Abstract
Objective
Ovarian low-grade serous carcinoma (LGSC) is a rare and indolent tumor. The utility of 18F-FDG PET/CT in monitoring patients with LGSC has not been established. We assessed the accuracy and clinical impact of 18F-FDG PET/CT in patients with ovarian LGSC after initial treatment.
Methods
A retrospective analysis was performed on patients with ovarian LGSC who had undergone 18F-FDG PET/CT scans during follow-up after primary treatment. The impact of 18F-FDG PET/CT on the management plan was assessed. The sensitivity, specificity, and accuracy of 18F-FDG PET/CT findings in the detection of recurrence were calculated. Total lesion glycolysis (TLG) was determined to assess metabolic activity of tumors. Potential prognostic factors for disease-free and overall survival after recurrence were assessed.
Results
Forty-eight patients were included in the analysis, 39 with recurrent disease and 9 without recurrence. A total of 91 18F-FDG PET/CT scans were performed, and 30% of these (27/91) had an impact on the management plan. Sensitivity, specificity, and accuracy in the detection of LGSC recurrence were 94%, 100%, and 97%, respectively, for 18F-FDG PET/CT; 89%, 95%, and 93%, respectively, for CT; and 68%, 89%, and 73%, respectively, for serum CA-125. There was no significant difference in sensitivity between PET/CT and CT. Survival after recurrence was poorer in patients with a TLG value greater than 67.7 g.
Conclusions
18F-FDG PET/CT may provide useful information during the follow-up of patients with LGSC after initial treatment. TLG may be a predictor of survival after recurrence.
Keywords: 18F-FDG PET/CT, low-grade ovarian carcinoma, total lesion glycolysis
Introduction
Ovarian carcinoma is the second most common gynecological malignancy in the United States and the most lethal; it accounted for nearly 15,500 deaths in the United States in 2012 [1]. Ovarian carcinoma is a heterogeneous disease including several distinct tumor subtypes. The serous subtype accounts for approximately 60% to 80% of ovarian cancer cases [2]. A two-tier grading system low-grade and high-grade for invasive ovarian serous carcinoma has been described [3]. Low-grade serous carcinoma (LGSC) accounts for fewer than 10% of ovarian serous carcinomas [4]. LGSC has different clinical behavior from high-grade serous carcinoma and is characterized by young age at diagnosis and prolonged overall survival [5].
Computed tomography (CT) and measurement of serum tumor marker antigen 125 (CA-125) are currently standard surveillance modalities in patients with ovarian cancer [6]. However, CA-125 poorly correlates with objective response and radiographic imaging, such as CT scans, often does not provide accurate information due to desmoplasia, calcification and fibrosis frequently associated with LGSC tumor nodules [7]. Since metabolic function may better discriminate active from treated tumor, we hypothesized that nuclear imaging, including 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET), would be a useful surveillance tool in patients diagnosed and treated for LGSC.
Materials and methods
This study was approved by the Institutional Review Board of The University of Texas MD Anderson Cancer Center and was performed in compliance with the Health Insurance Portability and Accountability Act. The Institutional Review Board waived the requirement for informed consent. We retrospectively reviewed the database of the Department of Gynecologic Oncology and Reproductive Medicine at MD Anderson and identified 71 consecutive patients with ovarian LGSC who were referred to our institution for treatment or follow-up between January 2002 and December 2011. Twenty-three patients were excluded because they had not undergone PET/CT during follow-up (n=10); had another active malignancy (n=2); or had visited our institution only once to obtain a second opinion (n=11). The remaining 48 patients were included in this study. Tumor specimens from all the patients were obtained from referring institutions, and diagnosis was confirmed as LGSC by an expert gynecologic pathologist at MD Anderson. PET/CT scans were performed at the primary physician’s discretion during follow-up after initial treatment.
18F-FDG PET/CT
For PET/CT imaging, patients were required to fast for 6 hours before 18F-FDG administration to achieve a blood glucose level of less than 120 mg/dL. 18F-FDG (185–370 MBq/injection; 5–10 mCi/injection) was administered intravenously, and then patients rested in a quiet room. Sixty minutes after 18F-FDG administration, imaging was performed. Integrated PET/CT systems were used to acquire imaging data (Discovery ST, STe, or RX; GE Healthcare). PET/CT was performed in accordance with guidelines published by the National Cancer Institute [8].
PET/CT datasets for eligible patients were reviewed for this study. Two reviewers (S.T., H.A.M.) experienced in PET/CT interpretation measured the highest metabolic activity within the tumor (maximum standard uptake value; SUVmax) and total lesion glycolysis (TLG), using an Advantage workstation (GE Healthcare). While SUVmax reflects only the highest point of metabolic activity within the tumor, it is hypothesized that TLG could better reflect tumor metabolic activity by taking into account the activity in the entire tumor. The SUV was defined as measured activity concentration (Bq/mL) multiplied by lean body mass (kg) divided by injected activity (Bq). TLG was defined as the average metabolic activity within the tumor multiplied by the tumor volume, with a threshold of 45% SUVmax in the volume of interest [9]. In the case of multiple recurrent lesions, the highest TLG was used for the analysis. For the patients who had PET/CT performed at an outside institution, available raw imaging data were obtained and input into an Advantage workstation, and SUVmax and TLG were defined by radiologists at our institution.
Evaluation of recurrence
On PET/CT, a positive finding was defined as a lesion diameter of more than 10 mm or a lesion diameter of 10 mm or less with FDG uptake. An SUVmax value of 2.0 g/mL was used as a cut-off between positive and negative findings. FDG activity only in areas of the physiologic tracer distribution and absence of sites of increased uptake were considered negative findings. On conventional CT, a positive finding was defined as a lesion more than 10 mm in diameter or with other signs of malignancy, such as central necrosis, characteristic shape (e.g., spherical lymph nodes), or abnormal contrast enhancement. Both PET/CT and conventional CT were performed within one month at the diagnosis of recurrence and were performed at physician’s discretion during follow-up. For CA-125 measurement, a CA-125 value greater than 35 U/mL was considered a positive finding. Serum CA-125 values had to have been obtained within 1 month of the confirmation of recurrence.
All PET/CT scans from follow-up of patients were correlated with histopathologic findings and/or subsequent imaging findings. PET/CT findings of recurrence were considered true-positive if they corresponded with histopathologically detected recurrence or with subsequent imaging findings positive for recurrence performed within 6 months after PET/CT. PET/CT findings were considered true-negative if they indicated no recurrence and subsequent imaging studies also showed no evidence of disease for at least 6 months after the initial PET/CT scan. The same parameters were used for CT scan.
The sensitivity, specificity, and accuracy of PET/CT, conventional CT, and CA-125 in the detection of recurrent disease were calculated using region-by-region comparison. For each imaging report, recurrences were divided into three regions: abdomen, pelvis, and distant site. For example, in the case of recurrent lesions in pelvis and mediastinum, a patient was considered to have two regions positive. If a patient had no recurrence, a patient was considered to have all three regions negative.
Impact of PET/CT on management plans
The impact of PET/CT on management plans was assessed. If the results of PET/CT prompted a change from one modality to another (e.g., from chemotherapy to surgery) or prompted changes in chemotherapy (e.g., regimen changes, dose changes, cessation, or a switch from chemotherapy to hormonal therapy), PET/CT was considered to have had an impact on management plans. For example, if CT revealed a suspicious lesion and subsequent PET/CT performed within one month confirmed a positive lesion and prompted a change in treatment then the PET/CT was considered to have had an impact on the management plan.
Statistical analysis
Statistical analyses were performed with S-PLUS 7.0 for Windows (Insightful Corp., Seattle, WA). Difference in performance between PET/CT and CT were compared with the McNemar test. Progression-free survival (PFS) and overall survival (OS) from the date of the first recurrence were calculated. Recurrence was defined as confirmation of recurrence by pathological or radiological diagnosis. For PFS, progressive disease and death were considered events. Patients who were alive at last follow-up were recorded as censored. The Kaplan-Meier method and a log-rank test were used to compare OS and PFS after recurrence stratified by various potential prognostic factors. The optimal cutpoint of TLG for PFS was determined using the methods described by Williams et al [10]. P < 0.05 was considered statistically significant.
Results
Patient characteristics
Patient characteristics are shown in Table 1. Of the 48 patients in the study, 47 had primary surgery and 1 had neoadjuvant chemotherapy as initial treatment. Thirty-nine patients had recurrence. As the diagnosis of recurrence, 22 patients (56%) were detected by PET/CT and/or CT, nine patients (23%) had an increasing CA-125 level, seven patients (18%) were symptomatic, one patient (3%) had abnormality detected by a pelvic examination. The median interval from initial treatment to disease recurrence was 29.2 months (range, 5.3–311.0). Thirty-four patients had recurrence in the abdomen, pelvis, or both. Five patients had recurrence at a distant site; in three of these, the distant recurrence was located in the mediastinum.
TABLE 1.
Patient characteristics
| Characteristic | Value |
|---|---|
| Number of patients | 48 |
| Age at initial diagnosis, years | |
| Median | 48.0 |
| SD | 14.1 |
| Range | 13–70 |
| Race/ethnicity, no. (%) | |
| White | 43 (90) |
| Latino | 3 (6) |
| African American | 2 (4) |
| Initial stage, no. (%) | |
| IIc | 2 (4) |
| IIIa | 4 (8) |
| IIIb | 10 (21) |
| IIIc | 25 (52) |
| Unknown | 7 (15) |
| Type of primary resection, no. (%) | |
| Optimal | 35 (73) |
| Suboptimal | 4 (8) |
| Unknown | 9 (19) |
| Adjuvant therapy, no. (%) | |
| Platinum-based | 36 (75) |
| Other regimen | 2 (4) |
| No treatment | 9 (19) |
| Unknown | 1 (2) |
| Recurrence, no. (%) | 39 (81) |
| Region of recurrence, no. (%)* | |
| Abdomen | 25 (52) |
| Pelvis | 24 (50) |
| Distant site | 5 (10) |
SD, standard deviation.
Some patients had recurrence in more than 1 region.
Impact of PET/CT on management plans
A total of 91 PET/CT scans and 218 conventional CT scans were performed in the 48 patients after initial therapy, respectively. Of those scans, 30% (27/91) of PET/CT had an impact on management plans. Details of the impact of PET/CT on management plans are presented in Table 2. Nineteen PET/CT scans were performed at outside institutions. Of those, 15 PET/CT scans did not have an impact on the management plan (13 scans indicated that current therapy should be continued; 2 scans showed no recurrence). The remaining 4 PET/CT scans prompted initiation of therapy: chemotherapy (n=2), hormonal therapy (n=1), or surgery (n=1).
TABLE 2.
Description of impact for the 27 PET/CT scans that had an impact on management plans
| Description of Impact | No. of Scans (%) | |
|---|---|---|
| PET/CT identified a recurrence not identified on CT | 2 (7) | |
| PET/CT identified a recurrence in a patient in whom CT identified a lesion with an intermediate probability of being malignant | 8 (30) | |
| PET/CT ruled out a recurrence in a patient in whom CT identified a lesion with an intermediate probability of being malignant | 1 (4) | |
| PET/CT prompted a change in therapy | Change in chemotherapy regimen | 10 (37) |
| Change from chemotherapy to hormonal therapy | 3 (11) | |
| Change from hormonal therapy to chemotherapy | 2 (7) | |
| Change from chemotherapy to surgery | 1 (4) |
Sensitivity, specificity, and accuracy
In the 39 patients with recurrence, recurrence was confirmed by biopsy in 14 patients and cytology of malignant pleural effusion in 1 patient. The remaining 24 patients had recurrence confirmed by an imaging study, demonstrating a new lesion or significant increase in existing lesions. A total of 144 regions (3 regions in each of the 48 patients) were evaluated with PET/CT and conventional CT. CA-125 data were available for 40 of the 48 patients. The median SUVmax value was 6.8 g/ml (range, 2.1–27.0).
The performance of PET/CT, conventional CT, and CA-125 in the detection of recurrence in patients with LGSC is summarized in Table 3. Sensitivity, specificity, and accuracy were 94% (95% confidence interval [CI]: 84–98%), 100% (95% CI: 94–100%), and 97% (95% CI: 93–99%), respectively, for PET/CT; 89% (95% CI: 78–96%), 95% (95% CI: 88–99%), and 93% (95% CI: 88–97 %), respectively, for CT; and 68% (95% CI: 49–83%), 89% (95% CI: 51–99%), and 73% (95% CI: 56–85%), respectively, for serum CA-125. There was no significant difference in sensitivity between PET/CT and CT (P = 0.13). There was no false-positive detected by PET/CT. Four patients had false-negative findings in one region each on PET/CT. In two patients, surgery revealed a metastasis of LGSC in the colon (1 patient) or in pelvis (1 patient) that was not detected by PET/CT. Another patient had a biopsy-proven metastasis in the vaginal cuff that was not detected by PET/CT. In the remaining patient, laparoscopy revealed liver implants that were not detected by PET/CT. The false-negative lesion in the vaginal cuff was 1 cm in diameter. Other three lesions were smaller than 1 cm.
TABLE 3.
Performance of PET/CT, conventional CT, and CA-125 in the detection of recurrence
| Modality | Result | Confirmed Recurrence* | Confirmed Lack of Recurrence* |
|---|---|---|---|
| PET/CT† | Positive for recurrence | 59 | 0 |
| Negative for recurrence | 4 | 81 | |
| CT† | Positive for recurrence | 51 | 4 |
| Negative for recurrence | 6 | 83 | |
| CA-125 | Positive for recurrence | 21 | 1 |
| Negative for recurrence | 10 | 8 |
Confirmed by findings on histopathologic analysis or subsequent imaging.
For each of the 48 patients, PET/CT and CT findings were evaluated for each of three regions: abdomen, pelvis, and distant site. Thus, the total number of results for PET/CT and CT is 48 × 3 = 144.
Predictive factors
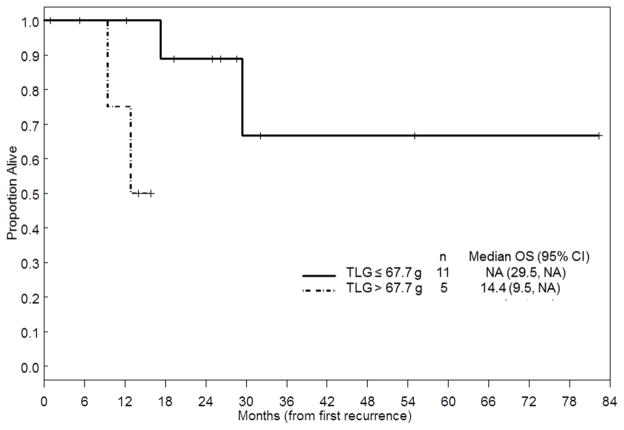
The results of univariate analysis of predictive factors for PFS and OS after the first recurrence are summarized in Table 4. Median follow-up time for all patients was 26.4 months (range, 1–315 months). As of this writing, 9 patients have died with progressive disease, 2 patients have died without progressive disease, 19 patients are alive with progressive disease, and 9 patients are alive without progressive disease. Optimal resection of recurrent tumor was associated with better PFS and OS. Multiple-lesion recurrence was associated with poorer PFS than single-lesion recurrence. CA-125 was not a significant predictor for PFS or OS. Of the 39 patients with recurrent disease, 19 had SUVmax values and 16 had TLG values at the first recurrence available in an Advantage workstation at MD Anderson. SUVmax was not a significant predictor of PFS or OS. On the other hand, TLG values greater than 67.7 g (the optimal cutoff value determined by our analysis) were significantly associated with poorer OS. Median survival was not met in patients with TLG values less than or equal to 67.7 g and was 14.4 months in patients with TLG values greater than 67.7 g (Figure 1). However, multivariate analysis could not be performed because of the small number of events.
TABLE 4.
Analysis of predictors of progression-free survival (PFS) and overall survival (OS) after recurrence
| Variable | N | PFS
|
OS
|
||||
|---|---|---|---|---|---|---|---|
| Events | 5-year PFS Probability | P value | Events | 5-year OS Probability | P value | ||
| Age at recurrence | |||||||
| > 50 years | 25 | 21 | 0.17 | 0.41 | 8 | 0.58 | 0.30 |
| ≤ 50 years | 14 | 9 | 0.24 | 3 | 0.75 | ||
| CA-125 level at recurrence | |||||||
| > 35 U/ml | 21 | 16 | 0.16 | 0.72 | 6 | 0.62 | 0.70 |
| ≤ 35 U/ml | 10 | 6 | N/A | 2 | N/A | ||
| Optimal resection of recurrent tumor | |||||||
| Yes | 19 | 14 | 0.25 | 0.028 | 4 | 0.78 | 0.044 |
| No | 20 | 16 | 0.12 | 7 | 0.46 | ||
| Number of recurrent lesion | |||||||
| Multiple | 28 | 23 | 0.05 | 0.008 | 10 | 0.51 | 0.063 |
| Single | 11 | 7 | 0.58 | 1 | 0.88 | ||
| SUVmax | |||||||
| > 10 g/ml | 5 | 4 | 0.27 | 0.71 | 1 | 0.50 | 0.84 |
| ≤ 10 g/ml | 14 | 8 | N/A | 4 | N/A | ||
| Total lesion glycolysis value | |||||||
| > 67.7 | 5 | 4 | 0.00 | 0.099 | 2 | N/A | 0.02 |
| ≤ 67.7 | 11 | 6 | 0.43 | 2 | 0.67 | ||
N/A, not assessable.
FIGURE 1.
Kaplan-Meier curve of overall survival after recurrence by TLG value.
Discussion
In this study, we found that PET/CT performed during follow-up after initial treatment had an impact on the management plan in 30% of patients with LGSC. We also found that sensitivity, specificity, and accuracy in the detection of LGSC recurrence were 94%, 100%, and 97%, respectively, for PET/CT. Furthermore, we showed that TLG at the time of diagnosis of recurrence may be a predictor of outcome.
Our findings regarding the impact of PET/CT on patient management agree with those of previous studies. The National Oncologic PET Registry (NOPR) was opened in 2006 to collect data on the clinical utility of PET and to assess how 18F-FDG PET affects decision-making. Using the NOPR system, Hillner et al found that the impact of PET on the management of known cancer cases was consistent across cancer types [11, 12]. According to these authors, 37.7% of 1971 PET scans had an impact on the management of ovarian cancer. However, the proportion of patients with ovarian cancer who had LGSC was unknown because NOPR does not include information on histopathologic subtypes.
CT and CA-125 are routinely used for monitoring recurrence and response to chemotherapy in patients with epithelial ovarian cancer. Previous studies showed that the sensitivity and specificity of CA-125 in the detection of recurrent ovarian cancer were 75% and 100%, respectively, and that the sensitivity and specificity of conventional CT in the detection of recurrent ovarian cancer were 55–66.6% and 93.3–100%, respectively [13, 14]. However, those studies did not include patients with LGSC. Gu et al published a systematic review of CA-125 and imaging modalities for diagnosing recurrent ovarian cancer [15]. According to their report, CA-125 had the highest pooled specificity, 93%, and PET/CT had the highest pooled sensitivity, 91%. However, like the aforementioned reports by Hillner et al, this report did not include information about histopathologic subtypes of ovarian cancer. Havrilesky et al reported that PET is a good modality in the detection of recurrence when the CA-125 level is elevated and findings on conventional imaging are negative or equivocal [16]. However, normal CA-125 values cannot exclude the presence of active disease because of CA-125’s low sensitivity. In our study, of the 31 patients with recurrent disease with available CA-125 data, 10 patients (32%) had normal CA-125 values.
SUVmax and TLG are measures of the metabolic activity of tumors determined on the basis of PET/CT images [17]. These parameters can have clinical value in evaluating tumor aggressiveness, proliferation, and outcome [9, 18]. A standard SUVmax threshold has not been established for evaluation of ovarian carcinoma; however, SUVmax values of 2.0–3.0 g/ml are commonly used thresholds in solid tumors. Because our study was conducted in patients with low-grade carcinoma, we chose a relatively low threshold. While SUVmax reflects only the point of greatest metabolic activity within the tumor, TLG is thought to more accurately reflect metabolic activity than SUVmax by taking into account the activity of the entire tumor. Recently, Chung et al. reported that the TLG value before initial treatment was significantly associated with a high rate of recurrence and shorter PFS in patients with epithelial ovarian cancer [19]. However, they did not mention how many patients with LGSC were included in their study. They used a TLG value of 563 as a cutoff value on the basis of the receiver operating characteristic analysis, whereas in this study we determined a TLG value of 67.7 as the cutoff for predicting survival at the time of recurrence. Our TLG values were derived from recurrent tumor volume, whereas the TLG values in the study by Chung et al were obtained before initial therapy and were much greater than those in our study. A change in TLG (ΔTLG) is also a predictor of survival in several types of malignancies because it corresponds to the change in the cell mass of the target lesion and reflects the global response of the entire tumor to treatment [9, 20, 21]. In our study, TLG data were available for only 16 patients with recurrent LGSC. Additionally, our OS data included many censored patients. ΔTLG could not be calculated as PET/CT was not always performed at the same interval after completion of treatment for recurrence. Hence, further study is necessary to verify whether TLG and ΔTLG are true predictors of survival in patients with LGSC.
Previous studies in women with LGSC have demonstrated that despite a significant decrease in CA-125, there is often minimal or no response by radiographic imaging [7]. We speculate that this may be due to the desmoplasia, calcification and fibrosis that our pathologists have observed associated with LGSC. In 8 cases in the current study, PET/CT noted lesions concerning for recurrence following an equivocal CT. Additional study is warranted to further investigate the use of PET/CT in distinguishing this fibrotic reaction from active tumor.
Our study had several potential limitations. First, since the prevalence of LGSC is less than 10% in patients with epithelial ovarian cancer, limited data were available. Second, TLG values could not be calculated for some patients who had PET/CT performed at an outside institution because raw imaging data were not available. Third, and the most important limitation, is that PET/CT scans were not performed at every recurrence. Most PET/CT scans were performed when the primary physician wanted more information about something that came up: a new lesion on CT, an increasing CA-125 level, or symptoms or findings on physical examination. The application of PET/CT to selected cases might have caused bias and influenced the study results. However, the strengths of our study lie in the number of patients included and the fact that this is the first study to show the accuracy of PET/CT in the detection of recurrence and the impact of PET/CT on management plans in follow-up of patients with ovarian LGSC.
In conclusion, PET/CT may provide useful information during the follow-up of patients with LGSC after initial treatment. TLG at the time of recurrence may be a predictor of survival. However, further prospective studies are necessary to confirm the utility of TLG as a prognostic factor.
Highlights.
PET/CT may impact clinical management in the setting of low-grade serous carcinoma in approximately 30% of patients.
PET/CT may be a useful tool in the detection of low-grade serous carcinoma recurrence.
Total lesion glycolysis may predict poorer overall survival after recurrence.
Acknowledgments
Sources of funding
This work was supported in part by the National Institutes of Health through MD Anderson’s Cancer Center Support Grant (NCI P30 CA016672); by the MD Anderson Cancer Center James E. Anderson Distinguished Professorship in Nuclear Medicine (to Dr. Macapinlac); by the Society of Nuclear Medicine and Molecular Imaging 2012/2014 Wagner-Torizuka Fellowship (to Dr. Takeuchi); and by the Ann Rife Cox Chair in Gynecology (to Dr. Coleman).
We thank the staff at MD Anderson Cancer Center for their assistance and especially Ms. Richelle D. Millican, Supervisor, Diagnostic Imaging, for data collection. This report was edited by Stephanie Deming in the Department of Scientific Publications at The University of Texas MD Anderson Cancer Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Satoshi Takeuchi, Email: STakeuchi@mdanderson.org.
Martin Lucchini, Email: drlucchini@gmail.com.
Kathleen M. Schmeler, Email: KSchmele@mdanderson.org.
Robert L. Coleman, Email: RColeman@mdanderson.org.
David M. Gershenson, Email: dgershen@mdanderson.org.
Mark F. Munsell, Email: mfmunsell@mdanderson.org.
Homer A. Macapinlac, Email: hmacapinlac@mdanderson.org.
Pedro T. Ramirez, Email: peramire@mdanderson.org.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Levanon K, Crum C, Drapkin R. New insights into the pathogenesis of serous ovarian cancer and its clinical impact. J Clin Oncol. 2008;26:5284–93. doi: 10.1200/JCO.2008.18.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Malpica A, Deavers MT, Lu K, Bodurka DC, Atkinson EN, Gershenson DM, et al. Grading ovarian serous carcinoma using a two-tier system. Am J Surg Pathol. 2004;28:496–504. doi: 10.1097/00000478-200404000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Seidman JD, Horkayne-Szakaly I, Cosin JA, Ryu HS, Haiba M, Boice CR, et al. Testing of two binary grading systems for FIGO stage III serous carcinoma of the ovary and peritoneum. Gynecol Oncol. 2006;103:703–8. doi: 10.1016/j.ygyno.2006.05.030. [DOI] [PubMed] [Google Scholar]
- 5.Gershenson DM, Sun CC, Lu KH, Coleman RL, Sood AK, Malpica A, et al. Clinical behavior of stage II–IV low-grade serous carcinoma of the ovary. Obstet Gynecol. 2006;108:361–8. doi: 10.1097/01.AOG.0000227787.24587.d1. [DOI] [PubMed] [Google Scholar]
- 6.NCCN Clinical Practice Guidelines in Oncology. Ovarian Cancer Version 1.2013. ( http://www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf)
- 7.Schmeler KM, Sun CC, Bodurka DC, Deavers MT, Malpica A, Coleman RL, et al. Neoadjuvant chemotherapy for low-grade serous carcinoma of the ovary or peritoneum. Gynecol Oncol. 2008;108:510–4. doi: 10.1016/j.ygyno.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 8.Shankar LK, Hoffman JM, Bacharach S, Graham MM, Karp J, Lammertsma AA, et al. Consensus recommendations for the use of 18F-FDG PET as an indicator of therapeutic response in patients in National Cancer Institute Trials. J Nucl Med. 2006;47:1059–66. [PubMed] [Google Scholar]
- 9.Costelloe CM, Macapinlac HA, Madewell JE, Fitzgerald NE, Mawlawi OR, Rohren EM, et al. 18F-FDG PET/CT as an indicator of progression-free and overall survival in osteosarcoma. J Nucl Med. 2009;50:340–7. doi: 10.2967/jnumed.108.058461. [DOI] [PubMed] [Google Scholar]
- 10.Williams BAMJ, Mandrekar SJ, Cha S, Furth AF. Mayo Clinic Department of Health Sciences Research, editor. Finding optimal cutpoints for continuous covariates with binary and time-to-event outcomes. Rochester: 2006. [Google Scholar]
- 11.Hillner BE, Siegel BA, Shields AF, Liu D, Gareen IF, Hunt E, et al. Relationship between cancer type and impact of PET and PET/CT on intended management: findings of the national oncologic PET registry. J Nucl Med. 2008;49:1928–35. doi: 10.2967/jnumed.108.056713. [DOI] [PubMed] [Google Scholar]
- 12.Hillner BE, Siegel BA, Liu D, Shields AF, Gareen IF, Hanna L, et al. Impact of positron emission tomography/computed tomography and positron emission tomography (PET) alone on expected management of patients with cancer: initial results from the National Oncologic PET Registry. J Clin Oncol. 2008;26:2155–61. doi: 10.1200/JCO.2007.14.5631. [DOI] [PubMed] [Google Scholar]
- 13.Torizuka T, Nobezawa S, Kanno T, Futatsubashi M, Yoshikawa E, Okada H, et al. Ovarian cancer recurrence: role of whole-body positron emission tomography using 2-[fluorine-18]-fluoro-2-deoxy- D-glucose. Eur J Nucl Med Mol Imaging. 2002;29:797–803. doi: 10.1007/s00259-001-0750-9. [DOI] [PubMed] [Google Scholar]
- 14.Prayer L, Kainz C, Kramer J, Stiglbauer R, Schurawitzki H, Baldt M, et al. CT and MR accuracy in the detection of tumor recurrence in patients treated for ovarian cancer. J Comput Assist Tomogr. 1993;17:626–32. doi: 10.1097/00004728-199307000-00021. [DOI] [PubMed] [Google Scholar]
- 15.Gu P, Pan LL, Wu SQ, Sun L, Huang G. CA 125, PET alone, PET-CT, CT and MRI in diagnosing recurrent ovarian carcinoma: a systematic review and meta-analysis. Eur J Radiol. 2009;71:164–74. doi: 10.1016/j.ejrad.2008.02.019. [DOI] [PubMed] [Google Scholar]
- 16.Havrilesky LJ, Kulasingam SL, Matchar DB, Myers ER. FDG-PET for management of cervical and ovarian cancer. Gynecol Oncol. 2005;97:183–91. doi: 10.1016/j.ygyno.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 17.Erdi YE, Macapinlac H, Rosenzweig KE, Humm JL, Larson SM, Erdi AK, et al. Use of PET to monitor the response of lung cancer to radiation treatment. Eur J Nucl Med. 2000;27:861–6. doi: 10.1007/s002590000258. [DOI] [PubMed] [Google Scholar]
- 18.Liao S, Penney BC, Wroblewski K, Zhang H, Simon CA, Kampalath R, et al. Prognostic value of metabolic tumor burden on 18F-FDG PET in nonsurgical patients with non-small cell lung cancer. Eur J Nucl Med Mol Imaging. 2012;39:27–38. doi: 10.1007/s00259-011-1934-6. [DOI] [PubMed] [Google Scholar]
- 19.Chung HH, Kwon HW, Kang KW, Park NH, Song YS, Chung JK, et al. Prognostic value of preoperative metabolic tumor volume and total lesion glycolysis in patients with epithelial ovarian cancer. Ann Surg Oncol. 2012;19:1966–72. doi: 10.1245/s10434-011-2153-x. [DOI] [PubMed] [Google Scholar]
- 20.Tateishi U, Gamez C, Dawood S, Yeung HW, Cristofanilli M, Macapinlac HA. Bone metastases in patients with metastatic breast cancer: morphologic and metabolic monitoring of response to systemic therapy with integrated PET/CT. Radiology. 2008;247:189–96. doi: 10.1148/radiol.2471070567. [DOI] [PubMed] [Google Scholar]
- 21.Gulec SA, Suthar RR, Barot TC, Pennington K. The prognostic value of functional tumor volume and total lesion glycolysis in patients with colorectal cancer liver metastases undergoing 90Y selective internal radiation therapy plus chemotherapy. Eur J Nucl Med Mol Imaging. 2011;38:1289–95. doi: 10.1007/s00259-011-1758-4. [DOI] [PubMed] [Google Scholar]