Abstract
Introduction
Vertebral fractures are an under-recognized complication of childhood glucocorticoid-treated illnesses. Our goal was to study the relationship among glucocorticoid exposure, lumbar spine areal BMD (LS BMD) and vertebral shape in glucocorticoid-treated children with new-onset nephrotic syndrome.
Methods
Lateral thoracolumbar spine radiography and lumbar spine bone mineral density (LS BMD) were performed in 80 children with nephrotic syndrome (median age 4.4 years; 46 boys) within the first 37 days of glucocorticoid therapy. Genant semi-quantitative grading was used as the primary method for vertebral morphometry; the Algorithm-Based Qualitative (ABQ) method was used for secondary vertebral deformity analysis.
Results
Six of the 78 children with usable radiographs (8%; 95% confidence interval 4 to 16%) manifested a single Genant Grade 1 deformity each. All deformities were mild anterior wedging (2 at each of T6, T7 and T8). Four of the 78 children (5%; 95% confidence interval 2 to 13%) showed one ABQ sign of fracture each (loss of endplate parallelism; 2 children at T6 and 2 at T8). Two of the children with ABQ signs also had a Genant Grade 1 deformity in the same vertebral body. None of the children with a Genant or ABQ deformity reported back pain. An inverse relationship was identified between LS BMD Z-score and glucocorticoid exposure.
Conclusions
Although we identified an inverse relationship between steroid exposure and LS BMD soon after glucocorticoid initiation for childhood nephrotic syndrome, there was only a low rate of vertebral deformities. The clinical significance of these findings requires further study.
Keywords: children, nephrotic syndrome, glucocorticoids, bone mineral density, vertebral deformities
Introduction
Childhood nephrotic syndrome (NS) is characterized by proteinuria, edema and hyperlipidemia. The annual incidence of NS varies from 1:15,000 to 1:50,000 [1]. Children with their first episode of idiopathic NS are treated with a widely accepted high-dose glucocorticoid (GC) regimen for 4 to 6 weeks (typically prednisone 60 mg/m2/day) followed by a reduced but still supra-physiological dose over a similar time interval (prednisone 40 mg/m2 every other day) [2]. With this initial regimen, one-third of patients will enter into permanent remission; another third will require re-initiation of GC therapy for up to six weeks’ duration at infrequent intervals, and the final third will either require frequent courses of pulse GC therapy or chronic daily immunosuppressive therapy [3].
GCs are known for their adverse effects on skeletal health, highlighted in a large, epidemiological study describing increased extremity fracture rates among children treated with GCs for a variety of underlying conditions [4]. Studies in adults treated with GCs for systemic disorders suggest that GC therapy impairs trabecular bone metabolism [5, 6], with estimates that up to 50% of adults receiving GCs for more than 1 year will develop osteoporotic (including vertebral) fractures [7, 8]. In adults with systemic disorders, vertebral fractures can occur early in the course of GC treatment, attributed to the rapid loss of bone mass in the first few months of therapy [6].
Similarly, reductions in lumbar spine areal bone mineral density (LS BMD) have been documented during the initial high-dose GC treatment phase in children with NS [9, 10], and modest deficits in LS bone mineral content have been shown following years of intermittent GC therapy for steroid-sensitive disease [11]. Overt bone fragility manifesting as vertebral fractures has also been reported in children with NS following the initial GC treatment phase in the context of a treatment study [10], and later in the disease course [12]. However, the frequency and characteristics of vertebral deformities in pediatric NS as well as their relationship to clinical factors has not been well studied. Therefore, we sought to determine the prevalence and characteristics of vertebral deformities around the time of GC initiation in an inception cohort of children with GC-treated NS. Other goals of the study were to describe the relationship between vertebral deformities and relevant clinical indices such as LS BMD, back pain, GC exposure and calcium and vitamin D intake.
Subjects and Methods
Patients and Study Design
Patients were recruited through the Canadian STeroid-associated Osteoporosis in the Pediatric Population (STOPP) research program, a national research initiative that studies bone morbidity in children with steroid-treated illnesses. The decision to initiate GC therapy was made clinically prior to consideration for study enrolment.
Patients from 1 month to 17 years of age were enrolled between January 1 2005 and December 31 2007 in 10 participating tertiary care children’s hospitals. All patients were targeted for evaluation within the first month of GC initiation. Children were included in the study if they met the clinical criteria for NS, including edema, proteinuria >960 mg/m2/day or urine protein/creatinine >0.2 g/mmol, and serum albumin <25 g/L. Idiopathic NS was diagnosed either clinically (following an appropriate response to GC treatment in the first month of therapy, presumed minimal change disease), or confirmed by renal biopsy (biopsy-confirmed minimal change disease). NS due to focal segmental glomerulosclerosis and membranoproliferative glomerulonephritis was also confirmed on renal biopsy. The diagnosis of Henoch-Schoenlein Purpura was made on clinical grounds.
Children were excluded if GCs had previously been used at any time for treatment of the underlying disease. Patients were also excluded if they had received intravenous or oral GCs for more than 14 consecutive days in the 12 months preceding study enrolment to treat any other medical condition (e.g. asthma), if they had received prior medication for osteoporosis or if they had received calcium or vitamin D supplementation that exceeded the Dietary Reference Intake for age [13].
The study was approved by the Research Ethics Board in each participating institution and informed consent/assent was obtained prior to study enrolment, as appropriate.
Clinical Evaluation
Demographic and anthropometric data were recorded. Raw values for height, weight and body mass index (BMI; weight (kg) divided by height squared (meters2)) were transformed into age- and gender-matched Z-scores according to the United States Center for Disease Control National Center for Health Statistics normative database [14] except for children under 2 years of age, for whom BMI Z-scores were calculated according to the World Health Organization child growth standards [15]. Pubertal staging was carried out according to the methods of Marshall and Tanner [16, 17]. The presence or absence of reported back pain in the 3 months preceding enrolment was recorded.
Calcium and vitamin D intake were assessed by a validated food frequency questionnaire [18]. Intake for each nutrient was expressed as the percent of the Adequate Intake value based on the nutrient’s Dietary Reference Intake [13]. Calcium and vitamin D intake by supplementation was added to the dietary intake to arrive at a total daily intake for both nutrients. For descriptive purposes the percentage of adequate intake scores were then classified as <50% of the age-related Dietary Reference Intake, ≥50 and <100% of the Dietary Reference Intake, or ≥100% of the Dietary Reference Intake. Calcium and vitamin D supplementation (yes/no) variables were chosen as clinically relevant covariates to include in the regression modeling. Physical Activity was assessed according to the Habitual Activity Estimation Scale as previously described [19]. For descriptive purposes, the number of very active weekend hours was compared to Canada’s recommended guidelines for daily physical activity for children [20]. Tertiles of very active weekend hours were included in the regression models.
Glucocorticoid (GC) Exposure
The dose of systemic GC therapy (oral and intravenous) was converted into prednisone equivalents and results were expressed in three ways, as previously described [21–23]: 1) cumulative GC Dose, defined as the amount of GC in prednisone equivalents (mg/m2) received during a given observation period; 2) GC dose intensity, defined as the cumulative dose in prednisone equivalents (mg/m2), divided by the number of days actually taking GCs; and 3) average GC dose, defined as the cumulative dose in prednisone equivalents (mg/m2) divided by the total number of days during the observation period.
Radiological Assessment
BMD was measured in the anterior-posterior direction at the LS (L1-L4) by dual-energy x-ray absorptiometry using either Hologic machines (QDR 4500, 3 centers; Discovery, 2 centers; Delphi, 1 center) or Lunar Prodigy (4 centers). Machines were cross-calibrated using a Hologic spine phantom (serial number 2603). The phantom was scanned 10 times on each machine without re-positioning and the mean value was used to derive cross-calibration factors. Data were converted to Hologic units and Z-scores were generated using the Hologic 12.4 normative database.
Bone age and second metacarpal morphometry on a left hand radiograph were also carried out as described in a previous publication by the Canadian STOPP Consortium [19].
Vertebral deformity assessment was carried out independently by two radiologists (NS, MM) from T4 to L4 [19, 24]. Discrepancies between the first two readers were resolved by a third expert radiologist (BL) who was blinded to the results of the other two, as previously described [19].
The Genant semi-quantitative method for vertebral morphometry, the primary spine film assessment method for this study, was performed in the following manner. Vertebral bodies were first assigned a severity score: grade 0 (normal), grade 1 (mild), grade 2 (moderate) or grade 3 (severe). The morphometric grading corresponded to the extent of the reduction in height ratios when the anterior vertebral height was compared to the posterior height (wedge deformity), the middle height to the posterior height (biconcave deformity), and the posterior height to the posterior height of the adjacent vertebral bodies (crush deformity). The scores corresponded to the following reduction in height ratios: Grade 0: 20% or less; Grade 1: > 20 to 25%; Grade 2: > 25 to 40%; Grade 3: >40%. Grade 0 was considered to be normal while higher grades were considered a deformity. Minimal physiological rounding of vertebral bodies in the mid-thoracic region of the spine, as can be seen in normal children, was assigned a grade 0 score [25].
As a secondary and exploratory assessment of vertebral deformities, lateral spine radiographs were also reviewed for radiological signs of fracture according to the Algorithm-Based Qualitative (ABQ) method [26], including loss of endplate parallelism, endplate depression and anterior cortical buckling.
Statistical Analyses
All analyses were conducted using SPSS 16.0 (SPSS Inc., Chicago IL). Categorical variables were summarized using frequency and percentage. Normally distributed continuous variables were summarized using mean and standard deviation. Non-normally distributed continuous variables were summarized using median and minimum, maximum. The 95% Confidence Intervals (CI) for the proportion of patients with vertebral deformities were calculated using the Wilson score method [27]. Z-score variables were compared to the healthy average (Z-score = 0) using a one-sample student’s t-test to assess whether the patient population significantly differed from the normal reference values. In addition, children with vertebral deformities were compared to those without using Wilcoxon Mann-Whitney and Fisher exact tests. Presented p-values are two-sided. A p-value ≤0.05 was considered significant.
Scatterplots were generated in order to visually explore relationships between clinical covariates and LS BMD Z-score, and to identify potential outliers. Multiple linear regressions were carried out to identify clinical parameters associated with LS BMD Z-score. Height and weight Z-scores were included in all linear regression models to adjust for bone size. GC exposure (in prednisone equivalents) was also included in the models since this was the clinical parameter of primary interest. The following covariates (in addition to the three mentioned above) were chosen a priori based on clinical relevance: age, gender, physical activity (very active weekend hours divided into tertiles), vitamin D and calcium supplementation and underlying NS diagnosis (minimal change disease versus other diagnoses).
Results
Patient Characteristics
Eighty children (46 boys, 58%) were enrolled in the study at a mean of 18.6 days (range: 0 to 37 days) following GC initiation (Table 1). Fifty-four percent of the children were White; the remainder was Aboriginal (10%), South Asian (10%), Black (1%) and Mixed or Other Ethnicity (25%). Height Z-scores were significantly above the normal average (p=0.028), as were weight (p<0.001) and BMI (p<0.001) Z-scores.
Table 1.
Description of an Inception Cohort of Children Recently Initiating Glucocorticoids for the Treatment of Nephrotic Syndrome
| Clinical Characteristics (n=80) | Results |
|---|---|
| Demographic Data | |
| Chronological age, median (min, max) in years | 4.4 (1.3, 16.9) |
| Male, n (%) | 46 (58) |
| Anthropometry and Pubertal Staging | |
| Height Z-score, mean (SD) | 0.24 (0.96)* |
| Weight Z-score, mean (SD) | 0.68 (1.06) * |
| BMI Z-score, mean (SD) | 0.81 (1.15) * |
| Pubertal stage, n (%) | |
| Stage 1 | 67 (84) |
| Stage 2–5 | 13 (16) |
| Bone age (n=77), median (min, max) in years | 4.5 (1.8, 17.5) |
| Chronological age to bone age difference, years, mean (SD) | 0.05 (0.73) |
| Diagnosis, n (%) | |
| Idiopathic NS without renal biopsy histology, presumed minimal change disease | 40 (50) |
| Minimal change disease, confirmed on renal biopsy | 20 (25) |
| Focal segmental glomerulosclerosis | 13 (16) |
| Nephrotic Syndrome due to Henoch-Schoenlein Purpura | 6 (8) |
| Other, Membranoproliferative glomerulonephritis Type 1 | 1 (1) |
| Glucocorticoid Treatment # | |
| Number of days between GC initiation and LS BMD, mean (SD) | 18.6 (9.9) |
| Cumulative GC dose (mg/m2) between GC initiation and LS BMD, median (min, max) | 974 (0, 3909) |
| GC dose intensity (mg/m2/day) between GC initiation and LS BMD, median (min, max) | 57 (0, 458) |
| Average GC dose (mg/m2/day) between GC initiation and LS BMD, median (min, max) | 55 (0, 458) |
| Physical Activity Level (n=74) | |
| Very Active Weekend Hours, n (%) of patients in each category& | |
| < 30 minutes | 23 (31) |
| >=30 minutes | 51 (69) |
| Total Vitamin D (n=78) and Calcium Intake (n=77) | |
| Total vitamin D daily intake (% of DRI), n (%) of patients in each category | |
| < 50 % | 19 (24) |
| 50 – <100 % | 19 (24) |
| >= 100% | 40 (52) |
| Vitamin D supplementation – Yes, n (%) | 26 (33) |
| Total calcium daily intake (% of DRI), n (%) of patients in each category | |
| < 50 % | 2 (3) |
| 50 – <100 % | 5 (6) |
| >= 100% | 70 (91) |
| Calcium supplementation – Yes, n (%) | 19 (24) |
| Second Metacarpal Morphometry, mean (SD) | |
| Metacarpal length Z-score (n=65) | 0.44 (0.92) * |
| Combined cortical thickness Z-score (n=65) | 0.30 (0.84) * |
| Percent cortical area Z-score (n=76) | 0.28 (0.81) * |
| Lumbar Spine (LS) BMD, mean (SD) | |
| LS BMD Z-score for chronological age | −0.54 (1.08)* |
| LS BMD Z-score for bone age (n=77) | −0.58 (1.07)* |
SD=Standard deviation, BMI=Body Mass Index, LS BMD= Lumbar Spine Bone Mineral Density, GC=Glucocorticoids, MCD=Minimal Change Disease
Z-score results significantly different compared to the healthy average
GC dose is reported in prednisone equivalents
30 minutes is the amount of vigorous activity per day recommended in Canada’s Physical Activity Guide for Children
Vertebral Deformity and Second Metacarpal Morphometry
Six of the 78 children with spine radiographs (8%; 95% CI 4 to 16%) manifested a single, Genant Grade 1 deformity each. All of these deformities were mild anterior wedging in the mid-thoracic region (2 each at T6, T7 and T8). Exploratory analyses of ABQ vertebral deformity signs found that 4/78 children (5%; 95% CI 2 to 13%) manifested ABQ signs of vertebral fracture (1 radiological sign each due to loss of endplate parallelism in all cases; 2 at T6 and 2 at T8), with the two children having a Genant Grade 1 deformity at T8 also manifesting an ABQ sign of fracture in the same vertebral body. A total of eight children (10%; 95% CI 5 to 19%) showed evidence of vertebral deformity by one or both methods; none of these reported back pain. Examples representative of the vertebral deformities detected in this cohort are presented in Figure 1 (panels A to C). Table 2 describes children with vertebral deformities and those without, as defined by the Genant method (our primary method for characterization of vertebral deformity).
Fig. 1.
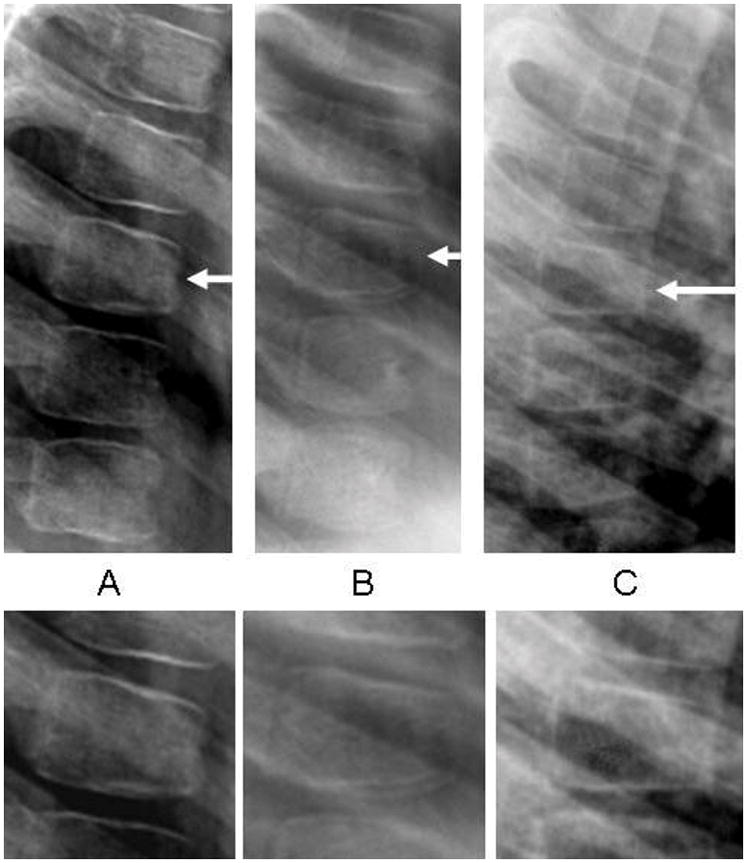
A-C Vertebral deformities that were representative of the spine changes observed in this cohort. A, A 5-year old girl with membranoproliferative glomerulonephritis and a Grade 1 anterior wedge deformity at T7; B, A 4-year old girl with minimal change NS and a Grade 1 anterior wedge deformity with loss of endplate parallelism at T8; C, An 8-year old boy with minimal change NS and a Grade 1 anterior wedge deformity at T6
Table 2.
Comparison of Children With and Without Vertebral Deformity According to the Genant Semi-Quantitative Method
| Clinical Characteristics | Children Without Vertebral Deformity n = 72 |
Children With Vertebral Deformity n = 6 |
p |
|---|---|---|---|
| Demographic Data | |||
| Chronological age, median (min, max) in years | 4.4 (1.3, 16.9) | 6.0 (3.2, 9.2) | 0.574 |
| Male, n (%) | 44 (61) | 1 (17) | 0.078 |
| Diagnosis, n (%) | |||
| Minimal Change NS | 53 (74) | 5 (83) | 1.000 |
| Glucocorticoid Treatment* | |||
| Cumulative GC dose (mg/m2) between GC initiation and LS BMD, median (min, max) | 943 (0, 3909) | 1737 (288, 2052) | 0.129 |
| GC dose intensity (mg/m2/day) between GC initiation and LS BMD, median (min, max) | 57 (0, 458) | 59 (41, 71) | 0.680 |
| Average GC dose (mg/m2/day) between GC initiation and LS BMD, median (min, max) | 55 (0, 458) | 58 (41, 70) | 0.793 |
| Total Vitamin D (n=76) and Calcium Intake (n=75) | |||
| Total vitamin D daily intake (% of DRI), n (%) of patients in each category | |||
| < 50 % | 15 (21) | 4 (67) | |
| 50 – <100 % | 17 (24) | 2 (33) | 0.012 |
| >= 100% | 38 (54) | 0 (0) | |
| Vitamin D Supplementation – Yes, n (%) | 25 (35) | 0 (0) | 0.169 |
| Total calcium daily intake (% of DRI), n (%) of patients in each category | |||
| < 50 % | 1 (2) | 0 (0) | |
| 50 – <100 % | 3 (4) | 2 (33) | 0.070 |
| >= 100% | 65 (94) | 4 (67) | |
| Calcium Supplementation – Yes, n (%) | 17 (24) | 1 (17) | 1.000 |
| Lumbar Spine (LS) BMD | |||
| LS BMD Z-score, mean (SD) | −0.52 (1.1) | −0.58 (1.4) | 0.851 |
SD=Standard deviation, LS BMD= Lumbar Spine Bone Mineral density, GC=Glucocorticoids
GC dose is reported in prednisone equivalents
Metacarpal Morphometry
Analysis of second metacarpal morphometry revealed above average metacarpal length Z-score (0.44, 95% CI 0.21 to 0.66, p<0.001), combined cortical thickness Z-score (0.30, 95% CI 0.09 to 0.51, p=0.005), percent cortical area Z-score (0.28, 95% CI 0.09 to 0.46, p=0.004) (Table 1).
Bone Densitometry
The mean LS BMD Z-score was significantly below the healthy average for the entire cohort (−0.54, 95% CI −0.78 to −0.30, p<0.001). LS BMD Z-scores were similar between patients with and without vertebral deformities (Table 2).
The relationship between LS BMD Z-score and clinical parameters was assessed by multiple regression analysis (Table 3). Cumulative GC dose was inversely related to LS BMD Z-score after adjusting for the clinical covariates chosen a priori as being the most clinically relevant. For every additional gram of cumulative GC dose per body surface area (m2), LS BMD Z-score was lower by 0.37 SD (95% CI, −0.69 to −0.04). Similar relationships were found for GC dose intensity (p=0.007), and average GC dose (p=0.006). One patient was identified as having an extreme outlying value (i.e. value exceeding the third quartile + 3*IQR) for GC dose intensity (458 mg/m2/day) and average GC dose (458 mg/m2/day); therefore, this outlying value was excluded from Models 2 and 3 (but not from Model 1). When the outlying value was included (models not shown), there was no association between GC exposure and LS BMD Z-score for GC Dose Intensity (β −0.003, 95% CI −0.008 to 0.002, p=0.19) or for Average GC Dose (β −0.003, 95% CI −0.008 to 0.001, p=0.17).
Table 3.
LS BMD Z-Score Multiple Linear Regression Analysis
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| Cumulative GC Dose* | GC Dose Intensity# | Average GC Dose& | ||||
|
| ||||||
| Clinical Parameters | β (95% CI) | p | β (95% CI) | p | β (95% CI) | p |
| Height Z-Score | 0.161 (−0.154, 0.476) | 0.311 | 0.240 (−0.076, 0.555) | 0.134 | 0.208 (−0.103, 0.519) | 0.186 |
| Weight Z-Score | 0.323 (0.038, 0.607) | 0.027 | 0.266 (−0.021, 0.554) | 0.069 | 0.283 (−0.002, 0.567) | 0.052 |
| Age | 0.022 (−0.039, 0.083) | 0.472 | 0.015 (−0.047, 0.076) | 0.634 | 0.018 (−0.042, 0.079) | 0.548 |
| Gender (female vs. male) | 0.449 (−0.016, 0.914) | 0.058 | 0.458 (−0.001, 0.918) | 0.051 | 0.434 (−0.023, 0.890) | 0.062 |
| Physical Activity 1 | 0.360 (−0.131, 0.851) | 0.148 | 0.363 (−0.126, 0.851) | 0.143 | 0.386 (−0.102, 0.874) | 0.119 |
| Vitamin D Supplementation | −0.058 (−0.747, 0.632) | 0.867 | −0.064 (−0.752, 0.624) | 0.853 | −0.091 (−0.778, 0.595) | 0.791 |
| Calcium Supplementation | 0.188 (−0.559, 0.935) | 0.616 | 0.188 (−0.546, 0.922) | 0.610 | 0.235 (−0.491, 0.962) | 0.519 |
| MCD vs. Other Diagnosis | 0.065 (−0.479, 0.610) | 0.812 | 0.049 (−0.489, 0.588) | 0.856 | 0.037 (−0.501, 0.575) | 0.891 |
| GC Exposure | −0.367 (−0.692, −0.041) | 0.028 | −0.017 (−0.029, −0.005) | 0.007 | −0.016 (−0.028, −0.005) | 0.006 |
| Model R2 | 0.303 | 0.332 | 0.336 | |||
Note: One patient identified as having an extreme outlier value for GC dose Intensity (458 mg/m2/day) and Average GC dose (458 mg/m2/day) was excluded from Models 2 and 3, as described in the results.
As measured by The Habitual Activity Estimation Scale. Highest Tertile of Very Active Weekend Hours versus Tertiles 1 and 2.
Total amount of GC received during the observation period relative to body surface area (g/m2)
Ratio between the total amount of GC received during the observation period and the number of days when GC treatment was received. This ratio was expressed relative to body surface area
Ratio between the total amount of GC received during the observation period and the number of days since the first GC was taken. This ratio was expressed relative to body surface area
Discussion
One of the key findings in this study was the observed inverse relationship between short-term GC exposure and LS spine BMD among children who recently initiated GCs for the treatment of NS. This relationship was consistent for all three methods used to quantify GC exposure, including cumulative GC dose, GC dose intensity and average GC dose. The possibility that even short-term, high-dose GC therapy can have a deleterious effect on spine BMD in pediatric NS is not inconceivable, given a number of supporting observations in the literature. First, it is well-known that GCs have a predilection for interference with trabecular bone architecture, both in humans [6] and in animal models [5]. Furthermore, bone resorption markers increase acutely in adults with NS following administration of GCs [28], and are associated with a significant decline in spine BMD after a few weeks of therapy in both adults [28] and children with NS [9]. In addition, spine BMD has been shown to decline rapidly in adults following GC initiation for organ transplantation [29]. These reports and our study attest to the potential for insult to spine BMD after short-term GC use, with the effects of longer-term administration highlighted in a large cross-sectional case-control study which showed after 4 years of GC exposure that spine bone mineral content in children with steroid-sensitive NS was reduced compared to controls [11].
In a recent study using peripheral quantitative computed tomography (pQCT) at the tibia, GC-treated children with NS showed greater cortical volumetric BMD and cortical area, and lower trabecular volumetric BMD compared to controls [30]. Similar results were reported by Hegarty et al, who found by pQCT a significant reduction in distal radial trabecular volumetric BMD, but no reduction in total volumetric BMD in young adults who had NS during childhood [31]. We did not use pQCT to assess the effects GCs on the trabecular and cortical compartments separately. However, we did observe a decrease in BMD at the spine (a trabecular-rich site) but increased cortical thickness at the second metacarpal. Our results are therefore in line with previous reports [30, 31] suggesting disparate effects of GCs on cortical and trabecular sites.
At the same time, we found the prevalence of vertebral deformities early in the course of GC treatment to be low, with the vast majority of patients in this cohort treated according to the standardized international protocol (prednisone 60 mg/m2/day for 4 to 6 weeks). Specifically, we have demonstrated a vertebral deformity prevalence rate of 8% (95% CI 4 to 16%) according to the Genant protocol, our primary vertebral assessment method. Indeed, we would not expect a high rate of vertebral deformity early in the course of GC exposure, since the path to fractured bone begins with alterations in bone architecture and density, with loss in bone strength observed following acute declines in LS BMD [6, 29].
The clinical significance of the observed vertebral deformities remains unclear and merits discussion, particularly since this is an inaugural study in its assessment of vertebral body height ratios early in the course of pediatric GC-treated NS. First of all, it is possible that these changes represent normal variants, especially given that none of the children with vertebral deformities reported back pain. At the same time, the lack of back pain does not negate the possibility that these deformities represent fractured bone, since vertebral fractures have been described without back pain in post-menopausal osteoporosis [32], in children with long-standing histories of rheumatic conditions [33], and in childhood acute lymphoblastic leukemia [19]. Furthermore, a recent pediatric study by Gaca et al. [34] showed 95% of healthy children had anterior wedging (the same deformity reported in our cohort) at the thoracolumbar junction that was represented by less than an 11% reduction in the anterior to posterior height ratio. The authors suggested that reductions in excess of 11% should raise the suspicion of vertebral injury. In our study, a reduction in height ratio of 20% or more was considered a vertebral deformity. At the same time, we note that the study by Gaca et al. [34] was carried out using a different imaging approach (computed tomography) and from T10 to L3 exclusively. Whether a normal cut-off around 11% would apply to the mid-thoracic region, the site of vertebral deformities in our cohort and also the site where minimal physiological rounding of vertebral bodies is frequently seen in children [35], remains to be determined. To date, there are no available normative data for vertebral morphometry by lateral radiograph among healthy children, a fact which renders interpretation of the clinical significance of our vertebral findings difficult.
Interestingly, the mid-thoracic region (T6 to T8) is the most frequent site for both mild and more severe vertebral fractures in adults [36–39], as well as in children with acute lymphoblastic leukemia [19] and rheumatic conditions [40]. This distribution is suggested to result from the relatively increased mechanical stresses on vertebrae at these sites imposed by the shape of the spine [41]. Furthermore, studies in adults have shown that Grade 1 deformities are clinically important, since they are associated with an increased risk for future (incident) vertebral fracture [42]. Whether the Grade 1 deformities in our report will be associated with increased risk for incident vertebral fractures in the face of ongoing bone health threats remains to be determined through further longitudinal assessment of our cohort.
We sought to explore the clinical significance of these findings by describing children with vertebral deformities compared to those without; however, the small number of children with vertebral deformities limited our power to detect differences. While the lack of statistical association between LS BMD, a key clinical parameter, and vertebral deformities could be a function of limited power, it is also possible that the time course following GC initiation was simply too short for vertebral fractures to manifest clinically, as previously mentioned. On the other hand, it should be noted that an absence of association between spine BMD and vertebral fractures has also been reported in post-menopausal women with GC-treated rheumatic disorders, where those with vertebral fractures had similar LS BMD results compared to those without [43]. The absence of a relationship between spine BMD and vertebral fractures among GC-treated patients has been postulated to result from alterations in bone mass or architecture that are not readily discernable by BMD testing in the anterior-posterior direction compared to the more sensitive width-adjusted approach which removes the dense posterior spinous processes from the projected scan [30, 44].
Limitations to our study merit consideration. While our overall research program is predicated upon within-subject change during longitudinal follow-up in key parameters such as vertebral morphometry and spine BMD, the description of this inception cohort at the time of study enrolment is based on uncontrolled, cross-sectional evaluation of spine status in relation to relevant clinical parameters. Another limitation is that to optimally assess the impact of GCs on skeletal health in the short-term, it would have been ideal to obtain the first study visit prior to GC initiation, as opposed to shortly thereafter. For logistical reasons this was not possible, as NS treatment would then have been delayed. In addition, we were unable to measure 25-hydroxyvitamin D levels in this study; beyond vitamin D intake (which we found to be frequently reduced in children with spine deformities compared to those without), the impact of circulating levels of 25-hydroxyvitamin D on bone strength in steroid-treated children with NS deserves further study. Finally, while this is the first study to assess vertebral morphometry in pediatric GC-treated NS, with only 6 patients harbouring deformities it is possible that some of the clinical variables were in fact related to the vertebral deformities but that these relationships went undetected due to insufficient power. Nevertheless, this study provides a novel description of vertebral morphometry in GC-treated NS and the basis for further longitudinal comparison.
In conclusion, we observed an inverse relationship between GC exposure and LS BMD Z-score in children following short-term GC treatment for NS and a low rate of vertebral deformity at this time-point. Additional studies are required before more definitive conclusions can be drawn about the clinical significance of the observed vertebral deformities and the impact of short-term steroids on spine health in children with NS. Further light will be shed through documentation of the clinical outcomes in these children with early deformities and through assessment of the incident vertebral deformity rate in the face of further GC exposure.
Acknowledgments
This study was primarily funded by an operating grant from the Canadian Institutes for Health Research (FRN 64285). Additional funding for this work has been provided by the Canadian Institutes for Health Research New Investigator Program (to Dr. Leanne Ward), the Canadian Child Health Clinician Scientist Career Enhancement Program (to Dr. Leanne Ward), the Children’s Hospital of Eastern Ontario and Women and Children’s Health Research Institute, University of Alberta.
In addition, the Canadian STOPP Consortium would like to thank the following individuals who contributed to the study: The children and their families who participated in the study, making the STOPP research program possible; Research Associates who managed the study at the co-ordinating center (the Children’s Hospital of Eastern Ontario Ottawa, Ontario): Elizabeth Sykes (STOPP Project Manager), Maya Scharke (STOPP Data Analyst and Database Manager), Monica Tomiak (Statistical Analyses), Victor Konji (STOPP Publications and Presentations Committee Liaison and hand morphometry measurements), Steve Anderson (Children’s Hospital of Eastern Ontario Pediatric Bone Health Program Research Manager), Catherine Riddell (STOPP National Study Monitor); Research Associates who took care of the patients from the following institutions: Alberta Children’s Hospital, Calgary, Alberta: Eileen Pyra; British Columbia Children’s Hospital, Vancouver British Columbia: Terry Viczko, Sandy Hwang; Children’s Hospital of Eastern Ontario, Ottawa, Ontario: Heather Cosgrove, Amanda George, Josie MacLennan, Catherine Riddell; Children’s Hospital of Western Ontario, London, Ontario: Leila MacBean, Mala Ramu; McMaster Children’s Hospital, Hamilton, Ontario: Susan Docherty-Skippen; IWK Health Center, Halifax, Nova Scotia: Aleasha Warner; Montréal Children’s Hospital, Montréal, Québec: Diane Laforte, Maritza Laprise, Mayito St-Pierre; Ste. Justine Hospital, Montréal, Québec: Claude Belleville, Stéphanie Pellerin, Natacha Gaulin Marion; Stollery Children’s Hospital, Edmonton, Alberta: Deborah Olmstead, Melissa Gabruck, Linda Manasterski; Toronto Hospital for Sick Children, Toronto, Ontario: Julie Lee, Karen Whitney; Winnipeg Children’s Hospital, Winnipeg, Manitoba: Dan Catte, Erika Bloomfield. The Research Nurses, Support Staff and all the STOPP collaborators from the various Divisions of Nephrology, Oncology, Rheumatology and Radiology who have contributed to the care of the children enrolled in the study.
Abbreviations
- ABQ
Algorithm-Based Qualitative
- BMI
Body Mass Index
- BMD
Bone mineral density
- CI
Confidence Interval
- GC
Glucocorticoid
- LS
Lumbar spine
- NS
Nephrotic Syndrome
The Canadian STeroid-associated Osteoporosis in the Pediatric Population (STOPP) Consortium (a pan-Canadian pediatric bone health working group)
Co-ordinating Center
Children’s Hospital of Eastern Ontario, Ottawa, Ontario: Leanne M. Ward#,*,§ (Study Principal Investigator), Janusz Feber*,§ (Nephrology), Isabelle Gaboury*,§ (Biostatistics, CHEO Clinical Research Unit at the time the research was being conducted), Jacqueline Halton*,§ (Oncology), Mary Ann Matzinger (Radiology, Central Radiograph Analyses), David Moher*,§ (Research Methods, Ottawa Hospital Research Institute), Johannes Roth (Rheumatology), Roman Jurencak (Rheumatology), Nazih Shenouda§ (Radiology, Central Radiograph Analyses)
Participating Centers
Alberta Children’s Hospital, Calgary, Alberta: David Stephure (Site Principal Investigator), Reinhard Kloiber (Radiology), Victor Lewis (Oncology), Julian Midgley (Nephrology), Paivi Miettunen (Rheumatology)
British Columbia Children’s Hospital, Vancouver, British Columbia: David Cabral* (Site Principal Investigator), David B. Dix (Oncology), Kristin Houghton (Rheumatology), Helen R. Nadel (Radiology)
British Columbia Women’s Hospital and Health Sciences Center, Vancouver, British Columbia: Brian C. Lentle§ (Radiology)
Brock University, Faculty of Applied Health Sciences, St. Catharines, Ontario: John Hay§ (Physical Activity Measurements)
Children’s Hospital of Western Ontario, London, Ontario: Cheril Clarson and Robert Stein (Site Principal Investigators), Elizabeth Cairney (Oncology), Guido Filler (Nephrology), Joanne Grimmer (Nephrology), Keith Sparrow (Radiology)
IWK Health Center, Halifax, Nova Scotia: Elizabeth Cummings (Site Principal Investigator), Conrad Fernandez (Oncology), Adam M. Huber§ (Rheumatology), Bianca Lang*,§ (Rheumatology), Kathy O’Brien (Radiology), Andrew Ross (Radiology)
McMaster Children’s Hospital, Hamilton, Ontario: Stephanie Atkinson*,§ (Site Principal Investigator), Steve Arora (Nephrology), Ronald Barr§ (Oncology), Craig Coblentz (Radiology), Peter B. Dent (Rheumatology), Maggie Larche (Rheumatology), Colin Webber* (DXA Methodology),
Montréal Children’s Hospital, Montréal, Québec: Celia Rodd§ (Site Principal Investigator), Sharon Abish (Oncology), Lorraine Bell (Nephrology), Rosie Scuccimarri (Rheumatology)
Shriners Hospital for Children, Montréal, Québec: Frank Rauch*,§ (Co-Chair, Publications and Presentations Committee), Francis Glorieux* (Chair, Ancillary Studies Committee)
Ste. Justine Hospital, Montréal, Québec: Nathalie Alos* (Site Principal Investigator), Josée Dubois (Radiology), Caroline Laverdière (Oncology), Véronique Phan (Nephrology), Claire Saint- Cyr (Rheumatology)
Stollery Children’s Hospital, Edmonton, Alberta: Robert Couch* (Site Principal Investigator), Janet Ellsworth (Rheumatology), Claire LeBlanc (Rheumatology), Maury Pinsk (Nephrology), Kerry Siminoski§ (Radiology), Beverly Wilson (Oncology)
Toronto Hospital for Sick Children, Toronto, Ontario: Ronald Grant* (Site Principal Investigator), Martin Charron (Radiology), Diane Hebert (Nephrology)
Winnipeg Children’s Hospital, Winnipeg, Manitoba: Shayne Taback§ (Site Principal Investigator), Tom Blydt-Hansen (Nephrology), Sara Israels (Oncology), Kiem Oen (Rheumatology), Martin Reed (Radiology)
Footnotes
Principal Investigator;
Executive Committee Member;
Publications and Presentations Committee Member
Conflicts of Interest
None of the authors has a conflict of interest
References
- 1.Schlesinger ER, Sultz HA, Mosher WE, Feldman JG. The nephrotic syndrome. Its incidence and implications for the community. Am J Dis Child. 1968;116:623–632. [PubMed] [Google Scholar]
- 2.Hodson EM, Knight JF, Willis NS, Craig JC. Corticosteroid therapy for nephrotic syndrome in children. Cochrane Database Syst Rev. 2001;2 doi: 10.1002/14651858.CD001533. [DOI] [PubMed] [Google Scholar]
- 3.Clark AG, Barratt TM. Steroid-responsive nephrotic syndrome. In: Barratt TM, Avner ED, Harmon WE, editors. Pediatric Nephrology. 4. 1999. pp. 731–747. [Google Scholar]
- 4.van Staa TP, Cooper C, Leufkens HG, Bishop N. Children and the risk of fractures caused by oral corticosteroids. J Bone Miner Res. 2003;18:913–918. doi: 10.1359/jbmr.2003.18.5.913. [DOI] [PubMed] [Google Scholar]
- 5.Dalle Carbonare L, Arlot ME, Chavassieux PM, Roux JP, Portero NR, Meunier PJ. Comparison of trabecular bone microarchitecture and remodeling in glucocorticoid-induced and postmenopausal osteoporosis. J Bone Miner Res. 2001;16:97–103. doi: 10.1359/jbmr.2001.16.1.97. [DOI] [PubMed] [Google Scholar]
- 6.Canalis E, Mazziotti G, Giustina A, Bilezikian JP. Glucocorticoid-induced osteoporosis: pathophysiology and therapy. Osteoporos Int. 2007;18:1319–1328. doi: 10.1007/s00198-007-0394-0. [DOI] [PubMed] [Google Scholar]
- 7.Lukert BP. Glucocorticoid-induced osteoporosis. South Med J. 1992;85:2S48–51. doi: 10.1097/00007611-199208001-00009. [DOI] [PubMed] [Google Scholar]
- 8.Ruegsegger P, Medici TC, Anliker M. Corticosteroid-induced bone loss. A longitudinal study of alternate day therapy in patients with bronchial asthma using quantitative computed tomography. Eur J Clin Pharmacol. 1983;25:615–620. doi: 10.1007/BF00542348. [DOI] [PubMed] [Google Scholar]
- 9.Bak M, Serdaroglu E, Guclu R. Prophylactic calcium and vitamin D treatments in steroid-treated children with nephrotic syndrome. Pediatr Nephrol. 2006;21:350–354. doi: 10.1007/s00467-005-2118-z. [DOI] [PubMed] [Google Scholar]
- 10.Acott PD, Wong JA, Lang BA, Crocker JF. Pamidronate treatment of pediatric fracture patients on chronic steroid therapy. Pediatr Nephrol. 2005;20:368–373. doi: 10.1007/s00467-004-1790-8. [DOI] [PubMed] [Google Scholar]
- 11.Leonard MB, Feldman HI, Shults J, Zemel BS, Foster BJ, Stallings VA. Long-term, high-dose glucocorticoids and bone mineral content in childhood glucocorticoid-sensitive nephrotic syndrome. N Engl J Med. 2004;351:868–875. doi: 10.1056/NEJMoa040367. [DOI] [PubMed] [Google Scholar]
- 12.Sbrocchi AM, Rauch F, Matzinger M, Feber J, Ward LM. Vertebral fractures despite normal spine bone mineral density in a boy with nephrotic syndrome. Pediatr Nephrol. 2010;26:139–142. doi: 10.1007/s00467-010-1652-5. [DOI] [PubMed] [Google Scholar]
- 13.Institute of Medicine. Dietary reference intakes for calcium, phosphorus, magnesium, Vitamin D, fluoride. Washington, DC: National Academy Press; 1997. [PubMed] [Google Scholar]
- 14.Ogden CL, Kuczmarski RJ, Flegal KM, Mei Z, Guo S, Wei R, Grummer-Strawn LM, Curtin LR, Roche AF, Johnson CL. Centers for Disease Control and Prevention 2000 growth charts for the United States: improvements to the 1977 National Center for Health Statistics version. Pediatrics. 2002;109:45–60. doi: 10.1542/peds.109.1.45. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization Multicentre Growth Reference Study Group. WHO Child Growth Standards: Length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: Methods and development. World Health Organization; Geneva: 2006. pp. 229–300. [Google Scholar]
- 16.Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child. 1969;44:291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child. 1970;45:13–23. doi: 10.1136/adc.45.239.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Musgrave KO, Giambalvo L, Leclerc HL, Cook RA, Rosen CJ. Validation of a quantitative food frequency questionnaire for rapid assessment of dietary calcium intake. J Am Diet Assoc. 1989;89:1484–1488. [PubMed] [Google Scholar]
- 19.Halton J, Gaboury I, Grant R, et al. Advanced vertebral fracture among newly diagnosed children with acute lymphoblastic leukemia: results of the Canadian Steroid-Associated Osteoporosis in the Pediatric Population (STOPP) research program. J Bone Miner Res. 2009;24:1326–1334. doi: 10.1359/jbmr.090202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Public Health Agency of Canada. [Last accessed March 31, 2010];Canada’s Physical Activity Guide for Children. http://www.phac-aspc.gc.ca/hp-ps/hl-mvs/pag-gap/cy-ej/children-enfants/index-eng.php.
- 21.van Staa TP, Leufkens HG, Cooper C. The epidemiology of corticosteroid-induced osteoporosis: a meta-analysis. Osteoporos Int. 2002;13:777–787. doi: 10.1007/s001980200108. [DOI] [PubMed] [Google Scholar]
- 22.Curtis JR, Westfall AO, Allison J, Bijlsma JW, Freeman A, George V, Kovac SH, Spettell CM, Saag KG. Population-based assessment of adverse events associated with long-term glucocorticoid use. Arthritis Rheum. 2006;55:420–426. doi: 10.1002/art.21984. [DOI] [PubMed] [Google Scholar]
- 23.Dubner SE, Shults J, Baldassano RN, Zemel BS, Thayu M, Burnham JM, Herskovitz RM, Howard KM, Leonard MB. Longitudinal assessment of bone density and structure in an incident cohort of children with Crohn’s disease. Gastroenterology. 2009;136:123–130. doi: 10.1053/j.gastro.2008.09.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Genant HK, Wu CY, van Kuijk C, Nevitt MC. Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res. 1993;8:1137–1148. doi: 10.1002/jbmr.5650080915. [DOI] [PubMed] [Google Scholar]
- 25.Keats TE, Smith TH. Year Book of Medical Publishers. 1977. An Atlas of Normal Developmental Anatomy. [Google Scholar]
- 26.Jiang G, Eastell R, Barrington NA, Ferrar L. Comparison of methods for the visual identification of prevalent vertebral fracture in osteoporosis. Osteoporos Int. 2004;15:887–896. doi: 10.1007/s00198-004-1626-1. [DOI] [PubMed] [Google Scholar]
- 27.Newcombe RG. Two-sided confidence intervals for the single proportion: comparison of seven methods. Statistics in Medicine. 1998;17:857–872. doi: 10.1002/(sici)1097-0258(19980430)17:8<857::aid-sim777>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 28.Fujita T, Satomura A, Hidaka M, Ohsawa I, Endo M, Ohi H. Acute alteration in bone mineral density and biochemical markers for bone metabolism in nephrotic patients receiving high-dose glucocorticoid and one-cycle etidronate therapy. Calcif Tissue Int. 2000;66:195–199. doi: 10.1007/s002230010039. [DOI] [PubMed] [Google Scholar]
- 29.Cohen A, Shane E. Osteoporosis after solid organ and bone marrow transplantation. Osteoporos Int. 2003;14:617–630. doi: 10.1007/s00198-003-1426-z. [DOI] [PubMed] [Google Scholar]
- 30.Wetzsteon RJ, Shults J, Zemel BS, Gupta PU, Burnham JM, Herskovitz RM, Howard KM, Leonard MB. Divergent effects of glucocorticoids on cortical and trabecular compartment bone mineral density in childhood nephrotic syndrome. J Bone Miner Res. 2008;24(3):503–513. doi: 10.1359/JBMR.081101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hegarty J, Mughal MZ, Adams J, Webb NJ. Reduced bone mineral density in adults treated with high-dose corticosteroids for childhood nephrotic syndrome. Kidney Int. 2005;68:2304–2309. doi: 10.1111/j.1523-1755.2005.00690.x. [DOI] [PubMed] [Google Scholar]
- 32.Dennison E, Cooper C. Epidemiology of osteoporotic fractures. Horm Res. 2000;54(Suppl 1):58–63. doi: 10.1159/000063449. [DOI] [PubMed] [Google Scholar]
- 33.Valta H, Lahdenne P, Jalanko H, Aalto K, Makitie O. Bone health and growth in glucocorticoid-treated patients with juvenile idiopathic arthritis. J Rheumatol. 2007;34:831–836. [PubMed] [Google Scholar]
- 34.Gaca AM, Barnhart HX, Bisset GS., 3rd Evaluation of wedging of lower thoracic and upper lumbar vertebral bodies in the pediatric population. AJR Am J Roentgenol. 2010;194:516–520. doi: 10.2214/AJR.09.3065. [DOI] [PubMed] [Google Scholar]
- 35.Ebel KD, Blickman H, Willich E, Richter E. Differential Diagnosis in Pediatric Radiology. Thieme Publishers; New York: 1999. Abnormalities in Vertebral Body Shape and Size. [Google Scholar]
- 36.Rea JA, Chen MB, Li J, Blake GM, Steiger P, Genant HK, Fogelman I. Morphometric X-ray absorptiometry and morphometric radiography of the spine: a comparison of prevalent vertebral deformity identification. J Bone Miner Res. 2000;15:564–574. doi: 10.1359/jbmr.2000.15.3.564. [DOI] [PubMed] [Google Scholar]
- 37.Vallarta-Ast N, Krueger D, Wrase C, Agrawal S, Binkley N. An evaluation of densitometric vertebral fracture assessment in men. Osteoporos Int. 2007;18:1405–1410. doi: 10.1007/s00198-007-0381-5. [DOI] [PubMed] [Google Scholar]
- 38.Wu C, van Kuijk C, Li J, Jiang Y, Chan M, Countryman P, Genant HK. Comparison of digitized images with original radiography for semiquantitative assessment of osteoporotic fractures. Osteoporos Int. 2000;11:25–30. doi: 10.1007/s001980050002. [DOI] [PubMed] [Google Scholar]
- 39.Jackson SA, Tenenhouse A, Robertson L. Vertebral fracture definition from population-based data: preliminary results from the Canadian Multicenter Osteoporosis Study (CaMos) Osteoporos Int. 2000;11:680–687. doi: 10.1007/s001980070066. [DOI] [PubMed] [Google Scholar]
- 40.Huber A, Gaboury I, Cabral DA, et al. Prevalent vertebral fractures among children initiating glucocorticoid therapy for the treatment of rheumatic disorders. Arth Care Res. 2010;62:516–526. doi: 10.1002/acr.20171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ismail AA, Cooper C, Felsenberg D, Varlow J, Kanis JA, Silman AJ, O’Neill TW. Number and type of vertebral deformities: epidemiological characteristics and relation to back pain and height loss. European Vertebral Osteoporosis Study Group. Osteoporos Int. 1999;9:206–213. doi: 10.1007/s001980050138. [DOI] [PubMed] [Google Scholar]
- 42.Delmas PD, Genant HK, Crans GG, Stock JL, Wong M, Siris E, Adachi JD. Severity of prevalent vertebral fractures and the risk of subsequent vertebral and nonvertebral fractures: results from the MORE trial. Bone. 2003;33:522–532. doi: 10.1016/s8756-3282(03)00241-2. [DOI] [PubMed] [Google Scholar]
- 43.Peel NF, Moore DJ, Barrington NA, Bax DE, Eastell R. Risk of vertebral fracture and relationship to bone mineral density in steroid treated rheumatoid arthritis. Ann Rheum Dis. 1995;54:801–806. doi: 10.1136/ard.54.10.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dubner SE, Shults J, Leonard MB, Zemel BS, Sembhi H, Burnham JM. Assessment of spine bone mineral density in juvenile idiopathic arthritis: impact of scan projection. J Clin Densitom. 2008;11:302–308. doi: 10.1016/j.jocd.2007.10.005. [DOI] [PubMed] [Google Scholar]


