Abstract
Objective
Roux-en-Y gastric bypass (RYGB) surgery causes greater weight loss than laparoscopic adjustable gastric banding (LAGB). We tested the hypothesis that RYGB has weight loss-independent effects on taste perception which influence eating behavior and contribute to the greater weight loss.
Design and Methods
Subjects were studied before and after ~20% weight loss induced by RYGB (n=17) or LAGB (n=10). We evaluated: taste sensitivity for sweet, salty and savory stimuli; sucrose and monosodium glutamate (MSG) preferences; sweetness palatability; eating behavior; and expression of taste-related genes in biopsies of fungiform papillae.
Results
Weight loss induced by both procedures caused the same decrease in: preferred sucrose concentration (−12±10%), perceived sweetness of sucrose (−7±5%), cravings for sweets and fast-foods (−22 ±5%), influence of emotions (−27±5%) and external food cues (−30±4%) on eating behavior, and expression of α-gustducin in fungiform papillae (all P-values <0.05). RYGB, but not LAGB, shifted sweetness palatability from pleasant to unpleasant when repetitively tasting sucrose (P=0.05). Neither procedure affected taste detection thresholds or MSG preferences.
Conclusions
LAGB and RYGB cause similar alterations in eating behaviors, when weight loss is matched. These changes in eating behavior were not associated with changes in taste sensitivity, suggesting other, as yet unknown, mechanisms are involved.
Keywords: taste, eating behavior, gastric bypass, laparoscopic banding, bariatric surgery
Introduction
Bariatric surgery is the most effective weight loss therapy for obesity because of its profound effects on eating behavior and food intake. Procedures that divert ingested nutrients away from the upper gastrointestinal tract, such as Roux-en-Y gastric bypass (RYGB), cause greater weight loss than those that simply restrict stomach size, such as laparoscopic adjustable gastric banding (LAGB).1 Data obtained from studies that used dietary recall methods and eating behavior questionnaires, suggest that patients who have had RYGB surgery decrease the proportion of their daily calorie intake from sweetened foods and beverages more than subjects who have had banded gastroplasty, 2–5 a behavior that could significantly contribute to the greater reduction in total energy intake observed after RYGB than LAGB surgery.
The mechanisms responsible for the decreased intake of sweetened foods after RYGB surgery are unknown, but could involve changes in taste perception. Taste perception involves two major psychological components, including a sensory-discriminative component and a hedonic component. 6 The sensory-discriminative component refers to taste quality (sweet, salty, savory, bitter and sour) and taste sensitivity, which ranges from what is the lowest concentration of taste stimuli that can be detected (taste detection thresholds) to how intense a taste stimuli is perceived (above-threshold responses). The hedonic component of taste perception accounts for how much the stimulus is liked or disliked.6
RYGB is associated with decreased hedonic value for sweet or highly palatable foods. 7–11 However, the effect of RYGB on taste sensitivity is unclear because of conflicting results from different studies, which have reported subjects became more sensitive (lower taste thresholds) to bitterness but not sweetness 12 or more sensitive to sweetness 13 but not bitterness 14 after RYGB surgery. An important limitation of these studies is that taste thresholds usually do not correlate with above-threshold sensory function; 15–16 therefore the consequence of having an altered taste threshold in food selection is unclear. In addition, it is not known whether changes in taste perception are due to altered food intake and weight loss itself or whether the anatomical alteration associated with RYGB has weight loss-independent effects on taste perception.
The primary purpose of the present study was to test the hypothesis that anatomical diversion of ingested nutrients from the upper gastrointestinal tract by RYGB has weight loss-independent and diet-independent effects on taste perception and eating behavior compared with LAGB. Accordingly, we evaluated the sensory-discriminative and hedonic components of taste perception, and eating behavior in obese women before and after subjects lost 20% of their body weight induced by either RYGB or LAGB surgery. We also evaluated the effects of surgery-induced weight loss on the cellular factors involved in the transduction of taste signals in fungiform papillae in a subsample of study subjects.
Methods and Procedures
Subjects
The study population consisted of 27 consecutive obese women who were scheduled to undergo either RYGB (n=17) or LAGB (n=10) procedures at Barnes-Jewish Hospital (St. Louis, MO, USA). Subjects who had LAGB served as a control group to account for the independent effects of weight loss and dietary intake on our study outcome measures. We only studied women because most patients who have bariatric surgery are women 17 and sex can affect taste perception 18. We excluded potential subjects who had diabetes, smoked cigarettes, were taking any medication that might affect taste, had previous intestinal surgery, inflammatory intestinal disease, signs of oral disease, a history of chronic rhinitis, or severe organ dysfunction. All subjects provided written informed consent before participating in this study, which was approved by the Washington University Institutional Review Board.
Experimental Procedures
Subjects were admitted to the Washington University School of Medicine Clinical Research Unit (CRU) after fasting for 12 h overnight at home. To avoid sensory system fatigue, the tests used in this study were administered in three separate 2-h sessions that were scheduled at least one day apart.
Sensory-discriminative component of taste perception
1. Detection Thresholds
Threshold sensitivities to sucrose, glucose, NaCl and monosodium glutamate (MSG; prototypical savory stimuli) were assessed separately by using a two-alternative, forced-choice staircase procedure as previously described.16
2. Above-threshold sensory function
After subjects were trained on the use the general Labeled Magnitude Scale (gLMS),19 we assessed intensity perception of a series of concentrations of sucrose, glucose, NaCl and MSG. Each taste stimulus was separately presented in two blocks of four concentrations and the four concentrations for each taste stimulus were presented in random order without replacement. The mean of the intensity at each concentration (for each taste quality) during the two-block series provided the estimate of subjects taste intensity perception.
Hedonic component of taste perception
1. Preference Tests
A forced-choice, paired-comparison, tracking technique, was used to determine subjects preferred concentration of sucrose and MSG. Subjects were presented with pairs of solutions that differed in concentration of the stimuli being assessed (sucrose or MSG) and preferences were determined as previously described.16, 20
2. Sweet Taste Palatability Test
Subjects were instructed to sip and taste 10 samples without swallowing (one every 2 min, with no interstimulus rinse). The samples, which were presented in medicine cups and contained 10 ml of a 24% w/v sucrose solution, were tasted for 10 s. Subjects rated changes in hedonic responses immediately after spitting out each sample, by answering two questions: 1) “How pleasant was the taste?” and 2) “How strong is your desire for a different taste?”. Subjects rated the first question by using the hedonic version of the gLMS and the second question by using a regular gLMS for intensity.
Taste stimuli
For detection threshold testing, sucrose, glucose, NaCl (all from Sigma-Aldrich, Inc., St Louis, MO, USA) and MSG (USB Corp., Cleveland, OH, USA) concentrations ranging from 1 to 1 × 10−4 M were prepared in quarter-log dilution steps. To test suprathreshold intensity perception, we used 0.00, 0.09, 0.36, and 1.05 M sucrose solutions, 0.00, 0.32, 0.56 and 1.00 M glucose solutions, 0.00, 0.056, 0.18 and 0.56 M NaCl solutions, and 0.00, 0.02, 0.06, and 0.18 M MSG solutions. All solutions were prepared using deionized water and presented at room temperature (22°C).
For preference testing, we used 0.09, 0.18, 0.35, 0.70, and 1.05 M sucrose and 0.018, 0.032, 0.056, 0.100, and 0.180 M MSG. The addition of MSG generally decreases palatability to room-temperature water solutions but increases palatability in foods such as soup.21 Therefore, we warmed the MSG solutions in water to 40°C and kept the solutions in thermal bottles until testing.
Eating behavior
Subjects completed the following standardized questionnaires: i) Dutch Eating Behavior Questionnaire (DEBQ), 22 ii) Food Craving Inventory (FCI), 23 iii) Sweet Taste Questionnaire (STQ), 24 and iv) Fat Preference Questionnaire (FPQ).25 The DEBQ measures three common psychological dimensions of eating behavior: 1) emotional eating (an inclination to eat in response to negative emotions such as depression or feelings of loneliness), 2) external eating (an inclination to eat in response to external food cues such as the smell of food), and 3) restrained eating (an inclination to consciously restrict food intake to control body weight). 22 The FCI is a validated measure of the frequency of overall food cravings as well as cravings for specific types of foods (high fats, sweets, carbohydrates/starches, and fast-food fats) during the past month.23 For the DEBQ and the FCI, subjects score their answers by using a 5-point Likert scale (1=never, 5=very often/always). The STQ identifies two factors associated with sweet food consumption: 1) sensitivity to the mood altering effects of sweets, and 2) impaired control over eating sweet foods. 24 In the STQ, subjects respond to 12 items using 7-point Likert scales ranging from “Strongly disagree” to “Strongly agree”. The FPQ is a validated instrument that assesses the preference for dietary fat by measuring the percentage of food sets in which high-fat were selected over lower-fat choices of the same food to “taste better” (TASTE score) and to be “eaten more often” (FREQ score).25
Tongue biopsy
Lingual fungiform papillae biopsies were obtained as described previously.26 Fungiform papillae were clipped from the dorsal surface of the anterior tongue using a curved spring micro-scissors (McPherson-Vannas type, Roboz, Rockville, MD, USA). A small Dumont forceps was used to place the papillae immediately in a tube containing RNAlaterTM (Ambion), which was stored overnight at 4°C and then transferred to −20°C until RNA extraction. Due to a delay in scheduling the training needed to perfom this technique (kindly provided by Drs. A. Spielman and J. Brand, Monell Chemical Senses Center, Philadelphia, PA); fungiform papillae biopsies were obtained in a subsample of 15 subjects (9 RYGB and 6 LAGB).
Surgical Procedures
Bariatric surgeries were performed using standard laparoscopic approaches. The RYGB procedure involved creating a small (~20 ml) proximal gastric pouch and a stapled gastrojejunostomy. A 75–150 cm Roux-Y limb was constructed by transecting the jejunum 30 cm distal to the ligament of Treitz and performing a stapled jejunojejunostomy at this site. 27 The standard pars flaccid technique was used for LAGB (Lap-Band, Allergan, Irvin, CA, USA).27
Diet Management after Surgery
Subjects participated in an individual supervised weight management program to help subjects in both groups consume a similar energy-deficit diet and achieve a 20% weight loss within 4–6 months after surgery. Subjects were instructed to consume a liquid diet for the first week after surgery, followed by a 2–4 week progression to a regular-food diet containing 1000–1200 kcal/day and 1.0 g of protein/kg body weight/day. A study dietician with expertise in weight management met with the subjects, or contacted them by phone, weekly to monitor body weight, review dietary intake, provide standard weight management behavioral education and adjust recommended energy intake as needed to achieve weight loss targets. After subjects achieved the targeted 20% weight loss, a weight maintenance diet was prescribed, and subjects maintained a stable body weight (<2% change) for at least 2 weeks before repeat studies were performed.
Tongue sample analyses
Quantitative PCR
RNA from tongue biopsy samples was isolated by using TRIzol reagent (Life Technologies, Carlsbad, CA, USA) cDNA was synthesized (Superscript VILO kit, Life Technologies) and gene specific amplification was performed utilizing SYBR Green chemistry on an ABI 7500 FAST qPCR cycler (Applied Biosystems, Foster City, CA, USA). Expression levels of each gene was determined by using the formula 2−ΔCt after correcting the threshold crossing (Ct) of each sample to the housekeeping control gene, acidic ribosomal phosphoprotein P0 (36B4). The primer sequences used are shown in Table 1. Fold changes were calculated by dividing the value post surgery by the value pre-surgery. Whenever the number was less than one, the (negative) reciprocal is shown (e.g. 0.3 or a drop of 75% from before surgery is reported as −3.3 fold change). Gene expression of α-gustducin could not be obtained in one RYGB subject before surgery and one LAGB subject after surgery, and gene expression of TAS1R1 could not be obtained in one LAGB subject after surgery.
TABLE 1.
Primer sequences used for quantitative PCR analysis of fungiform taste papillae
| Gene | Forward | Reverse |
|---|---|---|
| 36B4 | GTGATGTGCAGCTGATCAAGACT | GATGACCAGCCCAAAGGAGA |
| PLCB2 | TGCCAAGATGCCCAAGAGCCAGAA | TTGGCCGTCAGCGGATGTTTGA |
| αgustducin | TCCCAGAAGTGCAGAGGACCAA | TCAGCCAGTTGAGGTGTCATGC |
| TAS1R1 | GCGCACCATCCCCAATGACAAGTA | TAGTCGTCACTGCTGCCAACCAGA |
| TAS1R2 | ACATTTCCCGTGTGGTGGCTGT | TGGCGCTGTAGGTGATCTGTGGAA |
| TAS1R3 | TCTGACAACCAGAAGCCCGTGT | ATGTCGTCTGGGTTTTGCCGGT |
Statistical analyses
Two-way ANOVAs with group (RYGB and LAGB) as the between-subjects factor and time (before after surgery) were used to determine whether surgery-induced weight loss and the specific surgical procedure causes changes in: 1) taste detection thresholds, 2) perceived intensity of above-threshold concentrations, 3) degree of pleasure from tasting sweetness, 4) desire for tasting something non-sweet, and 5) eating behavior questionnaires scores. Sucrose and MSG detection thresholds were positively skewed and required logarithmic transformation to approximate a normal distribution, whereas perceived taste intensity of sucrose and MSG, and desire for a different taste required square root transformations.
Differences in the expression of taste-related genes in fungiform papillae were assessed by using Wilcoxon matched pairs tests. Pearson correlation coefficients were used to determine whether there was a relationship between changes in perceived sweetness intensity and the most preferred sucrose concentration before vs. after surgery. Data in the tables and figures are presented as means ± SD unless otherwise indicated, or medians with semi-interquartile range (SIQR= [75th – 25th percentile]/2) for skewed data sets. All analyses were performed with Statistica 8.0 (StatSoft Inc., Tulsa, OK), and criterion for statistical significance was P≤0.05.
Differences in taste detection thresholds were the primary outcome measures of this study. Based on reproducibility data from a previous study,28 we estimated that 10 subjects in each surgery group would be needed to detect a 60% difference in taste detection thresholds between the RYGB and the LAGB group with a β-value of 0.20 (i.e., 80% power) and an α-value of 0.05. This proposed difference was a reasonable expectation based on data from other taste perception studies conducted in women, which found obesity is associated with a 100% increase in detection thresholds for MSG.16
RESULTS
Characteristics of subjects
Characteristics that have been associated with sweetness preferences are presented in Table 2. There were no significant differences in age, BMI before and after surgery, race, co-morbidities, and years of education or income level between groups.
TABLE 2.
Subjects Characteristics
| RYGB (n=17) | LAGB (n=10) | P value | |
|---|---|---|---|
| Age (years) | 42.1 ± 8.4 | 46.8 ± 13.9 | 0.28 |
| Body weight (kg) | |||
| Before surgery | 123.8 ± 19.7 | 127.1 ± 31.0 | 0.74 |
| After surgery | 98.7 ± 15.6 | 103.7 ± 26.4 | 0.54 |
| Percent weight loss | 20.3 ±3.0 | 18.4 ± 2.0 | 0.11 |
| BMI (kg/m2) | |||
| Before surgery | 46.3 ± 7.7 | 48.5 ± 10.5 | 0.53 |
| After surgery | 36.9 ± 5.9 | 39.7 ±9.5 | 0.34 |
| Co-morbidities (%) | |||
| Hypercholesterolemia | 35 | 30 | 0.56 |
| Hypertension | 65 | 50 | 0.11 |
| Depression | 71 | 60 | 0.44 |
| Race (%) | |||
| White | 70 | 80 | |
| Black | 18 | 20 | 0.53 |
| Other/mixed | 12 | 0 | |
| Yearly income (%of group) | |||
| <$35,000 | 17.7 | 30.0 | |
| $35,000 – 75,000 | 52.9 | 40.0 | 0.72 |
| >$75,000 | 29.4 | 30.0 | |
| Years of education | 14.1 ± 2.0 | 14.3 ± 2.1 | 0.83 |
Values are means ± SD.
Effects that were similar between RYGB and LAGB
There were no differences in taste detection thresholds, in the above-threshold perception of taste intensity or taste preferences for sucrose or MSG between RYGB and LAGB groups, so the values from each surgical group were combined.
Sensory-discriminative component of taste perception
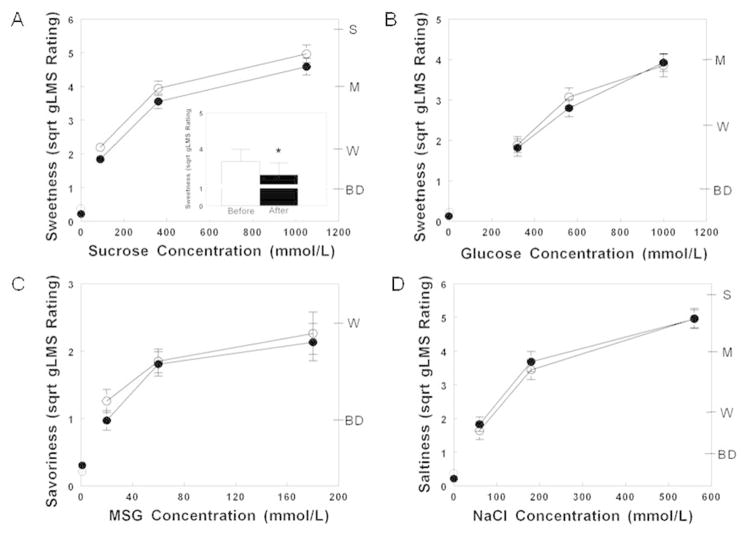
Taste detection thresholds after surgery-induced weight loss were not different than those measured before surgery (all P values >0.30; Table 2). The above-threshold perception of taste intensity increased progressively with an increase in stimulus concentration (sucrose sweetness: P<0.00001, glucose sweetness: P<0.0001; NaCl saltiness: P<0.00001 and MSG savoriness: P<0.00001) both before and after surgery. Sucrose was perceived as 7±5% less sweet after surgery than before surgery (P=0.03; Figure 1A). In contrast, the perceived sweetness of glucose, savoriness of MSG, and saltiness of NaCl did not change after surgery-induced weight loss (all P values >0.45; Figure 1B, 1C and 1D).
Figure 1.
Perceived sweetness of increasing concentrations of sucrose (A) and glucose (B), savoriness of increasing concentrations of MSG (C) and saltiness of increasing NaCl concentrations (D) before (open symbols) and after (closed symbols) 20% weight loss induced by bariatric surgery. Data are mean values ± SEM. * Significantly different from sucrose sweetness perception before surgery, P<0.05. MSG, monosodium glutamate; NaCl, sodium chloride; BD, barely detectable; W, weak; M, moderate; S, strong.
Hedonic component of taste perception
Preference
Lower sucrose concentrations were preferred after than before surgery-induced weight loss in both groups (P=0.008; Figure 2A). Furthermore, there was a negative association between changes in perceived sweetness intensity and the most preferred sucrose concentration (r= −0.53; P=0.004). Therefore, when the change in the perception of sweetness intensity was included as a covariate in the analysis of sucrose preference, the statistical significance of the effect of surgery on preferred sucrose concentration was even stronger (P=0.0002). In contrast, the preferred concentration of MSG did not change after surgery (P>0.37; Figure 2B).
Figure 2.
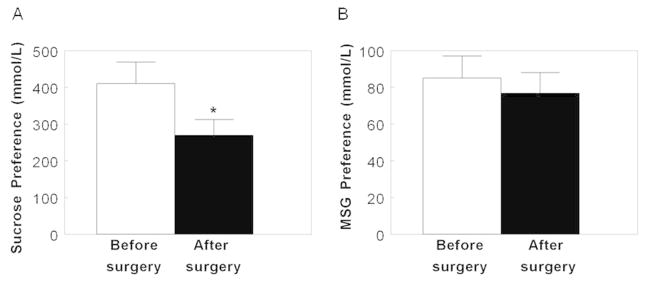
Sucrose (A) and MSG (B) preferences before (white bars) and after bariatric (black bars) 20% weight loss induced by bariatric surgery. Data are mean values ± SEM.* Significantly different from sucrose preference before surgery, P<0.05. MSG, monosodium glutamate.
Eating Behavior
RYGB tended to cause a greater decrease in the mood altering effects of sweets than did LAGB (P=0.07) (Table 2). Weight-loss induced by both surgical procedures was associated with a similar improvement in the control of eating sweets and a similar decrease in the frequency of cravings for sweets and fast-food, emotional eating behavior and external eating behavior (all P values <0.001; Table 2).
Cellular factors involved in taste signaling
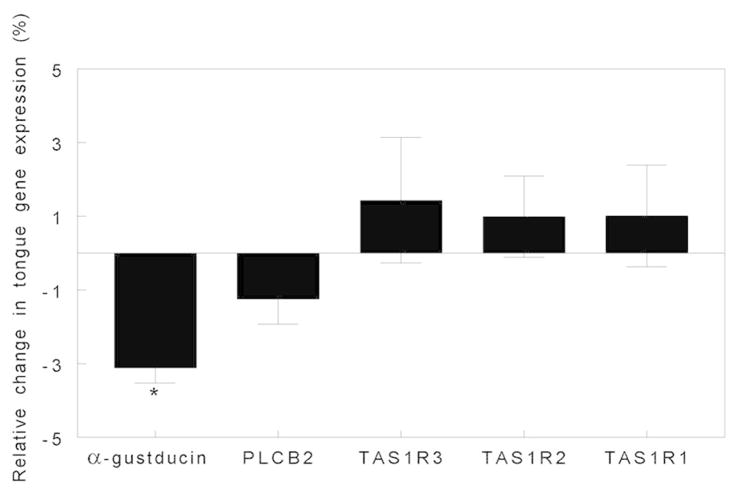
Weight loss induced by both surgical procedures caused a threefold decrease in lingual fungiform papillae gene expression of α-gustducin (P<0.05), a protein involved in the transduction pathways of sweet, bitter and savory sensing taste cells, but did not affect gene expression of the T1R family (i.e. T1R1+T1R3 for savory and T1R2+T1R3 for sweet receptors) or of phospholipase C-β2, a taste bud-specific phospholipase (Figure 4).
Figure 4.
Fold change in fungiform papillae gene expression of α-gustducin (a taste-specific G protein), PLCβ2 (a taste bud-specific phospholipase), and TAS1R1, TAS1R2 and TAS1R3 (taste receptor genes) after 20% weight loss induced by LAGB and RYGB surgeries. Data are median values ± semi-interquartile range ([75th–25th percentile]/2). *Significantly different from gene expression before surgery, P < 0.05. LAGB, laparoscopic adjustable gastric banding; RYGB, Roux-en-Y gastric bypass.
Effects that were dissimilar between RYGB and LAGB
Sweet Taste Palatability
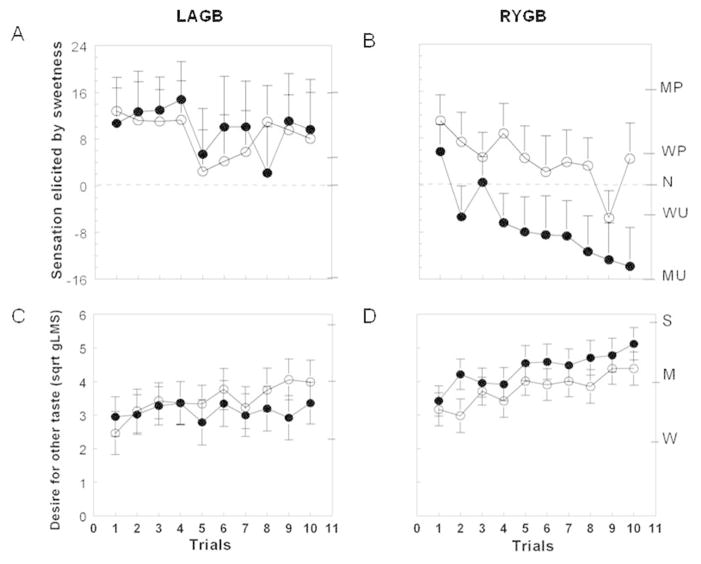
Both surgery groups experienced similar levels of pleasure which decreased slightly during repetitive tasting of sucrose before surgery. However, the hedonic value elicited by repetitive tasting of sucrose changed from pleasant to unpleasant after RYGB, but did not change after LAGB (P=0.05; Figure 3A and 3B). The desire to taste something different than sweet tended to increased more when repetitively tasting sucrose after RYGB than after LAGB (P=0.08; Figure 3C and 3D).
Figure 3.
Hedonic value (top panel) and desire for other taste (bottom panel) when tasting an unswallowed sucrose solution across 10 trials before (open symbols) and after (closed symbols) 20% weight loss induced by LAGB (A and C) or RYGB (B and D). Data are mean values ± SEM. LAGB, laparoscopic adjustable gastric banding; RYGB, Roux-en-Y gastric bypass; MP, moderately pleasant; WP, weakly pleasant; N, neutral; WU, weakly unpleasant; MU, moderately unpleasant; W, weak; M, moderate; S, strong.
Discussion
The primary findings of this study is that both RYGB and LAGB cause marked changes in eating behavior, including: i) decreased cravings for fast food and sweets, ii) decreased effect of eating sweets on mood, iii) increased control of eating sweets, iv) decreased preference for high sucrose concentration, and v) reduced influence of emotions and external cues of food on eating behavior. These results demonstrate that the same weight loss induced by either RYGB or LAGB causes similar alterations in eating behavior, which cannot be explained by changes in the sensory-discriminatory domain of taste perception. However, RYGB surgery, but not LAGB, affected the hedonic component of taste perception, characterized by a rapid shift in sweetness palatability from pleasant to unpleasant when repetitively tasting sucrose, which demonstrates a unique effect of RYGB that might further decrease the ingestion of sweet foods and increase adherence to a low-calorie diet.
Our study is not able to determine whether the changes in specific eating behaviors detected after bariatric surgery were caused by surgery itself or were a consequence of changes in dietary intake and weight loss. The observation that both RYGB and LAGB, which are radically different surgical procedures, caused such similar changes in eating behaviors suggests that changes in dietary intake and weight loss were primarily responsible for these changes. The reduction in food cravings that we found after both RYGB and LAGB is consistent with the results from a previous study that examined changes in food cravings after bariatric surgery. 29 Moreover, data from previous diet studies have found that calorie restriction, itself, diminishes food cravings,30 and foods that are restricted the most are those that are craved the least.31 In addition, decreasing the amount of sensory experience with a particular taste can reduce the preference for that taste.32–34 Therefore, the restriction of sweets and fast foods after bariatric surgery can reduce the preference and cravings for those foods. It is also possible that a weight-loss-induced increase in brain insulin sensitivity35 contributes to changes in eating behavior after bariatric surgery, because insulin regulates brain dopamine signaling in key areas that control appetite and reward.36 Additional studies are needed to fully elucidate the mechanisms responsible for the alterations in eating behaviors and the decrease in total energy intake that occur after bariatric surgery.
We found that RYGB, but not LAGB, affected the hedonic response to sweet taste, manifested by a more rapid and greater change from pleasant to unpleasant after repetitive tasting of sweetness. This observation is consistent with data from previous studies conducted in people that demonstrated RYGB-induced weight loss: 1) reduced neural activation in reward-related brain areas when looking at pictures of highly palatable foods,7,11 2) decreased the motivation to work for a sweet candy,8 and 3) lowered the hedonic drive to consume palatable food.10 Our results are also consistent with data from previous studies conducted in rodents, which found RYGB-induced weight loss leads to a shift in hedonic value, favoring low-sucrose over high-sucrose concentrations. 13; 37–38 Our results extend these previous findings by demonstrating that RYGB has effects on the hedonic component of sweet taste perception, independent of weight loss.
To our knowledge, this is the first study to assess the effects of bariatric surgery-induced weight-loss on above-threshold taste intensity ratings. The assessment of above-threshold taste is important because detection thresholds do not provide meaningful information about food palatability and preferences and do not predict above-threshold sensory function in real-world settings.15–16 The intensity of sweetness at sucrose concentrations above-threshold were perceived to be slightly weaker after both RYGB and LAGB induced weight loss than before surgery. It is possible that the decrease in lingual α-gustducin gene expression that we observed after surgery contributed to the decreased perception of sucrose intensity, because α-gustducin is involved in the transduction pathways of sweetness, 39 and sucrose perception is altered in α-gustducin knock-out animals.40
An important strength of this study is also a limitation. By controlling dietary intake and matching weight loss in the two surgical groups, we were able to eliminate the potential confounding effects of these factors on changes in taste perception and eating behavior. Therefore, our study evaluated whether upper gastrointestinal bypass itself has independent effects on our study outcomes. However, patients who have LAGB and RYGB might not consume similar diets and RYGB usually causes greater weight loss than does LAGB, so our results might not represent what actually occurs in clinical practice. In addition, we cannot completely exclude the possibility that weight loss induced by LAGB or RYGB has weight loss-independent effects on taste perception, because we did not study a non-surgical weight loss group. Finally, our study cannot determine whether taste perception and eating behavior in our obese subjects were different than lean subjects because we did not study a lean control group.
In conclusion, weight loss-induced by LAGB and RYGB surgeries are associated with similar modifications in eating behavior, when subjects are matched on the amount of weight lost. RYGB had independent effects on the hedonic value of sweetness, which could further contribute to consuming a low-calorie diet and weight loss. However, the changes in eating behavior and sweet taste palatability were not associated with changes in taste sensitivity. Additional studies are needed to understand the mechanism(s) responsible for the decreased intake of sweetened foods after bariatric surgery.
Table 3.
Taste detection thresholds and eating behavior questionnaires scores before and after bariatric surgery-induced weight loss.
| RYGB (n=17) | LAGB (n=10) | P value Group x Time interaction | |||
|---|---|---|---|---|---|
| Before | After | Before | After | ||
| Taste Detection Thresholds | |||||
| Glucose (mmol/L) | 27.6 ± 10.9 | 27.6 ± 9.2 | 28.6 ± 15.4 | 31.7 ± 4.7 | 0.19 |
| Sucrose (mmol/L) | 8.7 ± 4.6 | 6.5 ± 1.0 | 8.1 ± 2.5 | 7.5 ± 4.3 | 0.60 |
| NaCl (mmol/L) | 1.8 ± 1.2 | 1.6 ± 0.5 | 2.4 ± 1.5 | 1.9 ± 1.4 | 0.66 |
| MSG (mmol/L) | 1.2 ± 0.8 | 1.2 ± 0.7 | 1.2 ± 0.8 | 1.2 ± 0.4 | 0.93 |
| Food Cravings | |||||
| High fat | 2.2 ± 0.7 | 1.7 ± 0.6Ŧ | 2.0 ± 0.8 | 1.9 ± 0.7Ŧ | 0.18 |
| Carbohydrates | 2.3 ± 0.9 | 2.1 ± 0.8 | 2.2 ± 0.5 | 1.9 ± 0.6 | 0.84 |
| Sweets | 2.5 ± 0.7 | 1.7 ± 0.6* | 2.7 ± 0.7 | 2.3 ± 0.5* | 0.23 |
| Fast food | 3.0 ± 0.7 | 2.2 ± 0.6* | 2.6 ± 0.8 | 2.2 ± 0.5* | 0.15 |
| DEBQ | |||||
| Restrained | 2.8 ± 0.5 | 2.9 ± 0.7Ŧ | 2.8 ± 0.5 | 3.4 ± 1.0Ŧ | 0.22 |
| Emotional | 2.8 ± 0.8 | 1.9 ± 0.7* | 3.2 ± 1.0 | 2.3 ± 1.0* | 0.81 |
| External | 3.1 ± 0.5 | 2.1 ± 0.5* | 3.4 ± 0.5 | 2.4 ± 0.6* | 0.68 |
| Fat Preferences | |||||
| TASTE (better) | 70 ± 14% | 63 ± 19%Ŧ | 63 ± 17% | 54 ± 20%Ŧ | 0.84 |
| FREQ (often) | 54 ± 23% | 21 ± 14%* | 48 ± 20% | 18 ± 14%* | 0.72 |
| STQ | |||||
| Mood altering effect | 25.8 ± 7.5 | 14.3 ± 5.8* | 26.9 ± 6.0 | 21.3 ± 10.8* | 0.07 |
| Impaired control | 13.8 ± 6.1 | 4.1 ± 4.6* | 15.0 ± 5.7 | 9.5 ± 5.6* | 0.11 |
Values are means ± SD with the exception of taste detection thresholds values that are median ± SIQR. RYGB: roux-en Y gastric bypass; LAGB: laparoscopic adjustable gastric banding; DEBQ: Dutch Eating Behavior Questionnaire; MSG: monosodium glutamate; FREQ: eaten more frequently; STQ: Sweet Taste Questionnaire.
Values After surgery significant different from values Before surgery (P<0.001).
Trend for values After surgery to be different from values Before surgery (P=0.06).
What is already known about this subject
Roux-en-Y gastric bypass (RYGB) surgery causes greater weight loss than laparoscopic adjustable gastric banding (LAGB).
RYGB is associated with decreased hedonic value for sweet or highly palatable foods.
Findings from studies that measured thresholds to detect sweet taste suggest that taste sensitivity is increased after RYGB surgery and that as a consequence patients may reset their palates to like foods with less sugar.
What this study adds
Neither RYGB nor LAGB affect the discriminatory dimension of taste perception. There are no significant changes in sweet, salty or savory taste sensitivity (both at threshold and above-threshold sensory function) following weigh loss induced by RYGB or LAGB.
RYGB has weight loss-independent effects in the hedonic dimension of sweet taste perception. RYGB, but not LAGB, shift sweetness palatability from pleasant to unpleasant when repetitively tasting sucrose.
RYGB and LAGB cause similar decreases on the influence of emotions and external food cues on eating behavior, when weight loss is matched.
Acknowledgments
The authors thank Johanna Sonnenschein, Nancy Allen and Terri Pietka for technical assistance, Courtney Tiemann for helping with subject recruitment, and the study subjects for their participation. This study was supported by the National Institutes of Health grants DK 37948, UL1 TR000448 (Clinical and Translational Sciences Award), sub award KL2 TR000450, DK 56341 (Nutrition and Obesity Research Center), DK60022.
Footnotes
Clinical Trials.gov identifier: NCT01536197
Conflict of interest. S.K. is a shareholder of Aspire Bariatrics and serves on the Scientific Advisory Board for NovoNordisk, Takeda Pharmaceuticals, and the Egg Nutrition Council. No other authors declare a conflict of interest.
References
- 1.Buchwald H, Estok R, Fahrbach K, Banel D, Jensen MD, Pories WJ, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med. 2009 Mar;122:248–256. e5. doi: 10.1016/j.amjmed.2008.09.041. [DOI] [PubMed] [Google Scholar]
- 2.Sugerman HJ, Starkey JV, Birkenhauer R. A randomized prospective trial of gastric bypass versus vertical banded gastroplasty for morbid obesity and their effects on sweets versus non-sweets eaters. Ann Surg. 1987;205:613–624. doi: 10.1097/00000658-198706000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brolin RE, Robertson LB, Kenler HA, Cody RP. Weight loss and dietary intake after vertical banded gastroplasty and Roux-en-Y gastric bypass. Ann Surg. 1994;220:782–790. doi: 10.1097/00000658-199412000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kenler HA, Brolin RE, Cody RP. Changes in eating behavior after horizontal gastroplasty and Roux-en-Y gastric bypass. Am J Clin Nutr. 1990;52:87–92. doi: 10.1093/ajcn/52.1.87. [DOI] [PubMed] [Google Scholar]
- 5.Olbers T, Bjorkman S, Lindroos A, Maleckas A, Lonn L, Sjostrom L, et al. Body composition, dietary intake, and energy expenditure after laparoscopic Roux-en-Y gastric bypass and laparoscopic vertical banded gastroplasty: a randomized clinical trial. Ann Surg. 2006;244:715–722. doi: 10.1097/01.sla.0000218085.25902.f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Breslin PA, Spector AC. Mammalian taste perception. Curr Biol. 2008;18:R148–155. doi: 10.1016/j.cub.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 7.Ochner CN, Kwok Y, Concicao E, Pantazatos SP, Puma LM, Carnell S, et al. Selective reduction in neural responses to high calorie foods following gastric bypass surgery. Ann Surg. 2011;253:502–507. doi: 10.1097/SLA.0b013e318203a289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miras AD, Jackson RN, Jackson SN, Goldstone AP, Olbers T, Hackenberg T, et al. Gastric bypass surgery for obesity decreases the reward value of a sweet-fat stimulus as assessed in a progressive ratio task. Am J Clin Nutr. 2012;96:467–473. doi: 10.3945/ajcn.112.036921. [DOI] [PubMed] [Google Scholar]
- 9.Schultes B, Ernst B, Wilms B, Thurnheer M, Hallschmid M. Hedonic hunger is increased in severely obese patients and is reduced after gastric bypass surgery. Am J Clin Nutr. 2010;92:277–283. doi: 10.3945/ajcn.2009.29007. [DOI] [PubMed] [Google Scholar]
- 10.Ullrich J, Ernst B, Wilms B, Thurnheer M, Schultes B. Roux-en Y gastric bypass surgery reduces hedonic hunger and improves dietary habits in severely obese subjects. Obes Surg. 2013;23:50–55. doi: 10.1007/s11695-012-0754-5. [DOI] [PubMed] [Google Scholar]
- 11.Scholtz S, Miras AD, Chhina N, Prechtl CG, Sleeth ML, Daud NM, et al. Obese patients after gastric bypass surgery have lower brain-hedonic responses to food than after gastric banding. Gut. 2013 doi: 10.1136/gutjnl-2013-305008. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scruggs DM, Buffington C, Cowan GS., Jr Taste Acuity of the Morbidly Obese before and after Gastric Bypass Surgery. Obes Surg. 1994;4:24–28. doi: 10.1381/096089294765558854. [DOI] [PubMed] [Google Scholar]
- 13.Bueter M, Miras AD, Chichger H, Fenske W, Ghatei MA, Bloom SR, et al. Alterations of sucrose preference after Roux-en-Y gastric bypass. Physiol Behav. 2011;104:709–721. doi: 10.1016/j.physbeh.2011.07.025. [DOI] [PubMed] [Google Scholar]
- 14.Burge JC, Schaumburg JZ, Choban PS, DiSilvestro RA, Flancbaum L. Changes in patients’ taste acuity after Roux-en-Y gastric bypass for clinically severe obesity. J Am Diet Assoc. 1995;95:666–670. doi: 10.1016/S0002-8223(95)00182-4. [DOI] [PubMed] [Google Scholar]
- 15.Bartoshuk LM. The psychophysics of taste. Am J Clin Nutr. 1978;31:1068–1077. doi: 10.1093/ajcn/31.6.1068. [DOI] [PubMed] [Google Scholar]
- 16.Pepino MY, Finkbeiner S, Beauchamp GK, Mennella JA. Obese women have lower monosodium glutamate taste sensitivity and prefer higher concentrations than do normal-weight women. Obesity (Silver Spring) 2010;18:959–965. doi: 10.1038/oby.2009.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin M, Beekley A, Kjorstad R, Sebesta J. Socioeconomic disparities in eligibility and access to bariatric surgery: a national population-based analysis. Surg Obes Relat Dis. 2010;6:8–15. doi: 10.1016/j.soard.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 18.Fikentscher R, Roseburg B, Spinar H, Bruchmuller W. Loss of taste in the elderly: sex differences. Clinical otolaryngology and allied sciences. 1977;2:183–189. doi: 10.1111/j.1365-2273.1977.tb01356.x. [DOI] [PubMed] [Google Scholar]
- 19.Bartoshuk LM, Duffy VB, Green BG, Hoffman HJ, Ko CW, Lucchina LA, et al. Valid across-group comparisons with labeled scales: the gLMS versus magnitude matching. Physiol Behav. 2004;82:109–114. doi: 10.1016/j.physbeh.2004.02.033. [DOI] [PubMed] [Google Scholar]
- 20.Coldwell SE, Mennella JA, Duffy VB, Pelchat ML, Griffith JW, Smuitzer G, et al. Gustation assessment using the NIH Toolbox. Neurology. 2013;80:S20–S24. doi: 10.1212/WNL.0b013e3182872e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beauchamp GK, Vazquez de Vauera M, Pearson PB. Dietary status of human infants and their sensory responses to amino acid flavor. In: Kawamura Y, Kare MR, editors. Umami: a basic taste. Marcel Dekker, Inc; New York, NY, USA: 1987. pp. 125–138. [Google Scholar]
- 22.Van Strien T, Frijters ER, Bergers GPA, Defares PB. The Dutch Eating Behavior Questionnaire (DEBQ) for assessment of restrained, emotional, and external eating behavior. International Journal of Eating Disorders. 1986;5:295–315. [Google Scholar]
- 23.White MA, Whisenhunt BL, Williamson DA, Greenway FL, Netemeyer RG. Development and validation of the food-craving inventory. Obes Res. 2002;10:107–1014. doi: 10.1038/oby.2002.17. [DOI] [PubMed] [Google Scholar]
- 24.Kampov-Polevoy AB, Alterman A, Khalitov E, Garbutt JC. Sweet preference predicts mood altering effect of and impaired control over eating sweet foods. Eat Behav. 2006;7:181–187. doi: 10.1016/j.eatbeh.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 25.Ledikwe JH, Ello-Martin J, Pelkman CL, Birch LL, Mannino ML, Rolls BJ. A reliable, valid questionnaire indicates that preference for dietary fat declines when following a reduced-fat diet. Appetite. 2007;49:74–83. doi: 10.1016/j.appet.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 26.Spielman AI, Pepino MY, Feldman R, Brand JG. Technique to collect fungiform (taste) papillae from human tongue. Journal of visualized experiments: JoVE. 2010;(42) doi: 10.3791/2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Varela JE. Bariatric surgery: a cure for diabetes? Curr Opin Clin Nutr Metab Care. 2011;14:396–401. doi: 10.1097/MCO.0b013e3283468e50. [DOI] [PubMed] [Google Scholar]
- 28.Pepino MY, Mennella JA. Effects of cigarette smoking and family history of alcoholism on sweet taste perception and food cravings in women. Alcohol Clin Exp Res. 2007;31:1891–1899. doi: 10.1111/j.1530-0277.2007.00519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leahey TM, Bond DS, Raynor H, Roye D, Vithiananthan S, Ryder BA, et al. Effect of bariatric surgery on food cravings: do food cravings and the consumption of craved food “normalize” after surgery? Surgery for Obesity and Related Diseases. 2012;8:84–91. doi: 10.1016/j.soard.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martin CK, O’Neil PM, Pawlow L. Changes in food cravings during low-calorie and very-low-calorie diets. Obesity (Silver Spring) 2006;14:115–121. doi: 10.1038/oby.2006.14. [DOI] [PubMed] [Google Scholar]
- 31.Martin CK, Rosenbaum D, Han H, Geiselman PJ, Wyatt HR, Hill JO, et al. Change in food cravings, food preferences, and appetite during a low-carbohydrate and low-fat diet. Obesity (Silver Spring) 2011;19:1963–1970. doi: 10.1038/oby.2011.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bertino M, Beauchamp GK, Engelman K. Long-term reduction in dietary sodium alters the taste of salt. Am J Clin Nutr. 1982;36:1134–1144. doi: 10.1093/ajcn/36.6.1134. [DOI] [PubMed] [Google Scholar]
- 33.Beauchamp GK, Bertino M, Engelman K. Failure to compensate decreased dietary sodium with increased table salt usage. JAMA. 1987;258:3275–3278. [PubMed] [Google Scholar]
- 34.Mattes RD. Fat preference and adherence to a reduced-fat diet. Am J Clin Nutr. 1993;57:373–381. doi: 10.1093/ajcn/57.3.373. [DOI] [PubMed] [Google Scholar]
- 35.Tuulari JJ, Karlsson HK, Hirvonen J, Hannukainen JC, Bucci M, Helmio M, et al. Weight loss after bariatric surgery reverses insulin-induced increases in brain glucose metabolism of the morbidly obese. Diabetes. 2013 doi: 10.2337/db12-1460. e-pub ahead of print Mar 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Daws LC, Avison MJ, Robertson SD, Niswender KD, Galli A, Saunders C. Insulin signaling and addiction. Neuropharmacology. 2011;61:1123–1128. doi: 10.1016/j.neuropharm.2011.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hajnal A, Kovacs P, Ahmed T, Meirelles K, Lynch CJ, Cooney RN. Gastric bypass surgery alters behavioral and neural taste functions for sweet taste in obese rats. Am J Physiol Gastrointest Liver Physiol. 2010;299:G967–979. doi: 10.1152/ajpgi.00070.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shin AC, Zheng H, Pistell PJ, Berthoud HR. Roux-en-Y gastric bypass surgery changes food reward in rats. Int J Obes (Lond) 2011;35:642–651. doi: 10.1038/ijo.2010.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wong GT, Ruiz-Avila L, Margolskee RF. Directing gene expression to gustducin-positive taste receptor cells. J Neurosci. 1999;19:5802–5809. doi: 10.1523/JNEUROSCI.19-14-05802.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Glendinning JI, Bloom LD, Onishi M, Zheng KH, Damak S, Margolskee RF, et al. Contribution of alpha-gustducin to taste-guided licking responses of mice. Chem Senses. 2005;30:299–316. doi: 10.1093/chemse/bji025. [DOI] [PubMed] [Google Scholar]