Abstract
Background
Traumatic brain injury (TBI) is associated with a higher incidence of depression. The majority of individuals who suffer a TBI are juveniles and young adults and thus, the risk of a lifetime of depressive complications is a significant concern. The etiology of increased TBI-associated depression is unclear, but may be inflammatory-related with increased brain sensitivity to secondary inflammatory challenges (e.g., stressors, infection, and injury).
Methods
Adult male BALB/c mice received a sham (n=52) or midline fluid percussion injury (TBI) (n=57). Neuroinflammation, motor coordination (rotarod), and depressive behaviors (social withdrawal, immobility in the tail suspension test, and anhedonia) were assessed 4 h, 24 h, 72 h, 7 d, or 30 d later. Moreover, 30 d after surgery, sham and TBI mice received a peripheral injection of saline or lipopolysaccharide (LPS) and microglia activation and behavior were determined.
Results
Diffuse TBI caused inflammation, peripheral cell recruitment, and microglia activation immediately after injury coinciding with motor coordination deficits. These transient events resolved within 7 d. Nonetheless, 30 days post-TBI a population of de-ramified and major histocompatibility complex (MHC)II+ (primed) microglia were detected. After a peripheral LPS challenge, the inflammatory cytokine response in primed microglia of TBI mice was exaggerated compared to microglia of controls. Furthermore, this LPS-induced microglia reactivity 30 d after TBI was associated with the onset of depressive-like behavior.
Conclusions
These results implicate a primed and immune-reactive microglial population as a possible triggering mechanism for the development of depressive complications after TBI.
Keywords: Fluid percussion injury, Cytokines, Microglia, Major histocompatibility complex II, Depression, Lipopolysaccharide
INTRODUCTION
Traumatic brain injury (TBI) elicits immediate neuroinflammatory events that contribute to acute cognitive, motor, and behavioral disturbances (1–4). Despite resolution of these acute complications, depression can develop and persist years after TBI (5–7). Indeed, individuals who suffer a TBI are 5–10 times more likely to develop symptoms of depression compared to the general population (8). Depressive symptoms are diagnosed in 30–40% of individuals within the first year of TBI (5,9), in 60% of individuals within 8 years of TBI (10), and 50 years after TBI patients continue to report higher rates of depression (11). Moreover, most (69%; CDC, 2002–2006) brain injuries occur in juveniles (34.7%, 0–14 years) and young adults (34.3%, 15–34 years) implicating an increased risk for a lifetime of depressive complications that negatively affect quality of life and life-span (11,12). Furthermore, the limited number studies on anti-depressant therapies (e.g., amitriptyline, sertraline) after TBI show reduced efficacy in TBI patients (13,14). We propose that TBI-associated depression is inflammatory-based and associated with increased brain sensitivity to acute immune challenges.
In support of this premise, clinical and experimental data indicate a cause/effect relationship between inflammation and depression (15,16). Patients with higher inflammatory cytokine levels in circulation and within the central nervous system (CNS) report a higher incidence of treatment-resistant depression (15). These patients have elevated levels of the inflammatory cytokine interleukin (IL)-6 in circulation, and anti-depressant therapies fail to reduce tumor necrosis factor (TNF)α (17). Critically, TBI patients have increased levels of IL-6, IL-1β, and TNFα in cerebrospinal fluid (18,19) and serum (20) immediately after injury. Moreover, markers of neuroinflammation (e.g., CD68, CR3/43) persist in the brain parenchyma up to 16 years after TBI (21). Although several studies report increased neuroinflammation after TBI and others report increased depression after TBI, the extent to which prolonged brain inflammation contributes to neurobehavioral complications after TBI is unclear.
One potential consequence of heightened and prolonged brain inflammation after TBI is increased sensitivity to secondary challenges including subsequent injuries, stressors, and infections (22). In models of aging, stress, early life infection, sterile CNS injury, and preclinical neurologic disease increased sensitivity to inflammatory challenges corresponds with the development of a primed and more inflammatory microglia phenotype (e.g., increased major histocompatibility complex II [MHCII], IL-1β, CD68, complement receptor [CR]3) (23–27). Microglia are the innate immune cells of the CNS and responsible for interpreting and propagating inflammatory signals that affect neuronal function (28,29). Thus, enhanced microglial activation and amplified inflammatory cytokine production can impair normal neurologic function. In support of this idea, primed and MHCII+ microglia in the aged brain become hyper-reactive to a systemic injection of lipopolysaccharide (LPS) and produce exaggerated levels of IL-1β (23) corresponding to impaired cognitive performance (22,30,31), protracted sickness behavior (32,33), and depressive-like behavior (34). Relevant to the axonal and neuronal damage done during TBI, models of optic nerve crush also demonstrate microglia priming (CD68+) and exaggerated IL-1β, TNFα, and IL-6 expression after LPS challenge 28 days post injury (dpi) (27). Although not discussed in the context of microglial priming, increased MHCII (OX6) expression has also been detected in the brain 16 dpi in a rat model of cerebral contusion (35). Thus, a primed microglia phenotype after TBI may set the stage for exaggerated responses to acute challenges, precipitating the development of chronic neuropsychiatric disorders.
Based on these data, we hypothesize that a diffuse TBI induces microglial priming, and that an acute immune challenge weeks to months after injury results in a hyper-inflammatory microglia response triggering the development of depressive-like behavior. To test this hypothesis, the midline fluid percussion injury (FPI) model of TBI was used in mice. Midline FPI causes mild neuronal pathology including diffuse axonal injury (36) and transient neurological deficits (37) that recapitulate complications after mild to moderate concussive head injuries in humans (38). Here, we show that TBI caused immediate, but transient, neuroinflammation and behavioral impairments. Nonetheless, evidence of microglial priming was detected in the brain 30 dpi. Furthermore, activation of the peripheral immune system 30 days after TBI caused exaggerated microglial expression of IL-1β and TNFα corresponding with induction of depressive-like behavior. Collectively, these data support the premise that a diffuse TBI sensitizes the brain to secondary inflammatory challenges that may precipitate depression.
METHODS AND MATERIALS
Mice and LPS injections
Adult (3 mo) male BALB/c mice were obtained from a breeding colony at The Ohio State University (OSU). Mice were individually housed and maintained at 25° C under a 12 h light/12 h dark cycle with ad libitum access to food and water. For injections, mice were intraperitoneally (i.p.) injected 30 dpi with saline or LPS (0.33 mg/kg; serotype 0127:B8, Sigma) 1–2 h before the start of the dark phase (between 1700 and 1900) (32,39). All procedures were in accordance with the NIH Guidelines for the Care and Use of Laboratory Animals and were approved by the OSU Institutional Laboratory Animal Care and Use Committee.
Midline fluid percussion injury
Mice received a midline and diffuse TBI using a fluid percussion injury (FPI) apparatus (Custom Design & Fabrication) as previously described (40) and detailed in Supplemental Materials and Methods. This diffuse injury is well characterized and occurs in the absence of contusion, tissue cavitation, or gross neuronal loss, and causes diffuse axonal injury in the neocortex, hippocampus, and dorsolateral thalamus (41–43). Immediately after sham injury or TBI the injury hub was removed, dural integrity was confirmed, and mice were evaluated for injury severity using the self-righting test (2). Based on previous studies in FPI (44), self-righting inclusion criteria was modified for BALB/c mice as follows: sham≤60 s; 60 s<mild≤200 s; 200 s<moderate≤540 s; severe>540 s. Only mice with a moderate TBI were used.
Motor function and depressive-like behavior
Motor coordination was assessed using rotarod (Rotamex) as previously described (45) and detailed in the Supplementary Materials and Methods.
Activity was determined using an activity box paradigm (Open Field and Fusion software; AccuScan Instruments). Mice were placed into independent 8 × 8 inch chambers and automated software packaging was configured to determine the total distance traveled and total movement time for 10 min. A subset of mice was tested for 30 min and values were recorded in 10 min increments.
Sickness and depressive-like behavior were determined through un-motivated (locomotor) (32) and motivated (social exploratory behavior (24,32,46), TST (34,47), sucrose preference (48)) behavioral tests as described in the Supplemental Materials and Methods. For the TST, the same mice were tested at both 7 dpi and 30 dpi, with another subset of mice tested only at 30 dpi. At 30 dpi, immobility between the two subsets was not significantly different and data were collapsed.
Isolation of enriched brain CD11b+ cells
Enriched CD11b+ cells (microglia/peripheral myeloid cells) were isolated from whole brain homogenates as previously described (32). In brief, brains were homogenized and re-suspended in a discontinuous, isotonic Percoll gradient. Microglia were collected from the interphase of the 70% and 50% Percoll layers. We have previously characterized these cells as approximately 90% CD11b+/CD45+ “enriched CD11b+” cells (23,24).
RNA isolation and RT-PCR
RNA was isolated from individual brain regions or enriched brain CD11b+ cells using the Tri-Reagent protocol (Sigma), or the PrepEase kit (USB), respectively. RNA concentration was determined and RNA was reverse transcribed to cDNA. Real time PCR (RT-PCR) was performed using the Applied Biosystems Taqman® Gene Expression assay using an ABI PRISM 7300-sequence detection system as previously described (49). Data were analyzed using the comparative threshold cycle (ddCt) method and results are expressed as fold difference from controls.
Flow cytometry
Enriched brain CD11b+ cells were assayed for surface antigens by flow cytometry as described (23,39). In brief, cells were incubated with rat anti-mouse antibodies (eBioscience; CD11b-APC, CD45-PerCP-Cy5.5, and CD14-PE). Surface expression was determined using a Becton-Dickinson FACSCaliber four color Cytometer. Twenty thousand events were recorded and microglia (CD11b+/CD45low) and peripheral myeloid cells (PMCs) (CD11b+/CD45high) were identified by CD11b/CD45 expression (50). Gating was determined based on appropriate negative isotype controls. Data were analyzed using FlowJo software (Tree Star).
Immunohistochemistry
Fluorescent staining for glial fibrillary acidic protein (GFAP) and ionized binding association protein (Iba)-1 was performed as described in Supplemental Materials and Methods. Threshold staining was determined using NIH ImageJ and quantification was assessed for each image using digital image analysis (DIA) (51). Cell counts and cell body size were assessed using Metamorph Image (Metamorph Offline, 6.1) analysis. Results are reported as the average percent area for GFAP+ and Iba-1+ staining, number of cells / image, and average cell body size (μm2).
Statistical Analysis
Data were subjected to Shapiro-Wilk test using Statistical Analysis Systems (SAS) software. To determine significant main effects and interactions between main factors, data were analyzed using one-, two-, or three-way ANOVA. Body mass data were also assessed using repeated measures ANOVA. When appropriate, differences between treatment means were evaluated by Student’s t-test. All data are expressed as treatment means ± standard error of the mean (SEM).
RESULTS
Diffuse TBI promoted neuroinflammation in mice in a time-dependent manner
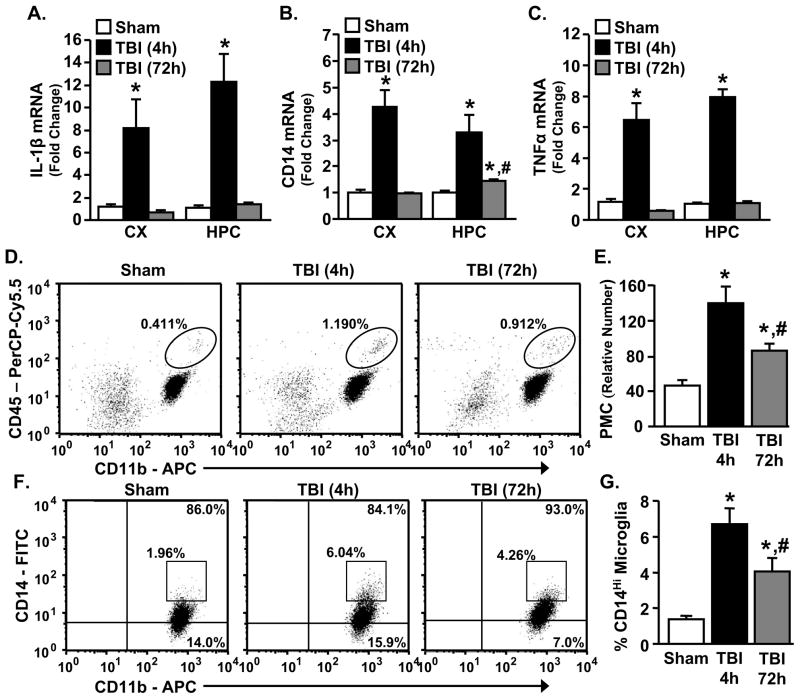
Coinciding with previous studies using midline FPI, moderate and diffuse TBI caused mild hematoma, limited brain edema, and transient blood brain barrier permeability in the absence of overt tissue cavitation or neurodegeneration (data not shown) (37,42,52–54). In addition, Fig. 1A–C show that the mRNA expression of several key inflammatory genes associated with microglia activation including IL-1β, CD14, and TNFα were increased in the cortex (CX) and hippocampus (HPC) 4 h after TBI (p<0.0006, for each). In the sham mice, inflammatory gene expression was not significantly different between the 4 and 72 h time points so the data were combined and presented as a single “sham” group. By 72 h after TBI, all markers were reduced in the CX, but CD14 remained elevated in the HPC compared to controls (p<0.0002). Other inflammatory genes had a similar expression pattern following TBI (Table 1). For example, chemokine ligand 2 (CCL2) mRNA was increased 4 h after TBI in the CX (p<0.009) and HPC (p<0.0008), and remained elevated 72 h after TBI in the CX (p<0.02). GFAP, a marker of astrocyte activation, tended to be increased 4 h after injury in the HPC (p=0.1) and was significantly increased 72 h after TBI in both the CX and HPC (p<0.02 for each). TBI also increased the expression of several anti-inflammatory genes 4 h after injury in the CX (arginase and IGF-1, p<0.05 for each), as well as 4 and 72 h after TBI in the HPC (IL-10, p<0.05) (Table 1). Overall, a moderate TBI elicited brain region-dependent inflammatory responses that were transient.
Figure 1. Diffuse TBI promoted neuroinflammation in mice in a time-dependent manner.
Four or 72 h after sham operation or TBI, mRNA levels of IL-1β, CD14, and TNFα were determined in the CX and underlying HPC (n=6–9), or enriched brain CD11b+ cells were collected and CD11b, CD45, and CD14 surface expression was determined by flow cytometry (n=3–5). A) There was a main effect of TBI on IL-1β expression in both the CX (F(2,21=11.01, p<0.0007) and HPC (F(2,22)=28.22, p<0.0006). B) There was a main effect of TBI on CD14 expression in both the CX (F(2,20)=33.23, p<0.0001) and HPC (F(2,19)=22.22, p<0.0001) and CD14 remained elevated in the HPC 72 h after TBI (p<0.0002). C) There was a main effect of TBI on TNFα in both the CX (F(2,20)=32.00, p<0.0001) and HPC (F(2,21)=284.89, p<0.0001). D) Representative bivariate dot plots of CD11b/CD45 staining of enriched brain CD11b+ cells. E) TBI increased the relative number of PMCs (CD11b+/CD45high) associated with the brain both 4 and 72 h after injury (F(2,12)=34.37, p<0.006). F) Representative bivariate dot plots of CD11b/CD14 staining on microglia (CD11b+/CD45low). G) TBI increased the percent of CD14high microglia present 4 and 72 h after injury (F(2,10)=28.23, p<0.01). Bars represent the mean ± SEM. Means with (*) are significantly different (p<0.05) from sham controls. Means with (#) are significantly different (p<0.05) from sham control and TBI-4h groups. CX = cortex; HPC = hippocampus.
Table 1.
| Cortex mRNA levels | |||
|---|---|---|---|
| Gene | Sham | TBI (4h) | TBI (72h) |
| GFAP | 1.14 ± 0.17 | 1.38 ± 0.23 | 2.47 ± 0.63* |
| CCL2 | 1.37 ± 0.42 | 12.1 ± 0.27* | 6.46 ± 1.78* |
| IFNγ | 1.19 ± 0.28 | 1.90 ± 0.40 | 2.27 ± 0.39* |
| iNOS | 1.06 ± 0.13 | 0.74 ± 0.05+ | 1.65 ± 0.14* |
| IL-4 | 1.03 ± 0.08 | 0.73 ± 0.15+ | 1.44 ± 0.22* |
| Arg | 1.04 ± 0.12 | 2.26 ± 0.51* | 0.93 ± 0.11 |
| IL-10 | 1.24 ± 0.27 | 1.26 ± 0.39 | 1.22 ± 0.39 |
| IGF-1 | 1.02 ± 0.06 | 1.19 ± 0.06* | 0.99 ± 0.04 |
| Hippocampus mRNA levels | |||
|---|---|---|---|
| Gene | Sham | TBI (4h) | TBI (72h) |
| GFAP | 1.02 ± 0.08 | 1.40 ± 0.19+ | 2.05 ± 0.24* |
| CCL2 | 1.03 ± 0.12 | 7.57 ± 5.43* | 1.41 ± 0.36 |
| IFNγ | 1.14 ± 0.21 | 0.91 ± 0.14 | 2.15 ± 0.74 |
| iNOS | 1.09 ± 0.14 | 0.78 ± 0.07+ | 1.59 ± 0.11* |
| IL-4 | 1.20 ± 0.22 | 0.59 ± 0.08+ | 2.04 ± 0.60+ |
| Arg | 1.06 ± 0.12 | 1.19 ± 0.21 | 1.08 ± 0.07 |
| IL-10 | 1.06 ± 0.14 | 4.57 ± 1.41* | 1.97 ± 0.38* |
| IGF-1 | 1.02 ± 0.07 | 1.17 ± 0.17 | 1.17 ± 0.09 |
Adult mice were subjected to a sham injury (sham) or a moderate midline fluid percussion injury (TBI) (n=6–9). After 4 or 72 h mRNA levels of inflammatory (grey) and anti-inflammatory (white) genes were determined in the CX and HPC. Values represent the mean ± SEM. Means with (*) are significantly different (p<0.05) and means with (+) tend to be different (p≤0.1) from sham controls.
Next, PMC (neutrophil, monocyte, macrophage) trafficking after TBI was determined. PMCs (CD11b+/CD45high) were differentiated from microglia (CD11b+/CD45low) based on CD45 expression (24). Fig. 1D&E show that the number of PMCs associated with the brain increased 2–3 fold 4 and 72 h after TBI (p<0.006). In the same set of samples, CD14 surface expression was determined on microglia (CD11b+/CD45low). Consistent with increased inflammatory markers after injury (Fig. 1; Table 1), the percentage of CD14high microglia was increased 4 and 72 h after TBI (Fig. 1F&G, p<0.01). Collectively these data indicate that TBI increased the number of brain associated PMCs and enhanced microglia activation for at least 72 h after injury.
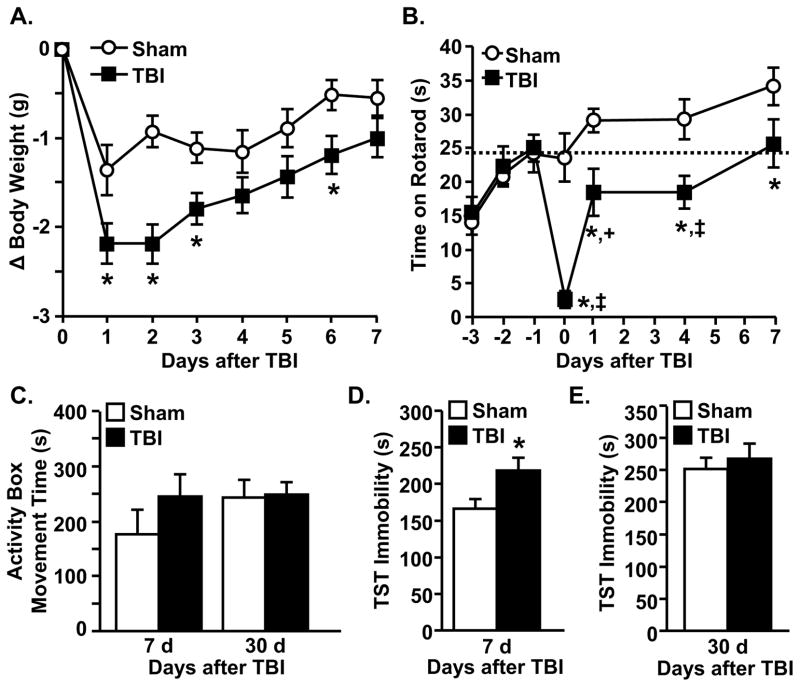
Diffuse TBI promoted transient deficits in body mass, motor coordination, and depressive-like behavior
To determine the degree to which diffuse TBI caused motor and behavior deficits, body weight, motor coordination, activity, and depressive-like behavior were determined at several time-points after TBI. Fig. 2A shows that TBI mice had exaggerated weight loss 1–3 dpi (p<0.004), but recovered to sham levels by 7 dpi. Fig. 2B shows that motor coordination was reduced following TBI (p<0.02) in time dependent manner (p<0.001). For example, TBI mice had impaired motor coordination 1 h and 1–4 dpi compared to baseline and sham levels, but recovered to baseline motor coordination by 7 dpi (Fig. 2B). Moreover, activity levels were equivalent in sham and TBI mice 7 dpi (Fig. 2C).
Figure 2. Diffuse TBI promotes transient deficits in body mass, motor coordination, and depressive-like behavior.
TBI caused A) reduced body weight 1–3 and 6 dpi (F(1,44)=3.41, p<0.004), but body weight recovered to sham levels by 7 dpi (n=22). B) Motor coordination was reduced by TBI (F(1,111)=23.46, p<0.0001) in a time manner (F(6,111)=5.40, p<0.0001) (n=8). The dashed, horizontal line indicates baseline motor function. C) Total movement time in the activity box did not differ between groups at either 7 or 30 dpi (n=7). D) TBI mice had increased immobility in the TST compared to sham mice 7 dpi (F(1,13)=5.4, p<0.04) (n=7). E) There was no significant difference in immobility in the TST at 30 dpi (n=16). Bars represent the mean ± SEM. Means with (*) are significantly different (p<0.05) from sham controls. Means with (‡) and (+) are significantly different (p<0.01) and tend to be different (p=0.09) from baseline levels, respectively.
Because depression can develop and persist after TBI (10,11), behavioral resignation was determined by immobility in the TST 7 dpi (55), a time when weight, motor coordination, and activity were not disrupted in TBI mice (Fig. 2A–C). TBI mice spent more time immobile in the TST 7 dpi compared to controls (Fig. 2D, p<0.04). By 30 dpi, however, immobility was similar between the groups (Fig. 2E). Increased immobility 7 dpi was not associated with exhaustion in TBI mice as activity levels were equivalent between groups over a 30 min testing period (data not shown). Taken together, TBI caused transient weight loss, motor dysfunction, and depressive-like behavior.
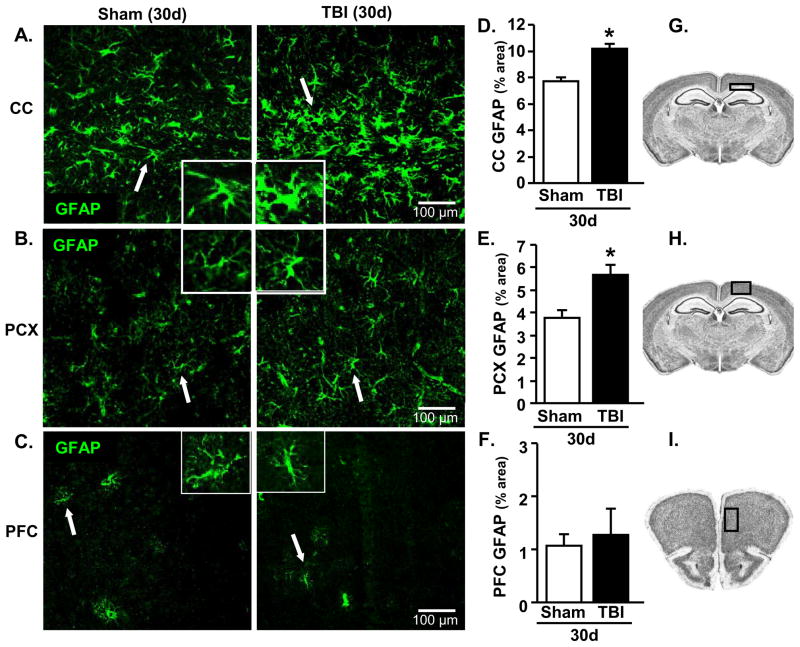
TBI-associated astrogliosis 30 dpi
Our data indicate that moderate TBI causes a transient increase in neuroinflammation corresponding with acute motor and behavioral impairments. We predict, however, that a degree of glial activation persists months to years after TBI. Thus, the relative level of astrocyte (GFAP+) activation was determined 30 dpi. GFAP immunoreactivity was increased in the corpus callosum (CC) (Fig. 3A&D, p<0.0001) and parietal cortex (PCX) (Fig. 3B&E, p<0.002). This increase in astrocyte activation occurred in the absence of tissue cavitation (data not shown). GFAP immunoreactivity was primarily restricted to white matter and gray matter directly beneath the injury site (Fig. 3G&H). Indeed, in brain regions more distal to the impact including the prefrontal cortex (PFC) (Fig. 3C&F), paraventricular nucleus (PVN), medial amygdala (MeA), and hippocampus (CA1 and DG), GFAP immunoreactivity was not increased (Table 2). Collectivity, evidence of astrocyte activation remained 30 dpi, but was restricted to areas proximal to the injury site.
Figure 3. TBI-associated astrogliosis 30 dpi.
Brains were collected 30 d after sham operation or TBI and proportional area of GFAP expression was determined (n=11–13). Three images / sample at 20x were collected. Representative images of GFAP staining in the A) CC, B) PCX, and C) PFC of sham and TBI mice. Inset includes enlarged image of GFAP+ cell indicated by white arrow. Proportional area for GFAP staining was increased in TBI mice compared to sham mice in the D) CC (F(1,23)=25.05, p<0.0001) and E) PCX (F(1,24)=12.60, p<0.002), but was not different between groups in the F) PFC. Schematics taken from the High Resolution Mouse Brain Atlas (Sidman et al.: http://www.hms.harvard.edu/research/brain/index.html) depicting where images were collected for the G) CC, H) PCX, and I) PFC. Bars represent the mean ± SEM. Means with (*) are significantly different (p<0.05) from sham controls. CC = corpus callosum; PCX = parietal cortex; PFC = prefrontal cortex
Table 2.
| GFAP Threshold (% Area) | ||
|---|---|---|
| Area | Sham | TBI |
| CA1 | 7.53 ± 0.52 | 7.20 ± 0.18 |
| PVN | 8.15 ± 0.75 | 8.88 ± 0.95 |
| MeA | 4.65 ± 0.25 | 4.03 ± 0.29 |
| DG | 12.92 ± 0.47 | 13.51 ± 0.46 |
| Iba-1 Threshold (% Area) | ||
|---|---|---|
| Area | Sham | TBI |
| CA1 | 4.30 ± 0.36 | 4.51 ± 0.37 |
| PVN | 3.98 ± 0.26 | 4.82 ± 0.34+ |
| MeA | 3.99 ± 0.30 | 4.16 ± 0.33 |
Adult mice were subjected to a sham injury (sham) or a moderate midline fluid percussion injury (TBI) (n=10–13). Brains were collected 30 d later and proportional area of GFAP or Iba-1 expression was determined. Results are presented as proportional area for GFAP or Iba-1 threshold staining. Values represent the mean ± SEM. Means with (*) are significantly different (p<0.05) and means with (+) tend to be different (p=0.06) from sham controls.
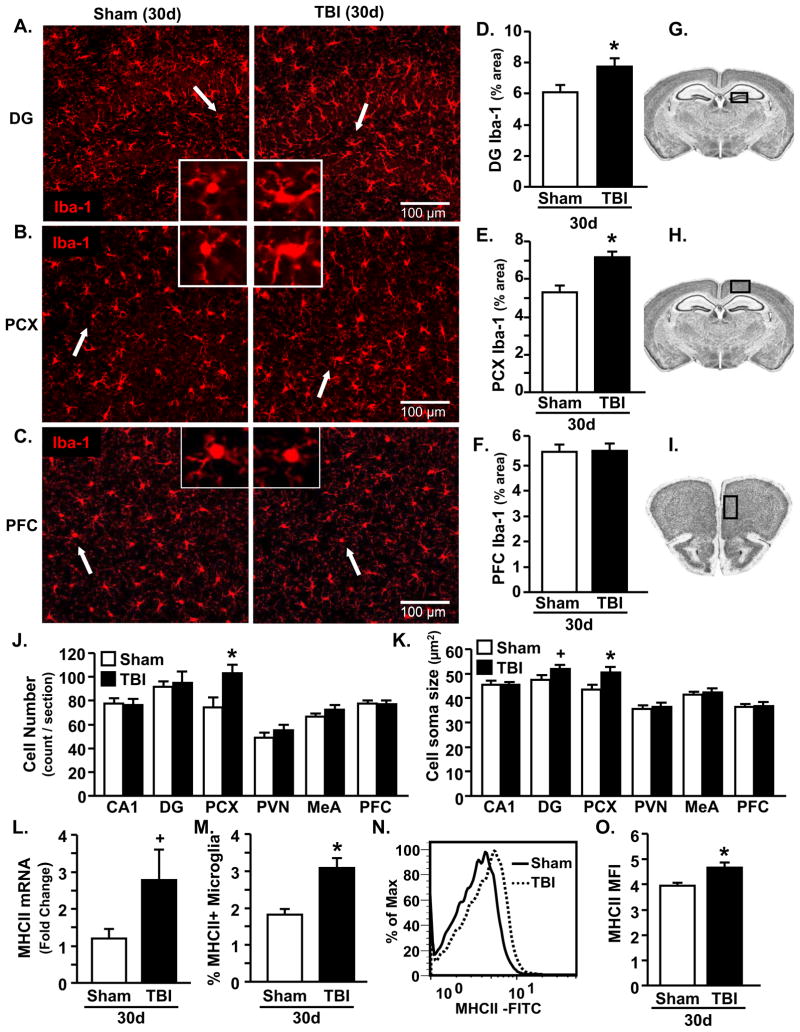
TBI-associated microglia priming 30 dpi
Next, the degree to which microglia retained a primed phenotype 30 dpi was determined. Representative images of Iba-1 labeling in the A) DG, B) PCX, and C) PFC of sham and TBI mice are shown. Iba-1 immunoreactivity was markedly increased 30 dpi in the DG (p<0.04, Fig. 4D) and PCX (p<0.0007, Fig. 4E), but was not different in the PFC (Fig. 4F). Iba-1 immunoreactivity tended to be increased in the PVN, but was unchanged in the CA1, or MeA (Table 2). In addition, the number of Iba-1+ cells was unchanged in brain regions examined with the exception of the PCX (p<0.02, Fig. 4J). Moreover, increased cell soma size was only detected in the DG (p=0.1) and PCX (p<0.03, Fig. 4K).
Figure 4. TBI-associated microglia priming 30 dpi.
Brains were collected 30 d after sham operation or TBI. In one cohort protein expression of Iba-1 was determined (n=10–11). In a separate cohort, CD11b+ cells were collected and mRNA and protein expression of MHCII was determined (n=9). A) Iba-1 staining in the DG of the HPC, B) PCX, and C) PFC of sham and TBI mice. Three images / sample at 20x were collected. Inset includes enlarged image of Iba-1+ cell indicated by white arrow. Proportional area for Iba-1 staining was increased in TBI mice compared to sham mice in the D) DG (F(1,19)=5.48, p<0.04) and E) PCX (F(1,18)=17.05, p<0.0007), but was not different between groups in the F) PFC. Schematics taken from the High Resolution Mouse Brain Atlas (Sidman et al.: http://www.hms.harvard.edu/research/brain/index.html) depicting where images were collected for the G) DG, H) PCX, and I) PFC. J) Average cell counts per section were only significantly different in the PCX (F(1,19)=6.81, p<0.02) and K) average cell soma size was increased in the DG (F(1,18)=2.51, p=0.1) and PCX (F(1,19)=5.61, p<0.03). Enriched CD11b+ cells from TBI mice had L) increased MHCII mRNA expression (F(1,15)= 3.4, p=0.09), M) and increased percentage of MHCII+ microglia (CD11b+/CD45low) (F(1,15)=16.18, p<0.002). N) Representative histogram of mean fluorescence intensity (MFI) for MHCII in microglia (CD11b+/CD45low). O) The average MFI for MHCII was increased in microglia (CD11b+/CD45low) of TBI mice (F(1,16)=9.13, p<0.009). Bars represent the mean ± SEM. Means with (*) are significantly different (p<0.05) and means with (+) tend to be different (p≤0.1) from sham controls. DG = dentate gyrus; HPC = hippocampus; PCX = parietal cortex; PVN = paraventricular nucleus; MeA = medial amygdala; PFC = prefrontal cortex
To further investigate this primed phenotype of microglia, MHCII mRNA and protein expression was determined in microglia from sham and TBI mice 30 dpi. Fig. 4L shows that MHCII mRNA expression was increased ~2.5 fold in enriched CD11b+ cells isolated from TBI mice compared to shams (p=0.09). Moreover, TBI increased the percentage of microglia (CD11b+/CD45low) that were MHCII+ (p<0.002, Fig. 4M), and increased the level of MHCII expression on a per cell basis by ~15% (p<0.009, Fig. 4N&O). Taken together, diffuse TBI induced a population of MHCII+ primed microglia 30 dpi.
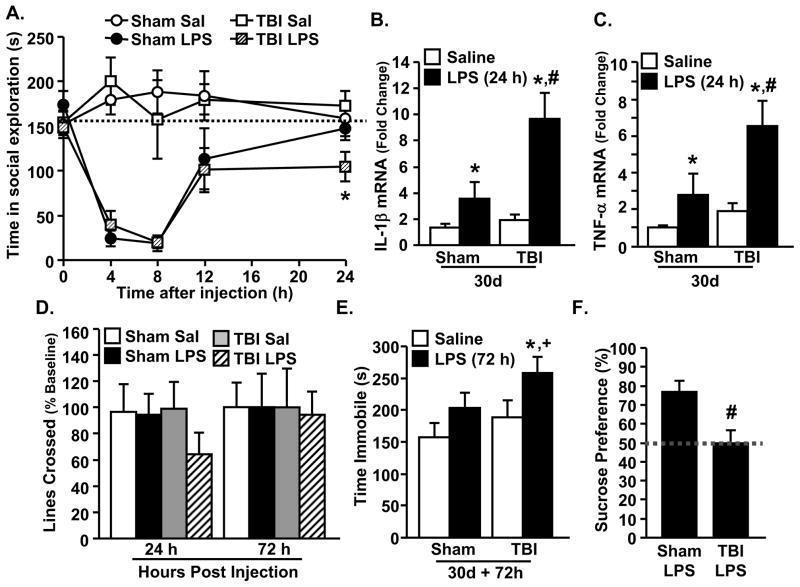
Peripheral LPS injection caused exaggerated microglial cytokine expression associated with protracted social withdrawal and depressive-like behavior in TBI mice
In several models, microglial priming or sensitization is associated with an exaggerated inflammatory cytokine response associated with cognitive impairment and the development of depressive-like behaviors (24,34,56). Therefore, we examined the degree to which primed microglia from TBI mice had an exaggerated response to peripheral LPS challenge 30 dpi. Fig. 5A shows that LPS injection reduced social exploratory behavior (p<0.0001) in a time dependent manner (p<0.001). The return to baseline social behavior 24 h after LPS was impaired in TBI-LPS mice compared to sham-LPS mice. For example, post-hoc analysis revealed that TBI mice injected with LPS had the lowest level of social exploration 24 h after injection compared to all other groups (p<0.02). After completing behavioral testing, enriched CD11b+ cells were isolated from whole brain homogenates and mRNA expression of IL-1β and TNFα was determined. IL-1β mRNA expression was increased in microglia 24 h after LPS in both sham and TBI mice (p<0.005), but was exaggerated in microglia of TBI mice injected with LPS (TBI-LPS) compared to all other groups (p<0.04, Fig. 5B). A similar pattern of TNFα expression was evident 24 h after LPS injection (p<0.02). Indeed post-hoc analysis showed that the highest expression of TNFα was in microglia from TBI-LPS mice (p<0.02, Fig. 5C). mRNA expression of iNOS, arginase, and IL-4Rα was also determined in enriched CD11b+ cells, but no significant differences were detected (Table 3).
Figure 5. Peripheral LPS injection caused exaggerated microglial cytokine expression associated with protracted social withdrawal and depressive-like behavior in TBI mice.
Mice were injected i.p. with saline or LPS (0.33 mg/kg) 30 d after sham operation or TBI. A) Social exploration was reduced by LPS (F(1,37)=87.88, p<0.0008) in a time dependent manner (F(4,196)=13.01, p<0.0001) (n=11–13). At 24 h after LPS, post-hoc analysis revealed that TBI-LPS mice had significantly reduced social exploration compared to all other groups (p<0.02). The dashed, horizontal line indicates baseline social exploratory behavior. B) Following the completion of the behavior tests (24 h), enriched brain CD11b+ cells were collected (n=9). mRNA expression of IL-1β was increased after LPS (F(1,32)=15.8, p<0.005), with the highest levels observed in TBI-LPS mice (TBI x LPS interaction: F(1,32)=4.96, p<0.04). C) mRNA expression of TNFα was increased after LPS (F(1,27)=10.1, p<0.004) and post-hoc analysis shows that the TBI-LPS group was significantly elevated compared to all other groups (p<0.02). D) Locomotor activity was determined at 0 (baseline), 24, and 72 h after injection (n=5–6). E) Depressive-like behavior was determined 72 h after LPS by time spent immobile in the TST (n=9–12). There was a main effect of TBI (F(1,41)=2.87, p≤0.1) and LPS (F(1,41)=5.31, p<0.03) and post-hoc analysis shows that the TBI-LPS group significantly differed from both sham-saline and TBI-saline groups (p<0.05 for both) and tended to be different from the sham-LPS group (p≤0.1). F) Depressive-like behavior was also assessed with a sucrose preference test for anhedonia starting 72 h after LPS. Whereas sham-LPS mice showed a strong preference for a 1% sucrose solution (77.0% ± 5.9%) (F(1,7)=8.98, p<0.03), TBI-LPS mice showed no preference (50.0% ± 6.8%). Bars represent the mean ± SEM. Means with (*) are significantly different (p<0.05) from sham-saline controls. Means with (#) are significantly different (p<0.05) from sham-saline and sham-LPS groups. Means with (+) tend to be different from sham-LPS groups.
Table 3.
| Enriched CD11b+ cell mRNA levels | ||||
|---|---|---|---|---|
| Gene | Sham Sal | Sham LPS | TBI Sal | TBI LPS |
| iNOS | 1.15 ± 0.23 | 0.96 ± 0.30 | 1.47 ± 0.26 | 1.74 ± 0.51 |
| Arg | 1.08 ± 0.14 | 1.75 ± 0.78 | 1.41 ± 0.22 | 1.01 ± 0.24 |
| IL-4Rα | 1.61 ± 0.59 | 3.67 ± 1.64 | 4.29 ± 2.20 | 1.90 ± 0.60 |
Adult mice were subjected to a sham injury (sham) or moderate midline fluid percussion injury (TBI) (n=8–10). After 30 d, mice were injected i.p. with saline or LPS (0.33 mg/kg). Following the completion of the behavior tests (24 h), enriched brain CD11b+ cells were collected. The mRNA expression of inflammatory-associated genes (iNOS) and anti-inflammatory-associated genes (Arg, IL-4Rα) were determined. Values represent the mean ± SEM. Arg = arginase 1.
In a related study, locomotor and depressive-like behaviors were evaluated in sham and TBI mice after LPS. Because generalized lethargy and malaise can confound behavioral tests of depression (34), locomotor activity was determined 24, 48, and 72 h after LPS. Fig. 5D shows that TBI mice had a ~60% reduction in locomotor activity 24 h after LPS indicating a maintained sickness response, but recovered to baseline activity by 72 h after LPS. Next, two major behavioral components of depression, resignation (TST) and anhedonia (sucrose preference), were determined 72 h after LPS. Fig. 5E shows that immobility in the TST was increased by both LPS (p<0.03) and TBI (p≤0.1), and that TBI-LPS mice had the highest immobility compared to all other groups (post-hoc: p<0.05 from sham-saline and TBI-saline; p≤0.1 from sham-LPS). Consistent with these results, sham-LPS mice preferred a 1% sucrose solution over water, but TBI-LPS mice did not (p<0.03, Fig. 5F). Taken together, LPS caused exaggerated IL-1β and TNFα expression in microglia of TBI mice (30 dpi) corresponding with prolonged social withdrawal, resignation, and anhedonia.
DISCUSSION
Previous studies in rodents indicate that early life infection, aging, sterile CNS injury, and pre-clinical neurodegenerative disease leads to the development of a primed and immune-reactive population of microglia (23,27,57–59). Here we defined primed microglia by increased Iba-1 labeling, increased cell soma size, increased MHCII mRNA and protein expression, and a hyperactive inflammatory response after acute immune challenge. We show that in a diffuse model of TBI (i.e., midline FPI), a population of primed microglia develops and persists in the brain for at least 30 dpi. Indeed, microglia from TBI mice (30 dpi) were hyperactive following an acute immune stimulus (i.p. LPS injection) associated with prolonged social withdrawal, resignation, and anhedonia. These are novel data that indicate diffuse TBI sensitizes microglia to an acute inflammatory challenge promoting depressive-like complications weeks after the initial injury.
An important element of this study was that diffuse TBI caused transient neuroinflammation, deficits in motor coordination, and depressive-like behavior. For example, TBI caused acute neuroinflammation (4–72 h) with increased mRNA expression of inflammatory mediators (IL-1β, TNFα, etc.) in the CX and HPC. Moreover, TBI resulted in a 2–3 fold increase in the number of PMCs that were associated with the brain. In focal models of CNS injury (e.g., spinal cord injury, axonal crush) these cells are thought to traffic to the site of injury and aid in clearance of debris or promote repair, but may also contribute to secondary damage (60). It is unclear what the roles of these cells are after a diffuse TBI, but they may contribute to increased neuroinflammation after injury. In the context of behavioral recovery, TBI mice were impaired in motor coordination on the rotarod immediately after injury and for at least 4 dpi, but recovered to baseline levels within 7 dpi. Although sham mice continued to have improved performance compared to TBI mice, this was likely an effect of training. A return to baseline motor coordination and body mass within 7 days after TBI is relevant because cognitive, balance, and motor impairments associated with mild-to-moderate TBI in humans are typically resolved within 7 dpi (1). Despite the recovery to baseline motor coordination and activity, TBI mice had increased resignation behavior 7 dpi. This is relevant because 30–40% of human TBI patients develop symptoms of depression acutely after injury (5,9). Increased depressive-like behavior in TBI mice, however, was no longer detected 30 dpi. Consistent with this result, TBI-related depression in humans is associated with periods of remission (5,10) and re-establishment (11). Therefore, the remission/re-occurrence of depression may point to secondary stimuli that can trigger the onset of these depressive-like symptoms.
Another key finding of the study was that primed microglia were detected in the brain of TBI mice 30 dpi. Indeed, expression of MHCII (mRNA and protein) was increased in microglia of TBI mice compared to sham controls. In addition, Iba-1 immunoreactivity and corresponding de-ramified morphology were increased in microglia of the HPC and PCX 30 dpi, consistent with previous studies investigating microglia activation 7 dpi (41). This hypertrophic (increased cell soma size) and increased Iba-1 profile in the DG and PCX is consistent with a primed and more inflammatory microglial phenotype (46,61). Notably, microglia activation and astrocyte activation did not occur in all of the same regions. This may suggest a larger role for astrocytes in responding to the primary injury whereas microglia may play a larger role in propagating the secondary injury. This microglial profile (increased Iba-1 and MHCII) in the brain of adult TBI mice is consistent with a primed phenotype detected in models of aging, early life infection, optic nerve crush, and pre-neurodegenerative disease (25,27,57,59,62,63). It is likely that these microglia will remain in a primed and hyper-reactive state for months to years after the injury. In support of this notion, positron emission tomography (PET) imaging studies on humans with a moderate to severe TBI show increased microglia activation by ligand [11C](R)PK11195 (PK) up to 17 years after injury (64). We interpret our data to indicate that the initial diffuse brain injury caused significant inflammation (cytokine expression, peripheral cell recruitment, etc.) preventing the resolution of microglia activation within the HPC. In support of this notion, increased CD14 mRNA expression persisted in the HPC 72 h after TBI coinciding with persistent microglia activation (CD14high). This is relevant because the HPC has a high proportion of inflammatory-associated receptors, glutamatergic neurons, and undergoes rapid remodeling making it more sensitive to inflammatory damage (65–67). Indeed, several models including aging, early life infection, pre-neurodegenerative disease, and social stress show that inflammatory-associated microglia priming is detected in the HPC (25,26,30,50,59). It is also relevant to highlight that regional specific priming may explain why we only observed a modest increase in MHCII mRNA and protein in microglia collected from whole brain homogenates. Taken together, these data provide increased evidence of the development of region-specific, primed microglial populations that persist after a diffuse TBI.
Related to the notion of microglia priming discussed above, acute activation of the immune system caused exaggerated expression of two key inflammatory cytokines, IL-1β and TNFα, in microglia of TBI mice. Microglia have an active role in the interpretation and propagation of cytokine signals that are initiated in the periphery. The production of these inflammatory cytokines by microglia normally results in an evolutionarily adaptive behavioral and physiological sickness response (32,68,69). Exaggerated or prolonged expression of these inflammatory mediators, however, can lead to increased neuronal damage and maladaptive behavioral responses including depression and delirium (26,27,34,58). For instance, exaggerated microglial activation in TBI mice after a peripheral LPS injection was associated with prolonged social withdrawal (24 h), and behavioral resignation and anhedonia (72 h). These behaviors are components of depressive-like behavior (55,70) and were undetectable in shams injected with LPS. Notably, TBI-associated microglia priming and the exaggerated inflammatory response to LPS were evident 30 dpi, which was well after these mice had returned to baseline activity and behavior. Thus, an acute systemic immune challenge unrelated to the initial head injury induced the re-occurrence of depression in TBI mice. The underlying mechanism of these inflammatory-associated cognitive and depressive complications is likely reduced long-term potentiation and neuronal firing within the HPC (71–73) in conjunction with increased metabolism of tryptophan to the neuroactive excitotoxin, quinolinic acid (74,75). This inflammatory-related depression may explain the higher percentage of TBI patients with resistance to conventional anti-depressant treatment (13,14). Thus, it will be important for future studies to investigate potential therapies that either prevent TBI-associated microglial priming or reverse priming once it has been established.
In conclusion, we provide the first evidence that an exaggerated response by primed microglia to a secondary inflammatory challenge is a potential trigger for the development/reoccurrence of neuropsychiatric complications. The critical component of this exaggerated microglia reactivity was the establishment of depressive-like behavior following an acute immune stimulus in TBI mice. Based on these data, we postulate that individuals who have suffered a TBI are more sensitive to inflammation caused by secondary stimuli (e.g., immune activation, stress, etc) resulting in the promotion of neuropsychiatric complications.
Supplementary Material
Acknowledgments
This research was supported by NIA grant R01-AG-033028 to J.P.G. A.M.F. was supported by a Med to Grad scholarship from the Howard Hughes Medical Institute (HHMI). The authors thank Todd Lash (OSU), Rachel Rowe (UA), and Kelley Hall (UK) for their technical assistance. The authors also thank Dr. Michelle Basso (OSU) for her consultation with the studies on motor coordination and locomotion after injury.
Footnotes
Supplemental information: Materials and Methods Supplementary
FINANCIAL DISCLOSURES
All authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McCrea M, Guskiewicz KM, Marshall SW, Barr W, Randolph C, Cantu RC, et al. Acute effects and recovery time following concussion in collegiate football players: The ncaa concussion study. JAMA. 2003;290:2556. doi: 10.1001/jama.290.19.2556. [DOI] [PubMed] [Google Scholar]
- 2.Lifshitz J, Witgen B, Grady M. Acute cognitive impairment after lateral fluid percussion brain injury recovers by 1 month: Evaluation by conditioned fear response. Beh Brain Res. 2007;177:347. doi: 10.1016/j.bbr.2006.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tang Y, Noda Y, Hasegawa T, Nabeshima T. A concussive-like brain injury model in mice (i): Impairment in learning and memory. J Neurotrauma. 1997;14:851–862. doi: 10.1089/neu.1997.14.851. [DOI] [PubMed] [Google Scholar]
- 4.Woodcock T, Morganti-Kossmann MC. The role of markers of inflammation in traumatic brain injury. Front Neurol. 2013;4:18. doi: 10.3389/fneur.2013.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jorge R, Robinson R, Arndt S, Starkstein S, Forrester A, Geisler F. Depression following traumatic brain injury: A 1 year longitudinal study. J Affect Disorders. 1993;27:233. doi: 10.1016/0165-0327(93)90047-n. [DOI] [PubMed] [Google Scholar]
- 6.Fleminger S. Long-term psychiatric disorders after traumatic brain injury. Eur J Anesth. 2008;25:123–130. doi: 10.1017/S0265021507003250. [DOI] [PubMed] [Google Scholar]
- 7.Kreutzer, Jeffrey S, Seel, Ronald T, Gourley, Eugene The prevalence and symptom rates of depression after traumatic brain injury: A comprehensive examination. Brain Injury. 2001;15:563–576. doi: 10.1080/02699050010009108. [DOI] [PubMed] [Google Scholar]
- 8.Gualtieri T, Cox DR. The delayed neurobehavioural sequelae of traumatic brain injury. Brain Injury. 1991;5:219–232. doi: 10.3109/02699059109008093. [DOI] [PubMed] [Google Scholar]
- 9.Jorge R, Robinson R, Moser D, Tateno A, Crespo-Facorro B, Arndt S. Major depression following traumatic brain injury. Arch Gen Psychiatry. 2004;61:42–50. doi: 10.1001/archpsyc.61.1.42. [DOI] [PubMed] [Google Scholar]
- 10.Hibbard MR, Uysal S, Kepler K, Bogdany J, Silver J. Axis i psychopathology in individuals with traumatic brain injury. J Head Trauma Rehab. 1998;13:24–39. doi: 10.1097/00001199-199808000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Holsinger T, Steffens DC, Phillips C, Helms MJ, Havlik RJ, Breitner JC, et al. Head injury in early adulthood and the lifetime risk of depression. Arch Gen Psychiatry. 2002;59:17–22. doi: 10.1001/archpsyc.59.1.17. [DOI] [PubMed] [Google Scholar]
- 12.Teasdale T, Engberg A. Suicide after traumatic brain injury: A population study. J Neurol Neurosurg Psychiatry. 2001;71:436–440. doi: 10.1136/jnnp.71.4.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saran A. Antidepressants not effective in headache associated with minor closed head injury. Int J Psychiat Med. 1988;18:75–83. doi: 10.2190/15ah-jb7q-u94t-jeaf. [DOI] [PubMed] [Google Scholar]
- 14.Ashman TA, Cantor JB, Gordon WA, Spielman L, Flanagan S, Ginsberg A, et al. A randomized controlled trial of sertraline for the treatment of depression in persons with traumatic brain injury. Arch Phys Med Rehab. 2009;90:733. doi: 10.1016/j.apmr.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 15.Miller AH, Raison CL. Immune system contributions to the pathophysiology of depression. Focus. 2008;6:36–45. [Google Scholar]
- 16.Raison CL, Capuron L, Miller AH. Cytokines sing the blues: Inflammation and the pathogenesis of depression. Trends Immunol. 2006;27:24. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lanquillon S, Krieg JC, Bening-Abu-Shach U, Vedder H. Cytokine production and treatment response in major depressive disorder. Neuropsychopharmacology. 2000;22:370. doi: 10.1016/S0893-133X(99)00134-7. [DOI] [PubMed] [Google Scholar]
- 18.Maier B, Schwerdtfeger K, Mautes A, Holanda M, Muller M, Steudel WI, et al. Differential release of interleukines 6, 8, and 10 in cerebrospinal fluid and plasma after traumatic brain injury. Shock. 2001;15:421–426. doi: 10.1097/00024382-200115060-00002. [DOI] [PubMed] [Google Scholar]
- 19.Fassbender K, Schneider S, Bertsch T, Schlueter D, Fatar M, Ragoschke A, et al. Temporal profile of release of interleukin-b in neurotrauma. Neuroscience Letters. 2000;284:135. doi: 10.1016/s0304-3940(00)00977-0. [DOI] [PubMed] [Google Scholar]
- 20.Kossmann T, Hans VH, Imhof HG, Stocker R, Grob P, Trentz O, et al. Intrathecal and serum interleukin-6 and the acute-phase response in patients with severe traumatic brain injuries. Shock (Augusta, Ga) 1995;4:311–317. doi: 10.1097/00024382-199511000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Gentleman SM, Leclercq PD, Moyes L, Graham DI, Smith C, Griffin WST, et al. Long-term intracerebral inflammatory response after traumatic brain injury. Forensic Sci Int. 2004;146:97. doi: 10.1016/j.forsciint.2004.06.027. [DOI] [PubMed] [Google Scholar]
- 22.Barrientos R, Frank M, Watkins L, Maier S. Memory impairments in healthy aging: Role of aging-induced microglial sensitization. Aging Dis. 2010;1:212. [PMC free article] [PubMed] [Google Scholar]
- 23.Henry C, Huang Y, Wynne AM, Godbout JP. Peripheral lipopolysaccharide (lps) challenge promotes microglial hyperactivity in aged mice that is associated with exaggerated induction of both pro-inflammatory il-1[beta] and anti-inflammatory il-10 cytokines. Brain Behav Immun. 2009;23:309. doi: 10.1016/j.bbi.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wohleb ES, Fenn AM, Pacenta AM, Powell ND, Sheridan JF, Godbout JP. Peripheral innate immune challenge exaggerated microglia activation, increased the number of inflammatory cns macrophages, and prolonged social withdrawal in socially defeated mice. Psychoneuroendocrino. 2012 doi: 10.1016/j.psyneuen.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bland ST, Beckley JT, Young S, Tsang V, Watkins LR, Maier SF, et al. Enduring consequences of early-life infection on glial and neural cell genesis within cognitive regions of the brain. Brain Behav Immun. 2010;24:329. doi: 10.1016/j.bbi.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cunningham C, Campion S, Lunnon K, Murray CL, Woods JFC, Deacon RMJ, et al. Systemic inflammation induces acute behavioral and cognitive changes and accelerates neurodegenerative disease. Biol Psychiatry. 2009;65:304. doi: 10.1016/j.biopsych.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palin K, Cunningham C, Forse P, Perry VH, Platt N. Systemic inflammation switches the inflammatory cytokine profile in cns wallerian degeneration. Neurobiology of Disease. 2008;30:19. doi: 10.1016/j.nbd.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 28.Jurgens HA, Johnson RW. Dysregulated neuronal-microglial cross-talk during aging, stress and inflammation. Exp Neurol. 2012;233:40. doi: 10.1016/j.expneurol.2010.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tremblay M-È, Stevens B, Sierra A, Wake H, Bessis A, Nimmerjahn A. The role of microglia in the healthy brain. J Neurosci. 2011;31:16064–16069. doi: 10.1523/JNEUROSCI.4158-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen J, Buchanan J, Sparkman N, Godbout J, Freund G, Johnson R. Neuroinflammation and disruption in working memory in aged mice after acute stimulation of the peripheral innate immune system. Brain Behav Immun. 2008;22:301. doi: 10.1016/j.bbi.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barrientos RM, Frank MG, Hein AM, Higgins EA, Watkins LR, Rudy JW, et al. Time course of hippocampal il-1 [beta] and memory consolidation impairments in aging rats following peripheral infection. Brain Behav Immun. 2009;23:46. doi: 10.1016/j.bbi.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Godbout J, Chen J, Abraham J, Richwine A, Berg B, Kelley K, et al. Exaggerated neuroinflammation and sickness behavior in aged mice following activation of the peripheral innate immune system. FASEB J. 2005;10:1329. doi: 10.1096/fj.05-3776fje. [DOI] [PubMed] [Google Scholar]
- 33.Huang Y, Henry C, Dantzer R, Johnson R, Godbout J. Exaggerated sickness behavior and brain proinflammatory cytokine expression in aged mice in response to intracerebroventricular lipopolysaccharide. Neurobiol Aging. 2008;29:1744. doi: 10.1016/j.neurobiolaging.2007.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Godbout J, Moreau M, Lestage J, Chen J, Sparkman N, O’Connor J, et al. Aging exacerbates depressive-like behavior in mice in response to activation of the peripheral innate immune system. Neuropsychopharmacol. 2008;33:2341. doi: 10.1038/sj.npp.1301649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holmin S, Mathiesen T, Shetye J, Biberfeld P. Intracerebral inflammatory response to experimental brain contusion. Acta Nirochlrurglca. 1995;132:110–119. doi: 10.1007/BF01404857. [DOI] [PubMed] [Google Scholar]
- 36.Lifshitz J, Kelley BJ, Povlishock JT. Perisomatic thalamic axotomy after diffuse traumatic brain injury is associated with atrophy rather than cell death. J Neuropathol Exp Neurol. 2007;66:218–29. doi: 10.1097/01.jnen.0000248558.75950.4d. [DOI] [PubMed] [Google Scholar]
- 37.Morales D, Marklund N, Lebold D, Thompson H, Pitkanen A, Maxwell W, et al. Experimental models of traumatic brain injury: Do we really need to build a better mousetrap? Neuroscience. 2005;136:971. doi: 10.1016/j.neuroscience.2005.08.030. [DOI] [PubMed] [Google Scholar]
- 38.Lifshitz J. Fluid percussion injury model. In: Chen J, Xu ZC, Xu X-M, Zhang JH, editors. Animal models of acute neurological injuries. Vol. 369 Humana Press; 2009. [Google Scholar]
- 39.Fenn AM, Henry CJ, Huang Y, Dugan A, Godbout JP. Lipopolysaccharide-induced interleukin (il)-4 receptor-alpha expression and corresponding sensitivity to the m2 promoting effects of il-4 are impaired in microglia of aged mice. Brain Behav Immun. 2012;26:766–777. doi: 10.1016/j.bbi.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Witgen B, Lifshitz J, Smith M, Schwarzbach E, Liang S, Grady M, et al. Regional hippocampal alteration associated with cognitive deficit following experimental brain injury: A systems, network and cellular evaluation. Neuroscience. 2005;133:1. doi: 10.1016/j.neuroscience.2005.01.052. [DOI] [PubMed] [Google Scholar]
- 41.Bachstetter AD, Rowe RK, Kaneko M, Goulding D, Lifshitz J, Van Eldik LJ. The p38[alpha] mapk regulates microglial responsiveness to diffuse traumatic brain injury. The Journal of Neuroscience. 2013;33:6143–6153. doi: 10.1523/JNEUROSCI.5399-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kelley BJ, Lifshitz J, Povlishock JT. Neuroinflammatory responses after experimental diffuse traumatic brain injury. J Neuropathol Exp Neurol. 2007;66:989–1001. doi: 10.1097/NEN.0b013e3181588245. [DOI] [PubMed] [Google Scholar]
- 43.Kelley BJ, Farkas O, Lifshitz J, Povlishock JT. Traumatic axonal injury in the perisomatic domain triggers ultrarapid secondary axotomy and wallerian degeneration. Experimental Neurology. 2006;198:350. doi: 10.1016/j.expneurol.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 44.Witgen B, Lifshitz J, Grady M. Inbred mouse strains as a tool to analyze hippocampal neuronal loss after brain injury: A stereological study. J Neurotraum. 2006;23:1320–1329. doi: 10.1089/neu.2006.23.1320. [DOI] [PubMed] [Google Scholar]
- 45.Dunham N, Miya T. A note on a simple apparatus for detecting neurological deficits in rats and mice. J Am Pharmac Ass. 1957;46:208–209. doi: 10.1002/jps.3030460322. [DOI] [PubMed] [Google Scholar]
- 46.Corona A, Huang Y, O’Connor J, Dantzer R, Kelley K, Popovich P, et al. Fractalkine receptor (cx3cr1) deficiency sensitizes mice to the behavioral changes induced by lipopolysaccharide. J Neuroinflamm. 2010;7:93. doi: 10.1186/1742-2094-7-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Corona AW, Norden DM, Skendelas JP, Huang Y, O’Connor JC, Lawson M, et al. Indoleamine 2,3-dioxygenase inhibition attenuates lipopolysaccharide induced persistent microglial activation and depressive-like complications in fractalkine receptor (cx3cr1)-deficient mice. Brain, Behavior, and Immunity. 2013;31:134. doi: 10.1016/j.bbi.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Henry C, Huang Y, Wynne A, Hanke M, Himler J, Bailey M, et al. Minocycline attenuates lipopolysaccharide (lps)-induced neuroinflammation, sickness behavior, and anhedonia. J Neuroinflamm. 2008;5:15. doi: 10.1186/1742-2094-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Paxinos G, Franklin K. The mouse brain in stereotaxic coordinates. 2 2004. [Google Scholar]
- 50.Wohleb ES, Hanke ML, Corona AW, Powell ND, Stiner LTM, Bailey MT, et al. [beta]-adrenergic receptor antagonism prevents anxiety-like behavior and microglial reactivity induced by repeated social defeat. J Neurosci. 2011;31:6277. doi: 10.1523/JNEUROSCI.0450-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Donnelly DJ, Gensel JC, Ankeny DP, van Rooijen N, Popovich PG. An efficient and reproducible method for quantifying macrophages in different experimental models of central nervous system pathology. J Neurosci Meth. 2009;181:36. doi: 10.1016/j.jneumeth.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hamm RJ. Neurobehavioral assessment of outcome following traumatic brain injury in rats: An evaluation of selected measures. J Neurotrauma. 2001;18:1207–16. doi: 10.1089/089771501317095241. [DOI] [PubMed] [Google Scholar]
- 53.McIntosh TK, Noble L, Andrews B, Faden AI. Traumatic brain injury in the rat: Characterization of a midline fluid-percussion model. Cent Nerv Syst Trauma. 1987;4:119–34. doi: 10.1089/cns.1987.4.119. [DOI] [PubMed] [Google Scholar]
- 54.Schmidt RH, Grady MS. Regional patterns of blood-brain barrier breakdown following central and lateral fluid percussion injury in rodents. J Neurotrauma. 1993;10:415–30. doi: 10.1089/neu.1993.10.415. [DOI] [PubMed] [Google Scholar]
- 55.El Yacoubi M, Bouali S, Popa D, Naudon L, Leroux-Nicollet I, Hamon M, et al. Behavioral, neurochemical, and electrophysiological characterization of a genetic mouse model of depression. P Natl Acad Sci USA. 2003;100:6227–6232. doi: 10.1073/pnas.1034823100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Williamson LL, Sholar PW, Mistry RS, Smith SH, Bilbo SD. Microglia and memory: Modulation by early-life infection. J Neurosci. 2011;31:15511–15521. doi: 10.1523/JNEUROSCI.3688-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bilbo SD, Schwarz JM. Early-life programming of later-life brain and behavior: A critical role for the immune system. Front Behav Neurosci. 2009;3:14. doi: 10.3389/neuro.08.014.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Murray C, Sanderson DJ, Barkus C, Deacon RMJ, Rawlins JNP, Bannerman DM, et al. Systemic inflammation induces acute working memory deficits in the primed brain: Relevance for delirium. Neurobiol Aging. 2010 doi: 10.1016/j.neurobiolaging.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cunningham C, Wilcockson DC, Campion S, Lunnon K, Perry VH. Central and systemic endotoxin challenges exacerbate the local inflammatory response and increase neuronal death during chronic neurodegeneration. The Journal of Neuroscience. 2005;25:9275–9284. doi: 10.1523/JNEUROSCI.2614-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.David S, Kroner A. Repertoire of microglial and macrophage responses after spinal cord injury. Nat Rev Neurosci. 2011;12:388–99. doi: 10.1038/nrn3053. [DOI] [PubMed] [Google Scholar]
- 61.Chen Z, Jalabi W, Shpargel KB, Farabaugh KT, Dutta R, Yin X, et al. Lipopolysaccharide-induced microglial activation and neuroprotection against experimental brain injury is independent of hematogenous tlr4. J Neurosci. 2012;32:11706–11715. doi: 10.1523/JNEUROSCI.0730-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Frank M, Barrientos R, Biedenkapp J, Rudy J, Watkins L, Maier S. Mrna up-regulation of mhc ii and pivotal pro-inflammatory genes in normal brain aging. Neurobiol Aging. 2006;27:717. doi: 10.1016/j.neurobiolaging.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 63.Streit WJ. Microglia and alzheimer’s disease pathogenesis. J Neurosci Res. 2004;77:1. doi: 10.1002/jnr.20093. [DOI] [PubMed] [Google Scholar]
- 64.Ramlackhansingh AF, Brooks DJ, Greenwood RJ, Bose SK, Turkheimer FE, Kinnunen KM, et al. Inflammation after trauma: Microglial activation and traumatic brain injury. Annals of Neurology. 2011;70:374. doi: 10.1002/ana.22455. [DOI] [PubMed] [Google Scholar]
- 65.Ban E, Milon G, Prudhomme N, Fillion G, Haour F. Receptors for interleukin-1 ([alpha] and [beta]) in mouse brain: Mapping and neuronal localization in hippocampus. Neuroscience. 1991;43:21. doi: 10.1016/0306-4522(91)90412-h. [DOI] [PubMed] [Google Scholar]
- 66.Cunningham ET, Wada E, Carter DB, Tracey DE, Battey JF, De Souza EB. In situ histochemical localization of type i interleukin-1 receptor messenger rna in the central nervous system, pituitary, and adrenal gland of the mouse. J Neurosci. 1992;12:1101–1114. doi: 10.1523/JNEUROSCI.12-03-01101.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Williamson LL, Bilbo SD. Chemokines and the hippocampus: A new perspective on hippocampal plasticity and vulnerability. Brain Behav Immun. 2013 doi: 10.1016/j.bbi.2013.01.077. [DOI] [PubMed] [Google Scholar]
- 68.Godbout JP, Johnson RW. Age and neuroinflammation: A lifetime of psychoneuroimmune consequences. Immunol Allergy Clin. 2009;29:321. doi: 10.1016/j.iac.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 69.Corona A, Fenn A, Godbout J. Cognitive and behavioral consequences of impaired immunoregulation in aging. J Neuroimmune Pharm. 2012;7:7. doi: 10.1007/s11481-011-9313-4. [DOI] [PubMed] [Google Scholar]
- 70.Beck A, Ward C, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 71.Maher FO, Nolan, Yvonne, Lynch, Marina A. Downregulation of il-4-induced signalling in hippocampus contributes to deficits in ltp in the aged rat. Neurobiol Aging. 2005;26:12. doi: 10.1016/j.neurobiolaging.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 72.Dantzer R, O’Connor J, Freund G, Johnson R, Kelley K. From inflammation to sickness and depression: When the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Frenois F, Moreau M, O’Connor J, Lawson M, Micon C, Lestage J, et al. Lipopolysaccharide induces delayed fosb/deltafosb immunostaining within the mouse extended amygdala, hippocampus and hypothalamus, that parallel the expression of depressive-like behavior. Psychoneuroendocrinology. 2007;32:516. doi: 10.1016/j.psyneuen.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Steiner J, Walter M, Gos T, Guillemin G, Bernstein H-G, Sarnyai Z, et al. Severe depression is associated with increased microglial quinolinic acid in subregions of the anterior cingulate gyrus: Evidence for an immune-modulated glutamatergic neurotransmission? J Neuroinflamm. 2011;8:94. doi: 10.1186/1742-2094-8-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dantzer R, O’Connor JC, Lawson MA, Kelley KW. Inflammation-associated depression: From serotonin to kynurenine. Psychoneuroendocrinology. 2011;36:426–36. doi: 10.1016/j.psyneuen.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.