Abstract
The development of glomerulonephritis causes glomerular injury and renal dysfunction and is thought to increase renin release thus activating the renin-angiotensin system (RAS). The aims of this study were to demonstrate activation of the intrarenal RAS and determine the effects of direct renin inhibition (DRI) on the progression of glomerulonephritis. Rats were treated with anti-Thy1.1 antibody with or without DRI, aliskiren (30 mg/kg/day). In the glomerulonephritic rats, protein, microalbumin excretion levels and urinary angiotensinogen excretion, glomerular expansion score, and intrarenal TGF-β and PAI-1 mRNA levels were augmented compared with control rats; however, hypertension was not observed in the glomerulonephritic rats and aliskiren treatment did not modify their blood pressure. The increases in urinary protein (94.7 ± 13.0 mg/day) and microalbumin (7.52 ± 2.6 mg/day) excretion were reduced by aliskiren (43.6 ± 4.5 mg/day of protein and 2.57 ± 0.7 mg/day of microalbumin). Furthermore, the progression of glomerular expansion and elevation of intrarenal TGF-β and PAI-1 levels were prevented by aliskiren. Importantly, aliskiren suppressed the augmentation of urinary angiotensinogen levels, the increased angiotensinogen expression in the kidneys and the increases in Ang II levels in renal medulla induced by the anti-Thy1.1 antibody. These results suggest that DRI with aliskiren prevents intrarenal RAS activation leading to mitigation of the development of glomerulonephritis. In addition, the renoprotective effects of DRI on glomerulonephritis occur in a blood pressure-independent manner. Accordingly, treatment with aliskiren may be an effective approach to treat glomerulonephritis as well as other intrarenal RAS associated kidney diseases.
Keywords: renin, angiotensinogen, kidney, renin inhibition, intrarenal angiotensin II
Introduction
The renin-angiotensin system (RAS) plays an important role in the development of hypertension and renal diseases1–8. Because components of the RAS are widely distributed in the brain9, heart10, adrenal glands11, vasculature, and kidneys2;12;13, the focus of interest has shifted toward the pleiotropic roles of the local RAS activation3;14. The intrarenal RAS not only regulates blood pressure (BP) but it also contributes to renal cell proliferation and the development of glomerulosclerosis and renal fibrosis15–17. Indeed, previous studies have shown that angiotensin-converting enzyme (ACE) inhibitors and/or angiotensin (Ang) II type 1 receptor (AT1R) blockers have beneficial effects in rats and humans with various renal diseases, and these effects are often considerably stronger than their suppressive effects on BP18–20.
Chronic kidney diseases (CKD) result in substantial renal damage and are frequently characterized by progression to end-stage renal disease. This process typically involves glomerulosclerosis and interstitial fibrosis. The mechanisms contributing to the progression of renal damage are not fully understood; however, they may be distinct from those responsible for the initial injury3. Glomerular hypertension, cell hypertrophy, and extracellular matrix accumulation all appear to be involved in the progression of CKD. In addition, numerous studies have implicated Ang II in this process21;22. Intrarenal Ang II levels are enhanced in CKD, which can stimulate glomerular cell hypertrophy, and augment extracellular matrix accumulation17;22;23. Blockers of AT1R or synthesis inhibitors mitigate and can even prevent the renal injury in experimental models with progressive CKD1;3;22;24. Studies on the efficacy of ACE inhibitors in patients with non-diabetic chronic renal failure indicate that these inhibitors have beneficial effects in preserving renal function25.
Various glomerulonephritis in humans including lupus nephritis, IgA nephropathy and Henoch-Schonlein purpura nephritis cause glomerular damage leading to renal dysfunction7. Anti-Thy1.1 glomerulonephritis is an established model of glomerulonephritis7;16;26. In this model, the immediate binding of the antibody to the antigen in mesangial cells activates severe immuno-mediated mesangiolysis. It has been demonstrated that treatment with AT1R blockers mitigates the progression of anti-Thy1.1 glomerulonephritis in rats7;16;27–29. Obvious mesangial cell proliferation, marked extracellular matrix accumulation, adhesion to Bowman’s capsule, glomerulosclerosis and tubulointerstitial fibrosis are observed in the anti-Thy1.1 glomerulonephritis. These pathological changes were less severe in rats chronically treated with ACE inhibitors or AT1R blockers16;27–29. These findings indicate that activation of the intrarenal RAS plays a pivotal role in the progression of glomerulonephritis.
The development of renin inhibitors has provided an opportunity to evaluate the effects of direct renin inhibition (DRI) as another means of RAS blockade. Aliskiren is the main inhibitor currently available30–32. The direct administration of aliskiren to kidneys by using a collagen matrix was shown to mitigate anti-Thy1.1 antibody-induced glomerulonephritis33; however, the effects of aliskiren on intrarenal RAS activity and its renoprotective effects when aliskiren is systemically administrated have not been established. The central hypothesis of this study is that activation of the intrarenal RAS plays a crucial role in the development of glomerulonephritis. In accordance with the hypothesis, the following aims were targeted: 1) To demonstrate the activation of intrarenal RAS34 using urinary angiotensinogen (uAGT) as an index of intrarenal Ang II levels, in rats with anti-Thy1.1 glomerulonephritis, and 2) to demonstrate that DRI with aliskiren, suppresses the activation of the intrarenal RAS in the glomerulonephritis model and prevents the progression of renal injury thus developing further a novel strategy for treatment of glomerulonephritis. The obtained results demonstrate that the intrarenal RAS is markedly activated during the early phases of glomerulonephritis, and that chronic systemic administration of aliskiren attenuates the increase in the intrarenal RAS activity and prevents the glomerular injury in anti-Thy1.1 glomerulonephritis.
Materials and Methods
Animals
The experiments were performed on Sprague-Dawley and Fischer male rats (230–430 g). The animal experimental protocol was approved by the Animal Care and Use Committee of Tulane University. The rats were housed in a constant temperature room with 12 hours dark and 12 hours light cycle with free access to food and water.
Anti-Thy1.1 Glomerulonephritis
A monoclonal antibody against rat anti-Thy1.1, OX-7, was purchased from Cedarlane Laboratories (Burlington, NC).
Experimental Design
Rats were divided into three groups; control group (n = 11), anti-Thy1.1 glomerulonephritis group (n = 19) and anti-Thy1.1 glomerulonephritis treated with aliskiren (30 mg/kg/day, n = 16). One group of rats was kept in individual metabolic cages with free access to food and tap water when urine samples were collected. Administration of aliskiren was started 4 days before the injection of anti-Thy1.1 antibody using an osmotic mini pump (Alzet osmotic pump, Alza, Mountain View, CA). Anti-Thy1.1 (200 µg/100 g body weight) was injected via a tail vein at day 0. Rats in the control group received the same volume of vehicle (isotonic saline). Blood and tissue samples were collected at 14 days after starting treatments by conscious decapitation.
Systolic Blood Pressure (SBP) and Renal Function
Systolic arterial blood pressure was measured by a tail-cuff system as previously described. Preliminary studies demonstrated that aliskiren treatment alone does not alter SBP in normal rats. In this group, after 10 days of aliskiren treatment, SBP averaged 114±2 mmHg compared to control measurement of 116±4 mmHg (n=6). Twenty-four hour urine samples were collected at days -4, 3, 5, 7, 10 and 14. Urinary protein was measured by the Pyrogallol red method (Wako chemical, Osaka, Japan). The level of microalbumin was determined by the rat albumin ELISA kit (ALPCO Diagnostics, Salem, NH). Plasma creatinine levels were measured by Jaffe method.
Urinary and Plasma Angiotensinogen (AGT) Measurements
Urinary and plasma concentrations of AGT were measured by using a commercially available ELISA kit (IBL America, Minneapolis, MN), as routinely used in our laboratory35. The results were normalized based on the 24-hour urine volumes and reported as uAGT excretion.
Plasma Renin Activity Assay
The blood samples were collected into tubes containing 5.0 mmol/l EDTA, and plasma renin activity (PRA) were assayed by using a commercially available kit (DiaSorin, Stillwater, MN). PRA was expressed as nanograms per milliliter per hour of generated Ang I.
Ang II Measurements in Plasma, Kidney Cortex and Medulla
For plasma Ang II determinations, blood (1ml) was added to 100% methanol. The supernatant was transferred, dried, and then assayed. For the Kidney Ang II determinations, the right kidney was sectioned into cortex and medulla. Each section was weighed and immediately minced into ice-cold 100% methanol. The tissue was homogenized with a tissue tearor and then centrifuged as mentioned above. The soluble homogenates were transferred, vacuum dried, extracted, and then assayed in the same manner as is routinely done in our lab.
Histological Analysis
Glomerular matrix expansion was evaluated by periodic acid-Schiff staining (PAS). Kidney tissues were fixed in 10% buffered formalin for 24 hours, embedded in paraffin and cut into 4 µm sections. Twenty glomeruli in a kidney section were randomly selected, and the PAS positive score was analyzed by using Image Pro-plus software. Thereafter, an average of the positive score in 20 glomeruli was calculated as a glomerular matrix expansion score in each animal.
Immunohistochemistry
Formalin fixed kidney sections (4 µm) were deparaffinized with xylene and dehydrated with ethanol. The samples were heated at 100° C for 60 min in citrate buffer, and rat AGT was detected by using a rabbit primary antibody against rat AGT (IBL America, Minneapolis, MN), a Vectastain ABC kit (VECTOR laboratories, Burlingame, CA) and 3,3’-diaminobenzidine substrate kits. Samples were co-stained with hematoxylin before analysis. The staining was performed with an Autostainer Plus (Dako, Carpinteria, CA). Immunoreactivity was semi-quantitatively evaluated in a blinded test as described in histological analysis section.
Western Blotting
Proteins were extracted from the renal cortex, and the protein concentrations were quantified by a bicinchoninic acid protein assays kit (Pierce, Rockford, IL). For AGT, ten µg of total protein and for TGF-β1, PAI-1 and PRR, 60 µg of total protein were separated by using a 4–12% SDS-PAGE (Invitrogen Life Technologies, Grand Island, NY) and then transferred to a nitrocellulose membrane. The membranes were incubated with a rabbit polyclonal anti-AGT antibody (IBL America, Minneapolis, MN), anti-TGF-β1 antibody (Santa Cruze Biotechnology, CA), anti-PAI-1 antibody (abcam, Cambridge, MA) or anti-ATP6AP2 antibody (SIGMA-ALDRICH, Saint lois, MO). A secondary fluorescent-goat anti-rabbit IgG antibody (IRDye 800 CW, Licor Biosciences, Lincoln, NE) was used to detect the target proteins. GAPDH or β-actin levels were also determined by using a mouse monoclonal anti-GAPDH antibody (Santa Cruze Biotechnology, CA) or a mouse monoclonal anti-β-actin antibody (abcam, Cambridge, MA). A secondary fluorescent-goat anti-mouse IgG antibody (IRDye 680 CW, Li-Cor Biosciences) was used for the detection of GAPDH and anti-β-actin. Immunoreactive proteins were detected by an Odyssey System (Li-Cor Bioscience).
Quantitative Real Time RT-PCR (qRT-PCR)
For total RNA isolation, approximately 10 mg of tissue stored in RNAlater (Ambion) was used. The tissues were homogenized, and total RNAs were isolated by using the RNeasy mini kit (Qiagen). Quantitative RT-PCR analysis was performed as described previously36;37. Twenty ng of total RNA was analyzed using the Mx3000P system (Stratagene) with the Brilliant Single-Step QRT-PCR Master Mix II Kit (Stratagene). The results were normalized on the basis of the mRNA expression level of β-actin. The sequence of the primers used in the study were as follows; transforming growth factor (TGF)-β1: forward primer, 5′-TACCATGCCAACTTCTGTC-3′; reverse primer, 5′-AAGGACCTTGCTGTACTGTGT-3′; and probe,6 FAM-CCCTACATTTGGAGCCTGGAC-BHQ1; plasminogen activator inhibitor (PAI)-1 forward primer, 5′-CTCCACAGCCATTCTAGTCT-3′; reverse primer, 5′-CCATGAAGAGGATTGTCTCT-3′; and probe, 6 FAM-ACCGATCCTTTCTCTTTGTGGTTC-BHQ1, PRR forward primer, 5′-ATCCTTGAGACGAAACAAGA-3′; reverse primer, 5′- AGCCAGTCATAATCCACAGT-3′; and probe, 6 FAM- ACACCCAAAGTCCCTACAACCTTG-BHQ1and β-Actin: forward primer, 5′-ATC ATG AAG TGT GAC GTT GA-3′; reverse primer, 5′-GAT CTT CAT GGT GCT AGG AGC-3′; and probe, 5′/HEX/TCT ATG CCA ACA CAG TGC TGT CTG GT/BHQ2/3′.
Statistical Analysis
All data are expressed as mean ± SE. Statistical analyses were performed using a one-way factorial ANOVA with GraphPad PRISM software (GraphPad Software, Inc., La Jolla, CA). Statistically significant differences were defined as P< 0.05.
Results
Systolic Blood Pressure (SBP) and Biological Parameters
All groups displayed similar food consumption, water intake, urine volumes, Na+ and K+ excretion rates (Table 1C) and body weight (Table 1A). SBP values (Table 1B) were not altered by the injection of anti-Thy1.1 antibody and were not significantly different throughout the 14 days. Likewise, SBP was not significantly altered by aliskiren treatment. Plasma creatinine values were also not significantly different in the 3 groups (Table 1C).
TABLE 1.
Body weight, systolic blood pressure (SBP) and plasma creatinine concentration in control, anti-Thy1.1 and anti-Thy1.1 + aliskiren-treated rats
| 0 Week | 1 Week | 2 Weeks | |
|---|---|---|---|
| Body weight | |||
| Control | 251.0 ± 7.2 | 305.5 ± 10.0 | 338.5 ± 12.0 |
| Anti-Thy1.1 GN | 228.9 ± 3.5 | 300.0 ± 3.6 | 327.8 ± 5.3 |
| Anti-Thy1.1 GN + Aliskiren | 265.0 ± 5.0 | 329.3 ± 10.5 | 364.3 ± 11.5 |
| Time cource of SBP (mm Hg) | |||
| Control | 109 ± 1.0 | 103 ± 1.5 | 112 ± 3.9 |
| Anti-Thy1.1 GN | 112 ± 3.9 | 106 ± 0.8 | 103 ± 3.0 |
| Anti-Thy1.1 GN + Aliskiren | 109 ± 2.0 | 104 ± 1.4 | 105 ± 1.4 |
| Plasma creatinine (µg/100mL) | |||
| Control | 394.8 ± 7.3 | ||
| Anti-Thy1.1 GN | 401.4 ± 73.8 | ||
| Anti-Thy1.1 GN + Aliskiren | 293.3 ± 18.6 | ||
Effects of the Aliskiren Treatment on Proteinuria and Microalbuminuria
In response to the anti-Thy1.1 antibody injection, proteinuria developed by day 3 following the anti-Thy1.1 antibody injection. The maximum augmentation of urinary protein excretion was observed at day 3 and decreased slightly at day 7 and 14 but remained elevated (Fig. 1A). Aliskiren treatment significantly attenuated the increase in urinary protein excretion at days 3, 5 and 7 compared with the group treated with the anti-Thy1.1 antibody alone (P< 0.01 vs. anti-Thy1.1 glomerulonephritis group) (Fig. 1A). The microalbumin excretion levels in the rats injected with the anti-Thy1.1 antibody alone were also much higher than those observed in the rats injected with the anti-Thy1.1 antibody and treated with aliskiren (Fig. 1B). The microablumin levels returned to control levels in the aliskiren treated group by day 14.
Figure 1.
Effects of aliskiren on urinary protein excretion (A) and urinary microalbumin excretion (B) in rats with glomerulonephritis and treated with aliskiren. Data are expressed as mean ± SE. Section (P < 0.05) and double section (p<0.01) indicate significant difference compared to day -4. Asterisk (P < 0.05) and double asterisk (P < 0.01) indicate significant difference compared to the control group. Pound (P < 0.05) and double pound (P < 0.01) indicate significant differences compared to the anti-Thy1.1 glomerulonephritis group.
Effects of Aliskiren Treatment on Glomerular Injury
The most prominent change in the anti-Thy1.1 glomerulonephritis group was mesangial matrix accumulation evaluated at 14 days after antibody administration. The glomerular matrix expansion score was significantly higher in the anti-Thy1.1 glomerulonephritis group compared to the control group (Fig. 2). Chronic treatment with aliskiren significantly suppressed the degree of glomerular matrix expansion.
Figure 2.
Effects of aliskiren on glomerular injury in anti-Thy1.1 glomerulonephritis rats. The sections were stained by Periodic acid-Schiff (PAS)-stained sections. Original magnification, ×200 (left). The positive staining (%) in glomeruli is shown in the right panel. Asterisk (P < 0.05) indicates significant difference compared to the control group. Pound (P < 0.05) indicates significant difference compared to the anti-Thy1.1 glomerulonephritis group.
Effect of Aliskiren on RAS Activation in Anti-Thy1.1 Glomerulonephritis
We examined the ability of aliskiren treatment to effectively suppress the activation of intrarenal RAS. uAGT excretion, AGT protein expression in the kidney and PRA levels were evaluated as markers for intrarenal RAS activity. The anti-Thy1.1 glomerulonephritis group exhibited markedly increased uAGT concentration (Fig. 3A) and excretion (Fig. 3B) on days 3 and 5. Treatment with aliskiren significantly suppressed the elevation of uAGT levels. In contrast, plasma AGT levels were not different among the three groups.
Figure 3.
Effects of aliskiren on uAGT elevation in anti-Thy1.1 glomerulonephritis rats. uAGT levels were measured by AGT ELISA. (A) uAGT concentration and (B) uAGT excretion levels. The results were normalized based on urine volume. Section (P < 0.05) and double section (P < 0.01) indicate significant differences compared to day -4. Asterisk (P < 0.01) indicates significant difference compared to the control group. Pound (P < 0.01) indicates significant difference compared to the anti-Thy1.1 glomerulonephritis group.
Figure 4 shows Western blot results demonstrating augmentation of AGT protein in the kidneys of the anti-Thy1.1 glomerulonephritis groups even at 14 days after initiating treatment. Aliskiren treatment prevented the increase in intrarenal AGT expression levels (Fig. 4A). Furthermore, immunohistological analyses demonstrated that the AGT protein expression in renal tubules was significantly higher in the anti-Thy1.1 group compared to the control group. Aliskiren treatment significantly attenuated the increase in AGT protein levels (Fig. 4B). As shown in Figure 5, both PRA and plasma Ang II levels were significantly increased in the anti-Thy1.1 group. Aliskiren treatment prevented the elevation of PRA and plasma Ang II levels. As shown in Figure 5C and 5D, kidney cortex and medulla Ang II contents were significantly higher in the rats with glomerulonephritis compared with the control group. However, while chronic aliskiren treatment significantly attenuated the Ang II content in medulla, Ang II content in the kidney cortex was not significantly reduced. PRR mRNA (Fig. 7A) and protein (Fig. 7C) levels in cortex were decreased in anti-Thy1.1 group. Aliskiren treatment did not normalize the suppression of PRR expression. PRR mRNA levels in anti-Thy1.1 with or without aliskiren groups were not significantly different.
Figure 4.
Effects of aliskiren on AGT expression level in the kidney. (A) AGT protein expression levels detected by Western blot and (B) AGT-immunoreactivity in kidney sections. Original magnification: × 200. Asterisk (P < 0.05) indicates significant difference compared to the control group. Pound (P < 0.05) indicates significant difference compared to the anti-Thy1.1 glomerulonephritis group.
Figure 5.
Effects of aliskiren on PRA, plasma Ang II levels, and kidney Ang II levels in anti-Thy1.1 glomerulonephritis rats. PRA (A), plasma Ang II (B), renal cortical Ang II (C) and renal medullary Ang II (D) levels were determined by radioimmunoassay. Kidney Ang II levels were normalized based on kidney weight. Data are expressed as mean ± SE. Asterisk (P < 0.05) and double asterisk (P < 0.01) indicate significant difference compared to the control group. Pound (P < 0.05) and double pound (P < 0.01) indicate significant differences compared to the anti-Thy1.1 glomerulonephritis group.
Figure 7.
Effects of aliskiren on PRR mRNA and PRR protein augmentation in anti-Thy1.1 glomerulonephritis rats. PRR mRNA in cortex (A) and PRR mRNA in medulla (B) levels were measured by qRT-PCR. PRR protein levels in kidney cortex (C) were measured by Western blot. Asterisk (P < 0.05) and double asterisk (P < 0.01) indicates significant difference compared to the control group.
Effects of Aliskiren on TGF- β1 and PAI-1 Levels
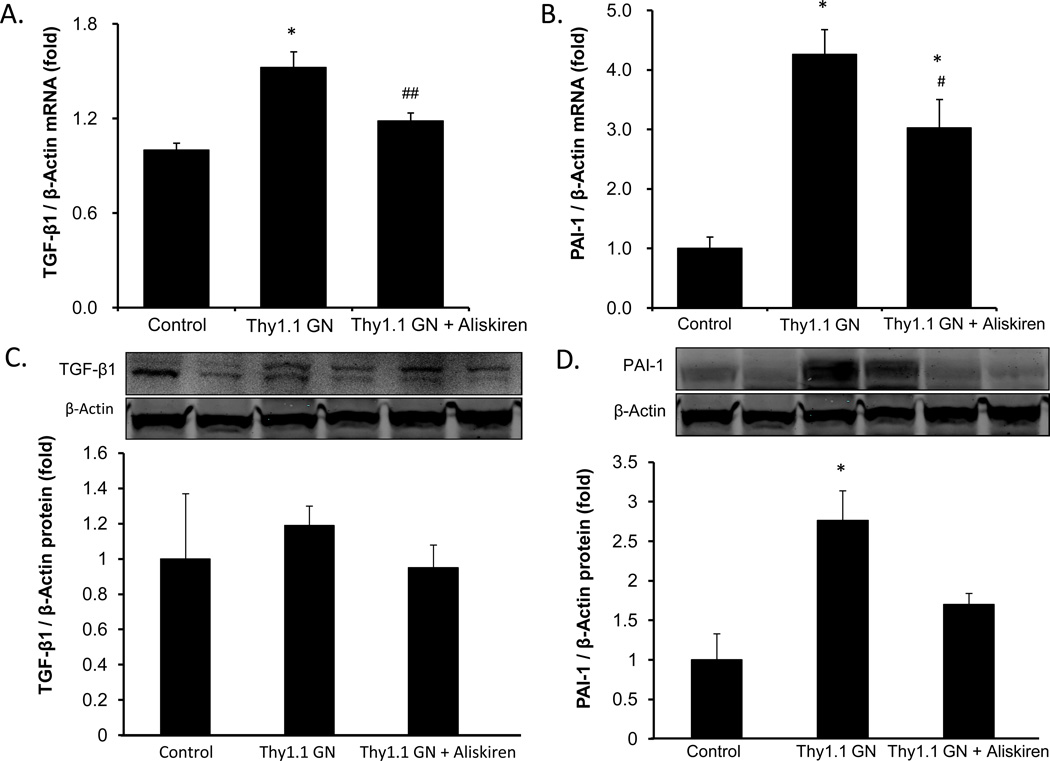
To determine the effects of aliskiren to mitigate the renal injury caused by the anti-Thy1.1 antibody, we quantified the expression of TGF-β mRNA and PAI-1 mRNA by qRT-PCR. The anti-Thy1.1 glomerulonephritis group showed elevation of TGF-β mRNA (Fig. 6A) expression and PAI-1 mRNA (Fig. 6B) expression compared with control rats. Chronic aliskiren treatment significantly attenuated the augmentation of TGF-β mRNA expression levels and PAI-1 mRNA expression compared with the anti-Thy1.1 alone (Fig. 6A and 6B). As shown in Figure 6D, Western blot results demonstrate augmentation of PAI-1 protein in the kidney of the anti-Thy1.1 glomerulonephritis group and aliskiren treatment prevented this increase. However, TGF-β1 protein levels were not significantly increased by the anti-Thy1.1 antibody or altered further by aliskiren treatment.
Figure 6.
Effects of aliskiren on TGF-β1 mRNA, PAI-1 mRNA, TGF-β1 protein and PAI-1 protein augmentation in anti-Thy1.1 glomerulonephritis rats. TGF-β1 mRNA (A) and PAI-1 mRNA (B) levels in the kidney cortex were measured by qRT-PCR. TGF-β1 protein (C) and PAI-1 protein (D) levels in the kidney were measured by Western blot. Asterisk (P < 0.01) indicates significant difference compared to the control group. Pound (P < 0.05) and double pound (P < 0.01) indicate significant difference compared to the anti-Thy1.1 glomerulonephritis group.
Discussion
There is a growing recognition that an inappropriately activated intrarenal RAS exerts a cardinal role in mediating or facilitating the progressive renal injury that occurs in glomerulonephritis7;13;27;29;38. Indeed, various studies have reported that blockade of the RAS with either ACE inhibitors or Ang II AT1R blockers ameliorate or markedly reduce the proteinuria and progressive renal injury in glomerulonephritis16;27;28;38–40. However, it has also been reported that ACE inhibition alone does not completely protect against the glomerular damage and renal fibrotic remodeling suggesting additional mechanisms41.
One specific model of glomerulonephritis that has been studied extensively is the anti-Thy1.1 antibody induced glomerulonephritis. Several recent studies have addressed the possible effects of direct renin inhibition on the development of glomerulonephritis. Zhang et al38 studied the effect of ACE inhibition alone or in combination with renin receptor inhibition. They demonstrated that ACE inhibition alone reduced the increased expression of TGF-β1, fibronectin and PAI-1 caused by injection of anti-Thy1.1 antibody. Suppressing the renin receptor with small interfering RNA alone also reduced the expression of fibrotic markers in a manner similar to ACE inhibition. Importantly, the combination of both treatments further reduced the expression of the fibrotic markers. The authors suggested that renin receptor activation could be responsible for the inability of ACE inhibition alone to completely suppress the expression of fibrotic markers and suggested that blocking renin or renin receptors might have additional therapeutic potential. However, our studies failed to show an increased PRR expression in response to anti-Thy1.1 treatment.
Huang et al42 showed that giving a high salt diet to anti-thymocyte nephritic rats increased the severity of the kidney fibrosis. Although plasma renin and Ang II levels were suppressed, there were increases in tubular prorenin and renin and there was redistribution of PRR from from the cytoplasm to the apical membrane along with elevated prorenin and Ang II in collecting ducts and connecting tubules of the nephritic rats. The authors suggested the involvement of the PRR in the augmentation of intrarenal Ang II and progression of kidney fibrosis in nephritic rats fed a high salt diet. In the present study, PRR levels were suppressed by injection of anti-Thy1.1 antibody in the kidneys, particularly in renal cortex. Since aliskiren treatment did not restore the PRR levels, the suppression of intrarenal PRR was not induced by renin inhibition. Thus, intrarenal PRR is unlikely to be involved in the development of glomerulonephritis, suggesting that the progression of glomerulonephritis occurs in an Ang II-dependent and PRR-independent manner.
Sato et al33 evaluated the effects of direct local renin inhibition on the progression of renal injury in anti-Thy1.1 glomerulonephritis. They accomplished local blockade by sub-renal capsular implantation of a collagen sponge containing aliskiren. This treatment significantly suppressed mesangial matrix expansion and ameliorated the glomerular sclerotic index. Although juxtaglomerular renin expression was enhanced, expression of α-smooth muscle actin and type 1 collagen were markedly decreased, thus suggesting an important renoprotective effect of aliskiren.
While these and other previous studies40 have provided substantial support for the involvement of the intrarenal RAS in the renal injury that occurs in glomerulonephritis, there has not been definitive evidence for specific enhancement of intrarenal RAS during the induction and development of anti-Thy1.1 antibody glomerulonephritis. Specifically, the effects of systemically administered aliskiren on renoprotection and intrarenal RAS activation in glomerulonephritis had not been evaluated. In the present study, we measured urinary protein excretion as a marker of renal injury and uAGT as an index of intrarenal and/or intratubular RAS activity. We observed that the greatest increases in urinary protein and microalbumin excretion occurred early at 3–5 days following injection of the antibody. Likewise, the greatest increase in uAGT excretion rates occurred in the 3–5 day period. Importantly, the rats treated with aliskiren had a marked attenuation of the increase in urinary protein and microalbuminuria excretion rates during the same period. Furthermore, the stimulation of uAGT caused by the antibody was suppressed to an even greater extent, thus demonstrating the efficacy of aliskiren to markedly reduce the extent of stimulation of the intrarenal RAS.
To evaluate the longer-term effects of aliskiren to mitigate or prevent the renal injury caused by the antibody, the rats were maintained for 13–14 days prior to harvesting the blood and tissue samples. At this time point, both the protein and AGT excretion rates had decreased markedly and were either the same or only slightly higher than the control values. Nevertheless, histological examination of the kidneys revealed sustained renal injury with increased glomerular matrix expansion and increased expression of AGT protein as well as kidney cortex and medullary Ang II contents. In addition, TGF-β1 and PAI-1 mRNA levels were still elevated at this time point, but only PAI-1 protein levels were augmented. Importantly, the rats that were also treated with aliskiren had either a reduced degree of injury or complete suppression of the effects caused by the antibody. The mesangial expansion and intrarenal AGT protein expression were almost completely suppressed and increases in TGF-β1 and PAI-1 mRNA expression were significantly lower. Recent studies have suggested that intratubular Ang II is generated by medullary renin43. These findings support a suppressing effect of aliskiren on the increases in renal medullary Ang II observed in this study.
As shown in Table 1, treatment with anti-Thy1.1 did not cause perceptible changes in SBP. Likewise, treatment with aliskiren did not modify the SBP values measured at 1 and 2 weeks after initiating treatment. Although Wagner et al44 reported a substantial increase in SBP following treatment with anti-Thy1.1, this response did not occur in our study and has not generally been substantiated by other studies26;28;29;42;45. Furthermore, while it may have been expected that aliskiren treatment would decrease SPB, this has not been observed in normal rats at doses that can normalize arterial pressure Cyp1a1-Ren2 transgenic rats46.
In summary, treatment of glomerulonephritis rats with aliskiren reduced or prevented the marked proteinuria and albuminuria as well as the increased uAGT levels that occurred in response to the anti-Thy1.1 antibody. Furthermore, the sustained renal injury that was demonstrated at 14 days was also substantially reduced by the aliskiren treatment. In addition, since the glomerulonephritis rats did not exhibit hypertension, one noteworthy finding in this study is that the renoprotective effect of aliskiren in the rats with glomerulonephritis occurs in a blood pressure-independent manner. These results clearly indicate the effectiveness of aliskiren in reducing or preventing the stimulation of the RAS and the development of renal injury that develops in anti-Thy1.1 antibody induced glomerulonephritis. They provide further evidence for the effectiveness of DRI beyond hypertension and diabetes.
Acknowledgements
We are grateful to Novartis Pharmaceutical Co. for supplying the aliskiren. We also appreciate the technical assistance provided by Porcha Davis, MS and Ayumi Kitano, MS. The current address for Dr. Maki Urushihara is Department of Pediatrics, Institute of Health Biosciences at the University of Tokushima Graduate School in Kuramoto, Tokushima, Japan. The current address for Dr. Hiroyuki Kobori is Department of Pharmacology at Kagawa Medical University, Kagawa, Japan. The current address for Dr. Daisuke Inui is Department of Anesthesiology, Shikoku Medical Center for Children and Adults, Kagawa, Japan.
Source of Support
This study was supported by a grant from Novartis Pharmaceuticals Corporation (CSPP100AUSNC06) and by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number 1P30GM103337.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Publisher's Disclaimer: Disclaimer
A preliminary report (abstract) of this study was submitted to the 2013 High Blood Pressure Research council meeting.
References
- 1.Anderson S, Rennke HG, Brenner BM. Therapeutic advantage of converting enzyme inhibitors in arresting progressive renal disease associated with systemic hypertension in the rat. J Clin Invest. 1986;77:1993–2000. doi: 10.1172/JCI112528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Navar LG, Kobori H, Prieto MC, Gonzalez-Villalobos RA. Intratubular renin-angiotensin system in hypertension. Hypertension. 2011;57:355–362. doi: 10.1161/HYPERTENSIONAHA.110.163519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev. 2007;59:251–287. doi: 10.1124/pr.59.3.3. [DOI] [PubMed] [Google Scholar]
- 4.Takamatsu M, Urushihara M, Kondo S, et al. Glomerular angiotensinogen protein is enhanced in pediatric IgA nephropathy. Pediatr Nephrol. 2008;23:1257–1267. doi: 10.1007/s00467-008-0801-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kobori H, Katsurada A, Ozawa Y, et al. Enhanced intrarenal oxidative stress and angiotensinogen in IgA nephropathy patients. Biochem Biophys Res Commun. 2007;358:156–163. doi: 10.1016/j.bbrc.2007.04.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ozawa Y, Kobori H. Crucial role of Rho-nuclear factor-kappaB axis in angiotensin II-induced renal injury. Am J Physiol Renal Physiol. 2007;293:F100–F109. doi: 10.1152/ajprenal.00520.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kondo S, Shimizu M, Urushihara M, et al. Addition of the antioxidant probucol to angiotensin II type I receptor antagonist arrests progressive mesangioproliferative glomerulonephritis in the rat. J Am Soc Nephrol. 2006;17:783–794. doi: 10.1681/ASN.2005050519. [DOI] [PubMed] [Google Scholar]
- 8.Ohashi N, Katsurada A, Miyata K, et al. Role of activated intrarenal reactive oxygen species and renin-angiotensin system in IgA nephropathy model mice. Clin Exp Pharmacol Physiol. 2009;36:750–755. doi: 10.1111/j.1440-1681.2009.05172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baltatu O, Silva JA, Jr, Ganten D, Bader M. The brain renin-angiotensin system modulates angiotensin II-induced hypertension and cardiac hypertrophy. Hypertension. 2000;35:409–412. doi: 10.1161/01.hyp.35.1.409. [DOI] [PubMed] [Google Scholar]
- 10.Dell'Italia LJ, Meng QC, Balcells E, et al. Compartmentalization of angiotensin II generation in the dog heart. Evidence for independent mechanisms in intravascular and interstitial spaces. J Clin Invest. 1997;100:253–258. doi: 10.1172/JCI119529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mazzocchi G, Malendowicz LK, Markowska A, Albertin G, Nussdorfer GG. Role of adrenal renin-angiotensin system in the control of aldosterone secretion in sodium-restricted rats. Am J Physiol Endocrinol Metab. 2000;278:E1027–E1030. doi: 10.1152/ajpendo.2000.278.6.E1027. [DOI] [PubMed] [Google Scholar]
- 12.Nakamura N, Soubrier F, Menard J, Panthier JJ, Rougeon F, Corvol P. Nonproportional changes in plasma renin concentration, renal renin content, and rat renin messenger RNA. Hypertension. 1985;7:855–859. doi: 10.1161/01.hyp.7.6.855. [DOI] [PubMed] [Google Scholar]
- 13.Wagner J, Volk S, Haufe CC, Ciechanowicz A, Paul M, Ritz E. Renin gene expression in human kidney biopsies from patients with glomerulonephritis or graft rejection. J Am Soc Nephrol. 1995;5:1469–1475. doi: 10.1681/ASN.V571469. [DOI] [PubMed] [Google Scholar]
- 14.Dzau VJ, Re R. Tissue angiotensin system in cardiovascular medicine. A paradigm shift? Circ. 1994;89:493–498. doi: 10.1161/01.cir.89.1.493. [DOI] [PubMed] [Google Scholar]
- 15.Kagami S, Border WA, Miller DE, Noble NA. Angiotensin II stimulates extracellular matrix protein synthesis through induction of transforming growth factor-B expression in rat glomerular mesangial cells. J Clin Invest. 1994;93:2431–2437. doi: 10.1172/JCI117251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mahmood J, Khan F, Okada S, Kumagai N, Morioka T, Oite T. Local delivery of angiotensin receptor blocker into the kidney ameliorates progression of experimental glomerulonephritis. Kidney Int. 2006;70:1591–1598. doi: 10.1038/sj.ki.5001872. [DOI] [PubMed] [Google Scholar]
- 17.Ruiz-Ortega M, Egido J. Angiotensin II modulates cell growth-related events and synthesis of matrix proteins in renal interstitial fibroblasts. Kidney Int. 1997;52:1497–1510. doi: 10.1038/ki.1997.480. [DOI] [PubMed] [Google Scholar]
- 18.Ruggenenti P, Perna A, Gherardi G, Gaspari F, Benini R, Remuzzi G. Renal function and requirement for dialysis in chronic nephropathy patients on long-term ramipril: REIN follow-up trial. Gruppo Italiano di Studi Epidemiologici in Nefrologia (GISEN). Ramipril Efficacy in Nephropathy. Lancet. 1998;352:1252–1256. doi: 10.1016/s0140-6736(98)04433-x. [DOI] [PubMed] [Google Scholar]
- 19.Horita Y, Tadokoro M, Taura K, et al. Low-dose combination therapy with temocapril and losartan reduces proteinuria in normotensive patients with immunoglobulin a nephropathy. Hypertens Res. 2004;27:963–970. doi: 10.1291/hypres.27.963. [DOI] [PubMed] [Google Scholar]
- 20.Ravid M, Brosh D, Levi Z, Bar-Dayan Y, Ravid D, Rachmani R. Use of enalapril to attenuate decline in renal function in normotensive, normoalbuminuric patients with type 2 diabetes mellitus. A randomized, controlled trial. Ann Intern Med. 1998;128:982–988. doi: 10.7326/0003-4819-128-12_part_1-199806150-00004. [DOI] [PubMed] [Google Scholar]
- 21.Ruiz-Ortega M, Bustos C, Egido J, Lorenzo O, Plaza JJ, Egido J. Angiotensin II participates in mononuclear cell recruitment in experimental immune complex nephritis through nuclear factor-KB activation and monocyte chemoattractant protein-1 synthesis. J Immunol. 1998;161:430–439. [PubMed] [Google Scholar]
- 22.Wolf G. The renin-angiotensin system and progression of renal diseases. Hamburg: Karger; 2002. [Google Scholar]
- 23.Kohan DE. Angiotensin II and endothelin in chronic glomerulonephritis. Kidney Int. 1998;54:646–647. doi: 10.1046/j.1523-1755.1998.00038.x. [DOI] [PubMed] [Google Scholar]
- 24.Lafayette RA, Mayer G, Park SK, Meyer TW. Angiotensin II receptor blockade limits glomerular injury in rats with reduced renal mass. J Clin Invest. 1992;90:766–771. doi: 10.1172/JCI115949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giatras I, Lau J, Levey AS. Effect of angiotensin-converting enzyme inhibitors on the progression of nondiabetic renal disease: a meta-analysis of randomized trials. Angiotensin-Converting-Enzyme Inhibition and Progressive Renal Disease Study Group. Ann Intern Med. 1997;127:337–345. doi: 10.7326/0003-4819-127-5-199709010-00001. [DOI] [PubMed] [Google Scholar]
- 26.Peters H, Wang Y, Loof T, et al. Expression and activity of soluble guanylate cyclase in injury and repair of anti-thy1 glomerulonephritis. Kidney Int. 2004;66:2224–2236. doi: 10.1111/j.1523-1755.2004.66012.x. [DOI] [PubMed] [Google Scholar]
- 27.Nagamatsu T, Oka T, Nagao T, Suzuki Y. Effects of KD3–671, an angiotensin II type 1 receptor antagonist, on anti-thy-1 nephritis in rats. Biol Pharm Bull. 2003;26:808–812. doi: 10.1248/bpb.26.808. [DOI] [PubMed] [Google Scholar]
- 28.Peters H, Ruckert M, Gaedeke J, et al. Angiotensin-converting enzyme inhibition but not beta-adrenergic blockade limits transforming growth factor-beta overexpression in acute normotensive anti-thy1 glomerulonephritis. J Hypertens. 2003;21:771–780. doi: 10.1097/00004872-200304000-00021. [DOI] [PubMed] [Google Scholar]
- 29.Mii A, Shimizu A, Masuda Y, et al. Angiotensin II receptor blockade inhibits acute glomerular injuries with the alteration of receptor expression. Lab Invest. 2009;89:164–177. doi: 10.1038/labinvest.2008.128. [DOI] [PubMed] [Google Scholar]
- 30.Jensen C, Herold P, Brunner HR. Aliskiren: the first renin inhibitor for clinical treatment. Nat Rev Drug Discov. 2008;7:399–410. doi: 10.1038/nrd2550. [DOI] [PubMed] [Google Scholar]
- 31.Nussberger J, Wuerzner G, Jensen C, Brunner HR. Angiotensin II suppression in humans by the orally active renin inhibitor Aliskiren (SPP100): comparison with enalapril. Hypertension. 2002;39:E1–E8. doi: 10.1161/hy0102.102293. [DOI] [PubMed] [Google Scholar]
- 32.Feldman DL. New insights into the renoprotective actions of the renin inhibitor aliskiren in experimental renal disease. Hypertens Res. 2010;33:279–287. doi: 10.1038/hr.2010.19. [DOI] [PubMed] [Google Scholar]
- 33.Sato A, Piao H, Nozawa Y, Morioka T, Kawachi H, Oite T. Local delivery of a direct renin inhibitor into the kidney ameliorates progression of experimental glomerulonephritis. Clin Exp Nephrol. 2012;16:539–548. doi: 10.1007/s10157-012-0601-y. [DOI] [PubMed] [Google Scholar]
- 34.Kobori H, Navar LG. Urinary angiotensinogen a a novel biomarker of intrarenal renin-angiotensin system in chronic kidney disease. International Review of Thrombosis. 2011;6:36–45. [PMC free article] [PubMed] [Google Scholar]
- 35.Kobori H, Katsurada A, Miyata K, et al. Determination of Plasma and Urinary Angiotensinogen Levels in Rodents by Newly Developed ELISA. Am J Physiol Renal Physiol. 2008;294:F1257–F1263. doi: 10.1152/ajprenal.00588.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kamiyama M, Farragut KM, Garner MK, Navar LG, Kobori H. Divergent localization of angiotensinogen mRNA and protein in proximal tubule segments of normal rat kidney. J Hypertens. 2012;30:2365–2372. doi: 10.1097/HJH.0b013e3283598eed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ohashi N, Urushihara M, Satou R, Kobori H. Glomerular angiotensinogen is induced in mesangial cells in diabetic rats via reactive oxygen species--ERK/JNK pathways. Hypertens Res. 2010;33:1174–1181. doi: 10.1038/hr.2010.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang J, Gu C, Noble NA, Border WA, Huang Y. Combining angiotensin II blockade and renin receptor inhibition results in enhanced antifibrotic effect in experimental nephritis. Am J Physiol Renal Physiol. 2011;301:F723–F732. doi: 10.1152/ajprenal.00271.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Urushihara M, Kobori H. Angiotensinogen Expression Is Enhanced in the Progression of Glomerular Disease. Int J Clin Med. 2011;2:378–387. doi: 10.4236/ijcm.2011.24064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakamura T, Obata J-E, Kimura H, et al. Blocking angiotensin II ameliorates proteinuria and glomerular lesions in progressive mesangioproliferative glomerulonephritis. Kidney Int. 1999;55:877–889. doi: 10.1046/j.1523-1755.1999.055003877.x. [DOI] [PubMed] [Google Scholar]
- 41.Westerweel PE, Joles JA, den OK, Goldschmeding R, Rookmaaker MB, Verhaar MC. ACE Inhibition in Anti-Thy1 Glomerulonephritis Limits Proteinuria but Does Not Improve Renal Function and Structural Remodeling. Nephron Extra. 2012;2:9–16. doi: 10.1159/000335750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang Y, Yamamoto T, Misaki T, et al. Enhanced intrarenal receptor-mediated prorenin activation in chronic progressive anti-thymocyte serum nephritis rats on high salt intake. Am J Physiol Renal Physiol. 2012;303:F130–F138. doi: 10.1152/ajprenal.00275.2011. [DOI] [PubMed] [Google Scholar]
- 43.Prieto MC, Gonzalez AA, Navar LG. Evolving concepts on regulation and function of renin in distal nephron. Pflugers Arch. 2013;465:121–132. doi: 10.1007/s00424-012-1151-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wagner J, Dechow C, Morath C, et al. Retinoic acid reduces glomerular injury in a rat model of glomerular damage. J Am Soc Nephrol. 2000;11:1479–1487. doi: 10.1681/ASN.V1181479. [DOI] [PubMed] [Google Scholar]
- 45.Wenzel UO, Wolf G, Jacob I, Thaiss F, Helmchen U, Stahl RA. Chronic anti-Thy-1 nephritis is aggravated in the nonclipped but not in the clipped kidney of Goldblatt hypertensive rats. Kidney Int. 2002;61:2119–2131. doi: 10.1046/j.1523-1755.2002.00354.x. [DOI] [PubMed] [Google Scholar]
- 46.Huang L, Howard CG, Mitchell KD. Chronic Direct Renin Inhibition With Aliskiren Prevents the Development of Hypertension in Cyp1a1-Ren2 Transgenic Rats With Inducible ANG II-Dependent Hypertension. Am J Med Sci. 2012;344:301–306. doi: 10.1097/MAJ.0b013e3182410d1e. [DOI] [PMC free article] [PubMed] [Google Scholar]