Abstract
The ability to regulate behaviors and emotions depends in part on the ability to flexibly monitor one’s own progress toward a goal. Atypical patterns of response monitoring have been reported in individuals with autism spectrum disorders (ASD). In the current study we examined the error related negativity (ERN), an electrophysiological index of response monitoring, in relation to behavioral, social cognitive, and emotional presentation in higher functioning children (8–16 years) diagnosed with autism (HFA: N = 38) and an age- and IQ-matched sample of children without autism (COM: N = 36). Both HFA and COM participants displayed larger amplitude responses to error compared to correct response trials and these amplitudes did not differ by diagnostic group. For participants with HFA, larger ERN amplitudes were associated with more parent-reported autistic symptoms and more self-reported internalizing problems. However, across the full sample, larger ERN amplitudes were associated with better performance on Theory of Mind tasks. The results are discussed in terms of the utility of electrophysiological measures for understanding essential moderating processes that contribute to the spectrum of behavioral expression in the development of Autism Spectrum Disorders.
Keywords: response monitoring, ERN, higher functioning autism, internalizing
Autism is a neurodevelopmental disorder characterized by varying degrees of social and communicative impairment, as well as restricted interests and repetitive behavior (APA 2000; World Health Organization 2007). The term Autism Spectrum Disorder (ASD) describes the widespread continuum of traits expressed by affected individuals. Particularly high levels of heterogeneity are noted among higher functioning individuals with autism (HFA), who despite having average or above average IQ, demonstrate adaptive functioning levels well below their typically developing peers (Kenworthy et al. 2010; Prior et al. 1998). Contributing to this heterogeneity are high rates of comorbidity with other psychological conditions including internalizing problems such as anxiety and depression. Youth with HFA may be particularly prone to internalizing problems because of their relatively intact abilities to monitor their own and others’ behaviors resulting in an increased awareness and sensitivity to their own interpersonal difficulties (Attwood 2000; Bellini 2004). The goal of the current study was to examine response monitoring in relation to the behavioral, social cognitive and emotional expression of HFA. Specifically, we examined an electrophysiological index of response monitoring, the error-related negativity (ERN), in a sample of children and adolescents with HFA and a comparison sample of children and adolescents without an ASD, with a focus on hypotheses about the relations between the ERN and (a) autism symptom severity, (b) advanced theory of mind, and (c) internalizing problems.
Response Monitoring, the ERN and Autism
The ability to monitor one’s own progress toward a goal is a higher-order cognitive process that supports behavioral and emotional flexibility, planning, and decision making (Pennington and Ozonoff 1996). The ERN is an event-related potential associated with the monitoring of goal-directed behaviors. It is elicited immediately following the commission of an error on speeded reaction time tasks such as the Flanker task (Falkenstein et al. 1991; Gehring et al. 1993). The ERN is maximal over midfrontal recording sites and is thought to reflect the early, preconscious performance monitoring function of the anterior cingulate cortex (ACC). According to both reinforcement-learning (Holroyd and Coles 2002) and conflict-monitoring (Yeung et al. 2004) theories, the ERN functions as an alerting system sensitive to actions that are either worse than expected or in conflict with planned actions. In healthy populations, the ERN triggers increased cognitive control supporting behavioral correction and self-regulation. The ERN is also highly sensitive to state and trait differences in affect and motivation. For example, the ERN is accentuated in groups of individuals characterized by hyper-sensitivities to conflict and error, such as those affected by anxiety, OCD, or depression, and diminished in populations characterized by hypo-sensitivities to similar cues, such as those affected by ADHD or schizophrenia (see Olvet and Hajcak 2008 for review).
The HFA phenotype is particularly interesting to consider from the perspective of response monitoring. On the one hand, individuals with autism show atypical patterns of self-related processing both behaviorally (Henderson et al. 2009) and neurally (Lombardo et al. 2010) which may contribute to difficulties monitoring their own, and others’, actions, thoughts and behaviors (Koegel et al. 1995; Russell and Jarrold 1998). Indeed, several recent electrophysiological and neuroimaging studies suggest that as a group, individuals with ASD show smaller amplitude ERNs (e.g., South et al. 2010) and less differentiation between error and correct trials in terms of ACC activation (Thakkar et al. 2008) relative to non-ASD comparison samples. On the other hand, individuals with HFA can also be described as engaging in excessive self-monitoring. Henderson et al. (2006) found that children with HFA who had high verbal IQs (but not those with average IQs) showed enhanced amplitude ERN responses and Goldberg et al. (2011) reported that children with HFA showed increased activation of the anterior medial prefrontal cortex and left superior temporal gyrus following errors relative to typically-developing children. These differences suggest that response monitoring may not be a global deficit associated with autism, but rather a process that influences variability in the behavioral, social cognitive, and emotional expression of autism spectrum disorders.
Response monitoring, the ERN and Theory of Mind
According to simulation theory (Gordon 1986), our own minds serve as a model for the minds of others. That is, we understand others’ actions, thoughts, and emotions by extrapolating our own internal experience and projecting them onto others. In support of such simulation processes, numerous studies have documented common neural networks activated during the direct or vicarious experience of motor actions (Decety et al. 1997), response monitoring (Bates et al. 2005), and emotion (Carr et al. 2003). In his shared manifold hypothesis, Gallese (2003) argued that shared neural representations of our own and others’ experiences, or a shared interpersonal space, functions to support interpersonal understanding. Based largely on simulation theory, others have proposed that the neurocognitive functions involved in self-monitoring are intricately related to those supporting theory of mind or the ability to accurately think about or ‘mentalize’ the intentions, beliefs and emotions that guide other people’s behaviors (see Frith and Frith 2001; Henderson and Mundy 2012; Mundy 2003; Rameson and Lieberman 2009).
Empirical support for the mapping of self-monitoring processes to the tendency to anticipate others’ cognitions and emotions comes from recent studies linking individual differences in response monitoring and the ERN to empathy in healthy adolescent boys (Santesso and Segalowitz 2009) and adults (Larson et al. 2010). In both cases, enhanced response monitoring, as indexed by larger amplitude ERN responses, was associated with higher self-reported Empathy Quotient scores, a measure tapping into mentalizing functions including understanding and predicting others’ emotional responses and perspective taking (Lawrence et al. 2004). Based on these findings, we hypothesized that enhanced response monitoring as indexed by larger amplitude ERN responses would be associated with better performance on higher-order theory of mind tasks, regardless of diagnostic group, but that this relation would be particularly strong in the HFA sample where we expected greater variability in theory of mind abilities.
Response monitoring, the ERN and Internalizing Symptoms
Internalizing symptomatology, a general category of emotion- and mood-related psychopathology, most commonly refers to symptoms of anxiety and depression (Zahn-Waxler et al. 2000). Parent- and self-reports indicate that children and adolescents with autism experience elevated, and often clinically-significant, levels of internalizing problems (Kim et al. 2000; Kuusiko et al. 2008). Children with HFA are rated by their parents as experiencing as much anxiety as children with anxiety disorders and significantly more anxiety than lower functioning children with autism (Mayes et al. 2010; Mazurek and Kanne 2010; Sukhodolsky et al. 2008). HFA children are also rated by their parents as experiencing significantly more depression than their typically developing peers as well as their lower functioning peers with autism (Mayes et al. 2010). Internalizing problems may arise in individuals with HFA given the joint influences of a desire for social interactions and moderate levels of social cognition and interpersonal insight, which together result in a heightened distress regarding their social impairments (Attwood 2000; Bellini 2004; Chamberlain et al. 2007).
Excessive performance monitoring has been noted in participants with subclinical and clinical elevations in internalizing problems. When an individual is hypersensitive to errors and errors are interpreted as threatening, a feedback loop may be created in which the regulatory function of response monitoring is diminished. There are many reports in the literature documenting enhanced ERN amplitudes in patients with anxiety disorders including obsessive-compulsive disorder (Gehring et al. 2000) and generalized anxiety disorder (Weinberg and Hajcak 2011) as well as in non-clinical samples characterized by high levels of worry (Hajcak et al. 2003) and anxiety (Olvet and Hajcak 2009). Some additional studies have reported patterns of generalized hypervigilance to both error and correct responses in individuals with obsessive compulsive symptoms (Hajcak and Simons 2002; Ursu et al. 2003), suggesting inflexible allocation of attention. There is also evidence of enhanced ERN amplitudes in depressed patients (Chiu and Deldin 2007; Holmes and Pizzagalli 2008), though this relation appears less robust than it is with anxiety (Vaidyanathan et al. 2012). In children with ASD, South et al. (2010) did not find an association between the ERN and anxiety symptoms and Henderson et al. (2006) noted a small effect that was reduced to non-significance when medication status was controlled for. Based on the extensive literature in other populations, though, we hypothesized that enhanced ERN amplitude would be associated with more internalizing symptoms, particularly among children with HFA who experience more internalizing problems than children without an ASD.
In summary, based on the existing literatures, we hypothesized that response monitoring is a unifying construct that might account for some of the observed heterogeneity among individuals with HFA. We hypothesized that as a group, relative to a comparison sample, participants with HFA would show reduced response monitoring, as indicated by smaller amplitude ERN responses. It was further hypothesized that enhanced amplitude ERN responses would be associated with better performance on theory of mind tasks but also more internalizing problems and that these relations would be particularly strong in the HFA sample relative to the comparison sample.
Method
Participants
Participants included 38 children and adolescents with HFA and 36 children and adolescents without autism (COM), aged 8 to 16 years. Participants were drawn from a larger sample (72 HFA; 94 COM) of children with verbal IQs in the average or above average range (VIQ ≥ 85) who were participating in a study of social emotional functioning in children with HFA. From this sample, 47 participants (18 HFA, 29 COM) were excluded because they did not complete the EEG assessment and 41 (16 HFA, 25 COM) participants were excluded because they did not have sufficient amounts of usable data (i.e., there was excessive artifact or because they committed fewer than 11 errors on the task). An additional four COM participants were excluded for failing to adequately match on IQ or gender. Included and excluded participants did not differ on the WISC-IV Verbal Comprehension Index, t(164) = .70, ns, Gender, χ2 (1, N = 166) = .70, ns, Ethnicity, χ2 (4, N = 160) = 5.55, ns, age, t(164) = −.46, ns, the Social Communication Questionnaire, t(159) = −1.41, ns, the Autism Symptom Screening Questionnaire, t(160) = −.56, ns, or the Autism Diagnostic Observation Schedule, t(150) = −1.26, ns.
The HFA and COM groups were matched on age and verbal IQ (see Table 1) and had comparable gender distributions (HFA: 34 male, 4 female; COM: 27 male, 9 female), Fisher’s Exact Test, p = .09. Participants in the HFA group were more likely to be prescribed psychotropic medication (n = 9) than were participants in the COM group (n=2), Fisher’s Exact Test, p = .047. The sample was 46.0% Caucasian, 38.2 % Hispanic, 4.8% Asian, 4.8% African American, 3.7% mixed race, and 2.5% unknown or not given and the ethnic distribution did not differ by diagnostic group, χ2(5, N = 74) = 4.29, ns.
Table 1.
Descriptive statistics by diagnostic group.
| HFA | COM | Analysis | ||||||
|---|---|---|---|---|---|---|---|---|
| N | Mean (SD) | Range | N | Mean (SD) | Range | t value | p value | |
| Age (yrs) | N = 38 | 13.16 (2.49) | 8.58–16.75 | N = 36 | 12.61(2.39) | 8.83–16.75 | −1.05 | 0.30 |
| VIQ | N = 38 | 104.50(12.11) | 87–130 | N = 36 | 108.61(10.72) | 89–128 | 1.54 | 0.13 |
| ADOS | N = 37 | 11.57(4.49) | 0–21 | N = 35 | 3.51(4.85) | 0–16 | −7.31 | <.001 |
| SCQ | N = 37 | 21.22(5.71) | 9–33 | N = 35 | 4.94(3.83) | 0–20 | −14.13 | <.001 |
| ASSQ | N = 38 | 28.00(9.84) | 10–47 | N = 35 | 3.16(9.84) | 0–11 | −14.05 | <.001 |
| Autism Symptom Severity | N = 37 | 0.03(1.76) | −3.06–3.28 | |||||
| Internalizing Problems | N = 35 | 55.06(9.11) | 37–77 | N = 35 | 45.26(6.88) | 36–63 | −5.08 | <.001 |
| Theory of Mind | N = 27 | −.11(2.13) | −3.56–3.90 | N = 31 | .63(1.30) | −1.41–3.09 | 1.58 | 0.12 |
Note: VIQ = Verbal IQ, ADOS = Autism Diagnostic Observation Schedule, SCQ = Social Communication Questionnaire, ASSQ = Autism Symptom Severity Questionnaire. Autism Symptom Severity is the mean of the SCQ and ASSQ total scores; Internalizing Problems is the BASC-2 Self-Report Broadband factor, Theory of Mind composite is mean of the Strange Stories correct responses and mental answers score.
Children in the HFA group were recruited from the {omitted for review} and had a community diagnosis of autism. The COM sample was recruited through the {omitted for review} school district and had never been diagnosed with autism. Diagnostic status was confirmed in the laboratory based on the following measures and associated cutoff scores: (1) parent report on the Social Communication Questionnaire (total score ≥ 12), (2) parent report on the Autism Symptom Screening Questionnaire (total score ≥ 13), and (3) direct observations using the Autism Diagnostic Observation Schedule (Communication and Social Interaction domain score ≥ 7). Children in the HFA sample were required to meet diagnostic criteria on at least 2 of the 3 diagnostic measures. Likewise, all children in the COM sample scored below the cutoff on at least 2 of the 3 measures. Five participants in the COM sample scored above the cutoff on the ADOS; however based on careful review of each case they were retained in the sample. Although these participants were difficult to engage, none of them had a previous diagnosis of autism or parent report responses that met cutoffs on either the SCQ or ASSQ. Further, neither the ADOS administrators nor the research staff reported any concerns about these participants having autism based on their other interactions in the lab. As expected, the HFA group scored significantly higher than the COM group on all diagnostic measures (see Table 1).
Procedure
All procedures were approved by the Institutional Review Board of the {omitted for review}. During an initial visit, parents provided written informed consent and children provided written informed assent. Also during this visit, participants completed standardized diagnostic measures and IQ testing while parents filled out questionnaires about their child’s symptoms, behaviors and emotions. During a second visit, participants completed measures about their behaviors and emotions and measures of advanced Theory of Mind. In addition, EEG was collected during a baseline period and during performance of a modified Flanker task.
Measures
Diagnostic and Intelligence Measures
The Autism Diagnostic Observation Schedule (ADOS; Lord et al. 2001) is a semi-structured observational assessment that measures social, communicative, cognitive, and self-regulatory behaviors. The ADOS consists of a series of standard play based activities designed to allow the examiner to observe social, communication, and repetitive behaviors. The ADOS includes multiple items rated on a qualitative scale of 0 (not abnormal) to 3 (most abnormal). Scores on the Communication and Social Interaction Domain were used to verify diagnostic status.
Parents completed the Social Communication Questionnaire (SCQ; Rutter et al. 2003) and the High-Functioning Autism Spectrum Screening Questionnaire (ASSQ; Ehlers et al. 1999). The SCQ is a 40-item, screening device, derived from the Autism Diagnostic Interview – Revised, that measures communication skills and social functioning in children diagnosed with autism. The ASSQ is a brief 27-item screening instrument used to identify symptoms associated with ASD in children and adolescents. Total Scores on the SCQ and ASSQ were used to verify diagnostic status and to index individual differences in symptom severity in the HFA sample. SCQ and ASSQ total scores were highly correlated within the HFA sample, r (37) = .57, p < .001, and therefore standardized and summed to form a composite measure of Autism Symptom Severity.
The Wechsler Intelligence Scale for Children, Fourth Edition (WISC-IV; Wechsler 2003) was used to assess intellectual functioning. Two subscales of the Verbal Comprehension Index (VCI; Vocabulary and Similarities Subscales) were administered to yield standardized VCI estimates. These scales have the highest loadings on the VCI factor, strong test-retest reliabilities, internal consistencies and the narrowest standard errors of measurement among the WISC-IV scales (Williams et al. 2003). The VCI was used to verify that all participants had at least Average (≥ 85) VIQ estimates.
Advanced Theory of Mind Tasks
The Children’s Eyes Test (Baron-Cohen et al. 2001) is a non-verbal theory of mind test in which children are presented with 28 photographs of the eye region of different faces and asked to choose one out of four words (e.g., surprised, embarrassed) to best describe what each person was thinking or feeling. Each trial is scored as correct or incorrect resulting in a total score ranging from 0 to 28.
The Strange Stories Task (Happé 1994) consists of 12 short vignettes and was used to assess participants’ abilities to attribute mental states to others in the context of stories involving a pretend event, a joke, a lie, a white lie, a figure of speech, and bluffing. Following each story the child was asked a simple question to confirm their understanding of the events in the story followed by an open-ended question in which they were asked to explain why the story events happened in that way. Variables of interest were the total number of correct responses to the simple and open-ended questions (possible range 0–24) and the number of mental explanations provided for the story (possible range 0–12). Two participants in the HFA sample scored more than 3 SDs below the sample mean on the Strange Stories task and were therefore removed from the individual difference analyses related to measure.
A composite measure of Theory of Mind was created by standardizing and summing the Eyes Test total score, and the number of correct responses and mental explanations on the Strange Stories Task, which were all positively and significantly correlated (rs .37–.61, ps < .003).
Internalizing Problems Measures
The Behavioral Assessment System for Children – Second Edition (BASC-2; Reynolds and Kamphaus 2004) is a self-report measure of behavior problems. Of interest for the current study is the broadband Internalizing Composite which is comprised of scores on the Atypicality, Locus of Control, Social Stress, Anxiety, Depression, and Sense of Inadequacy subscales. Children with ASD were included in the general and clinical normative samples as well as the reliability and validity studies for the BASC-2 (Reynolds and Kamphaus, 2004).
EEG Data Collection during the Modified Eriksen Flanker Task
EEG data were collected in a dimly lit, sound attenuated room. Participants were seated approximately 70 cm from the computer monitor. EEG was recorded during performance of a modified Eriksen Flanker task, in which participants used a two-button keypad to identify the direction of a central target arrow flanked by either compatible (> > > > > or < < < < <) or incompatible (< < > < < or > > < > >) arrows. Participants completed three blocks of 96 trials counterbalanced by compatible and incompatible stimulus displays, as well as left and right responses, for a total of 288 trials. Each trial consisted of a 200 ms warning cue (an asterisk), followed by a 300 ms delay, and then one of four targets displayed for 200 ms. Prior to EEG collection, participants completed 20 practice trials to ensure understanding of the instructions followed by an additional 20 trials (timing trials) used to individualize response time parameters for the task. Given the wide age range of participants, this strategy was employed in order to reduce age-related variability in task difficulty and to obtain a sufficient number of error and correct trials for ERP averaging. For children who performed at or above 70% accuracy on the timing trials, the allowable response time was set to their own median reaction time. For children who performed below 70%, the allowable reaction time was set to their own 75th percentile. As a result, across the sample the maximum allowable reaction time ranged from 350 to 800 ms following stimulus onset. The mean allowable reaction time did not differ across diagnostic groups (HFA mean 492 ms, SD = 111; COM mean 507 ms, SD = 95, t(72) = .62, p = .54). In addition, allowable reaction time was unrelated to error rates on the task, r(74) = −.04, p =.75, demonstrating the independence of the reaction time settings from the level of difficulty experienced by participants. Behavioral measures of interest from the Flanker Task were mean reaction times and error rates on compatible versus incompatible trials as well as correct versus incorrect trials.
Electrophysiological Recordings
EEG was collected using a 22-channel Lycra stretch Electrocap with electrodes placed according to the international 10/20 electrode system. EEG was recorded from the following sites: central (C3, Cz, C4), frontal (F7, F3, Fz, FCz, F4, F8), anterior temporal (T7, T8), parietal (P3, Pz, P4), occipital (O1, O2), and mastoid (M1, M2), with a ground electrode at site AFz. Data were collected referenced to Cz and then re-referenced offline to an average mastoid configuration. Electro-oculographic (EOG) eye movements were recorded at the supra- and suborbit of one eye, as well as the outer canthi of each eye. Data were collected with 0.1 Hz and 100 Hz filters and EEG and EOG signals were amplified by factors of 5000 and 2500, respectively.
Analysis of EEG data from Flanker Task
Analyses were conducted using the EEG/ERP Analysis System (James Long Company). Data were re-referenced offline to an average mastoid configuration and digitally re-filtered with a 30 Hz low-pass filter. The data were corrected for eye blink artifacts using a linear regression approach in which identified blinks are used to compute regression coefficients characterizing the linear relation between EOG activity and each EEG channel. The raw EEG data are transformed using the resulting regression coefficient matrix to remove blink-related activity from each EEG channel. A final visual inspection of the data was conducted and any remaining portions of data with artifact were removed.
The artifact-free EEG data were response-locked (correct/error) and averaged for each participant, using a minimum of 11 artifact-free trials. The data were baseline corrected using a 100 ms window spanning −150 ms to −50 ms. Errors of omission and error and correct trials with reaction times < 100 ms were excluded from the grand averages. On average, 37 trials were included in the error-related negativity (ERN) and 119 trials were included in the correct-related negativity (CRN). For each participant, the latency of the peak negativity within a −20 ms to 100 ms window was recorded and the amplitude of the ERN was computed as the mean amplitude in a +/− 20 ms window around the peak negativity. The latency and amplitude of the CRN were computed in a comparable fashion based on the correct response grand average waveform. Mean around the peak (rather than peak) scoring was deemed most appropriate because it is less susceptible to noise-related biases that arise when there are a relatively small and variable number of trials in the grand average waveform (Handy 2005; Luck 2005).
Amplitude and latency data from midline sites Fz and FCz were averaged to create frontal ERN and CRN composites.
Data Analysis Plan
Diagnostic group differences on behavioral (reaction times, error rates) and physiological (ERN/CRN amplitude, latency) responses on the Flanker task were examined using separate 2 (diagnostic group: HFA, COM) x 2 (response type: error, correct) repeated measures ANOVAs with Greenhouse-Geisser corrections for repeated measures, with partial-eta2 (ηp2) reported as a measure of effect size. The relations between the ERN and symptom severity were examined within the HFA group using Pearson correlations. To examine the relations between the ERN and Theory of Mind and Internalizing Problems, and the effect of diagnostic group on these associations, separate hierarchical regression models were analyzed following the guidelines of Aiken and West (1991) and with Cohen’s f2 reported as a measure of effect size. To avoid multi-collinearity, the predictors were mean centered and entered in the following order: (a) Diagnostic Group (coded −1 COM, 1 HFA), (b) frontal ERN amplitude, and (c) the interaction of diagnostic group and frontal ERN amplitude. Significant interactions were followed up by examining the significance of the slope relating the ERN amplitude to each outcome variable separately by diagnostic group. All regressions were run controlling for total error rates on the Flanker task and because effects were unchanged, the results of the simpler model without the covariate are reported below. To examine the specificity of the findings to the ERN, regressions were also run with the frontal CRN amplitude as a predictor. There were no significant main or interaction effects included the CRN so these models are not presented in detail. To
Results
Flanker Task: Behavioral Performance
A repeated measures ANOVA examining the effects of trial type (compatible, incompatible) and diagnostic group (HFA, COM) on participants’ reaction times revealed an expected main effect of compatibility, F(1, 72) = 16.55, p < .001, ηp2 = .19, showing slower responses on incompatible (M = 359 ms, SD = 86) compared to compatible (M = 347 ms, SD = 78) trials. The main effect of diagnostic group and the interaction of diagnostic group and compatibility were not significant. A similar analysis for error rates revealed a main effect of compatibility, F(1,72) = 307.69, p < .001, ηp2 = .81, with participants making fewer correct responses on incompatible (M = 54.67%, SD = 17.18) versus compatible (M = 87.66%, SD = 12.53) trials. There was also a main effect of diagnostic group, F(1,72) = 4.61, p = .04, ηp2 = .06, showing fewer correct responses for HFA (M = 68.2%, SD = 12.3) versus COM (M = 74.3%, SD = 12.6) participants across the entire task. An analysis of reaction times on error versus correct trials revealed an expected effect of response type with all participants responding more slowly on correct versus error trials, F(1, 72) = 159.23, p < .001. Diagnostic group was unrelated to RTs on correct, t(72) = 1.30, p = .20, or error, t(72) = .34, p=.73, trials.
In summary, although HFA participants made slightly more errors overall, a lack of interaction between stimulus type and diagnostic group on either error rates or reaction times, and a lack of diagnostic group differences on RTs on error versus correct trials demonstrates that participants were affected comparably by the demands of the Flanker task.
Flanker Task: Error Related Negativity and Correct Response Negativity
Grand average waveforms for correct and error trials averaged across frontal midline sites (Fz, FCz) are presented separately by diagnostic group in Figure 1. Preliminary analyses revealed that the frontal ERN and CRN amplitudes were unrelated to age and verbal IQ; however, error rates on the Flanker task tended to be associated with a smaller ERN amplitude, r(73) = .21, p = .075, and were therefore considered as a potential covariate in subsequent analyses.
Figure 1.
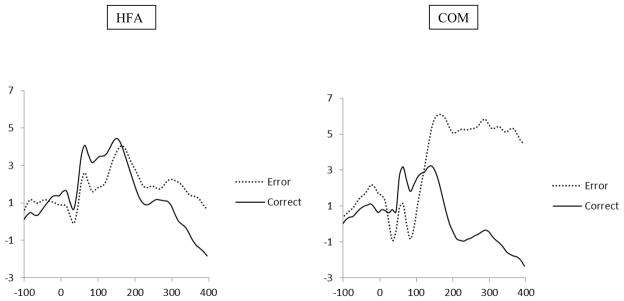
Grand Average Waveforms for Error and Correct Trials Separately by Diagnostic Group
A 2 (response type: correct vs. error) x 2 (diagnostic group: HFA vs. COM) repeated measures ANCOVA was conducted with ERN/CRN amplitude as the dependent variables and total error rates as a covariate. There was a main effect of response type, F(1, 71) = 14.23, p < .001, ηp2 = .17, with larger amplitude responses on error (−3.64 μV, SD = 5.59) versus correct (−1.13 μV, SD = 4.16) response trials. The main effect of diagnostic group and the response type by diagnostic group were not significant.
A 2 (response type: correct vs. error) x 2 (diagnostic group: HFA vs. COM) repeated measures ANCOVA with ERN/CRN latency as the dependent variables and total error rates as the covariate revealed no main or interaction effects. Together these data demonstrate that across the full sample, errors elicited larger negative amplitude responses than did correct responses. The latency of these responses did not vary based on response type or diagnostic group. Means and standard deviations for frontal ERN amplitude and latency are presented for the full sample and separately by diagnostic group in Table 2.
Table 2.
Mean (SD) amplitude and latency for the frontal ERN and CRN.
| HFA N = 38 | Comparison N = 36 | Full Sample N = 74 | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| ERN | CRN | ERN | CRN | ERN | CRN | |
| Amplitude (μV) | −2.59 (4.60) | −1.02 (3.65) | −4.76 (6.35) | −1.24 (4.69) | −3.64 (5.59) | −1.13 (4.16) |
| Latency (ms) | 48 (43) | 37 (50) | 60 (41) | 34 (44) | 54 (42) | 36 (47) |
The ERN in Relation to Symptoms, Social Cognition and Internalizing Problems
Zero-order correlations between the ERN, the CRN and Autism Symptom Severity, Internalizing Problems, and Theory of Mind composite measures are presented separately by diagnostic group in Table 3.
Table 3.
Interrelations among study variables by diagnostic group.
| HFA
| |||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| 1. Frontal ERN | |||||
| 2. Frontal CRN | .52*** | ||||
| 3. Autism Symptom Severity | −.45** | −.17 | |||
| 4. Theory of Mind | −.35t | −.07 | .23 | ||
| 5. Internalizing Problems | −.29t | −.17 | .26 | −.20 | |
| COM
| ||||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| 1. Frontal ERN | ||||
| 2. Frontal CRN | .65*** | |||
| 3. Theory of Mind | −.28 | −.32t | ||
| 4. Internalizing | .23 | .22 | −.20 | |
p < .10,
p ≤ .05,
p ≤ .01,
p ≤ .001
ERN and Autism Symptom Severity
Within the HFA sample, Autism Symptom Severity was inversely related to frontal ERN amplitude, r(37) = −.45, p = .005 but not the frontal CRN amplitude r(37) = −.17, p = .33. That is, larger amplitude ERN responses were associated with more parent-reported symptoms. The magnitude and significance of this association was unchanged when age, verbal IQ, medication status, or error rate on the Flanker task were considered as potential covariates.
ERN and Theory of Mind
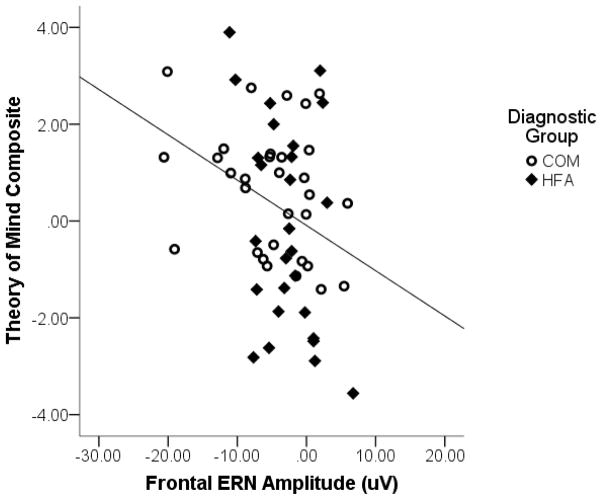
Results of the regression model predicting the Theory of Mind composite score are presented in Table 4. The full model was significant, F(3, 59) = 6.26, p = .001, total R2 = .15, f 2 = .18. After controlling for diagnostic group, there was a main effect of frontal ERN amplitude such that larger amplitude ERN responses were associated with higher Theory of Mind scores. This effect was comparable across diagnostic groups as indicated by a lack of interaction between ERN amplitude and diagnostic group. A scatterplot depicting the association between ERN amplitude and Theory of Mind is presented in Figure 2. A comparable analysis with frontal CRN amplitude as a predictor was not significant.
Table 4.
Diagnostic Group and Frontal ERN Amplitude as Predictors of Theory of Mind
| Dependent Variable/Step | β | t | Δ R2 | Total R2 |
|---|---|---|---|---|
| Theory of Mind Composite | .15 | |||
| Step 1: Diagnostic Group | −.14 | −1.10 | .05 | |
| Step 2: Frontal ERN Amplitude | −.38 | −2.55* | .08 | |
| Step 3: Diagnostic Group * Frontal ERN | −.19 | −1.34 | .03 |
p < .05.
Figure 2.
Frontal ERN in relation to Theory of Mind for full sample
ERN and Internalizing Problems
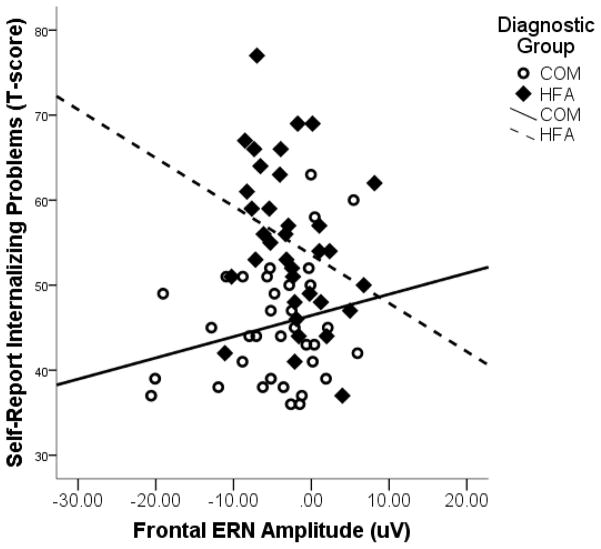
Results of the regression model predicting Internalizing Problems are presented in Table 5. The full model was significant, F(3, 69) = 10.78, p < .001, total R2 = .33, f 2= .49. Self-reported Internalizing Problems were predicted by a main effect of diagnostic group, such that HFA participants reported more Internalizing Problems than COM participants. This effect was qualified by an interaction between diagnostic group and frontal ERN amplitude. Figure 3 plots the significant interaction by showing the scatterplot and regression line for the relation between frontal ERN amplitude and Internalizing Problems separately for the HFA and COM samples. A post hoc analysis of the simple slopes revealed that for the HFA sample, larger frontal ERN amplitudes tend to be associated with higher levels of Internalizing Problems, β = −.34, t(68) = −1.97, p = .053. In contrast, for the COM sample, ERN amplitude is not related to Internalizing Problems, β = .15, t(68) = 1.29, p = .24. A comparable analysis with frontal CRN as a predictor revealed only a main effect of diagnostic group on Internalizing Problems but no effects of the CRN.
Table 5.
Diagnostic Group and Frontal ERN Amplitude as Predictors of Internalizing Problems
| Dependent Variable/Step | β | t | Δ R2 | Total R2 |
|---|---|---|---|---|
| BASC SRP Internalizing Problems | .33 | |||
| Step 1: Diagnostic Group | .56 | 5.40*** | .28 | |
| Step 2: Frontal ERN Amplitude | −.10 | −.90 | .00 | |
| Step 3: Diagnostic Group * Frontal ERN | −.24 | −2.29* | .05 |
p < .05,
p < .001.
Figure 3.
Frontal ERN in relation to Self-Reported Internalizing Problems by Diagnostic Group.
Discussion
The purpose of this study was to examine performance monitoring, as indexed by the error-related negativity, as a biomarker of individual differences in symptom presentation, theory of mind, and co-occurring internalizing problems in higher functioning children and adolescents with autism. Regardless of diagnostic group, participants clearly differentiated between error and correct responses both physiologically and behaviorally. Contrary to our hypothesis, however, diagnostic group was unrelated to the ERN amplitude. In terms of individual differences, as hypothesized, larger amplitude ERN responses were associated with more advanced theory of mind abilities. Although we hypothesized this association would be particularly strong for individuals with HFA, the association was comparable across both diagnostic groups. In addition, for individuals with HFA, larger amplitude ERN responses were associated with more parent-reported autistic symptoms and more self-reported Internalizing Problems. These findings are discussed below in terms of both the costs and benefits of enhanced response monitoring for higher functioning individuals with autism.
The ERN, but not the CRN, was associated with the severity of parent-reported ASD symptoms, such that larger amplitude ERN responses were associated with more parent-reported symptoms within the HFA sample. This relation was robust and held after controlling for a number of potential covariates including verbal IQ, age, medication status and task performance. Few studies have examined the relation between the ERN and autistic symptoms and those that have report mixed findings. For example, in a previous report on a smaller sample of children and adolescents with HFA, larger ERN peak amplitudes were associated with fewer parent-reported symptoms but only on the Social Behavior scale of the SCQ (Henderson et al. 2006). It is important to point out, however, that this difference was reduced to non-significance once medication status was taken into account. Our current finding is, however, consistent with a recent report by South et al. (2010) where the ERN-CRN difference score was inversely related to scores on the Social Behavior scale of the SCQ. The reliability of our current finding is further supported by the fact that our sample size is considerably larger than in prior studies and the fact that we used a composite measure of symptoms that includes current (ASSQ) and lifetime (SCQ) symptom ratings which likely provided a more reliable index than the individual subscores examined in prior studies.
A particularly interesting finding in the current study was that across the full sample, larger amplitude ERN responses were associated with better Theory of Mind performance. Based in part on simulation theory, Frith and Frith (1999, 2001) and Mundy (2003) speculated that deficits in early self-related processing set the cognitive and neural stage for difficulties processing information about others for children with autism. This is one of the first empirical studies we are aware of in which a preconscious index of self-monitoring, the ERN, is shown to relate to the understanding of others’ mental states. Importantly, this relation existed across the full sample, suggesting comparable mechanisms linking response monitoring to higher order social cognition in children with and without autism. This finding is consistent with a handful of studies with typically-developing populations demonstrating associations between the ERN and measures that tap into social cognition including empathy in adults (Larson et al. 2010; Santesso and Segalowitz 2009) and undersocialized behaviors in children including low levels of empathy and an indifference to social expectations (Santesso et al. 2005).
We hypothesized that the association between the ERN and theory of mind would be particularly strong for participants with HFA who we expected to perform less well on the theory of mind tasks. Contrary to this expectation, the association was comparable across groups which may be due to the fact that participants with HFA did not differ from participants in the COM group in terms of their performance on the Theory of Mind tasks. Moreover, for individuals with HFA Theory of Mind performance was not associated with symptom severity. These findings are consistent with several recent studies suggesting that higher functioning individuals with ASD, regardless of symptom severity, may understand the basic logic and principles of advanced mental state reasoning in theory (e.g., Scheeren et al. 2013). Given their well-developed verbal skills and their intact ability to analyze the logic and flow of social stories, individuals with HFA might be able to invoke mentalizing accounts for social stories. However, the accuracy of these accounts may suffer in more complex real-world contexts, where one must pick up on more subtle and non-verbal cues and track the dynamic nature of these cues. Although the ability to generate mental state explanations is necessary for effective and competent reciprocal social interactions, it is clearly not sufficient. The lack of association between Theory of Mind performance and symptom severity suggests that response monitoring and the ERN contribute to several relatively independent aspects of functioning which underlie the heterogeneous presentation of autism in higher functioning individuals.
Although response monitoring was associated with better theory of mind abilities, for individuals with HFA, larger amplitude frontal ERNs were also associated with more self-reported Internalizing Problems. This finding is consistent with many reports in the literature documenting relations between the ERN amplitude and clinical and subclinical elevations in anxiety. Again, this association was specific to the ERN, suggesting that a tendency to attend to negative performance outcomes, or outcomes that are worse than expected, might create a cycle of self-focused concern that is a central feature of internalizing symptoms including anxiety and depression. In examining the scatterplots relating the ERN to Internalizing Problems, the ERN amplitude appears to serve as a continuous indicator of relative risk for co-occurring internalizing symptoms, as shown by the fact that diagnostic group differences in internalizing problems were much more apparent when the amplitude of the ERN was relatively large. Given that approximately 50% of individuals with HFA experience clinically significant levels of internalizing problems, our findings are consistent with Olvet and Hajcak’s (2008) proposal that the ERN serves as an endophenotype for internalizing disorders.
There are several limitations to the current study that should be noted. First, several participants in the comparison sample scored at or above the diagnostic cutoff on the ADOS but were retained in the comparison sample because there was no corroborating support for an autism diagnosis based on developmental history or parent-report measures. We did not administer a full psychiatric assessment so it is possible that these participants had other undiagnosed conditions including anxiety and/or ADHD which are known to reduce the specificity of the ADOS (Molloy et al. 2011). Such conditions might also minimize or mask diagnostic group differences on study variables including the ERN and social anxiety. Second, although not a focus of the current paper, visual inspection of the ERP waveforms suggests that the Pe (the positive deflection 100 to 400 ms following an error) might discriminate between the diagnostic groups. The Pe is thought to signal behavioral change and correction following an error (Hajcak et al. 2003) and may be an important future avenue for mapping physiological and behavioral aspects of performance monitoring among individuals with autism. Finally, given the concurrent nature of our study, it is not possible to tease apart directions of effects among our variables. Our hypotheses and analyses were guided by an assumption that the ERN is a stable individual difference that serves as a marker of self-monitoring tendencies and that these tendencies influence, over time, the way children come to understand others and think about the self in relation to others. However, the opposite direction of effect is equally plausible. That is, deficits in thinking about others and the heightened self-focused attention that characterizes many internalizing problems may alter the development of preconscious response monitoring tendencies, their underlying neural systems, and patterns of connectivity and coherence with other brain systems underlying social behavior. The field is in need of longitudinal studies that include repeated assessments of both physiology and behavior over periods known to be crucial for the emergence of higher-order mentalizing abilities and the onset of internalizing problems. Such studies offer the potential of tracking trajectories of growth and change in EEG and/or ERP responses as predictors of comorbidity and developmental prognosis (e.g., Tierney et al. 2012).
In summary, the current findings support theoretical models positing the central role of response monitoring functions in regulating and integrating self- and other-directed attention both cognitively and affectively (Henderson and Mundy 2012). For individuals with HFA, response monitoring appears to support higher level social cognition but comes at the price of more internalizing problems. That is, in the context of the social deficits inherent to a diagnosis of autism, enhanced self-monitoring may increase one’s awareness and concern over others’ evaluations leading to significant levels of anxiety and depression. More generally, the findings demonstrate the importance of moving beyond thinking of electrophysiological measures solely as dependent measures used to describe diagnostic group differences in information processing. Rather, our results demonstrate that electrophysiological assessments can serve as an important source of information about moderators of phenotypic presentation that give rise to the wide spectrum of cognitive and emotional expression in Autism Spectrum Disorders (Burnette et al. 2011; Mundy et al. 2007).
Acknowledgments
The authors thank the participating families for their time and dedication to this research. We are grateful to the following individuals for their contributions to participant recruitment and data collection: Nicole Kojkowski Coman, Drew Coman, Nicole Zahka, Annie Inge, and Leena Mohapatra. This work was supported by NIH Grant R01 MH71273.
References
- Aiken LS, West SG. Multiple Regression: Testing and interpreting interactions. Newbury Park, CA: Sage; 1991. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders (text revision) 4. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- Attwood T. Asperger’s syndrome: A guide for parents and professionals. London: Jessica Kingsley; 2000. [Google Scholar]
- Baron-Cohen S, Wheelwright S, Hill J, Raste Y, Plumb I. The ‘Reading the Mind in the Eyes’ test revised version: A study of normal adults, and adults with Asperger syndrome or high functioning autism. Journal of Child Psychology and Psychiatry. 2001;42(2):241–251. [PubMed] [Google Scholar]
- Bates AT, Patel TP, Liddle PF. External behavior monitoring mirrors internal behavior monitoring. Journal of Psychophysiology. 2005;19(4):281–288. [Google Scholar]
- Bellini S. Social skill deficits and anxiety in high-functioning adolescents with autism spectrum disorders. Focus on Autism and Other Developmental Disabilities. 2004;19(2):78–86. [Google Scholar]
- Burnette CP, Henderson HA, Inge AP, Zahka N, Schwartz C, Mundy PC. Anterior EEG asymmetry and the modifier model of autism. Journal of Autism and Developmental Disorders. 2011;41:1113–1124. doi: 10.1007/s10803-010-1138-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr L, Iacoboni M, Dubeau MC, Mazziotta JC, Lenzi GL. Neural mechanisms of empathy in humans: a relay from neural systems for imitations to limbic areas. Proceedings of the National Academy of Sciences. 2003;100(9):5497–6602. doi: 10.1073/pnas.0935845100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain B, Kasari C, Rotheram-Fuller E. Involvement or isolation? The social networks of children with autism in regular classrooms. Journal of Autism and Developmental Disorders. 2007;37(2):230–242. doi: 10.1007/s10803-006-0164-4. [DOI] [PubMed] [Google Scholar]
- Chiu PH, Deldin PJ. Neural evidence for enhanced error detection in major depressive disorder. American Journal of Psychiatry. 2007;164:608–616. doi: 10.1176/ajp.2007.164.4.608. [DOI] [PubMed] [Google Scholar]
- Decety J, Grézes JJ, Costes NN, Perani DD, Jeannerod MM, Procyk EE, et al. Brain activity during observation of actions: Influence of action content and subject’s strategy. Brain: A Journal of Neurology. 1997;120(10):1763–1777. doi: 10.1093/brain/120.10.1763. [DOI] [PubMed] [Google Scholar]
- Ehlers S, Gillberg C, Wing L. A screening questionnaire for Asperger syndrome and other high-functioning autim spectrum disorders in school age children. Journal of Autism and Developmental Disorders. 1999;28:527–533. doi: 10.1023/a:1023040610384. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Hohnsbein J, Hoormann J, Blanke L. Effects of cross-modal divided attention on late ERP components: II. Error processing in choice reaction tasks. Electroencephalography and Clinical Neurophysiology. 1991;78:447–455. doi: 10.1016/0013-4694(91)90062-9. [DOI] [PubMed] [Google Scholar]
- Frith C, Frith U. Interacting minds-A biological basis. Science. 1999;286:1692–1695. doi: 10.1126/science.286.5445.1692. [DOI] [PubMed] [Google Scholar]
- Frith U, Frith C. The biological basis of social interaction. Current Directions in Psychological Science. 2001;10:151–155. [Google Scholar]
- Gallese V. The manifold nature of interpersonal relations: the quest for a common mechanism. Philosophical Transactions of the Royal Society of London Series B: Biological Sciences. 2003;358(1431):517–528. doi: 10.1098/rstb.2002.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring WJ, Goss B, Coles MGH, Meyer DE, Donchin E. A neural system for error detection and compensation. Psychological Science. 1993;4:385–390. [Google Scholar]
- Gehring WJ, Himle J, Nisenson LG. Action-monitoring dysfunction in obsessive-compulsive disorder. Psychological Science. 2000;11:1–5. doi: 10.1111/1467-9280.00206. [DOI] [PubMed] [Google Scholar]
- Goldberg MC, Spinelli S, Joel S, Pekar JJ, Denckla MB, Mostofsky SH. Children with high functioning autism show increased prefrontal and temporal cortex activity during error monitoring. Developmental Cognitive Neuroscience. 2011;1:47–56. doi: 10.1016/j.dcn.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon RM. Folk psychology as simulation. Mind and Language. 1986;1(2):158–171. [Google Scholar]
- Hajcak G, McDonald N, Simons RF. Error-related psychophysiology and negative affect. Brain and Cognition. 2003;56:189–197. doi: 10.1016/j.bandc.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Hajcak G, McDonald N, Simons RF. To err is autonomic: Error-related brain potentials, ANS activity, and post-error compensatory behavior. Psychophysiology. 2003;40:895–903. doi: 10.1111/1469-8986.00107. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Simons RF. Error-related brain activity in obsessive–compulsive undergraduates. Psychiatry Research. 2002;110:63–72. doi: 10.1016/s0165-1781(02)00034-3. [DOI] [PubMed] [Google Scholar]
- Handy TC. Basic Principles of ERP Quantification. In: Handy TC, editor. Event-Related Potentials: A Methods Handbook. Cambridge, MA: MIT Press; 2005. pp. 33–55. [Google Scholar]
- Happé F. An advanced test of theory of mind: Understanding the story characters thoughts and feelings by able autistic mentally handicapped and normal children and adults. Journal of Autism and Developmental Disorders. 1994;24:129–154. doi: 10.1007/BF02172093. [DOI] [PubMed] [Google Scholar]
- Henderson HA, Mundy PC. The integration of self and other in the development of self-regulation: Typical and atypical processes. In: Caplovitz Barrett K, Fox NA, Morgan GA, Fidler D, Daunhauer L, editors. Handbook of Self-Regulatory Processes in Development: New Directions and International Perspectives. New York: Psychology Press; 2012. pp. 113–134. [Google Scholar]
- Henderson HA, Schwartz C, Mundy P, Burnette C, Sutton S, Zahka N, et al. Response monitoring, the error-related negativity, and differences in social behavior in autism. Brain and Cognition. 2006;61(1):96–109. doi: 10.1016/j.bandc.2005.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson HA, Zahka NE, Kojkowski NM, Inge AP, Schwartz CB, Hileman CM, Coman DC, Mundy PC. Self-referenced memory, social cognition, and symptom presentation in autism. Journal of Child Psychology and Psychiatry. 2009;50(7):853–861. doi: 10.1111/j.1469-7610.2008.02059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes AJ, Pizzagalli DA. Spatiotemporal dynamics of error processing dysfunctions in major depressive disorder. Archives of General Psychiatry. 2008;65:179–188. doi: 10.1001/archgenpsychiatry.2007.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holroyd CB, Coles MGH. The neural basis of human error processing: Reinforcement learning, dopamine, and the error-related negativity. Psychological Review. 2002;109(4):679–709. doi: 10.1037/0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- Kenworthy L, Case L, Harms MB, Martin A, Wallace GL. Adaptive behavior ratings correlate with symptomatology and IQ among individual with high-functioning autism spectrum disorders. Journal of Autism and Developmental Disorders. 2010;40:416–423. doi: 10.1007/s10803-009-0911-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JA, Szatmari P, Bryson SE, Streiner DL, Wilson FJ. The prevalence of anxiety and mood problems among children with autism and Asperger syndrome. Autism. 2000;4:117–132. [Google Scholar]
- Koegel RL, Koegel LK, Parks DR. Teaching children with autism: Strategies for initiating positive interactions and improving learning opportunities. Baltimore, MD: Paul H. Brookes Publishing Company; 1995. [Google Scholar]
- Kuusikko S, Pollock-Wurman R, Jussila K. Social anxiety in high-functioning children and adolescents with autism and Asperger syndrome. Journal of Developmental Disorders. 2008;38(9):1697–1709. doi: 10.1007/s10803-008-0555-9. [DOI] [PubMed] [Google Scholar]
- Larson MJ, Fair JE, Good DA, Baldwin SA. Empathy and error processing. Psychophysiology. 2010;47:415–424. doi: 10.1111/j.1469-8986.2009.00949.x. [DOI] [PubMed] [Google Scholar]
- Lawrence EJ, Shaw P, Baker D, Baron-Cohen S, David AS. Measuring empathy: Reliability and validity of the Empathy Quotient. Psychological Medicine. 2004;34:911–919. doi: 10.1017/s0033291703001624. [DOI] [PubMed] [Google Scholar]
- Lombardo MV, Chakrabarti B, Bullmore ET, Sadek SA, Pasco G, Wheelwright SJ, Suckling J, Baron-Cohen S MRC AIMS Consortium. Atypical neural self-representation in autism. Brain. 2010;133(2):611–624. doi: 10.1093/brain/awp306. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, Dilavore P, Risi S. Manual: Autism diagnostic observation schedule. Los Angeles, CA: Western Psychological Services; 2001. [Google Scholar]
- Luck SJ. An introduction to the event-related potential technique. Cambridge, MA: MIT Press; 2005. [Google Scholar]
- Mayes SD, Calhoun SL, Murray MJ, Ahuja M, Smith LA. Anxiety, depression, and irritability in children with autism relative to children with other neuropsychiatric disorders and typical development. Autism Spectrum Disorders. 2010;5:474–485. [Google Scholar]
- Mazurek MO, Kanne SM. Friendship and internalizing symptoms among children and adolescents with ASD. Journal of Autism and Developmental Disorders. 2010;40:1512–1520. doi: 10.1007/s10803-010-1014-y. [DOI] [PubMed] [Google Scholar]
- Molloy CA, Murray DS, Akers R, Mitchell T, Manning-Courtney P. Use of the Autism Diagnostic Observation Schedule (ADOS) in a clinical setting. Autism. 2011;15(2):143–162. doi: 10.1177/1362361310379241. [DOI] [PubMed] [Google Scholar]
- Mundy P. Annotation: the neural basis of social impairments in autism: the role of the dorsal medial-frontal cortex and anterior cingulate system. Journal of Child Psychology and Psychiatry. 2003;44(6):793–809. doi: 10.1111/1469-7610.00165. [DOI] [PubMed] [Google Scholar]
- Mundy PC, Henderson HA, Inge AP, Coman DC. The modifier model of autism and social development in higher functioning children with autism. Research & Practice for Persons with Severe Disabilities. 2007;32:124–139. doi: 10.2511/rpsd.32.2.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olvet DM, Hajcak G. The error-related negativity (ERN) and psychopathology: Toward an endophenotype. Clinical Psychology Review. 2008;28:1343–1354. doi: 10.1016/j.cpr.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olvet DM, Hajcak G. The effect of trial-to-trial feedback on the error-related negativity and its relationship with anxiety. Cognitive, Affective, and Behavioral Neuroscience. 2009;9(4):427–433. doi: 10.3758/CABN.9.4.427. [DOI] [PubMed] [Google Scholar]
- Pennington BF, Ozonoff S. Executive functions and developmental psychopathology. Journal of Child Psychology and Psychiatry. 1996;37(1):51–87. doi: 10.1111/j.1469-7610.1996.tb01380.x. [DOI] [PubMed] [Google Scholar]
- Prior M, Eisenmajer R, Leekam S, Wing L, Gould J, Ong B, et al. Are there subgroups within the autistic spectrum? A cluster analysis of a group of children with autistic spectrum disorders. Journal of Child Psychology and Psychiatry. 1998;39(6):893–902. [PubMed] [Google Scholar]
- Rameson LT, Lieberman MD. Empathy: A social cognitive neuroscience approach. Social and Personality Psychology Compass. 2009;3(1):94–110. [Google Scholar]
- Reynolds CR, Kamphaus RW. BASC-2 behavioral assessment system for children manual. 2. Circle Pines, MN: American Guidance; 2004. [Google Scholar]
- Russell J, Jarrold C. Error-correction problems in autism: Evidence for a monitoring impairment. Journal of Autism and Developmental Disorders. 1998;28:177–188. doi: 10.1023/a:1026009203333. [DOI] [PubMed] [Google Scholar]
- Rutter M, Bailey A, Lord C. The social communication questionnaire. Los Angeles, CA: Western Psychological Services; 2003. [Google Scholar]
- Santesso DL, Segalowitz SJ. The error-related negativity is related to risk taking and empathy in young men. Psychophysiology. 2009;46:143–152. doi: 10.1111/j.1469-8986.2008.00714.x. [DOI] [PubMed] [Google Scholar]
- Santesso D, Segalowitz SJ, Schmidt LA. ERP correlates of error monitoring in 10 year olds are related to socialization. Biological Psychology. 2005;70:79–87. doi: 10.1016/j.biopsycho.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Scheeren AM, de Rosnay M, Koot HM, Begeer S. Rethinking theory of mind in high-functioning autism spectrum disorder. Journal of Child Psychology and Psychiatry. 2013;54:628–635. doi: 10.1111/jcpp.12007. [DOI] [PubMed] [Google Scholar]
- South M, Larson MJ, Krauskopf E, Clawson A. Error processing in high-functioning autism spectrum disorder. Biological Psychology. 2010;85(2):242–251. doi: 10.1016/j.biopsycho.2010.07.009. [DOI] [PubMed] [Google Scholar]
- Sukhodolsky D, Scahill L, Gadow K, Arnold L, Aman M, McDougle C, et al. Parent-rated anxiety symptoms in children with pervasive developmental disorders: Frequency and association with core autism symptoms and cognitive functioning. Journal of Abnormal Child Psychology. 2008;36(1):117–128. doi: 10.1007/s10802-007-9165-9. [DOI] [PubMed] [Google Scholar]
- Thakkar KN, Polli FE, Joseph RM, Tuch DS, Hadjikhani N, Barton JJ, Manoach DS. Response monitoring, repetitive behaviour and anterior cingulate abnormalities in autism spectrum disorders (ASD) Brain. 2008;131:2464–2478. doi: 10.1093/brain/awn099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tierney AL, Gabard-Durnam L, Vogel-Farley V, Tager-Flusberg H, Nelson CA. Developmental trajectories of resting EEG power: An endophenotype of autism spectrum disorder. PLoS One. 2012;7:e39127. doi: 10.1371/journal.pone.0039127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursu S, Stenger VA, Shear MK, et al. Overactive action monitoring in obsessive compulsive disorder: evidence from functional magnetic resonance imaging. Psychological Science. 2003;14:347–353. doi: 10.1111/1467-9280.24411. [DOI] [PubMed] [Google Scholar]
- Vaidyanathan U, Nelson LD, Patrick CJ. Clarifying domains of internalizing psychopathology using neurophysiology. Psychological Medicine. 2012;42:447–459. doi: 10.1017/S0033291711001528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg A, Hajcak G. Electrocortical evidence for vigilance–avoidance in generalized anxiety disorder. Psychophysiology. 2011;48:842–851. doi: 10.1111/j.1469-8986.2010.01149.x. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler intelligence scale for children. 4. San Antonio, TX: The Psychological Corporation; 2003. [Google Scholar]
- World Health Organization. International statistical classification of diseases and related health problems; ICD-10. 10. Geneva: WHO; 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung N, Botvinick MM, Cohen JD. The neural basis of error detection: Conflict monitoring and the error-related negativity. Psychological Review. 2004;111(4):931–959. doi: 10.1037/0033-295x.111.4.939. [DOI] [PubMed] [Google Scholar]
- Zahn-Waxler C, Klimes-Dougan B, Slattery MJ. Internalizing problems of childhood and adolescence: Prospects, pitfalls, and progress in understanding the development of anxiety and depression. Development and Psychopathology. 2000;12:443–466. [PubMed] [Google Scholar]