A., Rao S. M., Bernardin L., Grafman J., Yetkin F. Z., Lobeck L. Relationship between frontal lobe lesions and Wisconsin Card Sorting Test performance in patients with multiple sclerosis. Neurology. 1994;44((3 Pt 1):420–425. doi: 10.1212/wnl.44.3_part_1.420. doi:10.1212/WNL.44.3_Part_1.420. [DOI] [PubMed] [Google Scholar]
- Aron A. R., Behrens T. E., Smith S., Frank M. J., Poldrack R. A. Triangulating a cognitive control network using diffusion-weighted magnetic resonance imaging (MRI) and functional MRI. Journal of Neuroscience. 2007;27(14):3743–3752. doi: 10.1523/JNEUROSCI.0519-07.2007. doi:10.1523/JNEUROSCI.0519-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron A. R., Fletcher P. C., Bullmore E. T., Sahakian B. J., Robbins T. W. Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nature Neuroscience. 2003;6(2):115–116. doi: 10.1038/nn1003. doi:10.1038/nn1003. [DOI] [PubMed] [Google Scholar]
- Aron A. R., Poldrack R. A. The cognitive neuroscience of response inhibition: Relevance for genetic research in attention-deficit/hyperactivity disorder. Biological Psychiatry. 2005;57(11):1285–1292. doi: 10.1016/j.biopsych.2004.10.026. doi:10.1016/j.biopsych.2004.10.026. [DOI] [PubMed] [Google Scholar]
- Bari A., Mar A. C., Theobald D. E., Elands S. A., Oganya K. C., Eagle D. M. Prefrontal and monoaminergic contributions to stop-signal task performance in rats. Journal of Neuroscience. 2011;31(25):9254–9263. doi: 10.1523/JNEUROSCI.1543-11.2011. doi:10.1523/JNEUROSCI.1543-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunce D., Anstey K. J., Cherbuin N., Burns R., Christensen H., Wen W. Cognitive deficits are associated with frontal and temporal lobe white matter lesions in middle-aged adults living in the community. PLoS One. 2010;5(10):e13567. doi: 10.1371/journal.pone.0013567. doi:10.1371/journal.pone.0013567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers C. D., Bellgrove M. A., Stokes M. G., Henderson T. R., Garavan H., Robertson I. H. Executive “brake failure” following deactivation of human frontal lobe. Journal of Cognitive Neuroscience. 2006;18(3):444–455. doi: 10.1162/089892906775990606. doi:10.1162/089892906775990606. [DOI] [PubMed] [Google Scholar]
- Chen C. Y., Muggleton N. G., Tzeng O. J., Hung D. L., Juan C. H. Control of prepotent responses by the superior medial frontal cortex. Neuroimage. 2009;44(2):537–545. doi: 10.1016/j.neuroimage.2008.09.005. doi:10.1016/j.neuroimage.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Chevrier A. D., Noseworthy M. D., Schachar R. Dissociation of response inhibition and performance monitoring in the stop signal task using event-related fMRI. Human Brain Mapping. 2007;28(12):1347–1358. doi: 10.1002/hbm.20355. doi:10.1002/hbm.20355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark L., Blackwell A. D., Aron A. R., Turner D. C., Dowson J., Robbins T. W. Association between response inhibition and working memory in adult ADHD: A link to right frontal cortex pathology? Biological Psychiatry. 2007;61(12):1395–1401. doi: 10.1016/j.biopsych.2006.07.020. doi:10.1016/j.biopsych.2006.07.020. [DOI] [PubMed] [Google Scholar]
- Cohen J. A power primer. Psychological Bulletin. 1992;112(1):155–159. doi: 10.1037//0033-2909.112.1.155. doi:10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- Damasio H. Human brain anatomy in computerized images. New York: Oxford University Press; 1995. [Google Scholar]
- Damasio H., Damasio A. R. Lesion analysis in neuropsychology. New York: Oxford University Press; 1989. [Google Scholar]
- Eagle D. M., Bari A., Robbins T. W. The neuropsychopharmacology of action inhibition: Cross-species translation of the stop-signal and go/no-go tasks. Psychopharmacology. 2008;199(3):439–456. doi: 10.1007/s00213-008-1127-6. doi:10.1007/s00213-008-1127-6. [DOI] [PubMed] [Google Scholar]
- Eagle D. M., Baunez C., Hutcheson D. M., Lehmann O., Shah A. P., Robbins T. W. Stop-signal reaction-time task performance: Role of prefrontal cortex and subthalamic nucleus. Cerebral Cortex. 2008;18(1):178–188. doi: 10.1093/cercor/bhm044. doi:10.1093/cercor/bhm044. [DOI] [PubMed] [Google Scholar]
- Easdon C., Levine B., O'Connor C., Tisserand D., Hevenor S. Neural activity associated with response inhibition following traumatic brain injury: An event-related fMRI investigation. Brain and Cognition. 2004;54(2):136–138. [PubMed] [Google Scholar]
- Floden D., Stuss D. T. Inhibitory control is slowed in patients with right superior medial frontal damage. Journal of Cognitive Neuroscience. 2006;18(11):1843–1849. doi: 10.1162/jocn.2006.18.11.1843. doi:10.1162/jocn.2006.18.11.1843. [DOI] [PubMed] [Google Scholar]
- Gentry L. R., Godersky J. C., Thompson B. MR imaging of head trauma: Review of the distribution and radiopathologic features of traumatic lesions. AJR. American Journal of Roentgenology. 1988;150(3):663–672. doi: 10.2214/ajr.150.3.663. doi:10.2214/ajr.150.3.663. [DOI] [PubMed] [Google Scholar]
- Ghajar J., Ivry R. B. The predictive brain state: Timing deficiency in traumatic brain injury? Neurorehabilitation and Neural Repair. 2008;22(3):217–227. doi: 10.1177/1545968308315600. doi:10.1177/1545968308315600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gioia G. A., Isquith P. K., Guy S. C., Kenworthy L. Behavior rating inventory of executive function. Odessa, FL: Psychological Assessment Resources; 2000. [Google Scholar]
- Gioia G. A., Isquith P. K., Kenworthy L., Barton R. M. Profiles of everyday executive function in acquired and developmental disorders. Child Neuropsychology. 2002;8(2):121–137. doi: 10.1076/chin.8.2.121.8727. doi:10.1076/chin.8.2.121.8727. [DOI] [PubMed] [Google Scholar]
- Hogan A. M., Vargha-Khadem F., Saunders D. E., Kirkham F. J., Baldeweg T. Impact of frontal white matter lesions on performance monitoring: ERP evidence for cortical disconnection. Brain. 2006;129(Pt 8):2177–2188. doi: 10.1093/brain/awl160. doi:10.1093/brain/awl160. [DOI] [PubMed] [Google Scholar]
- Johnstone S. J., Dimoska A., Smith J. L., Barry R. J., Pleffer C. B., Chiswick D. The development of stop-signal and Go/Nogo response inhibition in children aged 7–12 years: Performance and event-related potential indices. International Journal of Psychophysiology. 2007;63(1):25–38. doi: 10.1016/j.ijpsycho.2006.07.001. doi:10.1016/j.ijpsycho.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Kaufman J., Birmaher B., Brent D., Rao U., Flynn C., Moreci P. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): Initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36(7):980–988. doi: 10.1097/00004583-199707000-00021. doi:10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Konrad K., Gauggel S., Manz A., Scholl M. Inhibitory control in children with traumatic brain injury (TBI) and children with attention deficit/hyperactivity disorder (ADHD) Brain Injury. 2000;14(10):859–875. doi: 10.1080/026990500445691. [DOI] [PubMed] [Google Scholar]
- Leblanc N., Chen S., Swank P. R., Ewing-Cobbs L., Barnes M., Dennis M. Response inhibition after traumatic brain injury (TBI) in children: Impairment and recovery. Developmental Neuropsychology. 2005;28(3):829–848. doi: 10.1207/s15326942dn2803_5. doi:10.1207/s15326942dn2803_5. [DOI] [PubMed] [Google Scholar]
- Levin H. S., Hanten G. Executive functions after traumatic brain injury in children. Pediatric Neurology. 2005;33(2):79–93. doi: 10.1016/j.pediatrneurol.2005.02.002. doi:10.1016/j.pediatrneurol.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Levin H. S., Song J., Scheibel R. S., Fletcher J. M., Harward H., Lilly M. Concept formation and problem-solving following closed head injury in children. Journal of the International Neuropsychological Society. 1997;3(6):598–607. [PubMed] [Google Scholar]
- Levin H. S., Wilde E. A., Chu Z., Yallampalli R., Hanten G. R., Li X. Diffusion tensor imaging in relation to cognitive and functional outcome of traumatic brain injury in children. Journal of Head Trauma Rehabilitation. 2008;23(4):197–208. doi: 10.1097/01.HTR.0000327252.54128.7c. doi:10.1097/01.HTR.0000327252.54128.7c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine B., Katz D. I., Dade L., Black S. E. Novel approaches to the assessment of frontal damage and executive deficits in traumatic brain injury. In: Stuss D. T., Knight R. T., editors. Principles of frontal lobe function. New York: Oxford University Press; 2002. pp. 448–465. [Google Scholar]
- Lipszyc J., Schachar R. Inhibitory control and psychopathology: A meta-analysis of studies using the stop signal task. Journal of the International Neuropsychological Society. 2010;16(6):1064–1076. doi: 10.1017/S1355617710000895. doi:10.1017/S1355617710000895. [DOI] [PubMed] [Google Scholar]
- Logan G. D. On the ability to inhibit thought and action: A users’ guide to the stop signal paradigm. In: Dagenbach D., Carr T. H., editors. Inhibitory processes in attention, memory, and language. San Diego: Academic Press; 1994. pp. 189–239. [Google Scholar]
- Madsen K. S., Baare W. F., Vestergaard M., Skimminge A., Ejersbo L. R., Ramsoy T. Z. Response inhibition is associated with white matter microstructure in children. Neuropsychologia. 2010;48(4):854–862. doi: 10.1016/j.neuropsychologia.2009.11.001. doi:10.1016/j.neuropsychologia.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Mangeot S., Armstrong K., Colvin A. N., Yeates K. O., Taylor H. G. Long-term executive function deficits in children with traumatic brain injuries: Assessment using the Behavior Rating Inventory of Executive Function (BRIEF) Child Neuropsychology. 2002;8(4):271–284. doi: 10.1076/chin.8.4.271.13503. doi:10.1076/chin.8.4.271.13503. [DOI] [PubMed] [Google Scholar]
- McAuley T., Chen S., Goos L., Schachar R., Crosbie J. Is the behavior rating inventory of executive function more strongly associated with measures of impairment or executive function? Journal of the International Neuropsychological Society. 2010;16(3):495–505. doi: 10.1017/S1355617710000093. doi:10.1017/S1355617710000093. [DOI] [PubMed] [Google Scholar]
- Miller E. K., Cohen J. D. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. doi:10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Muggleton N. G., Chen C. Y., Tzeng O. J., Hung D. L., Juan C. H. Inhibitory control and the frontal eye fields. Journal of Cognitive Neuroscience. 2010;22(12):2804–2812. doi: 10.1162/jocn.2010.21416. doi:10.1162/jocn.2010.21416. [DOI] [PubMed] [Google Scholar]
- Noppeney U., Friston K. J., Price C. J. Degenerate neuronal systems sustaining cognitive functions. Journal of Anatomy. 2004;205(6):433–442. doi: 10.1111/j.0021-8782.2004.00343.x. doi:10.1111/j.0021–8782.2004.00343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornstein T. J., Levin H. S., Chen S., Hanten G., Ewing-Cobbs L., Dennis M. Performance monitoring in children following traumatic brain injury. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2009;50(4):506–513. doi: 10.1111/j.1469-7610.2008.01997.x. doi:10.1111/j.1469-7610.2008.01997.x. [DOI] [PubMed] [Google Scholar]
- Rieger M., Gauggel S. Inhibition of ongoing responses in patients with traumatic brain injury. Neuropsychologia. 2002;40(1):76–85. doi: 10.1016/s0028-3932(01)00068-9. doi:10.1016/S0028-3932(01)00068-9. [DOI] [PubMed] [Google Scholar]
- Rieger M., Gauggel S., Burmeister K. Inhibition of ongoing responses following frontal, nonfrontal, and basal ganglia lesions. Neuropsychology. 2003;17(2):272–282. doi: 10.1037/0894-4105.17.2.272. doi:10.1037/0894-4105.17.2.272. [DOI] [PubMed] [Google Scholar]
- Schachar R., Levin H. S., Max J. E., Purvis K., Chen S. Attention deficit hyperactivity disorder symptoms and response inhibition after closed head injury in children: Do preinjury behavior and injury severity predict outcome? Developmental Neuropsychology. 2004;25(1–2):179–198. doi: 10.1080/87565641.2004.9651927. doi:10.1080/87565641.2004.9651927. [DOI] [PubMed] [Google Scholar]
- Schachar R., Logan G. D., Robaey P., Chen S., Ickowicz A., Barr C. Restraint and cancellation: Multiple inhibition deficits in attention deficit hyperactivity disorder. Journal of Abnormal Child Psychology. 2007;35(2):229–238. doi: 10.1007/s10802-006-9075-2. doi:10.1007/s10802-006-9075-2. [DOI] [PubMed] [Google Scholar]
- Schachar R., Mota V. L., Logan G. D., Tannock R., Klim P. Confirmation of an inhibitory control deficit in attention-deficit/hyperactivity disorder. Journal of Abnormal Child Psychology. 2000;28(3):227–235. doi: 10.1023/a:1005140103162. [DOI] [PubMed] [Google Scholar]
- Schutter D. J. L. G. Transcranial magnetic stimulation. In: Harmon-Jones E., Beer J. S., editors. Methods in social neuroscience. New York: Guilford Press; 2009. pp. 233–258. [Google Scholar]
- Seamans J. K., Lapish C. C., Durstewitz D. Comparing the prefrontal cortex of rats and primates: Insights from electrophysiology. Neurotoxicity Research. 2008;14(2–3):249–262. doi: 10.1007/BF03033814. doi:10.1007/BF03033814. [DOI] [PubMed] [Google Scholar]
- Shallice T., Burgess P. The domain of supervisory processes and temporal organization of behaviour. Philosophical Transactions of the Royal Society of London, Series B: Biological Sciences. 1996;351(1346):1405–1411. doi: 10.1098/rstb.1996.0124. discussion 1411–1402 doi:10.1098/rstb.1996.0124. [DOI] [PubMed] [Google Scholar]
- Slomine B. S., Gerring J. P., Grados M. A., Vasa R., Brady K. D., Christensen J. R. Performance on measures of executive function following pediatric traumatic brain injury. Brain Injury. 2002;16(9):759–772. doi: 10.1080/02699050210127286. doi:10.1080/02699050210127286. [DOI] [PubMed] [Google Scholar]
- Stancin T., Taylor H. G., Thompson G. H., Wade S., Drotar D., Yeates K. O. Acute psychosocial impact of pediatric orthopedic trauma with and without accompanying brain injuries. Journal of Trauma. 1998;45(6):1031–1038. doi: 10.1097/00005373-199812000-00010. doi:10.1097/00005373-199812000-00010. [DOI] [PubMed] [Google Scholar]
- Stewart J. A., Tannock R. Inhibitory control differences following mild head injury. Brain and Cognition. 1999;41(3):411–416. doi: 10.1006/brcg.1999.1141. doi:10.1006/brcg.1999.1141. [DOI] [PubMed] [Google Scholar]
- Swick D., Ashley V., Turken A. U. Left inferior frontal gyrus is critical for response inhibition. BMC Neuroscience. 2008;9:102. doi: 10.1186/1471-2202-9-102. doi:10.1186/1471–2202-9-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teasdale G., Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2(7872):81–84. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- Williams B. R., Ponesse J. S., Schachar R. J., Logan G. D., Tannock R. Development of inhibitory control across the life span. Developmental Psychology. 1999;35(1):205–213. doi: 10.1037//0012-1649.35.1.205. doi:10.1037/0012-1649.35.1.205. [DOI] [PubMed] [Google Scholar]
- Wilson J. T., Wiedmann K. D., Hadley D. M., Condon B., Teasdale G., Brooks D. N. Early and late magnetic resonance imaging and neuropsychological outcome after head injury. Journal of Neurology, Neurosurgery and Psychiatry. 1988;51(3):391–396. doi: 10.1136/jnnp.51.3.391. doi:10.1136/jnnp.51.3.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak J. R., Krach L., Ward E., Mueller B. A., Muetzel R., Schnoebelen S. Neurocognitive and neuroimaging correlates of pediatric traumatic brain injury: A diffusion tensor imaging (DTI) study. Archives of Clinical Neuropsychology. 2007;22(5):555–568. doi: 10.1016/j.acn.2007.03.004. doi:10.1016/j.acn.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T. C., Wilde E. A., Bigler E. D., Li X., Merkley T. L., Yallampalli R. Longitudinal changes in the corpus callosum following pediatric traumatic brain injury. Developmental Neuroscience. 2010;32(5–6):361–373. doi: 10.1159/000317058. doi:10.1159/000317058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeates K. O., Taylor H. G., Drotar D., Wade S. L., Klein S., Stancin T. Preinjury family environment as a determinant of recovery from traumatic brain injuries in school-age children. Journal of the International Neuropsychological Society. 1997;3(6):617–630. [PubMed] [Google Scholar]
- Young S. E., Friedman N. P., Miyake A., Willcutt E. G., Corley R. P., Haberstick B. C. Behavioral disinhibition: Liability for externalizing spectrum disorders and its genetic and environmental relation to response inhibition across adolescence. Journal of Abnormal Psychology. 2009;118(1):117–130. doi: 10.1037/a0014657. doi:10.1037/a0014657. [DOI] [PMC free article] [PubMed] [Google Scholar]