Abstract
Vitamin D derivatives, including its physiological form 1α,25(OH)2 vitamin D3 (1,25D), have anti-tumor actions demonstrated in cell culture and confirmatory epidemiological associations are frequently reported. However, their promise for use in the cancer clinic is still incompletely fulfilled, suggesting that a better understanding of the molecular events initiated by these compounds is needed for therapeutic advances. While ERK1/2 has been intensely investigated and is known to transmit signals for cell survival, growth, and differentiation, the role of other MAPK pathways has been studied sporadically. Therefore, we utilized acute myeloid leukemia (AML) cells in culture (HL60 and U937), to determine if ERK5 has a role in 1,25D-induced terminal differentiation which is distinct from the previously shown involvement of ERK1/2. We previously found that inhibition of kinase activity of ERK5 by specific pharmacological inhibitors BIX02189 or XMD8-92 results in higher expression of general myeloid marker CD11b, but a lower expression of the monocytic marker CD14. In contrast, the inhibition of the ERK1/2 pathway by PD98059 or U0126 reduced the expression of all differentiation markers studied. We report here for the first time that the differentiation changes induced by ERK5 inhibitors are accompanied by the inhibition of cell proliferation, and this occurs in the both G1 and G2 phases of the cell cycle. Of note, inhibition of ERK5 auto-phosphorylation by XMD8-92 results in a particularly robust cell cycle arrest in G2 phase in AML cells. This study provides a link between the 1,25D-elevated ERK5 pathway and changes in the cell cycle phase transitions in AML cells. Thus, combinations of vitamin D derivatives and ERK5 inhibitors may be more successful in cancer clinics than 1,25D or analogs alone.
Keywords: Vitamin D derivatives, ERK5, ERK1/2, MAPK inhibitors, acute myeloid leukemia (AML), cell differentiation
1. Introduction
The physiological form of vitamin D3, 1α, 25(OH)2 vitamin D3 (1,25D), and its synthetic analogs (VDDs) have anti-leukemic actions demonstrated in numerous cell culture studies (e.g. [1–3]). However, clinical trials performed so far were either inconclusive, or failed to show objective improvements, when VDDs were tested as sole therapeutic agents for several types of human cancer [4]. This suggests that a better understanding of the molecular events which are initiated by VDDs and lead to differentiation-associated cell cycle arrest of malignant cells is needed for the design of potential VDD-based regimens for cancer treatment. Such understanding may also lead to selection of patients who are likely to respond to VDDs in clinical trials.
ERK1/2 has been intensely investigated by oncologists as a target for kinase inhibitors in clinical trials of MEK1/2 inhibitors and some successes in solid tumors have been reported (e.g. [5]). A pathway that parallels ERK1/2 signaling is the MEK5-ERK5 pathway which also has been shown to transmit signals for cell survival, growth, epithelial-mesenchymal transition, differentiation, and angiogenesis (see [6] for a recent review). Both preclinical and clinical data suggest an association between increased activity of this signaling pathway and tumorigenesis as well as disease progression (e.g. [7]). However, in contrast to the MEK1/2-ERK1/2 pathway, targeting of the MEK5-ERK5 pathway in clinic has been only scarcely addressed.
Recently, we reported that 1,25D is capable of activating a proto-oncogene kinase Cot1 which results in the upregulation of ERK5, its downstream effector, in human AML cell lines [8]. Further, we found that inhibition of Cot1 either by a pharmacologic inhibitor or by Cot1-siRNA lead to increased cell differentiation and G1 cell cycle arrest in response to 1,25D [8]. However, the functional role of ERK5 in 1,25D-induced differentiation and inhibition of cell cycle progression of AML cells remained unclear.
2. Materials and Methods
2.1. Cell lines, cell culture, and inhibitors
HL60-G cells (FAB M2), subcloned from HL60 cells, and U937 monoblastic cells (FAB M4) were cultured in suspension under standard conditions [8]. For experiments, cells (75K/ml) were pre-treated with kinase inhibitors. These were the specific ERK1/2 inhibitors PD98059 (Selleckchem, Houston, TX) and U0126 (Selleckchem); as well as BIX02189 (Selleckchem), the inhibitor of MEK5 which phosphorylates ERK5 and MD8-92 (Santa Cruz, Dallas, TX), which inhibits autophosphorylation of ERK5 and its nuclear translocation. The cells were treated with the indicated concentrations of these inhibitors or with 0.1% DMSO (vehicle) for 1 h before the addition of 1,25D or 0.1% ethanol, followed by incubation for another 96 h.
2.2. Determination of differentiation markers
The expression of cell surface markers of myeloid differentiation was determined by dual labeling of the cells with 10 μL of PBS containing 0.5 μg anti-CD11b MO1-FITC and 0.5 μg anti-CD14 My4-RD-1 (Beckman Coulter) followed by two-parameter flow cytometric analysis, as described previously [8].
2.3. Cell proliferation assay
Cell number and viability were estimated on the basis of the trypan blue exclusion assay, by enumerating live and dead cells in a Vi-Cell XR cell viability analyzer (Beckman Coulter).
2.4. Cell cycle analysis
The DNA content of HL60 and U937 cells was determined by propidium iodide staining and analyzed using an EPICS or FC500 flow cytometer (Beckman Coulter) [8]. The resultant histogram of DNA content was analyzed using the Multicycle program (Phoenix Flow Systems) to determine the proportions of cells in each phase of the cell cycle.
2.5. Western blotting
Immunoblotting was performed by using total cell extracts according to standard procedures [8]. The following antibodies: P-p21 Ser146 (sc-12902), p21 (sc-397), P-p27 Ser10 (sc-12939), p27 (sc-528), and Crk-L (sc-319) were obtained from Santa Cruz Biotechnology (Dallas, TX).
2.6 Statistical analysis
Each experiment was repeated at least three times, and the results were expressed as the mean ± SD, and were subjected to paired student’s t-test using the Microsoft Excel program. A value of p < 0.05 was considered statistically significant.
3. Results and Discussion
3.1. Inhibitors of ERK5, but not of ERK1/2, alter the phenotype of AML cells during terminal differentiation
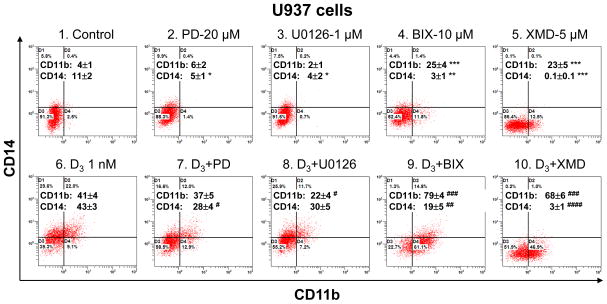
We have recently found that pretreatment of human AML cells with MEK5 inhibitor, BIX02189 [9], or ERK5 autophosphorylation inhibitor, XMD8-92 [10], leads to a marked decrease in 1,25D-induced expression of the monocytic differentiation marker CD14 and an increase in the expression of CD11b, a general marker of myeloid differentiation (in preparation). In the current report we focus on the role of ERK5 in cell cycle control of AML cells induced to terminal differentiation by 1,25D, and also contrast this with the role of ERK1/2 in this process. As shown in Fig. 1, pretreatment of U937 cells with BIX02189 or XMD8-92 had contrasting effects on cell phenotype when compared to treatment with the widely employed MEK1/2 kinase inhibitors, PD98059 and U0126 (compare panels 9 and 10 with panels 7 and 8 in Fig 1). These data indicate that ERK5 and ERK1/2 pathways have distinct contributions to 1,25D-induced terminal differentiation of AML cells.
Fig. 1. Reciprocal modulation of basal and 1,25D-induced expression of CD11b and CD14 by MEK5/ERK5 inhibitors contrasts with effects of MEK1/2 inhibitors.
Primary data from a typical experiment. Average CD11b and CD14 values are presented in each panel as the means ± SE (n = 3–9). *, P < 0.05; **, P < 0.01 and ***, P < 0.001 versus control. #, P < 0.05; ##, P < 0.01; ###, P < 0.001; and ####, P < 0.0001 versus 1,25D alone.
3.2. ERK5 is required for optimal AML cell proliferation and cell cycle transitions
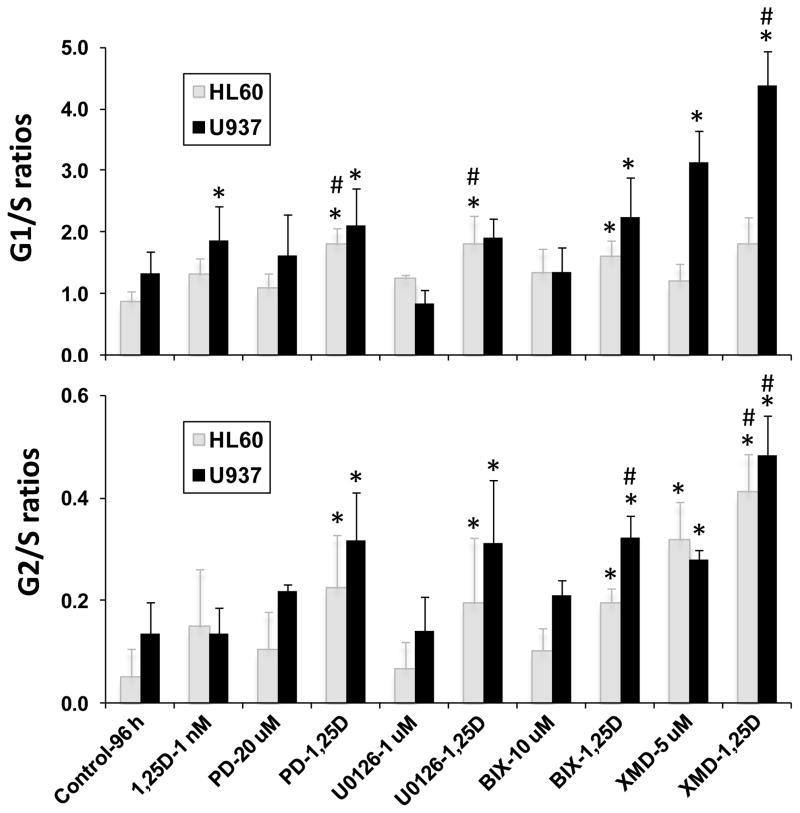
Inhibition of ERK5 autophosphorylation of its C-terminal by XMD8-92 had a greater effect on cell cycle transitions than inhibition of ERK5 phosphorylation by BIX02189 or inhibition of ERK1/2 by other MAPK inhibitors (Fig. 2). Although these differences between the two classes of inhibitors were numerically quite evident, especially in U937 cells, statistical significance was not reached in this small series, suggesting that further studies of these phenomena may provide a better insight into 1,25D-induced changes in AML phenotype. However, as shown in Fig 2, the differences resulting from the inhibitor treatment were generally significant when compared to the control or 1,25D-treated groups.
Fig. 2. Inhibition of MAPK activity enhances 1,25D-induced G1 and G2 cell cycle arrest in AML cells.
G1 to S and G2 to S phase ratios. Values are the means ± SD (n = 3). *, P < 0.05 versus control. #, P < 0.05 versus 1,25D alone.
The apparently greater effect of XMD8-92 on cell cycle transitions is probably due to the abrogation by XMD8-92 of nuclear translocation of ERK5 which requires phosphorylation on its C-terminal and thereby directs p27Kip1/p21Cip1 upregulation by nuclear transcription factors [10]. In contrast, N-terminal phosphorylation of ERK5 by MEK5 was insufficient for nuclear translocation, although activated cytoplasmic ERK5 can affect events such as AKT or serum and glucocorticoid-inducible kinase (SGK) signaling, but not p27/p21 upregulation [6, 11]. Accordingly, XMD8-92 is more effective than the other MAPK inhibitors studied here in inducing cell cycle arrest. Note that cell cycle inhibition was particularly marked when XMD8-92 was combined with 1,25D (Fig. 2), and inhibition of the ERK5 pathway was accompanied by a slower rate of cell proliferation (data not shown).
3.3. ERK5 dampens the expression of negative regulators of cell cycle progression
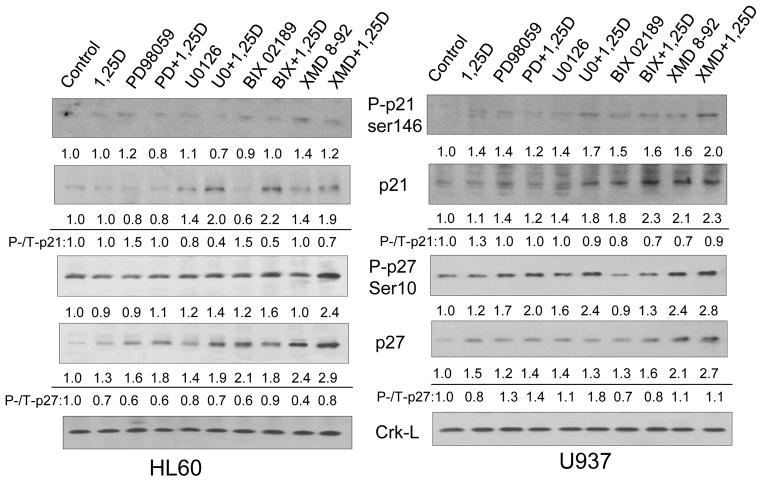
Treatment of AML cells with 1,25D is known to increase the levels of cell cycle negative regulators p27 and p21[12, 13], but at low 1,25D concentration these increases are modest in short experiments (Fig. 3). However, the addition of ERK1/2 and, especially, of ERK5 inhibitors together with 1,25D markedly increased the protein levels of p27 proteins and, to a lesser extent, the p21 proteins (Fig. 3). Consistent with the extent of cell cycle arrest, the inhibitor of ERK5 autophosphorylation, XMD8-92, induced the most marked increase in p27 in both cell lines. While the levels of phosphorylated p27 and p21 forms also increased under these conditions, particularly in U937 cells, the principal increases were at the level of protein abundance, not the phosphorylation of the cell cycle regulators.
Fig. 3. Inhibition of ERK5 activity by pharmacological inhibitors enhances the 1,25D-induced increase in the protein levels of cell cycle inhibitors p21Cip1 and p27Kip1.
Representative Western blots with relative optical densities (OD) under each signal. The ratios of phosphorylated to total protein expression are also indicated to show that phosphorylation is not the primary event. Crk-L is the protein loading control.
4. Conclusion
We report here for the first time that the cell cycle arrest following addition of ERK5 inhibitors BIX02189 or XMD8-92 to AML cells differentiating in the presence of 1,25D can take place in both G1 and G2 phases of the cell cycle. Of note, the inhibition of ERK5 auto-phosphorylation by XMD8-92 results in a particularly robust cell cycle arrest in the G2 phase in these cells, and correlates with the most marked effect of XMD8-92 on monocytic differentiation (Table 1). Thus, this study provides evidence that 1,25D- stimulated ERK5 pathway diminishes the ability of 1,25D to induce terminal differentiation of AML cells which is associated with cell cycle arrest. It therefore seems that combinations of vitamin D derivatives with ERK5 inhibitors may be more successful in cancer clinics than 1,25D or its analogs alone.
Table 1.
ERK pathways and monocytic differentiation of HL60 and U937 cells.
| Cell lines | HL60 | U937 | ||||
|---|---|---|---|---|---|---|
| Effects | ERK1/2 | ERK5 | Monocytic Diffn | ERK1/2 | ERK5 | Monocytic Diffn |
| Control-96 h | − | − | − | − | − | − |
| 1,25D-1 nM | ++ | + | ++ | ++ | + | ++ |
| PD-20 uM | − | − | − | − | − | − |
| PD-1,25D | − | + | + | − | + | + |
| U0126-1 uM | − | − | − | − | − | − |
| U0126-1,25D | − | + | + | − | + | + |
| BIX-10 uM | + | − | − | + | − | − |
| BIX-1,25D | ++ | − | + | ++ | − | + |
| XMD-5 uM | + | − | − | + | − | − |
| XMD-1,25D | ++ | − | − | ++ | − | − |
Note that ERK1/2 inhibitors PD 98059 (PD) and U0126 inhibit 1,25D-induced monocytic differentiation. Similarly, the MEK5 inhibitor BIX02189 (BIX) modestly reduces monocytic differentiation, while the ERK5 autophosphorylation inhibitor XMD8-92 (XMD) has a more marked inhibitory effect on differentiation.
Symbols: (−), 0–15%; (+), 16–30%; and (++), 31–50% differentiated cells.
Highlights.
ERK5 inhibitors combined with 1,25D arrest proliferation and cell cycle in AML cells
G1 and G2 cell cycle transitions are facilitated by ERK5 autophosphorylation
1,25D and ERK5 inhibitors increase p27 at the level of protein abundance
Acknowledgments
Supported by the NIH grant R01-CA 044722-22 from the National Cancer Institute to GPS, and by the Israel Science Foundation grant 635/11 to MD.
Abbreviations
- 1,25D
1α, 25(OH)2 vitamin D3
- AML
acute myeloid leukemia
- CC
cell cycle
- VDD
vitamin D derivative
- BIX
BIX02189
- PD
PD98059
- U0
U0126
- XMD
XMD8-92
Footnotes
The authors have no conflict of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abe E, Miyaura C, Sakagami H, Takeda M, Konno K, Yamazaki T, Yoshiki S, Suda T. Differentiation of mouse myeloid leukemia cells induced by 1a,25-dihydroxyvitamin D3. Proc Natl Acad Sci U S A. 1981;78:4990–4994. doi: 10.1073/pnas.78.8.4990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Studzinski GP, Bhandal AK, Brelvi ZS. A system for monocytic differentiation of leukemic cells HL 60 by a short exposure to 1,25-dihydroxycholecalciferol. Proc Soc Exp Biol Med. 1985;179:288–295. doi: 10.3181/00379727-179-42098. [DOI] [PubMed] [Google Scholar]
- 3.Norman AW, Manchand PS, Uskokovic MR, Okamura WH, Takeuchi JA, Bishop JE, Hisatake JI, Koeffler HP, Peleg S. Characterization of a novel analogue of 1alpha,25(OH)2-vitamin D3 with two side chains: interaction with its nuclear receptor and cellular actions. J Med Chem. 2000;43:2719–2730. doi: 10.1021/jm0000160. [DOI] [PubMed] [Google Scholar]
- 4.Harrison JS, Bershadskiy A. Clinical experience using vitamin d and analogs in the treatment of myelodysplasia and acute myeloid leukemia: a review of the literature. Leukemia research and treatment. 2012;2012:125814. doi: 10.1155/2012/125814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fremin C, Meloche S. From basic research to clinical development of MEK1/2 inhibitors for cancer therapy. Journal of hematology & oncology. 2010;3:8. doi: 10.1186/1756-8722-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nithianandarajah-Jones GN, Wilm B, Goldring CE, Muller J, Cross MJ. ERK5: structure, regulation and function. Cellular signalling. 2012;24:2187–2196. doi: 10.1016/j.cellsig.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 7.Montero JC, Ocana A, Abad M, Ortiz-Ruiz MJ, Pandiella A, Esparis-Ogando A. Expression of Erk5 in early stage breast cancer and association with disease free survival identifies this kinase as a potential therapeutic target. PLoS One. 2009;4:e5565. doi: 10.1371/journal.pone.0005565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang X, Gocek E, Novik V, Harrison JS, Danilenko M, Studzinski GP. Inhibition of Cot1/Tlp2 oncogene in AML cells reduces ERK5 activation and up-regulates p27Kip1 concomitant with enhancement of differentiation and cell cycle arrest induced by silibinin and 1,25-dihydroxyvitamin D3. Cell Cycle. 2010;9:4542–4551. doi: 10.4161/cc.9.22.13790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tatake RJ, O’Neill MM, Kennedy CA, Wayne AL, Jakes S, Wu D, Kugler SZ, Jr, Kashem MA, Kaplita P, Snow RJ. Identification of pharmacological inhibitors of the MEK5/ERK5 pathway. Biochemical and biophysical research communications. 2008;377:120–125. doi: 10.1016/j.bbrc.2008.09.087. [DOI] [PubMed] [Google Scholar]
- 10.Yang Q, Deng X, Lu B, Cameron M, Fearns C, Patricelli MP, Yates JR, 3rd, Gray NS, Lee JD. Pharmacological inhibition of BMK1 suppresses tumor growth through promyelocytic leukemia protein. Cancer Cell. 2010;18:258–267. doi: 10.1016/j.ccr.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Erazo T, Moreno A, Ruiz-Babot G, Rodriguez-Asiain A, Morrice NA, Espadamala J, Bayascas JR, Gomez N, Lizcano JM. Canonical and kinase activity-independent mechanisms for extracellular signal-regulated kinase 5 (ERK5) nuclear translocation require dissociation of Hsp90 from the ERK5-Cdc37 complex. Mol Cell Biol. 2013;33:1671–1686. doi: 10.1128/MCB.01246-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang QM, Jones JB, Studzinski GP. Cyclin-dependent kinase inhibitor p27 as a mediator of the G1-S phase block induced by 1,25-dihydroxyvitamin D3 in HL60 cells. Cancer Res. 1996;56:264–267. [PubMed] [Google Scholar]
- 13.Liu M, Lee MH, Cohen M, Bommakanti M, Freedman LP. Transcriptional activation of the Cdk inhibitor p21 by vitamin D3 leads to the induced differentiation of the myelomonocytic cell line U937. Genes Dev. 1996;10:142–153. doi: 10.1101/gad.10.2.142. [DOI] [PubMed] [Google Scholar]