Abstract
Problem
Human parturition is associated with an intrauterine pro-inflammatory environment in the choriodecidua. Evidence that some mediators of this signaling cascade also elicit responses leading to labor prompted us to characterize the cellular sources of these mediators in the human choriodecidua.
Method of study
Leukocyte-enriched preparations from human choriodecidua (ChL) and intervillous placental blood leukocytes (PL) were maintained in culture. Secretions of inflammatory cytokines, chemokines and MMP-9 were documented. Leukocyte phenotype of ChL and PL was determined by flow cytometry using specific fluorochrome-conjugated antibodies.
Results and Conclusions
ChL showed a distinct pro-inflammatory secretion pattern of cytokines and chemokines when compared with PL, including higher amounts of TNF-α and IL-6, and decreased secretions of IL-4 and IL-1ra. ChL also secreted more MIP-1α and MCP-1 and MMP-9 than PL. No significant differences were found in leukocytes subsets between compartments. Based on our findings, we propose that ChL isolated from fetal membranes at term are functionally different from PL and may collaborate to modulate the microenvironment linked to induction and progression of human labor.
Keywords: amniochorion, choriodecidua, fetal membranes, inflammation, labor
Introduction
The pathway of parturition is a complex process involving anatomical, biochemical, endocrinological and immunological factors1. Human labor appears as a sequence of events initiated by myometrium contractions, then the cervix ripens, the fetal membranes rupture and the fetus and placenta are expelled2. The mechanisms underlying the onset and progression of normal spontaneous labor remain unclear.
Increasing evidence shows that some components of the inflammatory pathway are involved in normal term labor3-5. The choriodecidual microenvironment during late gestation and during labor experiences functional modifications that include the active secretion of cytokines and chemokines, which results in the recruitment and activation of certain leukocytes subpopulations6-11. Identified components of this network include pro-inflammatory and anti-inflammatory cytokines and chemokines8-10, 12-18
This mediators may act as primary paracrine and autocrine signals, eliciting the local secretion of secondary mediators such as prostaglandins that act as uterotonics19 and matrix metalloproteinases (MMPs) such as 92 kDa type IV collagenase (MMP-9) which in turn is able to degrade the main extracellular matrix components of fetal membranes and promote their rupture20-23.
New and old evidence support that the phenotype of the leukocytes in the choriodecidual microenvironment changes during labor at term and T lymphocytes increase significantly in this site10, 14, 18. The arrival of a specific subset of lymphocytes may be linked to the choriodecidual activation observed at the term of gestation. In this paper, we analyzed the contribution of choriodecidual lymphocytes to the secretion of cytokines, chemokines and MMP-9, comparing the secretions of equivalent lymphocytes isolated from intervillous placenta blood, a nearby compartment.
Materials and methods
Patients and biological samples
Placentae and amniochorion samples were obtained from women at term gestation (38-40 weeks) undergoing indicated cesarean section without active labor and without clinical or microbiological infection determined by culture. Patients were recruited at Hospital Materno Infantil Inguarán, Secretaría de Salud del D.F. in México City, México. Participating women gave their informed consent and the project was accepted by the local IRB (Register No.101-010-08-09). All procedures described below were carried out within the first hour of collection of samples and under sterile conditions.
Isolation and culture of placental leukocytes
Leukocytes were obtained from intervillous placental blood (named placenta leukocytes or PL; n=9) as follow. After the placenta was delivered, intervillous blood was drained out by manually compressing the cotyledons and recovered in sterile tubes containing heparin as anticoagulant (Becton-Dickinson, Franklin Lakes, NJ, USA). PL were isolated by density gradient using Lymphoprep (Axis-Shield, Oslo, NOR). PL were then cultured in RPMI 1640 culture media supplemented with 0.2% lactoalbumin hidrolysate, 1% sodium pyruvate and 1% antibiotic-antimycotic (RPMI/HLA; Gibco BRL, Grand Island, NY, USA). Cell viability was confirmed to be over 95% by staining with trypan blue. Lastly, PL (1×106) were placed in 12-well plates (Corning Costar, NY, USA) with 700μL of RPMI/HLA and incubated for 24, 48 and 72 hours at 37 °C with 95% air/5% CO2.
Isolation and culture of choriodecidual cells. Fetal membranes (n=9) were collected after delivery and immediately washed to eliminate blood clots with saline isotonic solution in sterile conditions. Choriodecidual cells were obtained by gently scraping the chorionic side with a cell scraper (Sarstedt, Nümbrecht, Germany). Choriodecidual cell suspension was washed with phosphate buffered solution ((PBS); 10 mM sodium phosphate, 150 mM sodium chloride, pH 7.2)) (Life Technologies, Carlsbad, CA, USA) and filtered with a MACS pre-separation filter (30 μm) to eliminate tissue fragments (Miltenyi Biotec, Bergisch Gladbach, Germany)18. Choriodecidual cells were separated in Lymphoprep as described above. Gradient interphase including leukocytes was transferred into 25cm2 plastic flasks (Corning Costar, NY, USA) and incubated for 3.0h at 37°C in 95% air/5% CO2. Non-adherent choriodecidual cells, choriodecidual leukocyte-enriched preparation (ChL) hereinafter, (1×106 cells) were placed in 12-well plates (Corning Costar, NY, USA) in RPMI/HLA and incubated for 24, 48 and 72 hours at 37 °C with 95% air/5% CO2. Cell viability was confirmed to be over 95% by trypan blue staining.
Measurement of cytokines and chemokines in cell supernatants
After cell culture, ChL and PL conditioned media were collected and stored at -80°C until use. Samples were analyzed on a MagPix magnetic bead suspension array system (Luminex xMAP, Austin, TX, USA) using the multiplex sandwich immunoassay per manufacturer's protocols. A premixed human cytokine/chemokine magnetic bead assay kit (Milliplex MAG, Millipore, Billerica, MA, USA) was used to determine the concentration of TNFα, IL-6, Il-4, IL-1ra, MIP-1α and MCP-1. Other cytokines/chemokines were excluded using previous assays. All samples were performed in one-plate run modus. The detection limit was set at the lowest standard concentration for each cytokine (pg/ml): TNFα (0.7), IL-6 (0.9), IL4 (4.5), IL-1ra (8.3), MIP-1α (2.9) and MCP-1 (1.9).
Flow cytometry for leukocyte subsets
Leukocytes subsets were characterized in ChL and PL using the BD Multitest IMK kit following the manufacturer's protocol (BD Biosciences, CA, USA; Cat. No. 340504): total leukocytes/CD45+(clone 2D1-HLe-1), NK cells/CD16+ (clone B73.1), CD56+ (clone NCAM 16.2), B cells/CD19+ (clone SJ25C1), and monocytes/macrophages/CD14+ (clone HCD14). Subsets of T cells/CD3+ (clone SK7), CD8+ (clone SK1) and CD4+ (clone SK3). Leukocyte subsets were analyzed within the CD45+ gate using a FACSCalibur Flow Cytometer and data analysis was performed by BD Cell Quest software (BD Biosciences,CA,USA).
Zymography for gelatinolytic activity of MMP-9
Gelatinase activity in culture media was determined by SDS-PAGE containing 1% gelatin under non-denaturing conditions as described previously21. Culture supernatants (0.5 μg of total protein) were loaded into each well. Enzymatic activity standards for MMP-2 and MMP-9 were included using conditioned media o the U-937 promyelocyte cell line24.
Quantification of total and active forms of MMP-9
Specific quantification of active and total MMP-9 in culture supernatants of choriodecidual and peripheral leukocytes was done using the Biotrak MMP-9 Activity Assay System (General Electric Healthcare, Buckinghamshire, UK) following the protocol suggested by the manufacturer. In order to measure the total MMP-9 content, bound enzyme was activated with p-aminophenylmercuric acetate. The concentration of total and active MMP-9 in the samples is reported as nanograms (ng) of MMP-9 per μg of protein. Protein was measured by Bradford's method25.
Statistical analysis
For each variable, descriptive statistics (mean, standard deviation, standard error, median, and range) were obtained, and the data distribution was tested for normality using the Kolmogorov-Smirnoff and Shapiro-Wilk tests. Student's t-Test was performed to compare leukocytes subsets between ChL and PL. A p value ≤ 0.05 was considered to be statistically significant. Two way analysis of variance using repeated measurement model was used to compare cytokines/chemokines concentrations in the culture media from ChL and PL. Differences with p ≤ 0.05 were considered statistically significant. All statistical analyses were carried out using SPSS v.20 software (IBM Corporation, Armonk, NY, USA).
Results
The two-step method, using a density gradient followed by selection by plastic adherence, yielded in 133,000±3,500 choriodecidual leukocytes per cm2 of fetal membranes (n=18). According flow cytometry data, this method also allowed enriching and purifying (≥ 80%) choriodecidual leukocytes. Flow cytometry analysis revealed that T lymphocytes and Natural Killer cells were the major subsets in the ChL and PL preparations (Table I).
Table 1.
Percentages of leukocytes subsets in choriodecidua and intervillous placental blood.
| Subset | Choriodecidua (n=7) | Intervillous Placental Blood (n=7) |
|---|---|---|
| Total T lymphocytes (CD3+) | 35 (12.7-15.1) | 35.5 (32.3-38.5) |
| CD4+ T lymphocytes | 27.8 (27.3-28.3) | 26.9 (14.6-24.7) |
| CD8+ T lymphocytes | 11.1 (4.2-18.0) | 14.7 (11.7-17.8) |
| NK cells (CD56+) | 13.9 (12.7-15.1) | 10.5(10.33-10.7) |
| Monocytes/macrophages (CD14+) | 8.5 (8.0-9-0) | 4.9 (4.9-5.0) |
| B lymphocytes (CD19+) | 1.8 (0.9-2.8) | 1.9(1.47-2.4) |
Evaluation of leukocytes subsets found in choriodecidua and placental blood analyzed by flow cytometry. No significant differences were found between compartments. Median values (minimum-maximum) are reported. The phenotypes of leukocytes subsets were analyzed with in a CD45+ region. Choriodecidual leukocytes refer to non-adherent cells.
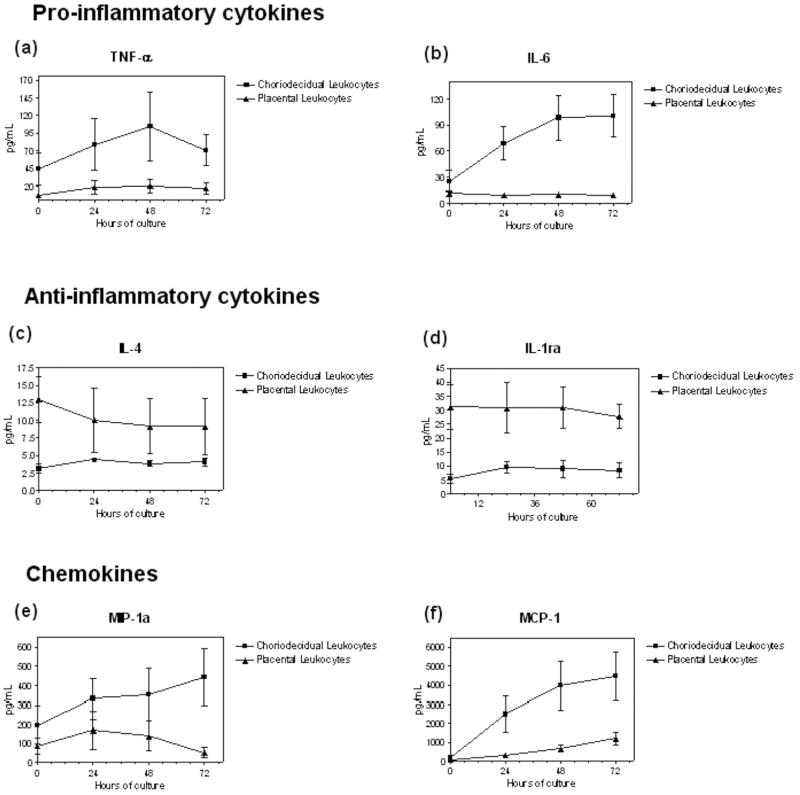
Choriodecidual leukocytes showed a distinct secretion pattern of cytokines and chemokines when compared with intervillous placental blood leukocytes (Figure 1). Overall, choriodecidual leukocytes secreted a pattern of pro-inflammatory cytokines which included higher amounts of TNF-α and IL-6 along the period of incubation (p< 0.001), and decreased secretions of IL-4 and IL-1ra, compared to intervillous placental blood leukocytes. Choriodecidual leukocytes also secreted more MIP-1α and MCP-1 than placental blood leukocytes (p<0.001) (Figure 1).
Figure 1. Characterization of the inflammatory profile of choriodecidual and intervillous placental blood leukocytes.
Choriodecidual and intervillous placental blood leukocytes were obtained and cultured up to 72 hours. Quantification of cytokines and chemokines are represented in individual panels: (a) Tumor necrosis factor α (TNF − α), p=0.009; (b) Interleukin-6 (IL-6), p=0.000; (c) Interleukin-4 (IL-4), p=0.007; (d) IL-1 receptor antagonist (IL-1ra), p=0.000 (e) inflammatory protein α (MIP − 1α), p=0.005; (f) monocyte chemotactic protein 1 (MCP-1), p=0.006. Data are shown as means ± SD of determination of 5 independent experiments in duplicate per group. Multivariate analysis revealed significant differences between groups; p<0.05.
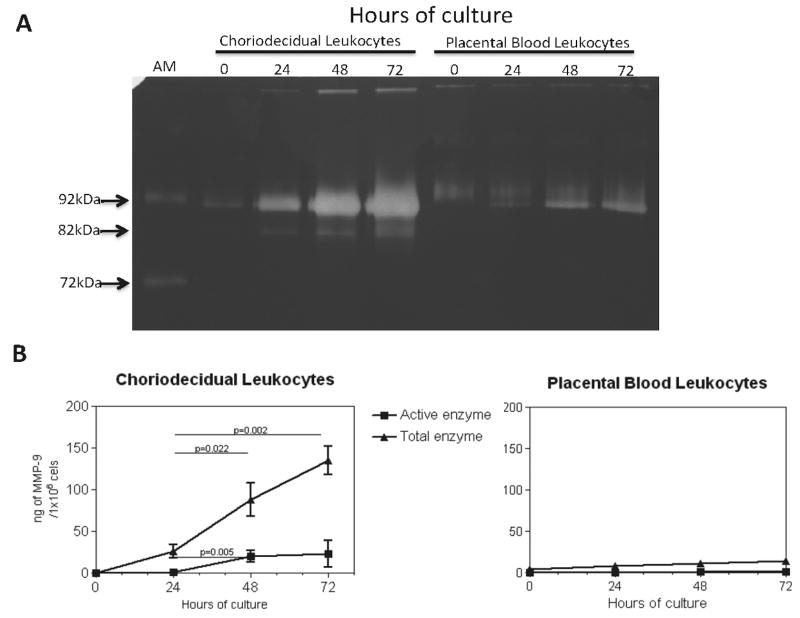
Placental and choriodecidual leukocytes secreted proMMP-9 (92 kDa) in culture after 24 hours as revealed by zymography. The total MMP-9 secretion of the choriodecidual leukocytes significantly increased from 24 to 72 hours of culture (n=15; p<0.01). Discrete and constant secretion of proMMP-9 was observed by placental leukocytes during the entire culture period. The active form of MMP-9 (82 kDa) was present from 24 hours and increased after 48 and 72 hours only in the media of choriodecidual leukocytes. Barely visible amounts of active MMP-9 were identified in the media culture of leukocytes isolated from placental blood during the culture period (Figure 2A).
Figure 2. MMP-9 activity by zymography and ELISA.
2A. Zimography. The culture media of choriodecidual and intervillous placental blood leukocytes (n=7) collected at 0, 24, 48, and 72 h were analyzed in gelatin-substrate gels. The electrophoretic positions of the gelatinolytic activities (clear bands) show the 92 kDa inactive enzyme and the 82 kDa active form of MMP-9, respectively. AM=culture medium of U937 cells as activity marker. 2B.ELISA. Active and total MMP-9 in the same media was quantitated. Each point represents the mean ± SD (n=3).
Quantitative determination of the total and active forms of MMP-9 also revealed a gradual significant increase in the active form of MMP-9 in choriodecidual leukocytes from 24 to 72 hours of culture (n=8; p<0.01). After 72 hours of culture, total secreted MMP-9 by choriodecidual leukocytes was statistically greater than the amount secreted by intervillous placental blood leukocytes (p=0.003). The active form of MMP-9 was barely detectable in the media culture of placental leukocytes (Figure 2B).
Discussion
Growing evidence suggests that some stages of the inflammatory response are present during initiation and/or progression of human parturition14, 26-28. These changes include the conditioning of a specific microenvironment in the choriodecidua characterized by migration and homing of specific populations of leukocytes and secretion of mediators resembling an intrauterine pro-inflammatory milieu8-10, 15, 29.
In this paper, we explored the functional properties of a choriodecidual leukocyte-enriched preparation isolated from fetal membranes, from pregnancies of at least 38 weeks of gestation in which the mother's underwent cesarean section without signs of spontaneous labor. We selected these tissues because they represent the prevalent conditions at the end of gestation, and evidence suggests that at this time of gestation many of the processes associated with initiation of labor are present. In order to assess the specific functional properties of choriodecidual leukocytes, we compared these cells to leukocytes isolated from intervillous maternal peripheral leukocytes of the same women.
Leukocytes isolated from term choriodecidua consisted mainly of a mix of T lymphocytes, NK cells and monocytes in a proportion similar to that in intervillous maternal peripheral blood. However, these cells showed remarkably different functional properties compared to equivalent subsets isolated from placenta circulating blood. In culture, choriodecidual leukocytes secreted a combination of modulators characterized by increased amounts of pro-inflammatory cytokines, including TNF-α and IL-6, and decreased amounts of anti-inflammatory signals (IL-10 and IL-1rα). The balance of this network of signaling molecules is clearly inclined to pro-inflammation. In addition, choriodecidual leukocytes secreted chemokines and active MMP-9. Based on these findings, we propose that term choriodecidua contains a potential cellular source of pro-inflammatory mediators and the enzymatic machinery required for amniochorion extracellular matrix degradation associated with normal delivery at the end of gestation. Characterization of the specific subsets of cells participating in the secretion of these compounds is currently under way in our laboratory.
These findings add functional meaning to old and new observations describing the infiltration of leukocytes in reproductive tissues near the time of labor10, 14, 18, 27, 28, 30. Our group recently provided evidence supporting that the choriodecidua cellular composition is actively and selectively modified at gestational term with the arrival of specific lymphocyte subsets, some of them expressing MMP-9, IL-1β and TNF-α 10, 17. Our findings using in vitro cultured choriodecidual leukocytes are also complementary to the previously reported in vivo presence of leukocytes in the choriodecidua expressing proinflammatory mediators, such as those described in this paper, in human tissues experiencing labor10, 18, 31.
Specific chemo-attraction and homing of leukocytes to term gestation choriodecidua has been proposed as the first step for conditioning a pro-inflammatory microenvironment resulting in the production of mediators for induction of labor at term pregnancy13, 32-34. Chemokines such as MIP-1α, MCP-1, IL-8 and RANTES are increased during labor in amniotic fluid and this increase correlates with cervical dilation33 and the number of leukocytes in reproductive tissues at term labor35-37. MIP-1α, IL-6 and MCP-1 are secreted by choriodecidual leukocytes8, 31 and these signals may attract and activate additional lymphocytes and monocytes, among other leukocytes34.
According to the current hypothesis, once homing of leukocytes to the choriodecidua is under way, activation of the inflammatory cascade by a non-identified modulator will result in the massive local liberation of mediators, including IL-1β, TNF-α and IL-64, 5, 9, 12. Increased concentrations of these cytokines have been documented during labor in different compartments, including umbilical cord blood, amniotic fluid and peripheral maternal blood3, 11, 16, 38. Choriodecidual cells may be a major source for these signals. These cytokines have been proposed as a first wave of signaling, acting on local cells and resulting in the production of a secondary wave of effector molecules7, 10, 18, 21. Even though we cannot totally integrate the resulting molecular cross-talk between leukocytes and the local cells in the choriodecidua, supporting experimental data allow us to suggest that cytokines secreted by choriodecidual leukocytes may explain secretion and activation of MMP-9 under the conditions in our experiments. Choriodecidual leukocytes may produce three times more MMP-9 than reference cell lines such as U93714 or amounts equivalent to those produced by some metastatic cancer lines. In addition to the above mentioned choriodecidual leukocyte functional properties, our data support the possibility that these cells could be contributing to the secondary wave of mediators, creating a microenvironment leading to collagenolysis, which could be related to the rupture of the fetal membranes10, 18.
In summary, our findings demonstrate that choriodecidual leukocytes isolated from fetal membranes at term are functionally different from cells in other compartments, and may collaborate to modulate the microenvironment linked to induction and progression of human labor.
Acknowledgments
Support for this work was provided partially by grant R01 ES016932 from the U.S. National Institute for Environmental Health Sciences and the National Institutes of Health. M.C.C. received a scholarship and financial support provided by the National Council of Science and Technology (CONACyT) and U.N.A.M. (PAPIIT IA200612-2). This paper constitutes a partial fulfillment of the Graduate Program in Biological Sciences of the National Autonomous University of México (UNAM). Marisol Castillo-Castrejon acknowledges the scholarship provided by the Consejo Nacional de Ciencia y Tecnologia (CONACyT No. 203418). N.G-L is funded by Wayne State University Research Initiative in Maternal, Perinatal and Child health (Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health). The authors thank Marie O'Neill for reviewing the manuscript prior to submission.
References
- 1.Smith R. Parturition. The New England Journal of Medicine. 2007;356:271–283. doi: 10.1056/NEJMra061360. [DOI] [PubMed] [Google Scholar]
- 2.Caldeyro-Barcia R, Schwarcz R, Belizán J, Martell M, Nieto F, Sabatino H, Tenzer S. Adverse perinatal effects of early amniotomy during labor. In: G L, editor. Modern Perinatal Medicine. Chicago,IL: Year Book; 1974. pp. 431–449. [Google Scholar]
- 3.Christiaens I, Zaragoza D, Guilbert L, Robertson S, Mitchell B, Olson D. Inflammatory processes in preterm and term parturition. Journal of Reproductive Immunology. 2008;79:50–57. doi: 10.1016/j.jri.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 4.Bollapragada S, Youssef R, Jordan F, Greer I, Norman J, Nelson S. Term labor is associated with a core inflammatory response in human fetal membranes,myometrium,and cervix. American Journal of Obstetrics and Gynecology. 2009;200:104,e101–111. doi: 10.1016/j.ajog.2008.08.032. [DOI] [PubMed] [Google Scholar]
- 5.Haddad R, Tromp G, Kuivaniemi H, Chaiworapongsa T, Kim Y, Mazor M, Romero R. Human spontaneous labor without histologic chorioamnionitis is characterized by an acute inflammation gene expression signature. American Journal of Obstetrics and Gynecology. 2006;195:394,e391–324. doi: 10.1016/j.ajog.2005.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.MacDonald P, Koga S, Casey L. Decidual activation in parturition: examination of amniotic fluid for mediators of the inflammatory response. Annals of the New York Academy of Sciences. 1991;622:315–330. doi: 10.1111/j.1749-6632.1991.tb37877.x. [DOI] [PubMed] [Google Scholar]
- 7.Estrada-Gutierrez G, Zaga V, Gonzalez-Jimenez MA, Beltran-Montoya J, Maida-Claros R, Giono-Cerezo S, Vadillo-Ortega F. Initial characterization of the microenvironment that regulates connective tissue degradation in amniochorion during normal human labor. Matrix biology : journal of the International Society for Matrix Biology. 2005;24:306–312. doi: 10.1016/j.matbio.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 8.Gomez-Lopez N, Estrada-Gutierrez G, Jimenez-Zamudio L, Vega-Sanchez R, Vadillo-Ortega F. Fetal membranes exhibit selective leukocyte chemotaxic activity during human labor. J Reprod Immunol. 2009;80:122–131. doi: 10.1016/j.jri.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 9.Gomez-Lopez N, Guilbert LJ, Olson DM. Invasion of the leukocytes into the fetal-maternal interface during pregnancy. J Leukoc Biol. 2010;88:625–633. doi: 10.1189/jlb.1209796. [DOI] [PubMed] [Google Scholar]
- 10.Gomez-Lopez N, Vadillo-Perez L, Hernandez-Carbajal A, Godines-Enriquez M, Olson DM, Vadillo-Ortega F. Specific inflammatory microenvironments in the zones of the fetal membranes at term delivery. Am J Obstet Gynecol. 2011;205:235 e215–224. doi: 10.1016/j.ajog.2011.04.019. [DOI] [PubMed] [Google Scholar]
- 11.Menon R, Swan K, Lyden T, Rote N, Fortunato S. Expression of inflammatory cytokines (interleukin-1 beta and interleukin-6) in amniochorionic membranes. American Journal of Obstetrics and Gynecology. 1995;172:493–500. doi: 10.1016/0002-9378(95)90562-6. [DOI] [PubMed] [Google Scholar]
- 12.Keelan J, Marvin K, Sato T, Coleman M, McCowan L, Mitchell M. Cytokine abundance in placental tissues: evidence of inflammatory activation in gestational membranes with term and preterm parturition. American Journal of Obstetrics and Gynecology. 1999;181:1530–1536. doi: 10.1016/s0002-9378(99)70400-x. [DOI] [PubMed] [Google Scholar]
- 13.Kelly R, Leask R, Calder A. Choriodecidual production of interleukin-8 and mechanisms of parturition. Lancet. 1992;339:776–777. doi: 10.1016/0140-6736(92)91896-g. [DOI] [PubMed] [Google Scholar]
- 14.Osman I, Young A, Ledingham M, Thomson A, Jordan F, Greer I, Norman J. Leukocyte density and pro-inflammatory cytokine expression in human fetal membranes, decidua, cervix and myometrium before and during labour at term. Molecular Human Reproduction. 2003;9:41–45. doi: 10.1093/molehr/gag001. [DOI] [PubMed] [Google Scholar]
- 15.Gomez-Lopez N, Laresgoiti E, Olson D, Estrada G, Vadillo-Ortega F. The role of chemokines in term and premature rupture of the fetal membranas: a Review. Biology of Reproduction. 2010;82:809–814. doi: 10.1095/biolreprod.109.080432. [DOI] [PubMed] [Google Scholar]
- 16.Nhan-Chang C, Romero R, Tarca A, Mittal P, Kusanovic J, Erez O, Mazaki-Tovi S, Chaiworapongsa T, Hotra J, Than N, Kim J, Hassan S, Kim C. Characterization of the transcriptome of chorioamniotic membranes at the site of rupture in spontaneous labor at term. American Journal of Obstetrics and Gynecology. 2010;202:462,e461–441. doi: 10.1016/j.ajog.2010.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gomez-Lopez N, Vadillo-Perez L, Nessim S, Olson DM, Vadillo-Ortega F. Choriodecidua and amnion exhibit selective leukocyte chemotaxis during term human labor. Am J Obstet Gynecol. 2011;204:364 e369–316. doi: 10.1016/j.ajog.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 18.Gomez-Lopez N, Vega-Sanchez R, Castillo-Castrejon M, Romero R, Cubeiro-Arreola K, Vadillo-Ortega F. Evidence for a role for the adaptive immune response in human term parturition. Am J Reprod Immunol. 2013;69:212–230. doi: 10.1111/aji.12074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garfield R. Physiology and electrical activity of uterine contractions. Seminars in Cellular Developmental Biology. 2007;18:289–295. doi: 10.1016/j.semcdb.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vadillo-Ortega F, Gonzalez-Avila G, Furth EE, Lei H, Muschel RJ, Stetler-Stevenson WG, Strauss JF., 3rd 92-kd type IV collagenase (matrix metalloproteinase-9) activity in human amniochorion increases with labor. Am J Pathol. 1995;146:148–156. [PMC free article] [PubMed] [Google Scholar]
- 21.Arechavaleta-Velasco F, Ogando D, Parry S, Vadillo-Ortega F. Production of matrix metalloproteinase-9 in lipopolysaccharide-stimulated human amnion occurs through an autocrine and paracrine proinflammatory cytokine-dependent system. Biology of Reproduction. 2002;67:1952–1958. doi: 10.1095/biolreprod.102.004721. [DOI] [PubMed] [Google Scholar]
- 22.Uchide K, Ueno H, Inoue M, Sakai A, Fujimoto N, Okada Y. Matrix metalloproteinase-9 and tensile strength of fetal membranes in uncomplicated labor. Obstetrics and Gynecology. 2000;95:851–855. doi: 10.1016/s0029-7844(00)00811-5. [DOI] [PubMed] [Google Scholar]
- 23.Vadillo-Ortega F, Estrada G. Role of matrix metalloproteinases in preterm labor. British Journal of Gynecology and Obstetrics. 2005;112:19–22. doi: 10.1111/j.1471-0528.2005.00579.x. [DOI] [PubMed] [Google Scholar]
- 24.Morodomi T, Ogata Y, Sasaguri Y, Morimatsu M, Nagase H. Purification and characterization of matrix metalloproteinase 9 from U-937 monocytic leukaemia and HT1080 fibrosarcoma cells. Biochemical Journal. 1992;285:603–611. doi: 10.1042/bj2850603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protei-dye binding. Analytical Biochemistry. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 26.Young A, Thomson A, Ledingham M, Jordan F, Greer I, Norman J. Immunolocalization of proinflammatory cytokines in myometrium, cervix, and fetal membranes during human parturition at term. Biology of Reproduction. 2002;66:445–449. doi: 10.1095/biolreprod66.2.445. [DOI] [PubMed] [Google Scholar]
- 27.Thomson A, Telfer J, Young A, Campbell S, Stewart C, Cameron I, Greer I, Norman J. Leukocytes infiltrate the myometrium during human parturition: further evidence that labour is an inflammatory process. Human Reproduction. 1999;14:229–236. [PubMed] [Google Scholar]
- 28.Keski-Nisula L, Aalto M, Katila M, Kirkinen P. Intrauterine inflammation at term: a histopathologic study. Human Pathology. 2000;31:841–846. doi: 10.1053/hupa.2000.8449. [DOI] [PubMed] [Google Scholar]
- 29.Marvin K, Keelan J, Eykholt R, Sato T, Mitchell M. Use of cDNA arrays to generate differential expression profiles for inflammatory genes in human gestational membranes delivered at term and preterm. Molecular Human Reproduction. 2002;8:339–408. doi: 10.1093/molehr/8.4.399. [DOI] [PubMed] [Google Scholar]
- 30.Junqueira L, Zugaib M, Montes G, Toledo O, Krisztan R, Shigihara K. Morphologic and histochemical evidence for the occurrence of collagenolysis and for the role of neutrophilic polymorphonuclear leukocytes during cervical dilation. American Journal of Obstetrics and Gynecology. 1980;138:273–281. doi: 10.1016/0002-9378(80)90248-3. [DOI] [PubMed] [Google Scholar]
- 31.Gomez-Lopez N, Hernandez-Santiago S, Lobb AP, Olson DM, Vadillo-Ortega F. Normal and Premature Rupture of Fetal Membranes at Term Delivery Differ in Regional Chemotactic Activity and Related Chemokine/Cytokine Production. Reprod Sci. 2012 doi: 10.1177/1933719112452473. [DOI] [PubMed] [Google Scholar]
- 32.Romero R, Ceska M, Avila C, Mazor M, Behnke E, Lindley I. Neutrophil attractant/activating peptide-1/interleukin-8 in term and preterm parturition. American Journal of Obstetrics and Gynecology. 1991;165:813–820. doi: 10.1016/0002-9378(91)90422-n. [DOI] [PubMed] [Google Scholar]
- 33.Tornblom S, Klimaviciute A, Bystrom B, Chromek M, Brauner A, Ekman-Orderberg G. Non-infected preterm parturition is related to increased concentrations of IL-6, IL-8 and MCP-1 in human cervix. Reproductive Biology and Endocrinology. 2005;3:39. doi: 10.1186/1477-7827-3-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gomez-Lopez N, Tanaka S, Zaeem Z, Metz GA, Olson DM. Maternal circulating leukocytes display early chemotactic responsiveness during late gestation. BMC pregnancy and childbirth. 2013;13(Suppl 1):S8. doi: 10.1186/1471-2393-13-S1-S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Romero R, Gomez R, Galasso M, Munoz H, Acosta L, Yoon B, Svinarich D, Cotton D. Macrophage inflammatory protein-1 alpha in term and preterm parturition: effect of microbial invasion of the amniotic cavity. American Journal of Reproductive Immunology. 1994;32:108–113. doi: 10.1111/j.1600-0897.1994.tb01101.x. [DOI] [PubMed] [Google Scholar]
- 36.Esplin M, Romero R, Chaiworapongsa T, Kim Y, Edwin S, Gomez R, Gonzalez R, Adashi E. Amniotic fluid levels of immunoreactive monocyte chemotactic protein-1 increase during term parturition. Journal of Maternal Fetal and Neonatal Medicine. 2003;14:51–56. doi: 10.1080/jmf.14.1.51.56. [DOI] [PubMed] [Google Scholar]
- 37.Athayde N, Romero R, Maymon E, Gomez R, Pacora P, Araneda H, Yoon B. A role for the novel cytokine RANTES in pregnancy and parturition. American Journal of Obstetrics and Gynecology. 1999;181:989–994. doi: 10.1016/s0002-9378(99)70337-6. [DOI] [PubMed] [Google Scholar]
- 38.Vega-Sanchez R, Gomez-Lopez N, Flores-Pliego A, Clemente-Galvan S, Estrada-Gutierrez G, Zentella-Dehesa A, Maida-Claros R, Beltran-Montoya J, Vadillo-Ortega F. Placental blood leukocytes are functional and phenotypically different than peripheral leukocytes during human labor. J Reprod Immunol. 2010;84:100–110. doi: 10.1016/j.jri.2009.08.002. [DOI] [PubMed] [Google Scholar]