Abstract
Background
Daughters of depressed mothers are at increased risk for developing a depressive disorder. We know relatively little, however, about the specific factors that contribute to this elevated risk. The present study investigated the effects of familial risk for depression and the 5-HTTLPR and COMT Val158Met polymorphisms, which have been associated with risk for depression, on biases in endorsement of and memory for positive and negative adjectives.
Methods
Following a negative mood induction, 60 girls between the ages of 10 and 14 who had recurrent depressed mothers (high risk for depression) and 91 age-matched daughters of never-disordered mothers (low risk for depression) completed a Self-Referent Encoding Task in which they decided whether negative and positive adjectives described them. Following the task they were asked to recall as many of the adjectives as they could.
Results
Despite the absence of significant group differences in endorsement of positive or negative adjectives, high-risk girls with the COMT Val158Met Val/Val polymorphism recalled more positive (but not negative) words that they had endorsed than did high-risk girls who were homozygous for the Met allele. COMT was not associated with recall of valenced adjectives in low-risk girls. Across risk groups, 5-HTTLPR polymorphism was not associated with recall of valenced adjectives.
Limitations
Even with over 150 participants, there were relatively small numbers in some of the cells of this study, limiting its statistical power.
Conclusions
These results suggest that assessing the interaction of familial risk status and COMT polymorphism is important in understanding the development of depressive disorders.
Keywords: depression, familial risk, COMT, memory, genetic
Children at Risk for Depression: Memory Biases, Self-Schemas, and Genotypic Variation Children of depressed parents are three to five times more likely to develop Major Depressive Disorder (MDD) during adolescence than are children with no family history of psychopathology (Gotlib & Hammen, 2009). Although environmental, psychological, and biological factors have been implicated as playing a role in this increased risk for the development of depression, the precise mechanisms are likely to be complex and multidimensional and are not yet fully understood.
Beck’s cognitive model of depression (Beck, Rush, Shaw, & Emery, 1979) posits that persons who are vulnerable to experience a depressive episode are characterized by a negative self-schema. This schema is hypothesized to be activated by adverse environmental factors and to lead to negatively biased distortions of the self, world, and future that increase the risk for depression (Beck et al., 1979). Investigators have found that depressed participants are characterized by biased recall of emotional material (see Gotlib and Joormann, 2010 for a review). Moreover, recall of negative information is significantly stronger in depressed adults if the information is self-relevant (Banos et al., 2001). Similarly, pre-adolescent depressed children recalled significantly fewer positive self-referential adjectives than did their never-depressed counterparts, but similar numbers of negative self-referential adjectives, documenting memory biases in depressed children for emotional stimuli (Timbremont and Braet, 2004).
Researchers have found that offspring of depressed parents also exhibit less positive self-schemas and biased cognitive processes (Jaenicke et al., 1987; Taylor and Ingram, 1999). Compared to offspring of never-depressed parents, children of depressed parents reported higher levels of self-criticism, and lower levels of self-worth (Garber and Robinson, 1997). Children of depressed mothers also had a more depressotypic attributional style than did children of never-depressed mothers. Girls with no personal history of psychopathology whose biological mothers had experienced recurrent depressive episodes have been found to attend selectively to negative information (Joormann et al., 2007). Although we do not know precisely why children of depressed parents manifest these cognitive biases, researchers have found that exposure to maternal depression is associated with elevated levels of morning cortisol in their offspring (Halligan et al., 2004; Mannie et al., 2007) and increased subjective stress (Jaenicke et al., 1987). This stress may contribute to the development of negative cognitive biases in the offspring through environmental factors such as the children’s direct experience of stress, through learned coping strategies, or through cognitive biases modeled by their mothers (Goodman and Gotlib, 1999).
Evidence also indicates that biological influences are important in understanding risk for depression (Gotlib and Hammen, 2002). In this context, researchers are examining associations between cognitive indices of vulnerability for the development of depression and specific candidate genes (Hariri et al., 2002; Hayden et al., 2008). In a landmark study. Caspi et al. (2003) found that individuals who were homozygous for the short allele of the 5-HTTLPR gene developed depression more frequently when exposed to stressful life events than did those without this genotype.1 To investigate whether Caspi et al.’s findings could be explained by differences in cognitive processing style as a function of 5-HTTLPR genotype, Hayden et al. (2008) studied self-referential information processing in a community sample of young children. Consistent with Caspi et al.’ findings, Hayden et al. found that, when primed with a negative mood induction, children homozygous for the 5-HTTLPR short allele exhibited significantly stronger processing of negative information.
Based on guidelines for genetic association studies in complex disease (Campbell and Rudan, 2002), the COMT Val158Met polymorphism has high “biological plausibility” and is a promising candidate through which to examine the effects of genes on cognitive indices for depression. COMT Val158Met polymorphism has been found to be associated with depression and to play a critical role in the regulation of pre-frontal dopamine (DA) activity (Massat et al., 2005; Tunbridge et al., 2004), a region that has been implicated in endorsement of and memory for self-referential information (Fossati et al., 2004).
Val158Met is a single nucleotide polymorphism (SNP; G → A transition at codon 158) of the catechol-O-methyltransferase (COMT) gene located on chromosome 22q11.2. The COMT SNP encodes activity of COMT, a key enzyme in the metabolism of DA and is a primary regulator of synaptic levels of DA in the prefrontal cortex (Gogos et al., 1998). A substitution of valine (Val) by methionine (Met) at codon 158 of the COMT gene affects the activity level of the COMT enzyme. The alleles are posited to be co-dominant, given that heterozygous individuals have enzyme activity that is halfway between their homozygous counterparts (Weinshilboum, et al., 1999). Compared with the Val allele of this polymorphism, the Met allele has been associated with reduced activity of the COMT enzyme and, therefore, higher DA levels in the prefrontal cortex (Chen et al., 2004). Compared with individuals with one or two Met alleles, persons with the homozygous Val/Val SNP have three to four times higher enzyme activity and increased DA catabolism (Chen et al, 2004). Consequently, persons homozygous for the Val allele have significantly lower synaptic DA levels following neurotransmitter release, ultimately reducing dopaminergic stimulation of the post-synaptic neuron and resulting in weaker prefrontal activation (Winterer et al., 2006). Importantly, in fMRI studies of self-referential processing, greater activation in the prefrontal cortex has been associated with both endorsement of and memory for positive self-relevant information (Fossati et al., 2004).
As a consequence of these differences in the availability of dopamine, investigators have documented distinct behavioral phenotypes for Val and Met carriers. The increased dopamine availability in the post-synaptic neuron that characterizes Met carriers, compared with homozygous COMT Val carriers, is associated with advantages in cognitive processing (Heinz and Smolka, 2006). Importantly, however, Smolka et al. (2005) found that Met carriers also exhibit difficulties in affective processing. For example, compared with homozygous Val carriers, homozygous Met carriers perform better on tests of working memory and attention, but are less efficient in their processing negative affective stimuli (Stein et al., 2006). Indeed, the COMT val158met genotype explained a significant proportion of the variance in BOLD response to unpleasant stimuli. These investigators found that increased prefrontal activation to negative stimuli in participants with one or two Met alleles contributed to the observed lower emotional resilience against negative mood states (Smolka et al. 2005). This increased activation, combined with the increased dopamine release in prefrontal regions associated with stress (Berridge, 2004), means that the load limit of systems concerned with emotional and cognitive behavior control will be reached earlier in Met carriers than in Val carriers, contributing to the lowered resilience of Met carriers against negative mood states (Smolka et al., 2005). Further evidence demonstrates that Val carriers are more efficient than are Met carriers in dopaminergic neurotransmission under conditions of stress and, therefore, have better working memory under stress than when they are not stressed (Mattay et al., 2003). Indeed under conditions of stress, Val carriers have been found to exhibit better performance on tasks assessing working memory than do Met carriers (Mattay et al., 2003).
The purpose of the present study is to examine the relation between two candidate genes – 5-HTTLPR and COMT, and cognitive biases that have been implicated as risk factors for the development of depression. The first aim was to examine whether the COMT Val158Met polymorphism had differential effects on biases in memory for valenced self-referential information in daughters at risk for depression compared to low-risk daughters. While prior studies have investigated the effects of risk status on memory for valenced information (Taylor and Ingram, 1999), or the relation of COMT genotype and memory (Heinz and Smolka, 2006; Mattay et al., 2003; Smolka et al., 2005), this is the first study to investigate the interactive effects of the COMT polymorphism and risk status on memory for self-referential information. Although speculative, we anticipated that COMT genotype would have differential effects on memory for valenced self-referential information in high vs. low risk girls. We based this hypothesis on research indicating that 1) COMT genotype is associated with depression (Massat et al., 2005); 2) COMT genotype controls dopamine availability in the region of the brain associated with endorsement of and memory for positive self-relevant information (Fossati et al., 2004); and 3) affective processing varies as a function of COMT genotype and stress exposure (Mattay et al., 2003). Given concerns in the field regarding replications of genetic studies (Duncan and Keller, 2011), the second aim of this study was to attempt to replicate Hayden et al.’s (2003) findings that children homozygous for the short allele of the 5-HTTLPR polymorphism are characterized by significantly stronger processing of negative information than are children with at least one long allele.
Methods
Participants
Participants were 151 girls between the ages of 9 and 14 (M=12.0, SD=1.5) with no current or past history of Axis I disorders included in the Diagnostic and Statistical Manual (DSM) of Mental Disorders, 4th edition (DSM-IV; American Psychiatric Association, 1994). Ninety-one of the girls had biological mothers with no current or past DSM-IV Axis I disorder, and 60 girls had biological mothers who had experienced at least two major depressive episodes during their daughter’s lifetime. Participants were recruited through advertisements in various local media (e.g., Craigslist).
Assessment of depression and risk
The diagnostic status of daughters was assessed by trained interviewers administering the Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime version (K-SADS-PL; Kaufman et al., 2000) separately to the daughters and to their mothers. The Structured Clinical Interview for the DSM-IV (SCID; First et al., 1995) was administered to the mothers. To assess inter-rater reliability, an independent rater evaluated 30% of the SCID and K-SAD-PL interviews by randomly selecting audiotapes of equal numbers of high-risk and low-risk pairs. In all cases these diagnoses matched the original interviewer.
Daughters met eligibility for the high-risk group if (1) they did not meet criteria for any past or current DSM-IV Axis-I disorder according to both the parent and child K-SADS-PL; (2) their mothers met DSM-IV criteria for at least two distinct episodes of MDD since the birth of their daughters but did not currently meet criteria for MDD; and (3) their mothers did not meet criteria for Bipolar I or II or current substance use or dependence disorders; and had not experienced any psychotic symptoms. Daughters met eligibility for the low-risk group if (1) they did not meet criteria for any past or current DSM-IV Axis-I disorder; and (2) their mothers did not meet criteria for any current or past DSM-IV Axis-I disorders. Mothers and daughters were excluded if either had experienced severe head trauma or diagnoses of learning disabilities.
Measures
To assess pubertal status, girls completed the five Tanner stages of maturity (Morris and Udry, 1980). This measure was found to be significantly correlated with physicians’ ratings (Rudolph, 2008).
To assess severity of depressive symptoms, girls completed the 10-item version of the Children’s Depression Inventory (CDI-S; Kovacs, 1992). Internal consistency for the CDI-S in this sample was α = .76.
To assess impact of the mood induction, we asked the participants to rate their mood before and after the induction on a visual analog scale (very sad = 1, very happy =5; Taylor & Ingram, 1999).
Finally, we administered the vocabulary section of the verbal subtest of the Wechsler Intelligence Scale for Children-IV (WISC-IV; Wechsler, 2003) to the girls. This test assesses knowledge of word meanings and language development.
Mood inductions
Taylor and Ingram (1999) found that memory biases were evident only when a negative mood state was induced, suggesting the presence of cognitive biases that are activated by negative affect. In the present study, participants were shown one of three randomly assigned 5- to 7-minute film clips before completing the SRET in order to induce a negative mood. The clips from Stepmom (Columbus, 1998), My Girl (Zeiff, 1991), and Dead Poet’s Society (Weir, 1989) all portray adolescents experiencing the loss of a loved one. Following the film clip, participants were instructed to think for two minutes about how they would feel if they experienced the situation in the film clip. No significant differences in self-reported mood state were found between or within groups.
SRET and incidental recall task
The SRET consisted of a set of 40 adjectives, half of which were negative and half of which were positive. The adjectives were selected from previous studies of information processing in depression (e.g. Gotlib et al., 2004).
When completing the SRET, participants were seated at a computer and were instructed to focus on a cross in the middle of the screen. For each trial, the words “Describes me?” replaced the cross for 500 ms, followed by a 250-ms pause, after which one of the stimulus words was presented. Participants indicated whether the displayed word described them. After the task, participants were asked to recall as many words as possible from the task. We used the proportion of endorsed positive and negative adjectives that were recalled to assess biases in memory.
Genetic data
DNA was analyzed using saliva collected with the Oragene Kit (DNA Genotek, Inc. Ottawa, Ontario, Canada). DNA extracted by this method permits genotyping with a high success rate (Rylander-Rudqvist et al., 2006).
DNA was amplified with the primers 5′-CCA GGT CTG ACA ACG GGT CA-3′ and 5′-CTC ATC ACC ATC GAG ATC AA-3′ to genotype the target COMT Val158Met polymorphism. Next, the 109-bp fragment was digested with NlaIII. The resulting digested bands were detected with ethidium bromide staining after gel electrophoresis (4% agarose).
Procedure
In the first session after mothers provided informed consent and daughters provided assent, both completed clinical interviews, self-report measures, and the WISC-IV vocabulary scale. Within one week, daughters completed a second session, which included a baseline mood measurement, followed by the mood induction, a second mood assessment, the SRET and incidental recall task.
Results
Participant characteristics
Demographic and clinical characteristics, categorized by risk group and COMT polymorphism, are presented in Table 1. A chi-square analysis conducted on race/ethnicity and COMT polymorphism yielded a significant effect, χ2 (5, N= 157) = 11.31, p < .05. Therefore, we included race/ethnicity as a covariate in subsequent analyses. A series of two-way analyses of variance (ANOVAs; risk group [low-risk, high-risk] × COMT group [Val/Val, Val/Met, Met/Met]) yielded no significant main effects of risk group or COMT group for age, pubertal status, or vocabulary scores (Fs < 2.7, ns). As indicated above, there was a main effect of risk group for CDI-S scores, F(1,145) = 14.05, p<.05, and CDI-S scores were used as a covariate for subsequent analyses. There were no significant interactions of risk group and COMT group for any of the variables (Fs < 0.71, ps > .05). There were no significant differences between the low- and high-risk girls or among the three COMT groups in changes in mood following the mood induction; there was also no significant interaction of risk group and COMT group (all Fs < 3.09, ps > .05).
Table 1.
Demographic and Clinical Characteristics of Participants in the COMT and Familial Risk Groups.
| Met/Met
|
Val/Met
|
Val/Val
|
||||
|---|---|---|---|---|---|---|
| High-Risk | Low-Risk | High-Risk | Low-Risk | High-Risk | Low-Risk | |
| Number of girls | 8 | 10 | 39 | 49 | 13 | 32 |
| Age M (SD) | 10.71 (1.50) | 12.30(.68) | 12.28 (1.61) | 11.88 (1.56) | 12.11 (1.71) | 11.80 (1.71) |
| Pubertal Status M (SD) | 2.81 (1.19) | 3.22 (1.28) | 3.42 (1.04) | 3.07 (0.99) | 3.18 (1.17) | 3.05 (0.96) |
| CDI-S M (SD) | 3.29 (1.80) | .90 (.99) | 2.17 (2.42) | 1.26 (1.51) | 3.60 (1.58) | 1.20 (1.56) |
| WISC M (SD) | 49.29 (9.18) | 55.80 (5.39) | 51.19 (6.78) | 47.09 (8.73) | 50.20 (9.76) | 48.64 (9.00) |
| Mood Change M (SD) | −1.57 (.79) | −1.9 (1.1) | −1.33 (1.14) | −1.43 (1.08) | −1.0 (.85) | −1.77 (1.12) |
| Ethnicity (%Caucasian) | 55.6 | 80 | 80.5 | 78.4 | 46.2 | 57.6 |
Note. CDI-S = Children’s Depression Inventory – Short Form; WISC-IV = Wechsler Intelligence Scale for Children-IV; M = mean; SD = standard deviation
Demographic and clinical characteristics, categorized by 5-HTTLPR, are presented in Table 2. A chi-square analysis conducted on race/ethnicity yielded a non-significant effect, χ2 (2, N= 110) = 2.735, p > .05. A series of one-way analyses of variance (ANOVAs) yielded no significant effects of 5-HTTLPR polymorphism for age, pubertal status, CDI-S, or vocabulary scores (Fs < 2.3, ns).
Table 2.
Demographic and Clinical Characteristics of Participants in the 5-HTTLPR polymorphism groups.
| Long/Long | Long/Short | Short/Short | |
|---|---|---|---|
| Number of girls | 22 | 69 | 19 |
| Age M (SD) | 11.86 (1.45) | 12.63 (1.60) | 11.93 (1.25) |
| Pubertal Status M (SD) | 2.70 (1.09) | 3.20 (1.03) | 2.85 (1.0) |
| CDI-S M (SD) | 1.48 (1.66) | 1.72 (2.17) | 1.05 (1.68) |
| WISC M (SD) | 45.95 (9.91) | 50.00 (8.34) | 50.47 (6.4) |
| Mood Change M (SD) | −1.43 (.98) | −1.47 (1.03) | −1.22(.94) |
| Ethnicity (% Caucasian) | 54.6 | 72.5 | 73.7 |
Note. CDI-S = Children’s Depression Inventory – Short Form; WISC-IV = Wechsler Intelligence Scale for Children-IV; M = mean; SD = standard deviation
SRET Recall
A two-way (risk group by COMT group) MANCOVA, covarying race/ethnicity and CDI-S scores, conducted on the proportion of endorsed positive and negative adjectives recalled by participants did not yield significant main effects of risk group or COMT group (both Fs<.91, ps > .05); however, it did yield a significant interaction of risk group and COMT group, F(4,278)= 2.58, p<.05, ηp2= .04.
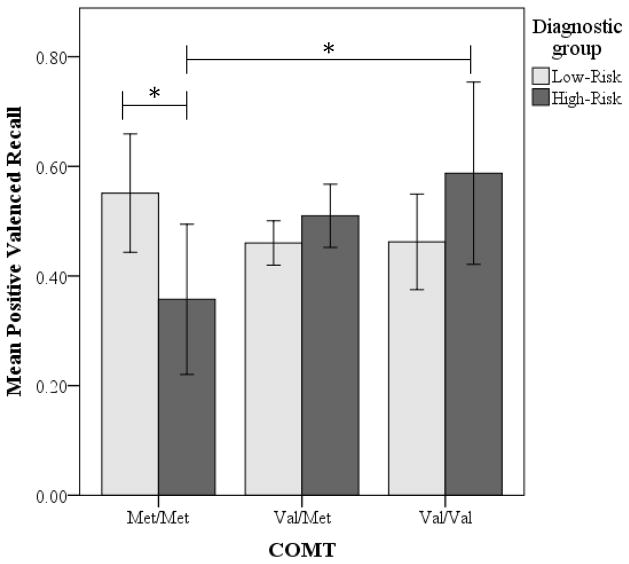
To examine this interaction, we conducted separate two-way (risk group by COMT group) ANCOVAs, covarying race/ethnicity and CDI-S scores, on recall of positive and negative endorsed adjectives. The analysis of recall of negative endorsed adjectives did not yield significant main effects or interactions, all Fs < .17, ps> .05. In contrast, the analysis of recall of positive endorsed adjectives yielded a significant interaction of risk group and COMT group, F(2,145) =4.29, p<.05, ηp2= .06. To probe this interaction, we first conducted two separate one-way ANCOVAs, covarying race/ethnicity and CDI-S scores, examining the effect of COMT genotype on recall of endorsed positive adjectives, within the low-risk and within the high-risk groups. These analyses yielded a significant main effect of COMT group in the high-risk group, F(2,57) = 3.27, p< .05 ηp2= .1, but not in the low-risk group, F(2, 88) = 1.07, p> .05, ηp2= .02. Fisher’s least significant difference (LSD) tests indicated that in the high-risk group, homozygous Met participants recalled a significantly lower proportion of positive endorsed adjectives (M = .36, SD = .16) than did the homozygous Val participants (M = .59, SD = .28), and a marginally lower proportion of positive endorsed adjectives than did the Val/Met participants (M = .51, SD = .18; see Figure 1). Second, using t-tests, we examined differences between low- and high-risk girls in the proportion of positive endorsed adjectives recalled within each of the Met/Met, Val/Met, and Val/Val groups. Low- and high-risk girls differed within the Met/Met group: high-risk girls had significantly poorer recall of positive endorsed adjectives than did low-risk girls, t(16)=2.61, p<.05, d=1.23; there were no differences between risk groups within the Val/Met or the Val/Val groups in the proportion of positive endorsed adjectives recalled.
Figure 1.
Mean recall of positive valenced endorsed words by COMT and risk groups. Bars are 95% confidence interval; * p<.05.
A one-way (5-HTTLPR group) multivariate analysis of covariance (MANCOVA) was conducted to examine differences in proportion of endorsed positive and negative adjectives recalled by participants across the three 5-HTTLPR polymorphisms. This analysis did not yield a significant effect of 5-HTTLPR polymorphism, F(2,104)= .445, p>.05, ηp2=.01. In order to try to replicate Hayden et al. (2008) results, we also conducted separate one-way analyses of covariance (ANCOVAs) on the proportion of endorsed positive, F(2,105)= .81, p>.05, ηp2=.015 (see Figure 2a) and negative adjectives, F(2,105)= .159, p>.05, ηp2=.003(see Figure 2b) recalled by participants across the three 5-HTTLPR polymorphisms; both of these analyses yielded non-significant results.3
Figure 2.
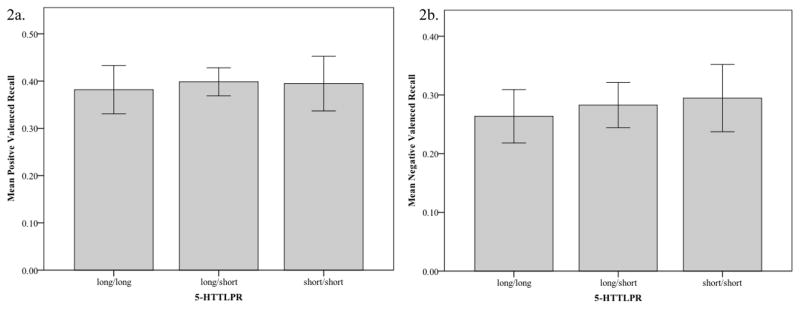
Mean recall of positively valenced (2a) and negatively valenced (2b) endorsed words by 5-HTTLPR polymorphism. Bars are 95% confidence interval.
Discussion and Conclusion
The present study was designed to examine memory biases in girls at familial and genetic risk for depression. We did find that girls with both a family history of depression and two COMT Met alleles recalled a lower proportion of positive endorsed adjectives than did high-risk girls with two COMT Val alleles and low-risk girls with two COMT Met alleles. These findings suggest that girls who are homozygous Met carriers and are at familial risk for depression have a less positive self-schema than do girls in the other risk/gene groups. In addition, high-risk girls who are homozygous Val carriers recalled a higher proportion of positive endorsed adjectives than did high-risk girls who are homozygous Met carriers, raising the possibility that this SNP is a marker of resilience in high-risk girls. We did not replicate Hayden et al.’s (2003) findings regarding 5-HTTLPR and the processing of valenced self-referential information.
The present findings regarding COMT and processing of positive self-referential information provide partial support for Stein et al.’s (2006) worrier-warrior model. Stein et al. noted that the Met allele has been associated with better memory and attentional functioning than the Val allele, but also with increased anxiety (worrier). In contrast, the Val allele is associated with poorer memory and attention but decreased anxiety and better emotion regulatory responses (warrior). Under conditions of increased negative affect, these cognitive patterns are reversed, so that Met-allele carriers perform more poorly on cognitive tasks than do Val-allele carriers. In the present study, in the processing of positive self-referential information, the high-risk girls exhibited a pattern of behavior consistent with what would be expected under conditions of negative affect; in contrast, the low-risk girls exhibited behavior that, according to Stein et al.’s hypothesis, would be expected in the absence of negative affect. As noted earlier, there were no significant differences between or within groups in self-reported mood state, indicating that the effect of COMT on levels of positive information processing in the high-risk girls is not merely a reflection of mood state. There is evidence indicating, however, that compared with low-risk girls, high-risk girls experience decreased positive affect and increased cortisol reactivity following a stressor (Waugh et al., 2012), as higher basal morning cortisol (Halligan et al., 2004; Mannie et al., 2007). Moreover, high-risk girls respond to negative mood inductions with stronger activation of regions involved in the experience of sadness and with more ventrolateral PFC activation (a region whose dopamine production is controlled by COMT enzyme activity; Joormann et al., 2012). In sum, although low- and high risk girls in the present study did not differ in measures of mood, they may have different experiences of stress and negative affect: the high-risk girls’ exposure to the mood induction may lead to increased cortisol reactivity and stronger activation of neural regions involved in the experience of sadness, possibly kindling negative schemas and offering an explanation for the finding that high risk girls exhibited a pattern of behavior consistent with what would be expected under conditions of negative affect while the low-risk girls exhibited behavior that would be expected in the absence of negative affect.
Conversely, in the processing of negative self-referential information, Stein’s worrier-warrior model was not supported. No significant differences were found in the processing of negative self-referential information when grouped by COMT polymorphism or risk group. The pattern of findings for the processing of negative information processing was similar to the positive self-referential information-processing pattern described above, but group differences did not reach statistical significance. It is important to note that 71% of the participants in this study endorsed three or fewer negative words, which likely contributed to lack of significant effects for this variable.
Although we did not find an association between 5-HTTLPR and the processing of valenced self-referential information, we should note that our sample differed in important respects from Hayden et al.’s (2003) sample. Whereas Hayden et al. studied a community sample of boys and girls between the ages of 6 and 8, we examined girls between 9 and 14 years of age who were comprehensively screened for the absence of current or past psychiatric disorders. It is possible that a study with a sample more similar to that studied by Hayden et al. would yield findings consistent with theirs.
We should note three limitations of this study. First, even with over 150 participants, there were relatively small numbers in some of the cells of this study, limiting its statistical power. Nevertheless, we did obtain statistically significant results with effect sizes that were consistent with our hypotheses. Moreover, the observed power to detect interaction effects was f = .76. Given current concerns regarding novel candidate gene-environment interactions with small samples and type I error (Duncan and Keller, 2011), it is important that the present findings be replicated with a larger sample. Second, the cell sizes were unequal and differed in mean age, which may have affected our results. Third, although our sample was ethnically heterogeneous, the relatively small size does not permit a reliable examination of whether the obtained pattern of findings was influenced by ethnicity; this will be an important question for future research to address.
Despite these limitations, the present findings suggest that individuals with a history of maternal depression who are at elevated risk for developing mood disorders but are not yet disordered are characterized by differential biases in their memory for self-relevant information dependent on their COMT genotype. These findings point to the possibility of developing a more personalized approach to intervention for, or prevention of, depression. Girls with the combined vulnerability of familial risk and homozygosity for the COMT Met allele may respond particularly well to interventions that focus on decreasing anxiety levels and that target dopamine transmission via COMT enzyme production. The results of the present study suggest that assessing the interaction of familial risk status and COMT polymorphism is important in understanding risk for depressive disorders.
Acknowledgments
This research was supported by National Institute of Mental Health Grant MH074849 awarded to Ian H. Gotlib
Footnotes
The Caspi et al. (2003) results have been replicated by some subsequent studies, however several failures to replicate have also been published (Zammit and Owen, 2006). Most recently a meta-analysis concluded that no evidence that the serotonin transporter genotype alone or in interaction with stressful life events is associated with an elevated risk of depression (Risch et al., 2009).
A critical issue in assessing the significance of associations with phenotypic measures is the likelihood of type I errors. The approach taken in this study was to select and analyze a single candidate functional polymorphism, chosen for its biological effect, in the context of a target phenotype that is likely to be affected by this polymorphism.
To investigate whether risk status interacted with 5-HTTLPR polymorphism, we also conducted a two-way (risk group by 5-HTTLPR polymorphism) MANCOVA, covarying for CDI-S scores, on the proportion of endorsed positive and negative adjectives recalled by participants did not yield significant main effects of risk group or 5-HTTLPR (both Fs<.91, ps > .05); and did not yield a significant interaction of risk group and 5-HTTLPR polymorphism, F(4,278)= 2.58, p<.05, ηp2= .04.
Contributors
Authors Ian Gotlib and Jutta Joormann designed the study and wrote the protocol. Author Lauren Asarnow managed the literature searches. Authors Lauren Asarnow and Renee Thompson undertook the statistical analysis, and author Lauren Asarnow wrote the first draft of the manuscript. All authors contributed to and have approved the final manuscript.
Conflict of Interests
The authors have no conflict of interests to disclose.
Role of Funding Source
NIMH had no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Lauren Asarnow had full access to all the data in the study and had final responsibility for the decision to submit for publication
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-IV. 4. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- Banos RM, Medina PM, Pascual J. Explicit and implicit memory biases in depression and panic disorder. Behav Res and Ther. 2001;39:61–74. doi: 10.1016/s0005-7967(99)00158-8. [DOI] [PubMed] [Google Scholar]
- Beck AT, Rush AJ, Shaw BF, Emery G. Cognitive therapy of depression. New York: Guilford Press; 1979. [Google Scholar]
- Berridge KC. Motivation concepts in behavioral neuroscience. Physiol Behav. 2004;81:179–209. doi: 10.1016/j.physbeh.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Campbell H, Rudan I. Interpretation of genetic association studies in complex disease. Pharmacogenomics. 2002;2:349–360. doi: 10.1038/sj.tpj.6500132. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Chen J, Lipska BK, Halim N, Ma QD, Matsumoto M, Melhem S, Weinberger DR. Functional analysis of genetic variation in catechol-O-methyltransferase (COMT): effects on mRNA, protein, and enzyme activity in postmortem human brain. Am J Hum Gen. 2004;75:807–821. doi: 10.1086/425589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Columbus C, Grazer GL, Nelson J, Rogers S, Hopkins KL, Bass R, et al. Stepmom. United States: Sony Pictures Entertainment; 1998. (pp. 14 film reels of 14 on 17 (ca. 124 min., ca.111,160 ft.)) [Google Scholar]
- Davis H. Self-reference and the encoding of personal information in depression. Cog Ther Res. 1979;3:97–110. [Google Scholar]
- Duncan LE, Keller MC. A critical review of the first 10 years of candidate gene-by-environment interaction research in psychiatry. Am J Psychiatry. 2011;168:1041. doi: 10.1176/appi.ajp.2011.11020191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM–IV Axis I Disorders—Patient Version (SCID–PV) Washington, DC: American Psychiatric Press; 1996. [Google Scholar]
- Fossati P, Hevenor SJ, Lepage M, Graham SJ, Grady C, Keightley ML, Mayber H. Distributed self in episodic memory: neural correlates of successful retrieval of self-encoded positive and negative personality traits. Neuroimage. 2004;22(4):1596–1604. doi: 10.1016/j.neuroimage.2004.03.034. [DOI] [PubMed] [Google Scholar]
- Garber J, Robinson NS. Cognitive vulnerability in children at risk for depression. Cog Emo. 1997;11:619–635. [Google Scholar]
- Gogos JA, Morgan M, Luine V, Santha M, Ogawa S, Pfaff D, et al. Catechol-O-methyltransferase-deficient mice exhibit sexually dimorphic changes in catecholamine levels and behavior. Procl Natl Acad Sci USA. 1998;95:9991–9996. doi: 10.1073/pnas.95.17.9991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib IH, Joormann J. Cognition and depression: current status and future directions. Ann Rev Clin Psychol. 2010;6:285–312. doi: 10.1146/annurev.clinpsy.121208.131305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib IH, Krasnoperova E, Neubauer DL, Joormann J. Attentional biases for negative interpersonal stimuli in clinical depression. J Abnorm Psychol. 2004;113:127–135. doi: 10.1037/0021-843X.113.1.121. [DOI] [PubMed] [Google Scholar]
- Halligan SL, Herbert J, Goodyer IM, Murray L. Exposure to postnatal depression predicts elevated cortisol in adolescent offspring. Biol Psychiatry. 2004;55:376–381. doi: 10.1016/j.biopsych.2003.09.013. [DOI] [PubMed] [Google Scholar]
- Hammen CL. In: Handbook of Depression. 2. Gotlib IH, Hammen CL, editors. New York, NY: Guilford Press; 2009. pp. 275–297. [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, Weinberger DR. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400–403. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- Hayden EP, Dougherty LR, Maloney B, Olino TM, Sheikh H, Durbin CE, Klein DN. Early-emerging cognitive vulnerability to depression and the serotonin transporter promoter region polymorphism. J Affect Disord. 2008;107:227–230. doi: 10.1016/j.jad.2007.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz A, Smolka MN. The effects of catechol O-methyltransferase genotype on brain activation elicited by affective stimuli and cognitive tasks. Rev Neurosci. 2006;17:359–368. doi: 10.1515/revneuro.2006.17.3.359. [DOI] [PubMed] [Google Scholar]
- Jaenicke C, Hammen C, Zupan B, Hiroto D, Gordon D, Adrian C, Burge D. Cognitive vulnerability in children at risk for depression. J Abnorm Child Psychol. 1987;15:559–572. doi: 10.1007/BF00917241. [DOI] [PubMed] [Google Scholar]
- Joormann J, Talbot L, Gotlib IH. Biased processing of emotional information in girls at risk for depression. J Abnorm Psychol. 2007;116:135–143. doi: 10.1037/0021-843X.116.1.135. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent DA, Ryan ND, Rao U. K-Sads-Pl. J Am Acad Child Adolesc Psychiatry. 2000;39:1208. doi: 10.1097/00004583-200010000-00002. [DOI] [PubMed] [Google Scholar]
- Kovacs M. Children’s depression inventory. New York, USA: MHS; 1992. [Google Scholar]
- Massat I, Souery D, Del-Favero J, Nothen M, Blackwood D, Muir W, Mendlewicz J. Association between COMT (Val158Met) functional polymorphism and early onset in patients with major depressive disorder in a European multicenter genetic association study. Mol Psychiatr. 2005;10:598–605. doi: 10.1038/sj.mp.4001615. [DOI] [PubMed] [Google Scholar]
- Mattay VS, Goldberg TE, Fera F, Hariri AR, Tessitore A, Egan MF, Weinberger DR. Catechol O-methyltransferase val158-met genotype and individual variation in the brain response to amphetamine. Proc Natl Acad Sci USA. 2003;100:6186–6191. doi: 10.1073/pnas.0931309100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris NM, Udry JR. Validation of a self-administered instrument to assess stage of adolescent development. J Youth Adolesc. 1980;9:271–280. doi: 10.1007/BF02088471. [DOI] [PubMed] [Google Scholar]
- Risch N, Herrell R, Lehner T, Liang KY, Eaves L, Hoh J, Merikangas KR. Interaction between the serotonin transporter gene (5-HTTLPR), stressful life events, and risk of depression. JAMA. 2009;301:2462–2471. doi: 10.1001/jama.2009.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph KD. Developmental influences on interpersonal stress generation in depressed youth. J Abnorm Psychol. 2008;117:673–679. doi: 10.1037/0021-843X.117.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rylander-Rudqvist T, Hakansson N, Tybring G, Wolk A. Quality and quantity of saliva DNA obtained from the self-administrated oragene method--a pilot study on the cohort of Swedish men. Cancer Epidemiol, Biomarkers Prev. 2006;15:1742–1745. doi: 10.1158/1055-9965.EPI-05-0706. [DOI] [PubMed] [Google Scholar]
- Smolka MN, Schumann G, Wrase J, Grusser SM, Flor H, Mann K, Heinz A. Catechol-O-methyltransferase val158met genotype affects processing of emotional stimuli in the amygdala and prefrontal cortex. J Neurosci. 2005;25:836–842. doi: 10.1523/JNEUROSCI.1792-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein DJ, Newman TK, Savitz J, Ramesar R. Warriors versus worriers: the role of COMT gene variants. CNS Spectrums. 2006;11:745–748. doi: 10.1017/s1092852900014863. [DOI] [PubMed] [Google Scholar]
- Taylor L, Ingram RE. Cognitive reactivity and depressotypic information processing in children of depressed mothers. J Abnorm Psychol. 1999;108:202–210. doi: 10.1037//0021-843x.108.2.202. [DOI] [PubMed] [Google Scholar]
- Timbremont B, Braet C. Cognitive vulnerability in remitted depressed children and adolescents. Behav Res Ther. 2004;42:423–437. doi: 10.1016/S0005-7967(03)00151-7. [DOI] [PubMed] [Google Scholar]
- Tunbridge EM, Bannerman DM, Sharp T, Harrison PJ. Catechol-o-methyltransferase inhibition improves set-shifting performance and elevates stimulated dopamine release in the rat prefrontal cortex. J Neurosci. 2004;24:5331–5. doi: 10.1523/JNEUROSCI.1124-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Patent No. San Antonio, TX: T. P. Corporation; 2003. [Google Scholar]
- Weinshilboum RM, Otterness DM, Szumlanski CL. Methylation pharmacogenetics: catechol O-methyltransferase, thiopurine methyltransferase, and histamine N-methyltransferase. Ann Rev Pharmacol Toxicol. 1999;39:19–52. doi: 10.1146/annurev.pharmtox.39.1.19. [DOI] [PubMed] [Google Scholar]
- Weir P. Dead Poets Society. United States: Buena Vista Pictures Distribution; 1989. (pp. 14 reels of 14 on 17 (ca. 11570 ft.)) [Google Scholar]
- Winterer G, Musso F, Vucurevic G, Stoeter P, Konrad A, Seker B, et al. COMT genotype predicts BOLD signal and noise characteristics in prefrontal circuits. Neuroimage. 2006;32(4):1722–1732. doi: 10.1016/j.neuroimage.2006.05.058. [DOI] [PubMed] [Google Scholar]
- Zieff H Copyright Collection (Library of Congress) My girl. United States: Columbia Pictures; 1991. [Google Scholar]
- Zammit S, Owen MJ. Stressful life events, 5-HTT genotype and risk of depression. Br J Psychiatry. 2006;188:199–201. doi: 10.1192/bjp.bp.105.020644. [DOI] [PubMed] [Google Scholar]