Abstract
Background
CHRONOS was a large naturalistic study designed to evaluate the effectiveness and safety of agomelatine in the management of patients with major depression in routine clinical practice.
Methods
Patients (n=6,276) with a moderate or severe major depressive episode without psychotic symptoms were treated initially as outpatients (80.2%) or in psychiatric facilities (19.8%) in 54 regions of the Russian Federation. Patients received a flexible-dosing regimen of agomelatine 25 mg or 50 mg once daily for 8 weeks, with frequent study visits (weeks 1, 2, 3, 4, 6, and 8).
Results
Patients (mean age 44 years, 72.6% female) showed progressive improvement on the 17-item Hamilton Rating Scale for Depression (HAMD-17) total score from 22±6.9 at baseline to 4.7±4.7 at week 8 (P<0.0001). The proportion of responders (HAMD-17 decrease of ≥50%) was 90.1% and the proportion of remitters (HAMD-17 <7) was 79.1% at week 8. All individual HAMD-17 item scores improved rapidly, and the change relative to baseline was significant (P<0.0001) at week 1 and at each subsequent visit in all cases. There were corresponding rapid improvements in Clinical Global Impression Severity and Improvement scores. In the subgroup of patients with more severe illness (HAMD-17 ≥21 at baseline; n=3,478), the proportions of responders and remitters were 92.4% and 72.8%, respectively, at week 8.
Conclusion
Agomelatine was effective and well tolerated in a large sample of depressed patients in an observational treatment setting, and showed a rapid onset of benefit across all HAMD-17 items.
Keywords: agomelatine, antidepressant, Hamilton Rating Scale for Depression, major depressive disorder, observational study
Introduction
Major depressive disorder is a common condition usually associated with substantial symptom severity and role impairment. It is a significant public health concern across all regions of the world, with both prevalence and impairment severity slightly higher in high-income countries than in low-income to middle-income countries.1 The 12-month prevalence of major depression across 30 European countries has been estimated at 6.9%,2 and the lifetime prevalence in the USA was estimated as 16.2% in the National Comorbidity Survey Replication.3 However, data obtained prospectively indicate that recall bias leads to substantial underestimation of the lifetime prevalence of depression in cross-sectional studies.4–6 Prospective estimates of prevalence during 7–13 years of follow-up in Canada were 24.2% in women and 14.2% in men,6 and it has been suggested that approximately half the population of the developed world can expect one or more episodes of depression in their lifetime.7
In a recent cross-national study, the average age of onset of a major depressive episode was approximately 25 years.1 The fact that many people are affected at an early age means that depression can impose a severe burden in terms of employment and role performance. Role impairment was rated as severe or very severe in 59% of 12-month cases of depression in the National Comorbidity Survey Replication,3 and major depressive episodes more than double the risk of transition from working to nonworking status.8 Persistent depressive symptoms, particularly in older people, are associated with a steep trajectory of worsening functional disability.9 An often quoted result published in 1997 was that depression was projected to rise to become the second greatest cause of disability worldwide, measured in terms of disability-adjusted life years, by 2020 (after ischemic heart disease).10 In fact, in the European Union, depression has already become by far the most burdensome of all diseases in terms of disability.2 The economic cost of major depression in Europe is very large, and was estimated at 92 billion Euros during 2010, and is predicted to increase.11
Agomelatine is a recently introduced antidepressant. It possesses both melatonergic agonist and 5-hydroxytryptamine2C antagonist properties.12 It has shown efficacy in both the acute and continuation phases of the treatment of depression in randomized, double-blind trials against placebo, and has shown efficacy at least comparable with that of established drugs such as sertraline, fluoxetine, escitalopram, and venlafaxine in active comparator studies.13,14 However, it is widely accepted that the benefits of treatment observed in randomized studies do not always translate into clinical practice. Therefore, it is of the utmost importance to evaluate the antidepressant efficacy and tolerability of agomelatine in clinical practice. Here we present the results of the multicenter observational CHRONOS study, in which a large sample of depressed patients were treated as inpatients or outpatients with a flexible-dosing regimen of agomelatine in everyday medical practice in the Russian Federation.
Materials and methods
Patients and study design
Male and female patients aged 18–65 years with a diagnosis of moderate or severe major depressive episode (single, recurrent, or bipolar) according to the International Classification of Diseases, 10th edition (ICD-10) without psychotic symptoms and with an indication for agomelatine monotherapy (according to the attending physician’s assessment) could be included. Appropriate ICD-10 diagnoses were F32.1, F32.2, F33.1, F33.2, F31.3, and F31.4. Patients could be treated as outpatients or as psychiatric facility inpatients. Use of a medical contraceptive method was required in women of childbearing potential. The main exclusion criteria were: presence of psychotic symptoms; significant suicide risk according to physician’s assessment; previous resistance to therapy with other antidepressants; known previous intolerance or poor response to agomelatine; need for concomitant mood stabilizer therapy; diagnosis of other mental disorder or disease; diagnosis of noncompensated serious somatic or neurological disease; history of dependence or abuse of psychoactive substances (including alcohol) within 5 years; impaired renal or hepatic function; and pregnancy or lactation.
All patients were planned to receive agomelatine for the 8-week treatment period. The starting dose was 25 mg once daily; if the treatment effect was judged by the investigator to be inadequate at 2 weeks, the dose could be increased to 50 mg once daily. Other antidepressants, antipsychotics, and mood stabilizing drugs were not permitted. Patients could receive anxiolytics (except alprazolam) or hypnotics, provided that only one drug was used and for not more than 7 days. Study visits were scheduled for weeks 1, 2, 3, 4, 6, and 8 after treatment initiation. The study was performed in 54 regions of the Russian Federation during the period between autumn 2008 and spring 2009. The study was performed in accordance with the Declaration of Helsinki and local ethical requirements. All patients gave written informed consent.
Efficacy and safety evaluation methods
Treatment efficacy was evaluated using the 17-item Hamilton Rating Scale for Depression (HAMD-17), the Clinical Global Impression Severity Scale (CGI-S), and the Clinical Global Impression Improvement Scale (CGI-I). The specific efficacy criteria used were: HAMD-17 total score; percentage of responders (patients whose HAMD-17 total score decreased by ≥50% from baseline); percentage of remitters (patients whose HAMD-17 total score was <7 during the treatment period, indicating they were free from depression); CGI-S score; CGI-I score; and individual HAMD-17 items. Response and remission were also evaluated by HAMD-17 total score in a subgroup of patients with more severe depression, defined as a HAMD-17 total score of ≥21 at baseline. Treatment tolerability was evaluated from adverse events and serious adverse events reported during the treatment period. No specific liver function monitoring was performed in the study since this was not required at the time by the Russian Federation Agency.
The Student’s t-test for paired samples was used for within-group comparisons of continuous variables that were normally distributed, and Wilcoxon’s test for those that were not normally distributed. The Student’s t-test for independent samples was used for between-group comparisons of continuous variables, and the Mann–Whitney test for those not normally distributed. The χ2 test was used for categorical variables. All tests were two-sided, and the type I error rate was 5%.
Results
Baseline characteristics
A total of 6,276 patients were included in the study by 1,910 participating psychiatrists. The majority of patients (80.2%) were treated initially as outpatients and 19.8% as inpatients in psychiatric facilities. The average patient age was 44.4 years, and the majority (72.6%) were female (Table 1). Most patients had major depressive disorder (91.4%), either as a first episode (44.7%) or in the context of recurrent depressive disorder (42.7%); 17.2% had experienced three or more previous depressive episodes. A minority of patients had a depressive episode in the context of bipolar affective disorder (5.1%). Considering all patients, 86.6% of depressive episodes were diagnosed as moderate and 9.9% as severe. The mean HAMD-17 total score at baseline was 22.4, and a majority of patients (68.5%) had a baseline CGI-S score of 4, corresponding to moderate illness severity. The duration of the current depressive episode ranged widely, from 0.1 to 96 months, with a mean of 3.2 months. The duration of depressive illness prior to inclusion in the study also ranged widely, from 0.05 years to 41 years, with a mean of 3.2 years.
Table 1.
Demographic and clinical characteristics of patients at baseline
| Age, years, mean ± SD (range) | 44.4±11.8 (17–65) |
| <30 years, n (%) | 772 (12.3%) |
| 30–59 years, n (%) | 4,797 (76.4%) |
| ≥60 years, n (%) | 688 (11.0%) |
| Male, n (%) | 1,683 (26.8%) |
| Female, n (%) | 4,553 (72.6%) |
| Treatment setting, n (%) | |
| Outpatient | 5,030 (80.2%) |
| Inpatient | 943 (15.0%) |
| Diagnosis (according to ICD-10), n (%) | |
| F32.1 (single episode, moderate) | 2,792 (44.5%) |
| F32.2 (single episode, severe) | 264 (4.2%) |
| F33.1 (recurrent episode, moderate) | 2,382 (38.0%) |
| F33.2 (recurrent episode, severe) | 300 (4.8%) |
| F31.3 (bipolar disorder, current episode moderate) | 261 (4.2%) |
| F31.4 (bipolar disorder, current episode severe) | 58 (0.92%) |
| Duration of current episode, months, mean ± SD (range) | 3.2±4.2 (0.1–96) |
| Duration of depressive disease, years, mean ± SD (range) | 3.2±4.8 (0.05–41) |
| HAMD-17 total score, mean ± SD Clinical Global Impression Severity scores, n (%) | 22.4±6.9 |
| 3 (mildly ill) | 736 (11.7%) |
| 4 (moderately ill) | 4,302 (68.6%) |
| 5 (markedly ill) | 813 (13.0%) |
Notes: Percentages are calculated relative to the full study population; data were not available for some patients.
Abbreviations: HAMD-17, 17-item Hamilton Rating Scale for Depression; ICD-10, International Classification of Diseases, 10th edition; SD, standard deviation.
Patient disposition
The great majority of patients (5,781 patients, 92.1%) completed the full 8-week course of treatment. The most frequent reasons for withdrawal from the study were adverse events (108 patients, 1.7%), failure to complete questionnaires (67 patients, 1.1%) and lack of therapeutic effect (63 patients, 1.0%).
All patients initially received agomelatine 25 mg once daily, and 4,993 (79.6%) continued on this dose throughout therapy. The remaining 1,283 patients (20.4%) switched to 50 mg once daily at some time during the study, with most (690, 53.8% of those who switched) changing dose at the week 2 visit.
Although forbidden by the protocol, small numbers of patients took other antidepressants (15 patients, 0.24%), antipsychotic drugs (74 patients, 1.18%), or mood stabilizers (eleven patients, 0.18%) during the treatment period. Anxiolytics (other than alprazolam) and hypnotics were permitted, but were taken by a relatively small proportion of patients (19.4%); the most frequently used drugs were phenazepam (5.2% of patients), diazepam (3.2%), and hydroxyzine (2.0%). Alprazolam, although forbidden, was taken by 1.6% of patients.
Assessment of depressive symptom severity
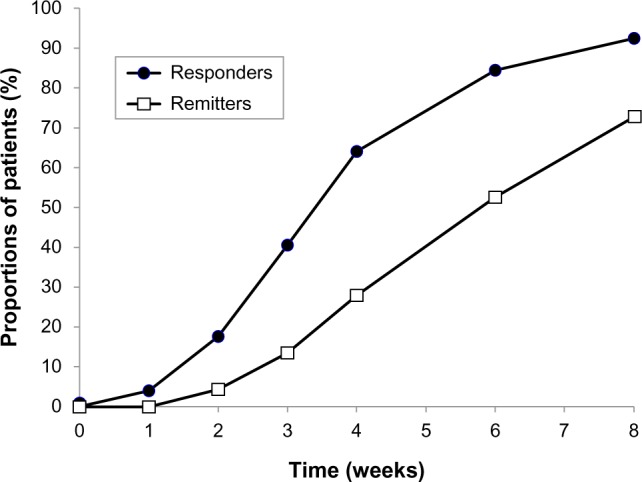
The HAMD-17 total score decreased substantially and progressively throughout the treatment period, from 22.5±6.9 at baseline to 4.7±4.7 at 8 weeks (P<0.0001; Figure 1). The improvement was significant at the week 1 study visit (P<0.0001) and at all subsequent visits. The proportion of patients showing a response to treatment (HAMD-17 total score decreased by ≥50% from baseline) increased slightly between baseline and the second week of treatment, and rapidly thereafter, reaching 90.1% by week 8 (Figure 2). The proportion of patients showing remission from depression (HAMD-17 total score <7) increased progressively throughout the treatment period, reaching 25.3% at week 3 and 79.1% at week 8 (Figure 2).
Figure 1.
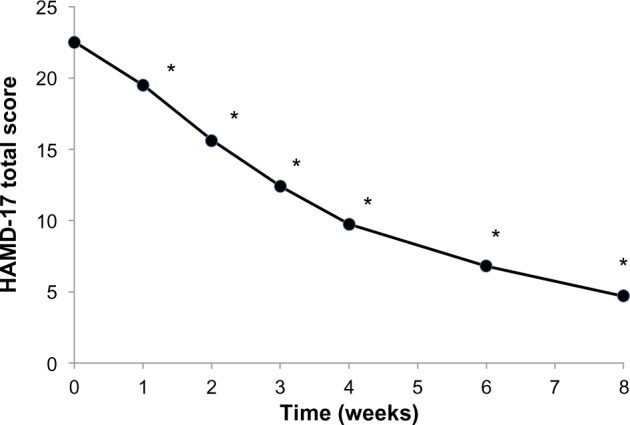
Mean 17-item Hamilton Rating Scale for Depression (HAMD-17) total score during the 8-week treatment period.
Note: *P<0.0001.
Figure 2.
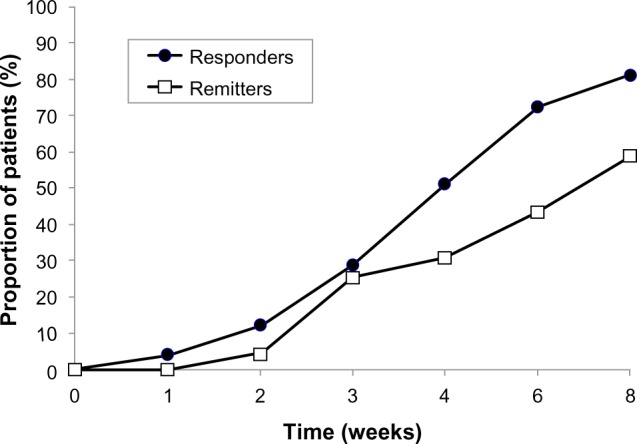
Proportions of patients showing response to treatment (responders: HAMD-17 total score decreased by ≥50% from baseline) and remission (remitters: HAMD-17 total score <7 during the treatment period, indicating they were free from depression) during the 8-week treatment period.
Abbreviation: HAMD-17, 17-item Hamilton Rating Scale for Depression.
When the single HAMD-17 item scores were considered individually, it was found that every item score decreased substantially during the treatment period (P<0.0001 in every case). Notable among these were concomitant improvements in both symptoms relating to daytime activities and those relating to sleep. Thus, HAMD-17 items “work and activities” (item 7) and “psychomotor retardation” (item 8) improved from 2.0±0.86 to 0.49±0.63 and from 1.19±0.84 to 0.22±0.47, respectively, between baseline and week 8 (Figure 3). Similarly, items 4, 5, and 6, relating to early, middle-of-the-night, and late insomnia, respectively, improved from 1.45±0.69, 1.39±0.67, and 1.27±0.76 at baseline to 0.21±0.43, 0.15±0.39, and 0.21±0.45, respectively, at week 8. There were also marked improvements in symptoms of anxiety in depression, with “psychic anxiety” (item 10) and “somatic anxiety” (item 11) improving from 1.88±0.94 and 1.70±0.87 at baseline to 0.41±0.60 and 0.43±0.59 at week 8, respectively. A notable feature was that the onset of improvement was rapid for every HAMD-17 item, and the improvement relative to baseline was significant at one week of treatment (P<0.0001 in every case) and at every visit thereafter.
Figure 3.
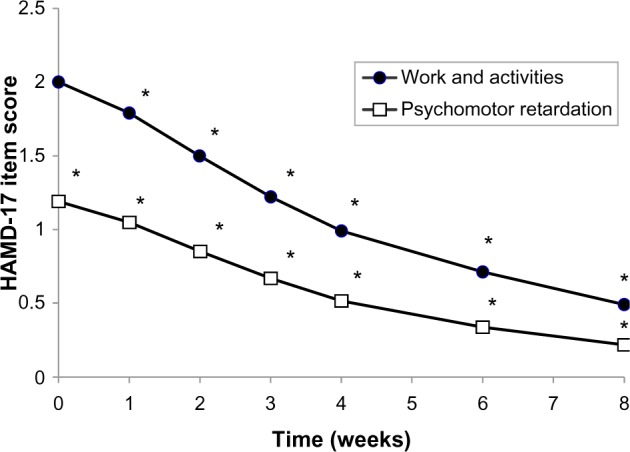
Mean single Hamilton Rating Scale for Depression item scores for “work and activities” (item 7) and “psychomotor retardation” (item 8) during the 8-week treatment period.
Note: *P<0.0001.
Abbreviation: HAMD-17, 17-item Hamilton Rating Scale for Depression.
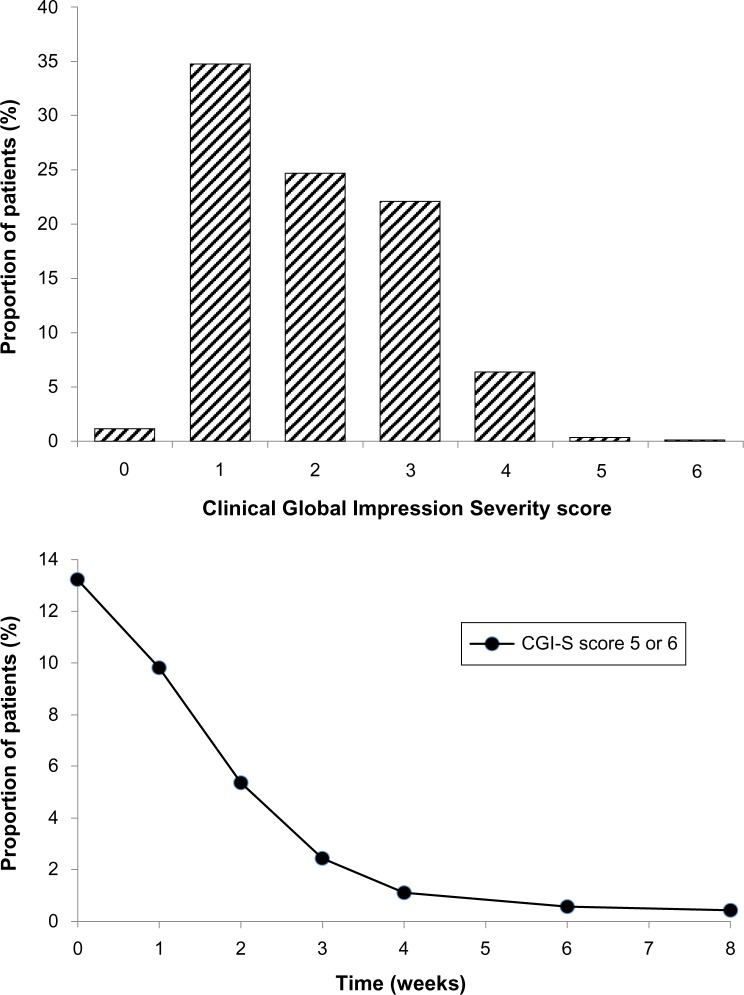
Severity of depressive illness, as evaluated by the CGI-S score, decreased markedly throughout the treatment period. At baseline, the mean CGI-S score was 3.93, and a majority of patients (68.9%) had a CGI-S score of 4, indicating moderate illness severity. At the end of the treatment period (week 8), the mean CGI-S score was 2.02; the modal CGI-S score was 1, indicating normal (not at all ill), and this category accounted for 34.8% of patients (Figure 4). A further 24.7% of patients had a CGI-S score of 2, indicating only borderline illness at the end of the study. Marked improvements were also seen in patients who were markedly or severely ill at baseline (CGI-S scores 5 or 6); the proportion of patients in these categories decreased from 13.2% at baseline to 0.42% at 8 weeks. A notable feature was the rapidity of improvement in these patients: the proportion of markedly or severely ill patients fell from 13.2% to 2.4% after only 3 weeks of treatment (Figure 4, lower panel).
Figure 4.
Upper panel: frequency distribution of Clinical Global Impression Severity Scale (CGI-S) scores after 8 weeks of treatment.
Notes: Lower panel: proportion of patients either markedly or severely ill (CGI-S score 5 or 6) throughout the treatment period.
The improvement in patients’ condition was reflected in CGI-I scores throughout the treatment period. At 8 weeks, 64.1% of patients were much or very much improved, relative to baseline, with CGI-I scores of 5 or 6. Again, the rapidity of onset of improvement was notable: 41.5% were improved (CGI-I scores 4, 5, or 6) at week 1 and 71.0% were improved by the week 2 visit.
The efficacy of agomelatine was also high in the subgroup of more severely depressed patients (HAMD-17 total score ≥21 at baseline; n=3,478). By week 8, the proportions of responders and remitters in this subgroup according to HAMD-17 total scores were 92.4% and 72.8%, respectively (Figure 5). The onset of antidepressant efficacy was also rapid in this subgroup, with 4.0% of patients being responders at the week 1 visit and 17.6% at the week 2 visit. Remission was apparent throughout the treatment period (Figure 5).
Figure 5.
Subgroup of more severely ill patients. Proportions of patients showing response to treatment (responders: HAMD-17 total score decreased by ≥50% from baseline) and remission (remitters: HAMD-17 total score <7 during the treatment period, indicating they were free from depression) in the subgroup of patients (n=3,478) with HAMD-17 total score ≥21 at baseline.
Abbreviation: HAMD-17, 17-item Hamilton Rating Scale for Depression.
Tolerability
Adverse events were reported in 1,321 (21.1%) of patients. The most frequent adverse events were nausea (4.0%), dizziness (3.1%), and headache (3.0%, Table 2). Most adverse events were typical symptoms of depressive episodes or those frequently observed in studies of psychotropic drugs. Serious adverse events were reported in 51 patients (0.81%). These included eleven cases (0.18%) of hospitalization for exacerbation of existing nonpsychiatric chronic diseases, three severe injuries (motor vehicle accident, arm fracture, and hip fracture due to a fall), two cases of diagnosis of serious disease (brain tumor, temporal lobe epilepsy), and two instances in which patients called for an ambulance (abrupt increase in blood pressure, tongue numbness, swelling, and edema). There were 22 instances (0.35%) of exacerbation of mental illness and admission to a psychiatric hospital, including three (0.05%) unsuccessful suicide attempts and two (0.03%) cases of inversion of affect.
Table 2.
Adverse events reported by ≥0.25% of patients
| Nausea | 251 (4.0%) |
| Dizziness | 197 (3.1%) |
| Headache | 186 (3.0%) |
| Morning or afternoon sleepiness | 147 (2.3%) |
| Increased anxiety | 129 (2.1%) |
| Sleep disturbance | 125 (2.0%) |
| Xerostomia | 1.03 (1.6%) |
| Diarrhea | 49 (0.78%) |
| Weakness | 46 (0.73%) |
| Nightmares | 44 (0.70%) |
| Abdomen heaviness and pain | 40 (0.64%) |
| Pruritus | 32 (0.51%) |
| Epigastric pain or heaviness | 28 (0.45%) |
| Irritability | 28 (0.45%) |
| Palpitations | 23 (0.37%) |
| Anxiety | 21 (0.33%) |
| Retardation | 19 (0.30%) |
| Lethargy | 18 (0.29%) |
| Blood pressure increase | 17 (0.27%) |
| Decreased appetite | 17 (0.27%) |
| Decreased libido | 16 (0.25%) |
As part of the safety evaluation, all patients underwent standard laboratory tests, including blood and urine analyses, according to the routine practice of participating hospitals and outpatient clinics, and any deviations from normal value ranges were recorded. No case of clinically significant increase in transaminase was reported.
There were two deaths (0.03%) during the study, one due to a motor vehicle accident, and one due to acute heart failure in a patient with diabetes mellitus and hypertension.
Discussion
The principal findings of the observational CHRONOS study in 6,276 depressed patients without psychotic symptoms were that treatment with a flexible-dose regimen of agomelatine resulted in a reduction in HAMD-17 total score from a mean of 22.5±6.9 at baseline to 4.7±4.7 at the end of the 8-week treatment period. Marked and significant improvements were observed in each item of the HAMD-17 when considered separately, and improvements were rapid in onset, being detectable and significant after only one week of treatment. The proportion of patients showing response to treatment (≥50% decrease in HAMD-17 score) was 90.1% at week 8, by which time 79.1% had achieved remission (HAMD-17 total score <7). Results were similar in the subgroup of patients with more severe depression (HAMD-17 total score ≥21 at baseline; n=3,478), with 92.4% and 72.8% of patients achieving response and remission, respectively, at week 8.
The efficacy of agomelatine in the treatment of major depressive disorder has been evaluated in several randomized, double-blind trials, both against placebo and against active comparator drugs.13 In particular, the flexible-dose regimen used in the present study has shown antidepressant efficacy that was superior to placebo in terms of HAMD-17 total score in two trials,15,16 and efficacy similar to that of venlafaxine17 and escitalopram14 and superior to sertraline.18 The efficacy of agomelatine was also shown to be superior to that of fluoxetine in a trial in patients with major depressive disorder of severe intensity.19
The CHRONOS study complements the recent observational VIVALDI (Valdoxan Improves depressiVe symptoms And normaLizes circaDIan rhythms) study of agomelatine, which was performed in 3,356 depressed inpatients treated in everyday medical practice in Germany.20 In CHRONOS, the treatment period was shorter than in VIVALDI (8 weeks versus 12 weeks) and assessment visits were more frequent (six versus three follow-up visits), with the first visit only one week after treatment initiation. Direct comparison of efficacy results between the two studies is complicated by the fact that a modified Montgomery-Asberg Depression Rating Scale (MADRS) was used for detailed assessment of depressive symptom severity in VIVALDI, rather than the HAMD-17 instrument used in CHRONOS, and because patients in the VIVALDI study had more severe depressive symptoms at baseline. In VIVALDI, the mean CGI-S score decreased from 4.7 to 3.2 during the 12-week treatment period, compared with a decrease from 3.9 to 2.0 over 8 weeks in CHRONOS. In VIVALDI, 66% of patients showed response to treatment (≥50% reduction in MADRS total score) and 55% achieved remission (MADRS total score ≤12). In CHRONOS, the corresponding values (by HAMD-17 criteria) were 90% for response and 79% for remission at 8 weeks.
The efficacy results from the present CHRONOS study can be compared with those from two other large trials of antidepressant treatment in everyday medical practice which involved patients with similar depressive symptom severity at baseline. In a recent naturalistic study in 1,014 patients treated in psychiatric hospitals in Germany,21 the mean HAMD-17 total score decreased from 22.3 to 8.8 during the acute treatment phase (mean duration 53.6 days), compared with the decrease from 22.5 to 4.7 at 8 weeks in CHRONOS. In the STAR*D (Sequenced Treatment Alternatives to Relieve Depression) study of 2,876 depressed outpatients without psychotic symptoms in the “real world” setting,22 the mean HAMD-17 total score was 21.8 at baseline, and treatment with a flexible-dosing regimen of citalopram for an average of 10 weeks produced a remission rate of 27.5%, which was similar regardless of whether patients were treated in primary (26.6%) or psychiatric (28.0%) care.
In our study, the large sample size and frequent follow-up assessments allowed changes in individual HAMD-17 item scores and scores at individual time points during treatment to be evaluated reliably and their significance assessed. Two important findings emerged from this analysis. Firstly, there were significant improvements in every HAMD-17 item. The efficacy of agomelatine in improving the daytime condition in depressed patients has been demonstrated in randomized studies, including objective assessments of reaction time14 and subjective assessments of daytime alertness.17,18 In CHRONOS, HAMD-17 items relating to work and activities (item 7) and psychomotor retardation (assessing concentration, motor activity and speed of thought and speech; item 8) were also markedly improved. These improvements are of particular importance in light of the frequently severe impact of depression in terms of functioning and role impairment. Improvements in the sleep–wake cycle during depressive episodes have been evaluated specifically in randomized, double-blind trials,14,17,18 and were confirmed in a naturalistic setting by the marked improvements in all three insomnia-related HAMD-17 items in the present study. Agomelatine also improved the HAMD-17 items relating to agitation (item 9) and psychological and somatic anxiety (items 10 and 11), indicating that agomelatine treatment was effective in both the “anxiety–agitation” and the “depression–retardation” components of depression, which may be somewhat opposed.23
Secondly, the improvements in HAMD-17 total and individual item scores were detectable and statistically significant as early as one week after commencement of treatment. Relatively slow onset of efficacy has been identified as an important limitation of most current antidepressant drugs.24,25 Perceived improvement early in treatment may be important in maintaining patient confidence in the prescribed treatment, leading to greater adherence and persistence with treatment and improved outcome while minimizing work and role impairments.
A further important result was that agomelatine showed substantial and rapid efficacy in patients with more severe depressive symptoms. In the subgroup with HAMD-17 total score ≥21 at baseline, rates of response were similar to and remission only slightly lower than in the full study population. Additionally, the proportion of patients with CGI-S scores of 5 or 6, indicating they were markedly or severely ill, decreased rapidly from 13.2% at baseline to 2.4% at 3 weeks and to 0.42% at week 8. These results suggest that the efficacy of agomelatine is high and rapid in onset in patients with any level of depression severity.
The tolerability profile of agomelatine in the present CHRONOS study was very similar to that in the previous VIVALDI study and in the randomized trials of the flexible-dose regimen for agomelatine. The most frequent adverse events in CHRONOS were nausea, dizziness, and headache, and these were also the three most frequent adverse events in the VIVALDI study.20 The overall incidence of adverse events reported was lower in CHRONOS (21.1%) than in the randomized trials (42%–66%). In the randomized studies, the incidences with agomelatine were similar to those with placebo (agomelatine 42.4% versus placebo 42.5%)16 and with the comparator antidepressant sertraline (agomelatine 48.0% versus sertraline 49.1%),18 and lower than with the comparators venlafaxine (agomelatine 51.2% versus venlafaxine 57.1%)17 and escitalopram (agomelatine 66.2% versus escitalopram 81.8%).14 During CHRONOS, 108 patients (1.7%) discontinued agomelatine due to adverse events, compared with 8.6% during first step treatment with citalopram in the STAR*D trial.22 The proportion of patients admitted to hospital for psychiatric reasons during CHRONOS (22 patients, 0.35%) also compared favorably with citalopram in STAR*D (2%).
It should be noted that no cases of a clinically significant increase in transaminases was reported. As mentioned above, no specific liver function monitoring was performed in the study since this was not required at the time by the Russian Federation Agency. However, our data are at least partly in line with the results of liver toxicity assessments for agomelatine in the observational VIVALDI study.20 In VIVALDI, an increase to a value >3 times the upper limit of the normal range for alanine aminotransferase and aspartate aminotransferase was less frequently observed than in controlled clinical trials. The authors mentioned that this might be due to the difference between spontaneous reporting of adverse drug reactions in a naturalistic setting and systematic evaluation of adverse events in controlled clinical studies, where intensive monitoring is standard. This explanation is probably true for the CHRONOS study.
Antidepressant monotherapy for bipolar disorder is not the rule in clinical practice today. It is well known that monoaminergic antidepressants have been associated with high rates of affect inversion in bipolar patients, and are often used in combination with mood stabilizing drugs.26 In CHRONOS, mood stabilizers were forbidden by the protocol, and were taken by only a very small number of patients (eleven, 0.18%). Unfortunately, we do not have more detailed information about the reasons for monotherapy with agomelatine in patients with bipolar depression in the CHRONOS study. Anyway, these preliminary data suggest that agomelatine may be less likely to trigger mania switch in patients with bipolar depression compared with monoaminergic antidepressants, probably due to the atypical mode of action, and this issue should be addressed in future randomized controlled trials.
The main strengths of the CHRONOS trial were its very large sample size of over 6,000 patients and the frequent follow-up visits, starting at week 1. Its main limitations were those also highlighted by the authors of the STAR*D study,22 namely its open treatment design and the lack of a placebo control group. However, such observational studies document the course of the depressive episode with treatment as experienced by the patient, and also provide information that complements the results of randomized studies in at least two areas. First, observational studies give information on the effectiveness of treatments when administered in everyday clinical practice. Second, they generally involve a more representative patient population, often with more comorbidities and taking a wider range of concomitant medications than is usual in randomized studies. Further limitations of the CHRONOS study were the short-term nature of the treatment and the fact that there were no follow-up visits after the end of the treatment period. However, it should be pointed out that previous randomized, double-blind trials have demonstrated the efficacy of long-term treatment with agomelatine in prevention of relapse27 and the absence of discontinuation symptoms following abrupt cessation of treatment with agomelatine.28
Another limitation of the CHRONOS study was the small number of appropriate scales addressing important characteristics of depressed patients, such as mania scales and functional outcome. This was due to providing the least time-consuming protocol to encourage practitioners to make out of routine assessments needed for study data collection.
Overall, the CHRONOS study confirmed the effectiveness of a flexible agomelatine dosing regimen in the acute treatment of depressive episodes in a large sample of patients in a naturalistic setting. Rates of response and remission were high in both the overall population and in the subgroup of more severely depressed patients. Notable features were the rapidity of onset of benefits (significant at one week) and the significant improvements observed in each of the HAMD-17 items. Agomelatine was well tolerated, with a low rate of withdrawals from treatment due to adverse events. These results suggest that agomelatine can be highly effective in the treatment of a depressive episode in the “real world” clinical setting.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Bromet E, Andrade LH, Hwang I, et al. Cross-national epidemiology of DSM-IV major depressive episode. BMC Med. 2011;9:90. doi: 10.1186/1741-7015-9-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wittchen HU, Jacobi F, Rehm J, et al. The size and burden of mental disorders and other disorders of the brain in Europe 2010. Eur Neuropsychopharmacol. 2011;21(9):655–679. doi: 10.1016/j.euroneuro.2011.07.018. [DOI] [PubMed] [Google Scholar]
- 3.Kessler RC, Berglund P, Demier O, et al. National Comorbidity Survey Replication The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) JAMA. 2003;289(23):3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- 4.Palsson SP, Ostling S, Skoog I. The incidence of first-onset depression in a population followed from the age of 70 to 85. Psychol Med. 2001;31(7):1159–1168. doi: 10.1017/s0033291701004524. [DOI] [PubMed] [Google Scholar]
- 5.Kruijshaar ME, Barendregt J, Vos T, de Graaf R, Spijker J, Andrews G. Lifetime prevalence estimates of major depression: an indirect estimation method and a quantification of recall bias. Eur J Epidemiol. 2005;20(1):103–111. doi: 10.1007/s10654-004-1009-0. [DOI] [PubMed] [Google Scholar]
- 6.Patten SB. Accumulation of major depressive episodes over time in a prospective study indicates that retrospectively assessed lifetime prevalence estimates are too low. BMC Psychiatry. 2009;9:19. doi: 10.1186/1471-244X-9-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andrews G, Poulton R, Skoog I. Lifetime risk of depression: restricted to a minority of waiting for most? Br J Psychiatry. 2005;187:495–496. doi: 10.1192/bjp.187.6.495. [DOI] [PubMed] [Google Scholar]
- 8.Patten SB, Wang JL, Williams JV, Lavorato DH, Bulloch A, Eliasziw M. Prospective evaluation of the effect of major depression on working status in a population sample. Can J Psychiatry. 2009;54(12):841–845. doi: 10.1177/070674370905401207. [DOI] [PubMed] [Google Scholar]
- 9.Lenze EJ, Schulz R, Martire LM, et al. The course of functional decline in older people with persistently elevated depressive symptoms: longitudinal findings from the Cardiovascular Health Study. J Am Geriatr Soc. 2005;53(4):569–575. doi: 10.1111/j.1532-5415.2005.53202.x. [DOI] [PubMed] [Google Scholar]
- 10.Murray CJL, Lopez AD. Alternative projections of mortality and disability by cause 1990–2020: Global Burden of Disease Study. Lancet. 1997;349(9064):1498–1504. doi: 10.1016/S0140-6736(96)07492-2. [DOI] [PubMed] [Google Scholar]
- 11.Olesen J, Gustavsson A, Svensson M, Wittchen HU, Jönsson B, CDBE2010 study group; European Brain Council The economic cost of brain disorders in Europe. Eur J Neurol. 2012;19(1):155–162. doi: 10.1111/j.1468-1331.2011.03590.x. [DOI] [PubMed] [Google Scholar]
- 12.de Bodinat C, Guardiola-Lemaitre B, Mocaër E, Renard P, Muñoz C, Millan MJ. Agomelatine, the first melatonergic antidepressant: discovery, characterization and development. Nat Rev Drug Discov. 2010;9(8):628–642. doi: 10.1038/nrd3140. [DOI] [PubMed] [Google Scholar]
- 13.Demyttenaere K. Agomelatine: a narrative review. Eur Neuropsychopharmacol. 2011;21(Suppl 4):S703–S709. doi: 10.1016/j.euroneuro.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 14.Quera-Salva MA, Hajak G, Philip P, et al. Comparison of agomelatine and escitalopram on nighttime sleep and daytime condition and efficacy in major depressive disorder patients. Int Clin Psychopharmacol. 2011;26(5):252–262. doi: 10.1097/YIC.0b013e328349b117. [DOI] [PubMed] [Google Scholar]
- 15.Kennedy SH, Emsley R. Placebo-controlled trial of agomelatine in the treatment of major depressive disorder. Eur Neuropsychopharmacol. 2006;16(2):93–100. doi: 10.1016/j.euroneuro.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 16.Olié JP, Kasper S. Efficacy of agomelatine, a MT1/MT2 receptor agonist with 5-HT2C antagonistic properties, in major depressive disorder. Int J Neuropsychopharmacol. 2007;10(5):661–673. doi: 10.1017/S1461145707007766. [DOI] [PubMed] [Google Scholar]
- 17.Lemoine P, Guilleminault C, Alvarez E. Improvement in subjective sleep in major depressive disorder with a novel antidepressant, agomelatine: randomized, double-blind comparison with venlafaxine. J Clin Psychiatry. 2007;68(11):1723–1732. doi: 10.4088/jcp.v68n1112. [DOI] [PubMed] [Google Scholar]
- 18.Kasper S, Hajak G, Wulff K, et al. Efficacy of the novel antidepressant agomelatine on the circadian rest-activity cycle and depressive and anxiety symptoms in patients with major depressive disorder: a randomized, double-blind comparison with sertraline. J Clin Psychiatry. 2010;71(2):109–120. doi: 10.4088/JCP.09m05347blu. [DOI] [PubMed] [Google Scholar]
- 19.Hale A, Corral RM, Mencacci C, Ruiz JS, Severo CA, Gentil V. Superior antidepressant efficacy results of agomelatine versus fluoxetine in severe MDD patients: a randomized, double-blind study. Int Clin Psychopharmacol. 2010;25(6):305–314. doi: 10.1097/YIC.0b013e32833a86aa. [DOI] [PubMed] [Google Scholar]
- 20.Laux G, VIVALDI Study Group The antidepressant agomelatine in daily practice: results of the non-interventional study VIVALDI. Pharmacopsychiatry. 2012;45(7):284–291. doi: 10.1055/s-0032-1309003. [DOI] [PubMed] [Google Scholar]
- 21.Seemüller F, Riedel M, Obermeier M, et al. Outcomes of 1014 naturalistically treated in patients with major depressive episode. Eur Neuropsychopharmacol. 2010;20(5):346–355. doi: 10.1016/j.euroneuro.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 22.Trivedi MH, Rush AJ, Nierenberg AA, et al. STAR*D Study Team Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163(1):28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- 23.Katz MM, Bowden CL, Frazer A. Rethinking depression and the actions of antidepressants: uncovering the links between the neural and behavioural elements. J Affect Disord. 2010;120(1–3):16–23. doi: 10.1016/j.jad.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 24.Machado-Vieira R, Salvadore G, Luckenbaugh DA, Manji HK, Zarate CA., Jr Rapid onset of antidepressant action: a new paradigm in the research and treatment of major depressive disorder. J Clin Psychiatry. 2008;69(6):946–958. doi: 10.4088/jcp.v69n0610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang T, Fava M. The future of psychopharmacology of depression. J Clin Psychiatry. 2010;71(8):971–975. doi: 10.4088/JCP.10m06223blu. [DOI] [PubMed] [Google Scholar]
- 26.Perlis RH, Ostacher MJ, Goldberg JF, et al. Transition to mania during treatment of bipolar depression. Neuropsychopharmacology. 2010;35(13):2545–2552. doi: 10.1038/npp.2010.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goodwin GM, Emsley R, Rembry S, Rouillon F, Agomelatine Study Group Agomelatine prevents relapse in patients with major depressive disorder without evidence of a discontinuation syndrome: a 24-week randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2009;70(8):1128–1137. doi: 10.4088/JCP.08m04548. [DOI] [PubMed] [Google Scholar]
- 28.Montgomery SA, Kennedy SH, Burrows GD, Lejoyeux M, Hindmarch I. Absence of discontinuation symptoms with agomelatine and occurrence of discontinuation symptoms with paroxetine: a randomized, double-blind, placebo-controlled discontinuation study. Int Clin Psychopharmacol. 2004;19(5):271–280. doi: 10.1097/01.yic.0000137184.64610.c8. [DOI] [PubMed] [Google Scholar]