Abstract
A 145-kDa tyrosine-phosphorylated protein that becomes associated with Shc in response to multiple cytokines has been purified from the murine hemopoietic cell line B6SUtA1. Amino acid sequence data were used to clone the cDNA encoding this protein from a B6SUtA1 library. The predicted amino acid sequence encodes a unique protein containing an N-terminal src homology 2 domain, two consensus sequences that are targets for phosphotyrosine binding domains, a proline-rich region, and two motifs highly conserved among inositol polyphosphate 5-phosphatases. Cell lysates immunoprecipitated with antiserum to this protein exhibited both phosphatidylinositol 3,4,5-trisphosphate and inositol 1,3,4,5-tetrakisphosphate polyphosphate 5-phosphatase activity. This novel signal transduction intermediate may serve to modulate both Ras and inositol signaling pathways. Based on its properties, we suggest the 145-kDa protein be called SHIP for SH2-containing inositol phosphatase.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexandropoulos K., Cheng G., Baltimore D. Proline-rich sequences that bind to Src homology 3 domains with individual specificities. Proc Natl Acad Sci U S A. 1995 Apr 11;92(8):3110–3114. doi: 10.1073/pnas.92.8.3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltensperger K., Kozma L. M., Cherniack A. D., Klarlund J. K., Chawla A., Banerjee U., Czech M. P. Binding of the Ras activator son of sevenless to insulin receptor substrate-1 signaling complexes. Science. 1993 Jun 25;260(5116):1950–1952. doi: 10.1126/science.8391166. [DOI] [PubMed] [Google Scholar]
- Batty I. R., Nahorski S. R., Irvine R. F. Rapid formation of inositol 1,3,4,5-tetrakisphosphate following muscarinic receptor stimulation of rat cerebral cortical slices. Biochem J. 1985 Nov 15;232(1):211–215. doi: 10.1042/bj2320211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batzer A. G., Blaikie P., Nelson K., Schlessinger J., Margolis B. The phosphotyrosine interaction domain of Shc binds an LXNPXY motif on the epidermal growth factor receptor. Mol Cell Biol. 1995 Aug;15(8):4403–4409. doi: 10.1128/mcb.15.8.4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buday L., Downward J. Epidermal growth factor regulates p21ras through the formation of a complex of receptor, Grb2 adapter protein, and Sos nucleotide exchange factor. Cell. 1993 May 7;73(3):611–620. doi: 10.1016/0092-8674(93)90146-h. [DOI] [PubMed] [Google Scholar]
- Burgering B. M., Coffer P. J. Protein kinase B (c-Akt) in phosphatidylinositol-3-OH kinase signal transduction. Nature. 1995 Aug 17;376(6541):599–602. doi: 10.1038/376599a0. [DOI] [PubMed] [Google Scholar]
- Craparo A., O'Neill T. J., Gustafson T. A. Non-SH2 domains within insulin receptor substrate-1 and SHC mediate their phosphotyrosine-dependent interaction with the NPEY motif of the insulin-like growth factor I receptor. J Biol Chem. 1995 Jun 30;270(26):15639–15643. doi: 10.1074/jbc.270.26.15639. [DOI] [PubMed] [Google Scholar]
- Cullen P. J., Hsuan J. J., Truong O., Letcher A. J., Jackson T. R., Dawson A. P., Irvine R. F. Identification of a specific Ins(1,3,4,5)P4-binding protein as a member of the GAP1 family. Nature. 1995 Aug 10;376(6540):527–530. doi: 10.1038/376527a0. [DOI] [PubMed] [Google Scholar]
- Cutler R. L., Liu L., Damen J. E., Krystal G. Multiple cytokines induce the tyrosine phosphorylation of Shc and its association with Grb2 in hemopoietic cells. J Biol Chem. 1993 Oct 15;268(29):21463–21465. [PubMed] [Google Scholar]
- Damen J. E., Liu L., Cutler R. L., Krystal G. Erythropoietin stimulates the tyrosine phosphorylation of Shc and its association with Grb2 and a 145-Kd tyrosine phosphorylated protein. Blood. 1993 Oct 15;82(8):2296–2303. [PubMed] [Google Scholar]
- Damen J. E., Mui A. L., Puil L., Pawson T., Krystal G. Phosphatidylinositol 3-kinase associates, via its Src homology 2 domains, with the activated erythropoietin receptor. Blood. 1993 Jun 15;81(12):3204–3210. [PubMed] [Google Scholar]
- Egan S. E., Giddings B. W., Brooks M. W., Buday L., Sizeland A. M., Weinberg R. A. Association of Sos Ras exchange protein with Grb2 is implicated in tyrosine kinase signal transduction and transformation. Nature. 1993 May 6;363(6424):45–51. doi: 10.1038/363045a0. [DOI] [PubMed] [Google Scholar]
- Feig L. A. The many roads that lead to Ras. Science. 1993 May 7;260(5109):767–768. doi: 10.1126/science.8484117. [DOI] [PubMed] [Google Scholar]
- Gale N. W., Kaplan S., Lowenstein E. J., Schlessinger J., Bar-Sagi D. Grb2 mediates the EGF-dependent activation of guanine nucleotide exchange on Ras. Nature. 1993 May 6;363(6424):88–92. doi: 10.1038/363088a0. [DOI] [PubMed] [Google Scholar]
- Hu Q., Klippel A., Muslin A. J., Fantl W. J., Williams L. T. Ras-dependent induction of cellular responses by constitutively active phosphatidylinositol-3 kinase. Science. 1995 Apr 7;268(5207):100–102. doi: 10.1126/science.7701328. [DOI] [PubMed] [Google Scholar]
- Jackson S. P., Schoenwaelder S. M., Matzaris M., Brown S., Mitchell C. A. Phosphatidylinositol 3,4,5-trisphosphate is a substrate for the 75 kDa inositol polyphosphate 5-phosphatase and a novel 5-phosphatase which forms a complex with the p85/p110 form of phosphoinositide 3-kinase. EMBO J. 1995 Sep 15;14(18):4490–4500. doi: 10.1002/j.1460-2075.1995.tb00128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson A. B., Majerus P. W. Properties of type II inositol polyphosphate 5-phosphatase. J Biol Chem. 1995 Apr 21;270(16):9370–9377. doi: 10.1074/jbc.270.16.9370. [DOI] [PubMed] [Google Scholar]
- Kavanaugh W. M., Turck C. W., Williams L. T. PTB domain binding to signaling proteins through a sequence motif containing phosphotyrosine. Science. 1995 May 26;268(5214):1177–1179. doi: 10.1126/science.7539155. [DOI] [PubMed] [Google Scholar]
- Kavanaugh W. M., Williams L. T. An alternative to SH2 domains for binding tyrosine-phosphorylated proteins. Science. 1994 Dec 16;266(5192):1862–1865. doi: 10.1126/science.7527937. [DOI] [PubMed] [Google Scholar]
- Koch C. A., Anderson D., Moran M. F., Ellis C., Pawson T. SH2 and SH3 domains: elements that control interactions of cytoplasmic signaling proteins. Science. 1991 May 3;252(5006):668–674. doi: 10.1126/science.1708916. [DOI] [PubMed] [Google Scholar]
- Li N., Batzer A., Daly R., Yajnik V., Skolnik E., Chardin P., Bar-Sagi D., Margolis B., Schlessinger J. Guanine-nucleotide-releasing factor hSos1 binds to Grb2 and links receptor tyrosine kinases to Ras signalling. Nature. 1993 May 6;363(6424):85–88. doi: 10.1038/363085a0. [DOI] [PubMed] [Google Scholar]
- Lioubin M. N., Myles G. M., Carlberg K., Bowtell D., Rohrschneider L. R. Shc, Grb2, Sos1, and a 150-kilodalton tyrosine-phosphorylated protein form complexes with Fms in hematopoietic cells. Mol Cell Biol. 1994 Sep;14(9):5682–5691. doi: 10.1128/mcb.14.9.5682. [DOI] [PMC free article] [PubMed] [Google Scholar]
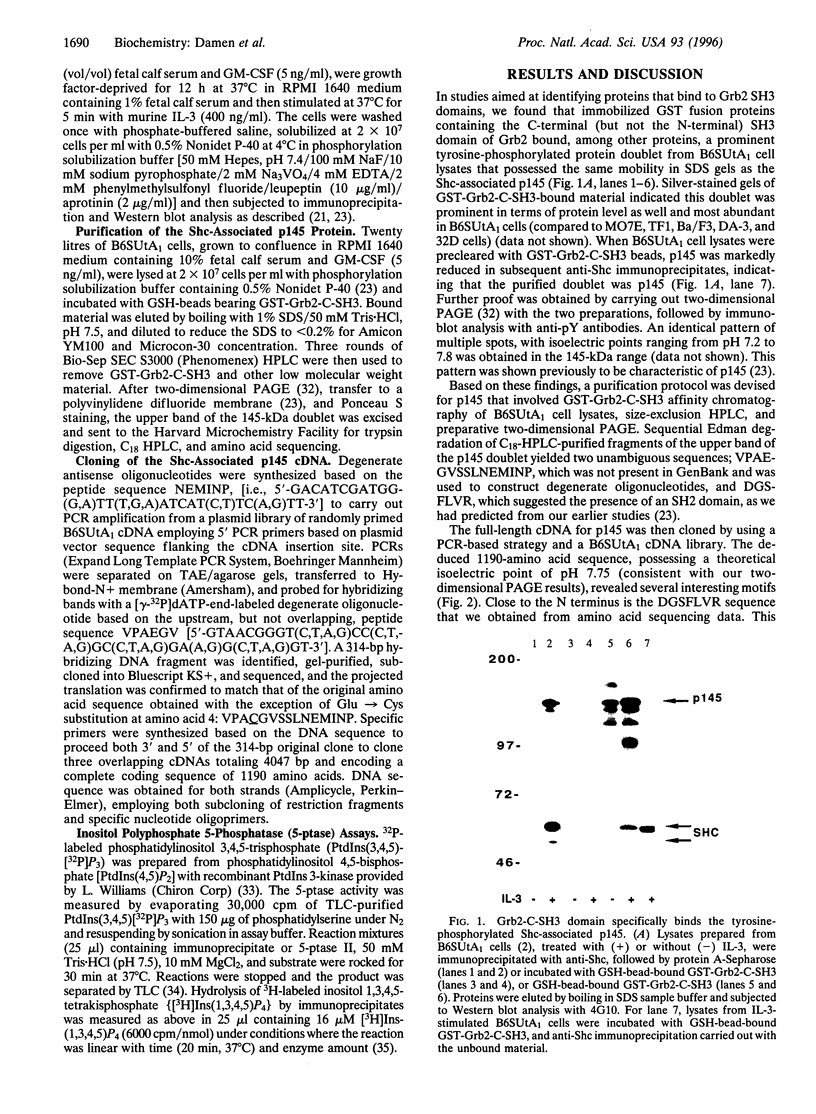
- Liu L., Damen J. E., Cutler R. L., Krystal G. Multiple cytokines stimulate the binding of a common 145-kilodalton protein to Shc at the Grb2 recognition site of Shc. Mol Cell Biol. 1994 Oct;14(10):6926–6935. doi: 10.1128/mcb.14.10.6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuguchi T., Salgia R., Hallek M., Eder M., Druker B., Ernst T. J., Griffin J. D. Shc phosphorylation in myeloid cells is regulated by granulocyte macrophage colony-stimulating factor, interleukin-3, and steel factor and is constitutively increased by p210BCR/ABL. J Biol Chem. 1994 Feb 18;269(7):5016–5021. [PubMed] [Google Scholar]
- Medema R. H., Bos J. L. The role of p21ras in receptor tyrosine kinase signaling. Crit Rev Oncog. 1993;4(6):615–661. [PubMed] [Google Scholar]
- Mitchell C. A., Connolly T. M., Majerus P. W. Identification and isolation of a 75-kDa inositol polyphosphate-5-phosphatase from human platelets. J Biol Chem. 1989 May 25;264(15):8873–8877. [PubMed] [Google Scholar]
- Murthy S. C., Sorensen P. H., Mui A. L., Krystal G. Interleukin-3 down-regulates its own receptor. Blood. 1989 Apr;73(5):1180–1187. [PubMed] [Google Scholar]
- Norris F. A., Auethavekiat V., Majerus P. W. The isolation and characterization of cDNA encoding human and rat brain inositol polyphosphate 4-phosphatase. J Biol Chem. 1995 Jul 7;270(27):16128–16133. doi: 10.1074/jbc.270.27.16128. [DOI] [PubMed] [Google Scholar]
- Norris F. A., Majerus P. W. Hydrolysis of phosphatidylinositol 3,4-bisphosphate by inositol polyphosphate 4-phosphatase isolated by affinity elution chromatography. J Biol Chem. 1994 Mar 25;269(12):8716–8720. [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Pawson T. Protein modules and signalling networks. Nature. 1995 Feb 16;373(6515):573–580. doi: 10.1038/373573a0. [DOI] [PubMed] [Google Scholar]
- Pelicci G., Lanfrancone L., Grignani F., McGlade J., Cavallo F., Forni G., Nicoletti I., Grignani F., Pawson T., Pelicci P. G. A novel transforming protein (SHC) with an SH2 domain is implicated in mitogenic signal transduction. Cell. 1992 Jul 10;70(1):93–104. doi: 10.1016/0092-8674(92)90536-l. [DOI] [PubMed] [Google Scholar]
- Ravichandran K. S., Burakoff S. J. The adapter protein Shc interacts with the interleukin-2 (IL-2) receptor upon IL-2 stimulation. J Biol Chem. 1994 Jan 21;269(3):1599–1602. [PubMed] [Google Scholar]
- Ravichandran K. S., Lee K. K., Songyang Z., Cantley L. C., Burn P., Burakoff S. J. Interaction of Shc with the zeta chain of the T cell receptor upon T cell activation. Science. 1993 Nov 5;262(5135):902–905. doi: 10.1126/science.8235613. [DOI] [PubMed] [Google Scholar]
- Ravichandran K. S., Lorenz U., Shoelson S. E., Burakoff S. J. Interaction of Shc with Grb2 regulates association of Grb2 with mSOS. Mol Cell Biol. 1995 Feb;15(2):593–600. doi: 10.1128/mcb.15.2.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozakis-Adcock M., Fernley R., Wade J., Pawson T., Bowtell D. The SH2 and SH3 domains of mammalian Grb2 couple the EGF receptor to the Ras activator mSos1. Nature. 1993 May 6;363(6424):83–85. doi: 10.1038/363083a0. [DOI] [PubMed] [Google Scholar]
- Rozakis-Adcock M., McGlade J., Mbamalu G., Pelicci G., Daly R., Li W., Batzer A., Thomas S., Brugge J., Pelicci P. G. Association of the Shc and Grb2/Sem5 SH2-containing proteins is implicated in activation of the Ras pathway by tyrosine kinases. Nature. 1992 Dec 17;360(6405):689–692. doi: 10.1038/360689a0. [DOI] [PubMed] [Google Scholar]
- Saxton T. M., van Oostveen I., Bowtell D., Aebersold R., Gold M. R. B cell antigen receptor cross-linking induces phosphorylation of the p21ras oncoprotein activators SHC and mSOS1 as well as assembly of complexes containing SHC, GRB-2, mSOS1, and a 145-kDa tyrosine-phosphorylated protein. J Immunol. 1994 Jul 15;153(2):623–636. [PubMed] [Google Scholar]
- Schumacher C., Knudsen B. S., Ohuchi T., Di Fiore P. P., Glassman R. H., Hanafusa H. The SH3 domain of Crk binds specifically to a conserved proline-rich motif in Eps15 and Eps15R. J Biol Chem. 1995 Jun 23;270(25):15341–15347. doi: 10.1074/jbc.270.25.15341. [DOI] [PubMed] [Google Scholar]
- Smit L., de Vries-Smits A. M., Bos J. L., Borst J. B cell antigen receptor stimulation induces formation of a Shc-Grb2 complex containing multiple tyrosine-phosphorylated proteins. J Biol Chem. 1994 Aug 12;269(32):20209–20212. [PubMed] [Google Scholar]
- Toker A., Meyer M., Reddy K. K., Falck J. R., Aneja R., Aneja S., Parra A., Burns D. J., Ballas L. M., Cantley L. C. Activation of protein kinase C family members by the novel polyphosphoinositides PtdIns-3,4-P2 and PtdIns-3,4,5-P3. J Biol Chem. 1994 Dec 23;269(51):32358–32367. [PubMed] [Google Scholar]
- Trüb T., Choi W. E., Wolf G., Ottinger E., Chen Y., Weiss M., Shoelson S. E. Specificity of the PTB domain of Shc for beta turn-forming pentapeptide motifs amino-terminal to phosphotyrosine. J Biol Chem. 1995 Aug 4;270(31):18205–18208. doi: 10.1074/jbc.270.31.18205. [DOI] [PubMed] [Google Scholar]
- Witthuhn B. A., Quelle F. W., Silvennoinen O., Yi T., Tang B., Miura O., Ihle J. N. JAK2 associates with the erythropoietin receptor and is tyrosine phosphorylated and activated following stimulation with erythropoietin. Cell. 1993 Jul 30;74(2):227–236. doi: 10.1016/0092-8674(93)90414-l. [DOI] [PubMed] [Google Scholar]
- York J. D., Chen Z. W., Ponder J. W., Chauhan A. K., Mathews F. S., Majerus P. W. Crystallization and initial X-ray crystallographic characterization of recombinant bovine inositol polyphosphate 1-phosphatase produced in Spodoptera frugiperda cells. J Mol Biol. 1994 Feb 18;236(2):584–589. doi: 10.1006/jmbi.1994.1167. [DOI] [PubMed] [Google Scholar]
- Zhang X., Jefferson A. B., Auethavekiat V., Majerus P. W. The protein deficient in Lowe syndrome is a phosphatidylinositol-4,5-bisphosphate 5-phosphatase. Proc Natl Acad Sci U S A. 1995 May 23;92(11):4853–4856. doi: 10.1073/pnas.92.11.4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Geer P., Pawson T. The PTB domain: a new protein module implicated in signal transduction. Trends Biochem Sci. 1995 Jul;20(7):277–280. doi: 10.1016/s0968-0004(00)89043-x. [DOI] [PubMed] [Google Scholar]