Abstract
Tendinopathy is a clinical syndrome of pain, tendon thickening, and increased blood flow. The current review highlights evidence supporting an underlying role of neuropeptides in the etiology, clinical presentation, and treatment of painful overuse tendinopathy. Painful tendons demonstrate an increased presence of Substance P-containing nerves which are strongly implicated as a potential source of pain, but which also play important roles in the tendon’s attempt to self-repair. Recent findings have identified potential roles of additional sensory and autonomic neuropeptides which regulate pain, tissue remodeling, and vascular flow, including acetylcholine, noradrenaline and neuropeptide Y. Neuropeptide production within tendons is stimulated by mechanical load and exercise, and both direct and indirect neuropeptide effects may be responsible for the potential benefits of heavy-load eccentric loading. A model is presented which delineates the physiologic basis for signalling pathways between tenocytes, mast cells and sensory and autonomic nerves, with implications for understanding the mechanisms of traditional as well as emerging treatment strategies including sclerosing therapy and nitric oxide.
Keywords: Tendinopathy, neuropeptides, neuromodulators, neurotransmitters, tendon, overuse injury, Review
2. INTRODUCTION
Tendinopathy is a clinical syndrome of tendon pain and thickening. In many patients, tendinopathy presents gradually following prolonged periods of overuse, with most demonstrating long-standing symptoms recalcitrant to traditional therapies (e.g. non-steroidal anti-inflammatory drugs, corticosteroids) (1–3). The underlying histology is best characterized as a “failed healing” response, with ongoing, unsuccessful attempts by the tendon to restore the normal tissue structure (4, 5). Whether the initiating event is due to collagen fibril and microvessel failure, or rather to load-induced responses in tenocytes, remains a subject of debate (6, 7). However, the chronic pathological changes in the tendon and paratendon can be substantial and include an aberrant increase of microvessels and sensory nerves, abnormal quality and accelerated remodeling of the extracellular matrix, and increased local metabolism with excessive lactate production (8–12).
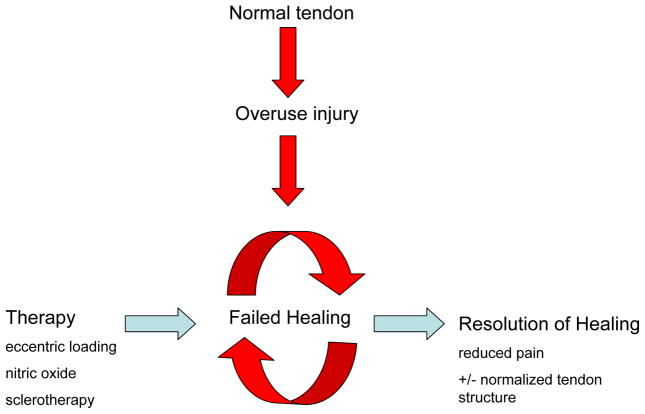
There is encouraging new evidence that novel conservative treatments may stimulate a healing response in tendons, and promote resolution of symptoms (Figure 1). These new strategies include heavy-load eccentric exercise, sclerosing injections and nitric oxide therapy. Interestingly, two of these treatments (exercise and sclerosing injections) result in an acute increase in tendon blood flow (above and beyond the baseline blood flow seen in painful tendons), followed in many instances by gradual normalization of tendon structure (13–16). Nitric oxide, a potent vasodilator (17), may have a similar effect.
Figure 1.
Working model of tendinopathy. The underlying histopathology and chemical environment suggest an ongoing attempt to repair the tendon, with involvement of neurovascular ingrowth and local delivery of neuropeptides such as Substance P. Successful therapies may exploit the ability of neuropeptides to further increase blood flow to the tissue, thereby promoting the resolution of healing and regression of excessive neurovascular elements.
Given the importance of blood flow in tendinopathy and its management, increasing attention is being paid to the role of the tendon’s microvessels and their accompanying innervation. In particular, tendon vasculature is associated with autonomic nerves that may regulate blood flow, as well as sensory nerves that are strongly implicated as a source of tendon pain (11, 18, 19).
We outline the neuroanatomy and potential roles of nerves and associated neuropeptides in tendinopathy. In addition, intriguing new data in support of a primary role for tenocytes in tendinopathy suggests that there may be a substantial intrinsic source of neuropeptides within tendons – namely, the tenocytes themselves. We also discuss the implications of the above for conservative management.
3. NORMAL TENDON SENSORY INNERVATION
Tendons are sparsely innervated, as the majority of sensory and autonomic nerves run in close proximity to the vascular supply in the paratendon and endotendon (11, 18, 20). Extensive studies of patellar, Achilles and extensor carpi radialis brevis tendon innervation have recently been conducted (11, 18, 20). In all the tendons examined, a general nerve marker (protein gene product /PGP 9.5) has demonstrated that the paratendinous tissue is well supplied with nerve fibres and fascicles. Many of these nerves are closely adjacent to arteries and arterioles (21). To a lesser extent, PGP 9.5 is also present in nerve fibres and fascicles within the tendon proper itself, i.e. not associated with vessels (11, 20, 22).
Many of the nerves in the paratendon can be identified as sensory types (displaying immunoreactivity for Substance P (SP) and Calcitonin Gene Related Peptide (CGRP) (18). The same is true in the tendon proper, but again to a much lesser extent (18). At a finer level of detail, all four types of sensory nerve endings have been identified within human tendons, including Ruffini corpuscles, Vater-Pacini corpuscles, Golgi tendon organs, and free nerve endings or pain receptors (23).
4. NORMAL TENDON AUTONOMIC INNERVATION
Autonomic innervation is heavily implicated in the dynamic regulation of tendon blood flow during exercise (24). The human tendon nerve supply demonstrates sympathetic markers including tyrosine hydroxylase and neuropeptide Y (11). Acetylcholine esterase reactions have also been identified in fine nerve fibers associated with small blood vessels in the patellar and Achilles tendons, demonstrating the existence of a parasympathetic innervation (20). Acetylcholine is well known as a vasodilator, while sympathetic neuropeptides mediate vasoconstriction. Indeed, tendon blood vessels express receptors both for sympathetic (alpha-adrenergic) as well as for parasympathetic (M2 muscarinic) neuropeptide receptors (11, 20). Vasoactive intestinal peptide (a vasodilatory parasympathetic neuropeptide) has also been demonstrated in rat tendons via immunohistochemistry and radioimmunoassay (25). Thus, the tendon blood supply is capable of dynamic regulation by both vasodilatory and vasoconstrictory neuropeptides.
5. OPIOID SYSTEM IN PARATENDON NERVES
A single study in the rat Achilles tendon has demonstrated the presence of an endogenous opioid system via radioimmunoassay, immunohistochemistry and in vitro naloxone binding assays (26). Enkephalins were localized exclusively in the paratendon (not in the tendon proper), in varicose nerve fibres within vessel walls and also in small peripheral nerve terminals suggesting localization in unmyelinated C-fibres. The distribution of δ-opiod receptors was similar, with positive fibres and terminals located in vessel walls and the loose connective tissue of the paratendon. The significance of these findings has not yet been explored, but the study opens the possibility for peripherally acting anti-nociceptive regulation of tendon pain (26). It also reinforces the concept that the paratendon (and/or endotendon with which it is continuous in larger tendons such as the human Achilles) may be the likely source of pain in many tendinopathies (27).
6. ABNORMAL INNERVATION IN TENDINOPATHY
Several studies have reported aberrations in the distribution and type of nerves present in tendinopathic tissue. Sanchis-Alfonso and co-workers (28) demonstrated the presence of pathological neural changes at the patellar tendon-bone junction in young athletes with patellar tendinopathy. Using S-100, a general mesenchymal and nerve cell marker, they showed an increase in the vascular innervation, as well as a histologic pattern of “sprouting” free nerve endings in about half the cases examined. Four out of seventeen symptomatic cases demonstrated neuromatous changes. A similar pattern of nerve sprouting has also been reported (19) in Achilles tendinopathy (Figure 2). The abnormal nerve endings in the Achilles tendon were positive for the neuropeptide SP. Given the role of SP in nociception, the results indicate that neural sprouting may be related to the development of pain in tendinopathy.
Figure 2.
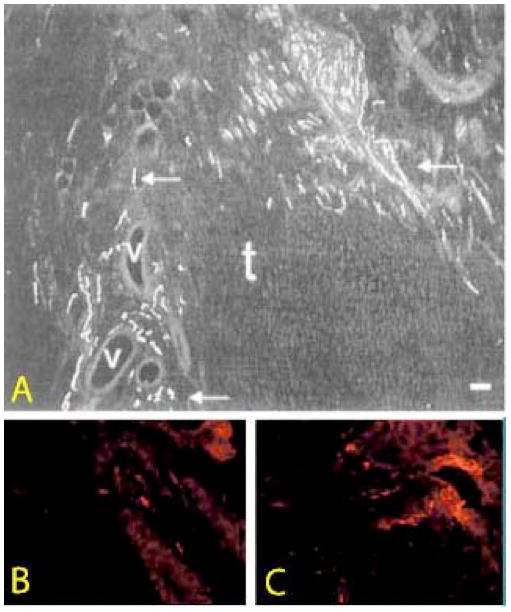
Sensory nerve ingrowth in injured Achilles tendon (T). (A) Note numerous positive fibres (arrows) in association with vessels (V) as well as free nerve endings. Rat tendon stained with antisera to a sensory marker (calcitonin gene related peptide). Reproduced from Ackermann et al, (36) with permission. (B, C) Human control (A) and tendinopathy (B) showing increased presence of Substance P-positive nerve fibres adjacent to a vessel in tendinopathy. Reproduced from Schubert et al (19) with permission.
An increased number of microvessels is a feature of tendinopathy -- microvessel area may be increased up to 300% (12). Many of these vessels, especially the larger and more mature ones including arterioles, appear to have an accompanying nerve supply (11). The distribution of autonomic innervation is also reported to be affected in tendinopathy. Lian and coworkers (29) reported a reduced presence of sympathetic (tyrosine hydroxylase positive) nerve fibres in patients with patellar tendinopathy. The authors suggest that a reduced sympathetic regulation of tendinopathy microvessels may contribute to an increased blood flow observed on colour Doppler ultrasound.
However, not all groups have consistently reported the above alterations in tendinopathy innervation. Danielson et al reported that there was no difference in the distribution of either sensory or sympathetic innervation between normal and tendinopathic tissue from the patellar tendon, although nerves and microvessels were both more prominent in tendinopathy (11). They further noted that quantitative comparisons between normal and tendinopathic tissue were difficult because of the differences in extent of innervation at different tissue depths (the deeper in the tendon, the sparser the innervation), and differences in the length of time since onset of injury or symptoms. Nonetheless, semi-quantitative grading demonstrated a greater presence of adrenoreceptors within blood vessel walls in patellar tendinopathy. This increase was related both to an increased intensity of immunoreactions, as well as an increase in the number of vessels present. These data suggest an increased sympathetic regulation of tendinopathy vessels.(11)
7. RELATIONSHIP OF NEURAL INGROWTH AND PAIN
The presence of nerve remodeling has not been studied in detail in human tendons. In rats, the Achilles tendon is reported to be essentially devoid of nerves within the tendon proper, but following injury, the paratendinous nerves sprout and penetrate the repairing scar tissue within the tendon midsubstance (30). This neural ingrowth is strongly correlated with pain. Although this correlation of neural ingrowth and pain in the rat model does not prove a causal relationship, it is likely that the sprouting nerve endings in the healing tendon contributes to the sensation of pain originating from the injured area (30). Notable differences in tendon healing between rats and humans make it difficult to determine the clinical relevance of the above findings. In the rat model, as repair progresses, the nerves and vessels withdraw and the nociceptive reflex declines in concert with the restoration of normal tendon structure after several weeks. In contrast, the human patellar tendon remains hypervascular with disorganized collagen for years following harvest of its central one third for anterior cruciate ligament reconstruction (31). More importantly, the hypervascular and disorganized appearance of healed human tendon is essentially the same as that which has been described as “tendinopathy”, although the tendons are successfully healed and pain free (32). Thus, there is less concordance between histology and pain in humans than in rats.
Overall, the rather sparse sensory innervation of the tendon proper is consistent with the fact that quite substantial tendon abnormalities may be identified on ultrasound or magnetic resonance imaging while the patient remains asymptomatic (33). In other words, one could hypothesize that early microtrauma in tendon may not necessarily trigger pain sensation, due to the sparse nerve supply within the tendon proper. The events that underlie the transition from symptom-free lesions to painful ones are completely unknown. Much information could be gained from a comparison of neuropeptide distribution in healed tendon vs tendinopathic tendon, as the presence of pro- or anti-nociceptive neuropeptides may hold the key as to why one type of “failed healing response” is painful (tendinopathic) and the other is not.
8. ESSENTIAL ROLE OF NEUROPEPTIDES IN TENDON HEALING
Despite the relatively rare occurrence of nerve fibres and fascicles within the deeper portions of the tendon itself, animal studies suggest that an intact tendon innervation is essential for successful healing. Denervation (sciatic neurectomy) leads to inferior healing of the rat Achilles tendon, as evidenced by irregular collagen alignment, higher cell to matrix ratio, and reduced ultimate tensile stress and stiffness (34). Exogenously administered SP stimulates early reparative events following Achilles tendon transection, including fibroblast proliferation, angiogenesis and collagen organization in a rat model (35).
The mechanism by which neuropeptides may contribute to tendon healing has also been studied in the rat. Following Achilles tendon injury, a nerve growth marker (growth associated protein 43) was expressed specifically in association with tissue repair (36). The majority of ingrowing nerve endings 2–6 weeks after injury contained the sensory peptide CGRP. In contrast, sympathetic (vasoconstrictory) nerves were not identified until later (week 8), and only in areas peripheral to the repair site (36). Thus, the neuropeptide CGRP (and its likely partner SP) may increase the healing response of the tissue by increasing blood flow via both vasodilation and angiogenesis. Later, sympathetic fibres may down-regulate the blood flow to the area as healing progresses and metabolic requirements decline.
9. STIMULATION OF NEUROPEPTIDE DELIVERY BY MECHANICAL LOAD AND EXERCISE
If neuropeptides play a role in promoting tendon healing, then treatments which can induce their expression in the tissue may be beneficial. Mechanical loading of healing rat tendon (delivered via intermittent pneumatic compression) increased tendon blood flow concomitant with an increase in the expression of SP and CGRP (37). In the mechanically loaded group, accelerated early repair events were observed, including angiogenesis, fibroblast proliferation and collagen alignment. Wheel running following Achilles tendon transection resulted in a similar improvement in repair and an accelerated neuronal remodeling observed at 4 weeks following injury (38).
One disadvantage to the approach of increasing SP expression in tendon via mechanical load or exercise is that it may exacerbate pain or edema. Neurokinin-1 receptors (the preferred SP receptor) are present on nerve fibres in human tendon, and SP has a well known nociceptive role (22). Indeed, SP levels are strongly correlated to rotator cuff tendon pain (39). Accordingly, tendon pain may be exacerbated by heavy mechanical loading regimes, particularly if the loading is combined with too high a volume of training (40).
10. CONVERGENT SIGNALING BY NEUROPEPTIDES AND NITRIC OXIDE
The vasodilatory effect of SP is mediated by induction of nitric oxide synthase (NOS) in endothelial cells, downstream of the Neurokinin-1 receptors. Thus, an alternate approach to achieving the beneficial effects of SP and other neuropeptides without exacerbating pain may be the use of a nitric oxide (NO) donor patch directly over the painful site (41). NO produced by the induced nitric oxide synthases (NOS) diffuses and exerts a localized vasodilation through intracellular signaling pathways in smooth muscle cells. Interestingly, all three isoforms of NOS are upregulated by tendon overuse, including inducible NOS (iNOS), endothelial NOS (eNOS) and neuronal NOS (iNOS) (42). In several randomized controlled trials, treatment with NO via patches reduce tendon pain and improved function (17, 43). This improvement in pain and repair may be at least partly due to a local vasodilatory effect, however this possibility has not yet been examined in detail. NO has the added benefit of directly stimulating collagen production by tenocyte cell cultures (44).
11. PRODUCTION AND RESPONSE OF NEUROTRANSMITTERS IN TENDON
Several recent studies have demonstrated that neurotransmitters in tendon, including catecholamines, acetylcholine, and glutamate, may be produced not only by nerves, but also by local tenocytes. Furthermore, these neurotransmitters appear to exist to a greater degree in tendinopathic tissue.
First, there is evidence of catecholamine production and a functional catecholamine response system in tenocytes. Immunohistochemistry and in situ hybridization have both shown that tyrosine hydroxylase is expressed by tenocytes within the human patellar tendon (11, 45, 46). Additionally, these same tenocytes demonstrate immunoreactions for alpha-adrenergic receptors (11). Similar findings have been reported for avian flexor tenocytes, which demonstrated mRNA expression of alpha-adrenergic receptors (47). Stimulation of avian tenocyte cultures with noradrenaline resulted in a wave of calcium signaling within tenocytes (47). It is therefore possible that noradrenaline may lead to downstream responses such as tenocyte proliferation and/or modulation of extracellular matrix production. Most intriguingly, immunoreactions for both tyrosine hydroxylase and adrenergic receptors were more prominent in tenocytes with an abnormal (enlarged) appearance, in keeping with their proposed role in the failed healing/increased metabolic activity model of tendinopathy (Figure 1) (11).
Markers of acetylcholine production have also been identified in immunohistochemical studies of human patellar tendinopathy, again most prominently in enlarged or abnormally appearing tenocytes (20). Choline acetyltransferase and vesicular acetylcholine transporter were identified in tenocytes, along with the acetylcholine (M2) receptor itself (20). The functional importance of this finding has not yet been examined. However, acetylcholine modulates cellular proliferation and extracellular matrix metabolism in closely related (fibroblastic) cells (48).
Finally, the potential role of the neurotransmitter glutamate has been investigated in tendinopathy. Alfredson and colleagues detected elevated levels of free glutamate from within painful Achilles tendons (49, 50). The glutamate within tendons is likely to derive from the tenocytes themselves, as extensive expression of a vesicular glutamate transporter (VGlut2) has been observed at the protein and mRNA levels (51). VGlut2 expression was more widespread and prominent in Achilles and patellar tendinopathy tissue compared to normals (51). Furthermore, glutamate receptors (N-methyl d-aspartate receptor, NMDAR-1) have been identified both on tendon nerves as well as on tenocytes themselves (52, 53). Glutamate, acting via NMDAR-1, is a well known trigger for pain and vasodilation, which has led to the hypothesis that glutamate may be the neuropeptide responsible for pain. However, microdialysis conducted on successfully treated (i.e. pain-free) tendons demonstrated persistently high glutamate levels, suggesting that other neuropeptides may play a more definitive role in symptom production (54).
12. POTENTIAL SIGNALING PATHWAYS AMONG NERVES AND TENOCYTES
The evidence outlined above provides the anatomic basis for a complex and as yet largely unexplored interplay between nerves and tenocytes, with neuropeptides (both neuromodulators and neurotransmitters) as the mediators (see Tables 1 and 2). As an example of nerve-tenocyte signaling, SP is produced and released by sensory nerves, then diffuses and modulates the expression of gene products in tenocytes, including growth factors, proteinases and proteinase inhibitors (55, 56). This may represent a mechanism whereby ingrowing nerves reinforce the tendon’s attempts at repair. As another example, acetylcholine and adrenaline/noradrenaline released by nerves may influence tenocyte behaviour via as-yet largely uncharacterized pathways. Conversely, these same mediators released by tenocytes may modulate pain and vascular function (Figure 3).
Table 1.
Distribution of neuropeptides (or enzyme markers) in human tendinopathy
| Neuropeptide | Distribution in tendinopathy |
|---|---|
| Substance P | Sensory nerves (18, 19, 22, 29) |
| Calcitonin Gene Related Peptide | Sensory nerves (11, 18, 22) |
| Neuropeptide Y | Sympathetic nerves |
| Adrenaline / Noradrenaline (TH1) | Sympathetic nerves; tenocytes (11, 29, 45) |
| Acetylcholine (AchE2, ChAT3) | Cholinergic nerves; tenocytes (20) |
| Glutamate (VGluT24) | Tenocytes (51) |
tyrosine hydroxylase,
acetylcholine esterase,
choline acetyltransferase,
vesicular gluatamate transporter 2
Table 2.
Distribution of neuropeptide receptors in human tendinopathy
| Receptor | Distribution in tendinopathy |
|---|---|
| NK-1R1 (Substance P receptor) | Nerves; blood vessels; tenocytes (22, 55, 62) |
| M2R2 (Acetylcholine receptor) | Blood vessels; tenocytes (20) |
| Alpha-adrenergic (Catecholamine receptor) | Sympathetic nerves; blood vessels; tenocytes (11, 45) |
| NMDAR-13 (Glutamate receptor) | Sensory nerves; tenocytes (53, 63) |
neurokin receptor 1,
muscarinic receptor 2,
N-methyl d-aspartate receptor-1
Figure 3.
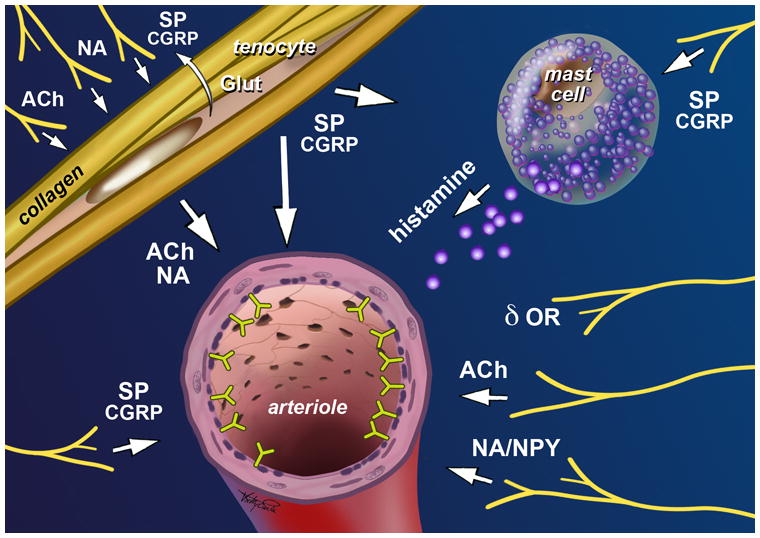
Neuropeptides as mediators of vascular function and pain in tendon. Nerve fibres in normal and pathological tendons include sympathetic (NA: noradrenaline / adrenaline; NPY: neuropeptide Y), parasympathetic (Ach: acetylcholine), and sensory (SP: substance P; CGRP: calcitonin gene related peptide) nerve fibres, as well as free nerve endings expressing delta opioid receptors (δ OR). Tenocytes have been identified as both source and target of neuropeptide signals. Vascular regulation may occur via both direct and mast cell- or tenocyte-mediated mechanisms. Reproduced from Scott (BJSM, in press), with permission.
13. ROLE OF MAST CELLS IN TENDINOPATHY
Another possibility which has been considered is that mast cells may represent an important target of neuropeptides in tendon, as well as a potential source of neurotrophic substances (56). When specific immunolabeling is performed, mast cells are found to be more prominent in tendinopathy tissue, mostly in association with microvessels (57). These mast cells express tryptase, a potent angiogenic factor (57). Mast cells are also a prominent source of nerve growth factor, which may play a role in the neural sprouting described above for acute and chronic tendon injuries (58). SP, vasoactive intestinal peptide and CGRP can all activate mast cells through specific receptors, causing the mast cells to degranulate and release histamine, which results in an axonal stimulation that stimulates adjoining sensory peptide-containing nerve endings. This axonal stimulation leads to further neuropeptide release in an escalating process known as neurogenic inflammation (58). Given the increased levels of SP in combination with prominent mast cells observed in tendinopathies, a neurogenic inflammation is a distinct possibility.
14. POTENTIAL ROLE OF NEUROPEPTIDES IN THE RESPONSE TO PARATENDINOUS SCLEROSING INJECTIONS
Sclerosing polidocanol injections are targeted under ultrasound guidance to the point where the blood supply (and sensory/autonomic nerves) enters the tendon – anterior to the Achilles tendon, and posterior to the patellar tendon (59, 60). This approach can result in a rather dramatic return to pain-free, high intensity loading (61). The speed with which the tendon pain is reduced in some cases suggests that the injections may be interfering with the local nerve supply, essentially creating a partial denervation of the tendon midportion. The treatment also results in an acute increase in tendon blood flow, possibly from alternate collateral circulation (i.e. musculotendinous and osseotendinous) (16). Thus, the effect of this treatment on neuropeptide distribution and neurovascular regulation warrants investigation. A combination of prospective and mechanistic studies will shed further light on the response of tendon pain to this treatment.
15. CONCLUSION AND PERSPECTIVES
In summary, neuropeptides modulate important aspects of tendinopathy including pain, vascular flow, and tissue remodeling. Substance P has emerged as a potential source of tendon pain and tissue abnormality. Neural sprouting of Substance P-positive fibres accompanies the vascular hyperplasia which characterizes tendinopathies. Neuropeptides represent an important and often overlooked aspect of tendon pathology, as well as a potential opening for therapeutic interventions.
Acknowledgments
The authors would like to acknowledge Karim Khan for helpful editorial comments. RB was visiting professor at the Centre for Hip Health during the writing of this manuscript. AS was supported by a CIHR Fellowship and the Canada Scandinavia Foundation.
Abbreviations
- Ach
acetylcholine
- AchE
acetylcholine esterase
- ChAT
choline acetyltransferase
- CGRP
calcitonin gene related peptide
- δ OR
delta opioid receptor
- GAP
growth associated protein 43
- Glut
glutamate
- mRNA
messenger ribonucleic acid
- M2R
muscarinic receptor 2
- NA
noradrenaline
- NO
nitric oxide
- NOS
nitric oxide synthase
- NK-1R
neurokinin-1 receptor
- NMDAR-1
N-methyl d-aspartate receptor-1
- NPY
neuropeptide Y
- PGP 9.5
protein gene product 9.5
- SP
substance P
- TH
tyrosine hydroxylase
- VgluT2
vesicular glutamate transporter 2
References
- 1.Lian OB, Engebretsen L, Bahr R. Prevalence of jumper’s knee among elite athletes from different sports: a cross-sectional study. Am J Sports Med. 2005;33:561–567. doi: 10.1177/0363546504270454. http://dx.doi.org/10.1177/0363546504270454. [DOI] [PubMed] [Google Scholar]
- 2.Maffulli N, Wong J, Almekinders LC. Types and epidemiology of tendinopathy. Clin Sports Med. 2003;22:675–692. doi: 10.1016/S0278-5919(03)00004-8. http://dx.doi.org/10.1016/S0278-5919(03)00004-8. [DOI] [PubMed] [Google Scholar]
- 3.Bisset L, Paungmali A, Vicenzino B, Beller E. A systematic review and meta-analysis of clinical trials on physical interventions for lateral epicondylalgia. Br J Sports Med. 2005;39:411–422. doi: 10.1136/bjsm.2004.016170. http://dx.doi.org/10.1136/bjsm.2004.016170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kraushaar BS, Nirschl RP. Tendinosis of the elbow (tennis elbow). Clinical features and findings of histological, immunohistochemical, and electron microscopy studies. J Bone Joint Surg Am. 1999;81:259–278. No doi match found. [PubMed] [Google Scholar]
- 5.Budoff JE, Kraushaar BS, Ayala G. Flexor carpi ulnaris tendinopathy. J Hand Surg [Am] 2005;30:125–129. doi: 10.1016/j.jhsa.2004.07.018. http://dx.doi.org/10.1016/j.jhsa.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 6.Wang JH, Thampatty BP, Lin JS, Im HJ. Mechanoregulation of gene expression in fibroblasts. Gene. 2007;391:1–15. doi: 10.1016/j.gene.2007.01.014. http://dx.doi.org/10.1016/j.gene.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schechtman H, Bader DL. Fatigue damage of human tendons. J Biomech. 2002;35:347–353. doi: 10.1016/S0021-9290(01)00177-4. http://dx.doi.org/10.1016/S0021-9290(01)00177-4. [DOI] [PubMed] [Google Scholar]
- 8.Alfredson H, Bjur D, Thorsen K, Lorentzon R, Sandstrom P. High intratendinous lactate levels in painful chronic Achilles tendinosis. An investigation using microdialysis technique. J Orthop Res. 2002;20:934–938. doi: 10.1016/S0736-0266(02)00021-9. http://dx.doi.org/10.1016/S0736-0266(02)00021-9. [DOI] [PubMed] [Google Scholar]
- 9.Kvist M, Jozsa L, Jarvinen MJ, Kvist H. Chronic Achilles paratenonitis in athletes: a histological and histochemical study. Pathology. 1987;19:1–11. doi: 10.3109/00313028709065127. No doi match found. [DOI] [PubMed] [Google Scholar]
- 10.Riley GP, Curry V, DeGroot J, van El B, Verzijl N, Hazleman BL, Bank RA. Matrix metalloproteinase activities and their relationship with collagen remodelling in tendon pathology. Matrix Biol. 2002;21:185–195. doi: 10.1016/S0945-053X(01)00196-2. http://dx.doi.org/10.1016/S0945-053X(01)00196-2. [DOI] [PubMed] [Google Scholar]
- 11.Danielson P, Alfredson H, Forsgren S. Studies on the importance of sympathetic innervation, adrenergic receptors, and a possible local catecholamine production in the development of patellar tendinopathy (tendinosis) in man. Microsc Res Tech. doi: 10.1002/jemt.20413. (In Press) No doi match found. [DOI] [PubMed] [Google Scholar]
- 12.Scott A, Lian O, Roberts CR, Cook JL, Handley CJ, Bahr R, Samiric T, Ilic MZ, Parkinson J, Hart D, Duronio V, Khan KM. Increased versican content is associated with tendinosis pathology in the patellar tendon of athletes with jumper’s knee. Scand J Med Sci Sports. doi: 10.1111/j.1600-0838.2007.00735.x. (In Press) No doi match found. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohberg L, Lorentzon R, Alfredson H. Eccentric training in patients with chronic Achilles tendinosis: normalised tendon structure and decreased thickness at follow up. Br J Sports Med. 2004;38:8–11. doi: 10.1136/bjsm.2001.000284. http://dx.doi.org/10.1136/bjsm.2001.000284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cook JL, Kiss ZS, Ptasznik R, Malliaras P. Is vascularity more evident after exercise? Implications for tendon imaging. Am J Roentgenol. 2005;185:1138–1140. doi: 10.2214/AJR.04.1205. http://dx.doi.org/10.2214/AJR.04.1205. [DOI] [PubMed] [Google Scholar]
- 15.Lind B, Ohberg L, Alfredson H. Sclerosing polidocanol injections in mid-portion Achilles tendinosis: remaining good clinical results and decreased tendon thickness at 2-year follow-up. Knee Surg Sports Traumatol Arthrosc. 2006;14:1327–1332. doi: 10.1007/s00167-006-0161-3. http://dx.doi.org/10.1007/s00167-006-0161-3. [DOI] [PubMed] [Google Scholar]
- 16.Alfredson H, Ohberg L. Increased intratendinous vascularity in the early period after sclerosing injection treatment in Achilles tendinosis : a healing response? Knee. Surg Sports Traumatol Arthrosc. 2006;14:399–401. doi: 10.1007/s00167-006-0720-7. http://dx.doi.org/10.1007/s00167-006-0720-7. [DOI] [PubMed] [Google Scholar]
- 17.Paoloni JA, Appleyard RC, Nelson J, Murrell GA. Topical glyceryl trinitrate treatment of chronic noninsertional achilles tendinopathy. A randomized, double-blind, placebo-controlled trial. J Bone Joint Surg [Am] 2004;86-A:916–922. doi: 10.2106/00004623-200405000-00005. No doi match found. [DOI] [PubMed] [Google Scholar]
- 18.Danielson P, Alfredson H, Forsgren S. Distribution of general (PGP 9.5) and sensory (substance P/CGRP) innervations in the human patellar tendon. Knee Surg Sports Traumatol Arthrosc. 2006;14:125–132. doi: 10.1007/s00167-005-0636-7. http://dx.doi.org/10.1007/s00167-005-0636-7. [DOI] [PubMed] [Google Scholar]
- 19.Schubert TE, Weidler C, Lerch K, Hofstadter F, Straub RH. Achilles tendinosis is associated with sprouting of substance P positive nerve fibres. Ann Rheum Dis. 2005;64:1083–1086. doi: 10.1136/ard.2004.029876. http://dx.doi.org/10.1136/ard.2004.029876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Danielson P, Alfredson H, Forsgren S. Immunohistochemical and histochemical findings favoring the occurrence of autocrine/paracrine as well as nerve-related cholinergic effects in chronic painful patellar tendon tendinosis. Microsc Res Tech. 2006;69:808–819. doi: 10.1002/jemt.20351. http://dx.doi.org/10.1002/jemt.20351. [DOI] [PubMed] [Google Scholar]
- 21.Maffulli N, Testa V, Capasso G, Oliva F, Sullo A, Benazzo F, Regine R, King JB. Surgery for Chronic Achilles Tendinopathy Yields Worse Results in Nonathletic Patients. Clin J Sport Med. 2006;16:123–128. doi: 10.1097/00042752-200603000-00007. http://dx.doi.org/10.1097/00042752-200603000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Ljung BO, Alfredson H, Forsgren S. Neurokinin 1-receptors and sensory neuropeptides in tendon insertions at the medial and lateral epicondyles of the humerus. Studies on tennis elbow and medial epicondylalgia. J Orthop Res. 2004;22:321–327. doi: 10.1016/S0736-0266(03)00183-9. http://dx.doi.org/10.1016/S0736-0266(03)00183-9. [DOI] [PubMed] [Google Scholar]
- 23.Jozsa L, Kannus P. Human tendons : anatomy, physiology, and pathology. Champaign, IL: Human Kinetics; 1997. No doi match found. [Google Scholar]
- 24.Hannukainen J, Kalliokoski KK, Nuutila P, Fujimoto T, Kemppainen J, Viljanen T, Laaksonen MS, Parkkola R, Knuuti J, Kjaer M. In vivo measurements of glucose uptake in human Achilles tendon during different exercise intensities. Int J Sports Med. 2005;26:727–731. doi: 10.1055/s-2005-837458. http://dx.doi.org/10.1055/s-2005-837458. [DOI] [PubMed] [Google Scholar]
- 25.Ackermann PW, Li J, Finn A, Ahmed M, Kreicbergs A. Autonomic innervation of tendons, ligaments and joint capsules. A morphologic and quantitative study in the rat. J Orthop Res. 2001;19:372–378. doi: 10.1016/S0736-0266(00)90029-9. http://dx.doi.org/10.1016/S0736-0266(00)90029-9. [DOI] [PubMed] [Google Scholar]
- 26.Ackermann PW, Spetea M, Nylander I, Ploj K, Ahmed M, Kreicbergs A. An opioid system in connective tissue: a study of achilles tendon in the rat. J Histochem Cytochem. 2001;49:1387–1395. doi: 10.1177/002215540104901107. No doi match found. [DOI] [PubMed] [Google Scholar]
- 27.Kvist MH, Lehto MU, Jozsa L, Jarvinen M, Kvist HT. Chronic achilles paratenonitis. An immunohistologic study of fibronectin and fibrinogen. Am J Sports Med. 1988;16:616–623. doi: 10.1177/036354658801600611. http://dx.doi.org/10.1177/036354658801600611. [DOI] [PubMed] [Google Scholar]
- 28.Sanchis-Alfonso V, Rosello-Sastre E, Subias-Lopez A. Neuroanatomic basis for pain in patellar tendinosis (“jumper’s knee”): a neuroimmunohistochemical study. Am J Knee Surg. 2001;14:174–177. No doi match found. [PubMed] [Google Scholar]
- 29.Lian O, Dahl J, Ackermann PW, Frihagen F, Engebretsen L, Bahr R. Pronociceptive and antinociceptive neuromediators in patellar tendinopathy. Am J Sports Med. 2006;34:1801–1808. doi: 10.1177/0363546506289169. http://dx.doi.org/10.1177/0363546506289169. [DOI] [PubMed] [Google Scholar]
- 30.Ackermann PW, Li J, Lundeberg T, Kreicbergs A. Neuronal plasticity in relation to nociception and healing of rat achilles tendon. J Orthop Res. 2003;21:432–441. doi: 10.1016/S0736-0266(02)00207-3. http://dx.doi.org/10.1016/S0736-0266(02)00207-3. [DOI] [PubMed] [Google Scholar]
- 31.Svensson M, Kartus J, Christensen LR, Movin T, Papadogiannakis N, Karlsson J. A long-term serial histological evaluation of the patellar tendon in humans after harvesting its central third. Knee Surg Sports Traumatol Arthrosc. 2005;13:398–404. doi: 10.1007/s00167-004-0590-9. http://dx.doi.org/10.1007/s00167-004-0590-9. [DOI] [PubMed] [Google Scholar]
- 32.Khan KM, Cook JL, Bonar F, Harcourt P, Astrom M. Histopathology of common tendinopathies. Update and implications for clinical management. Sports Med. 1999;27:393–408. doi: 10.2165/00007256-199927060-00004. No doi match found. [DOI] [PubMed] [Google Scholar]
- 33.Cook JL, Khan KM, Kiss ZS, Purdam CR, Griffiths L. Prospective imaging study of asymptomatic patellar tendinopathy in elite junior basketball players. J Ultrasound Med. 2000;19:473–479. doi: 10.7863/jum.2000.19.7.473. No doi match found. [DOI] [PubMed] [Google Scholar]
- 34.Yeung CK, Guo X, Ng G. Denervation impairs achilles tendon healing in rat model. Paper presented at: International Society of Biomechanics XXth Conference - American Society of Biomechanics 29th Annual Meeting; 2005; Clevelah, Ohio. Reference not parsed. [Google Scholar]
- 35.Burssens P, Steyaert A, Forsyth R, van Ovost EJ, De Paepe Y, Verdonk R. Exogenously administered substance P and neutral endopeptidase inhibitors stimulate fibroblast proliferation, angiogenesis and collagen organization during Achilles tendon healing. Foot Ankle Int. 2005;26:832–839. doi: 10.1177/107110070502601008. No doi match found. [DOI] [PubMed] [Google Scholar]
- 36.Ackermann PW, Ahmed M, Kreicbergs A. Early nerve regeneration after achilles tendon rupture--a prerequisite for healing? A study in the rat. J Orthop Res. 2002;20:849–856. doi: 10.1016/S0736-0266(01)00159-0. http://dx.doi.org/10.1016/S0736-0266(01)00159-0. [DOI] [PubMed] [Google Scholar]
- 37.Dahl J, Li J, Bring DK, Renstrom P, Ackermann PW. Intermittent pneumatic compression enhances neurovascular ingrowth and tissue proliferation during connective tissue healing: a study in the rat. J Orthop Res. 2007;25:1185–1192. doi: 10.1002/jor.20390. http://dx.doi.org/10.1002/jor.20390. [DOI] [PubMed] [Google Scholar]
- 38.Bring DK, Kreicbergs A, Renstrom PA, Ackermann PW. Physical activity modulates nerve plasticity and stimulates repair after Achilles tendon rupture. J Orthop Res. 2007;25:164–172. doi: 10.1002/jor.20257. http://dx.doi.org/10.1002/jor.20257. [DOI] [PubMed] [Google Scholar]
- 39.Gotoh M, Hamada K, Yamakawa H, Inoue A, Fukuda H. Increased substance P in subacromial bursa and shoulder pain in rotator cuff diseases. J Orthop Res. 1988;16:618–621. doi: 10.1002/jor.1100160515. http://dx.doi.org/10.1002/jor.1100160515. [DOI] [PubMed] [Google Scholar]
- 40.Visnes H, Hoksrud A, Cook J, Bahr R. No effect of eccentric training on jumper’s knee in volleyball players during the competitive season: a randomized clinical trial. Clin J Sport Med. 2005;15:227–234. doi: 10.1097/01.jsm.0000168073.82121.20. http://dx.doi.org/10.1097/01.jsm.0000168073.82121.20. [DOI] [PubMed] [Google Scholar]
- 41.Murrell GA. Using nitric oxide to treat tendinopathy. Br J Sports Med. 2007;41(4):227–231. doi: 10.1136/bjsm.2006.034447. http://dx.doi.org/10.1136/bjsm.2006.034447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Szomor ZL, Appleyard RC, Murrell GA. Overexpression of nitric oxide synthases in tendon overuse. J Orthop Res. 2006;24:80–86. doi: 10.1002/jor.20009. http://dx.doi.org/10.1002/jor.20009. [DOI] [PubMed] [Google Scholar]
- 43.Paoloni JA, Appleyard RC, Nelson J, Murrell GA. Topical nitric oxide application in the treatment of chronic extensor tendinosis at the elbow: a randomized, double-blinded, placebo-controlled clinical trial. Am J Sports Med. 2003;31:915–920. doi: 10.1177/03635465030310062901. No doi match found. [DOI] [PubMed] [Google Scholar]
- 44.Xia W, Szomor Z, Wang Y, Murrell GA. Nitric oxide enhances collagen synthesis in cultured human tendon cells. J Orthop Res. 2006;24:159–172. doi: 10.1002/jor.20060. http://dx.doi.org/10.1002/jor.20060. [DOI] [PubMed] [Google Scholar]
- 45.Danielson P, Alfredson H, Forsgren S. In situ hybridization studies confirming recent findings of the existence of a local nonneuronal catecholamine production in human patellar tendinosis. Microsc Res Tech. 2007;70:908–911. doi: 10.1002/jemt.20495. http://dx.doi.org/10.1002/jemt.20495. [DOI] [PubMed] [Google Scholar]
- 46.Andersson G, Danielson P, Alfredson H, Forsgren S. Nerve-related characteristics of ventral paratendinous tissue in chronic Achilles tendinosis. Knee Surg Sports Traumatol Arthrosc. 2007;15:1272–1279. doi: 10.1007/s00167-007-0364-2. http://dx.doi.org/10.1007/s00167-007-0364-2. [DOI] [PubMed] [Google Scholar]
- 47.Wall ME, Faber JE, Yang X, Tsuzaki M, Banes AJ. Norepinephrine-induced calcium signaling and expression of adrenoceptors in avian tendon cells. Am J Physiol Cell Physiol. 2004;287:C912–918. doi: 10.1152/ajpcell.00099.2004. http://dx.doi.org/10.1152/ajpcell.00099.2004. [DOI] [PubMed] [Google Scholar]
- 48.Oben JA, Yang S, Lin H, Ono M, Diehl AM. Acetylcholine promotes the proliferation and collagen gene expression of myofibroblastic hepatic stellate cells. Biochem Biophys Res Commun. 2003;300:172–177. doi: 10.1016/S0006-291X(02)02773-0. http://dx.doi.org/10.1016/S0006-291X(02)02773-0. [DOI] [PubMed] [Google Scholar]
- 49.Alfredson H, Thorsen K, Lorentzon R. In situ microdialysis in tendon tissue: high levels of glutamate, but not prostaglandin E2 in chronic Achilles tendon pain. Knee Surg Sports Traumatol Arthrosc. 1999;7:378–381. doi: 10.1007/s001670050184. http://dx.doi.org/10.1007/s001670050184. [DOI] [PubMed] [Google Scholar]
- 50.Alfredson H, Ljung BO, Thorsen K, Lorentzon R. In vivo investigation of ECRB tendons with microdialysis technique--no signs of inflammation but high amounts of glutamate in tennis elbow. Acta Orthop Scand. 2000;71:475–479. doi: 10.1080/000164700317381162. http://dx.doi.org/10.1080/000164700317381162. [DOI] [PubMed] [Google Scholar]
- 51.Scott A, Alfredson H, Forsgren S. VGluT2 expression in painful Achilles and patellar tendinosis: evidence of local glutamate release by tenocytes. J Orthop Res. doi: 10.1002/jor.20536. (In Press). No doi match found. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alfredson H, Forsgren S, Thorsen K, Lorentzon R. In vivo microdialysis and immunohistochemical analyses of tendon tissue demonstrated high amounts of free glutamate and glutamate NMDAR1 receptors, but no signs of inflammation, in Jumper’s knee. J Orthop Res. 2001;19:881–886. doi: 10.1016/S0736-0266(01)00016-X. http://dx.doi.org/10.1016/S0736-0266(01)00016-X. [DOI] [PubMed] [Google Scholar]
- 53.Alfredson H, Forsgren S, Thorsen K, Fahlstrom M, Johansson H, Lorentzon R. Glutamate NMDAR1 receptors localised to nerves in human Achilles tendons. Implications for treatment? Knee Surg Sports Traumatol Arthrosc. 2001;9:123–126. doi: 10.1007/s001670000188. http://dx.doi.org/10.1007/s001670000188. [DOI] [PubMed] [Google Scholar]
- 54.Alfredson H, Lorentzon R. Intratendinous glutamate levels and eccentric training in chronic Achilles tendinosis: a prospective study using microdialysis technique. Knee Surg Sports Traumatol Arthrosc. 2003;11:196–199. doi: 10.1007/s00167-003-0360-0. No doi match found. [DOI] [PubMed] [Google Scholar]
- 55.Hart DA, Reno C. Pregnancy alters the in vitro responsiveness of the rabbit medial collateral ligament to neuropeptides: effect on mRNA levels for growth factors, cytokines, iNOS, COX-2, metalloproteinases and TIMPs. Biochim Biophys Acta. 1998;1408:35–43. doi: 10.1016/s0925-4439(98)00051-9. No doi match found. [DOI] [PubMed] [Google Scholar]
- 56.Hart D, Frank CB, Kydd A, Iveie T, Sciore P, Reno C. Neurogenic, mast cell and gender variables in tendon biology: potential role in chronic tendinopathy. In: Maffulli N, Renstrom P, Leadbetter W, editors. Tendon Injuries: Basic Science and Clinical Medicine. London: Springer; 2005. pp. 0–48. Reference not parsed. [Google Scholar]
- 57.Scott A, Lian O, Hart D, Bahr R, Duronio V, Khan K. Elevated mast cell numbers in human patellar tendinosis: correlation with symptom duration and vascular hyperplasia. Br J Sports Med. doi: 10.1136/bjsm.2007.040212. (In Press) No doi match found. [DOI] [PubMed] [Google Scholar]
- 58.Scott A, Khan KM, Roberts CR, Cook JL, Duronio V. What do we mean by the term “inflammation”? A contemporary basic science update for sports medicine. Br J Sports Med. 2004;38:372–380. doi: 10.1136/bjsm.2004.011312. http://dx.doi.org/10.1136/bjsm.2004.011312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Alfredson H, Ohberg L. Neovascularisation in chronic painful patellar tendinosis--promising results after sclerosing neovessels outside the tendon challenge the need for surgery. Knee Surg Sports Traumatol Arthrosc. 2005;13:74–80. doi: 10.1007/s00167-004-0549-x. http://dx.doi.org/10.1007/s00167-004-0549-x. [DOI] [PubMed] [Google Scholar]
- 60.Alfredson H, Ohberg L. Sclerosing injections to areas of neo-vascularisation reduce pain in chronic Achilles tendinopathy: a double-blind randomised controlled trial. Knee Surg Sports Traumatol Arthrosc. 2005;13:338–344. doi: 10.1007/s00167-004-0585-6. http://dx.doi.org/10.1007/s00167-004-0585-6. [DOI] [PubMed] [Google Scholar]
- 61.Gisslen K, Ohberg L, Alfredson H. Is the chronic painful tendinosis tendon a strong tendon? A case study involving an Olympic weightlifter with chronic painful jumper’s knee. Knee Surg Sports Traumatol Arthrosc. 2006 doi: 10.1007/s00167-006-0054-5. No doi match found. [DOI] [PubMed] [Google Scholar]
- 62.Forsgren S, Danielson P, Alfredson H. Vascular NK-1 receptor occurrence in normal and chronic painful Achilles and patellar tendons: studies on chemically unfixed as well as fixed specimens. Regul Pept. 2005;126:173–181. doi: 10.1016/j.regpep.2004.09.008. http://dx.doi.org/10.1016/j.regpep.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 63.Molloy TJ, Kemp MW, Wang Y, Murrell GA. Microarray analysis of the tendinopathic rat supraspinatus tendon: glutamate signaling and its potential role in tendon degeneration. J Appl Physiol. 2006;101:1702–1709. doi: 10.1152/japplphysiol.00386.2006. http://dx.doi.org/10.1152/japplphysiol.00386.2006. [DOI] [PubMed] [Google Scholar]