Abstract
Here, we report a patient with gastric adenosquamous carcinoma (ASC) with human epidermal growth factor receptor-2 (HER2) overexpression who was successfully treated with trastuzumab-based chemotherapy. The patient was a 66-year-old man preoperatively diagnosed with gastric adenocarcinoma with no evidence of distant metastases. On histopathological examination, the curatively resected tumor was identified as ASC with mixed adenocarcinoma and squamous cell carcinoma components. Multiple liver metastases developed 2.5 months after surgery. Because immunohistochemical staining for HER2 was strong in both components, combination chemotherapy with capecitabine, cisplatin, and trastuzumab was initiated. A partial response was confirmed after 6 treatment cycles and PET and CT scans performed after 13 cycles revealed disease resolution with no uptake in the metastatic lesions. No evidence of disease progression has been observed 16 months after initial chemotherapy. This report suggests the potential utility of trastuzumab-based chemotherapy for HER2-positive gastric ASC.
Key Words: Adenosquamous carcinoma, Stomach, Human epidermal growth factor receptor-2, Trastuzumab
Introduction
Adenosquamous carcinoma (ASC) of the stomach is a rare malignancy, accounting for <1% of all gastric carcinomas [1, 2, 3, 4], and is characterized by a mixture of two components: adenocarcinoma and squamous cell carcinoma. In the adenocarcinoma component, the differentiated type is more frequently observed than the poorly differentiated type [1, 2, 5], and the clinicopathological features of this tumor are dependent on the histological type of the adenocarcinoma component [5]. On the other hand, lymph node and liver metastases are frequently observed in ASC, and these metastases also tend to be found in advanced stages at diagnosis [1, 2, 3, 4, 5, 6, 7]; hence, this malignancy seems to a have a poorer prognosis than conventional gastric adenocarcinoma [1, 5].
Human epidermal growth factor receptor-2 (HER2), a membrane-associated receptor with an intracellular tyrosine kinase domain, dimerizes with other HER family members and mediates signal transduction pathways linked to cell growth and survival. HER2 is overexpressed in approximately 20% of gastric adenocarcinoma cases and is considered a key therapeutic target for HER2-positive gastric adenocarcinoma. In the Trastuzumab for Gastric Cancer (ToGA) trial, trastuzumab, a monoclonal antibody targeting HER2, significantly improved overall survival when combined with chemotherapy in the treatment of advanced HER2-positive gastric adenocarcinoma [8]. However, to date, there are no reports regarding immunoreactivity for HER2 and its role as a therapeutic target in gastric ASC.
Here, we report a patient with gastric ASC with HER2 overexpression who achieved a dramatic durable response with trastuzumab-based chemotherapy, presumably because of strong HER2 immunoreactivity in the tumor. The findings of the present report warrant further investigation to clarify the role of HER2 in this rare tumor.
Case Report
A 66-year-old man was referred to our hospital with a diagnosis of gastric adenocarcinoma. Upper gastrointestinal endoscopy revealed type 3 advanced gastric cancer of the lesser curvature of the upper to lower gastric body (fig. 1a, b), and histological analysis of biopsy samples showed poorly-differentiated adenocarcinoma. Serum concentrations of carcinoembryonic antigen (CEA) and carbohydrate antigen 19–9 (CA19–9) were highly elevated (86.8 ng/ml and 6,970 U/ml, respectively). An abdominal CT scan revealed thickening of the gastric wall and enlargement of the abdominal lymph nodes, but no evidence of distant metastasis. A total gastrectomy with lymph node dissection (D2) was performed with a curative intent. In the postoperative period, the patient developed a pancreatic fistula with a subphrenic abscess, which was subsequently drained.
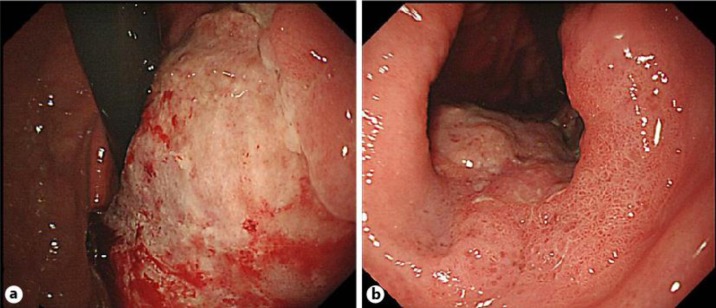
Fig. 1.
a, b An endoscopic examination revealed an ulcerated protruding tumor that occupied the lesser curvature of the upper to lower gastric body. This tumor was diagnosed as advanced type 3 gastric cancer.
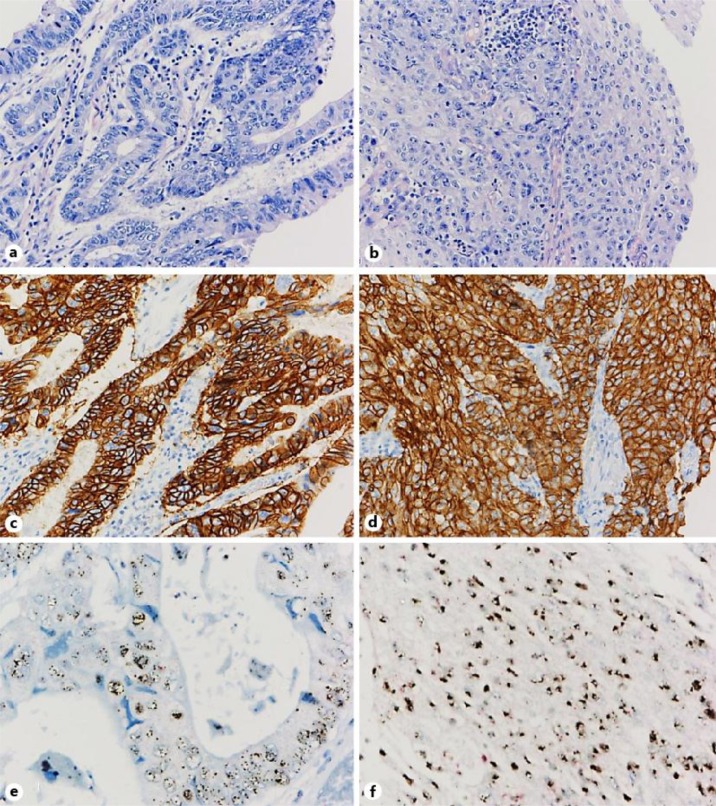
A macroscopic examination revealed a type 1 protruded lesion occupying the lesser curvature of the gastric body, measuring 87 × 82 mm at its largest dimensions, which invaded beyond the serosal layer. A pathological diagnosis of the resected ASC specimens showed that it was composed of a mixture of adenocarcinoma and squamous cell carcinoma components (fig. 2a, b). Both components were moderately differentiated; the adenocarcinoma component showed tubular structures and the squamous cell carcinoma component showed partly differentiated keratinizing cells. The individual components constituted nests adjacent to one another. Venous and lymphatic invasion were identified (v3 and ly2, respectively). Metastatic lesions were identified in 6 of 49 dissected lymph nodes, in which only an adenocarcinoma component was observed. According to the 14th edition of the Japanese Classification of Gastric Carcinoma [9], the cancer was graded as T4aN2M0 (stage IIIB). Immunohistochemical staining for the membrane-bound HER2 protein using a polyclonal antibody (HercepTestTM; Dako A/S, Glostrup, Denmark) showed that both the squamous carcinoma and adenocarcinoma components of the primary tumor were strongly positive (score, 3+) for HER2 (fig. 2c, d). A high level of HER2 gene amplification in both components was also detected by dual-color chromogenic in situ hybridization (INFORM HER2 Dual ISH; Ventana Medical Systems Inc., Oro Valley, Ariz., USA) (fig. 2e, f).
Fig. 2.
a, b The pathological diagnosis of the resected specimens was adenosquamous cell carcinoma with a mixture of both adenocarcinoma (a) and squamous cell carcinoma components (b). c, d Immunohistochemical analysis showed strong positivity for HER2 (HerceptestTM) in both components. e, f Dual-color chromogenic in situ hybridization revealed high amplification of the HER2 gene (INFORM HER2 Dual ISH; red, chromosome 17 centromere; black, HER2) in both components.
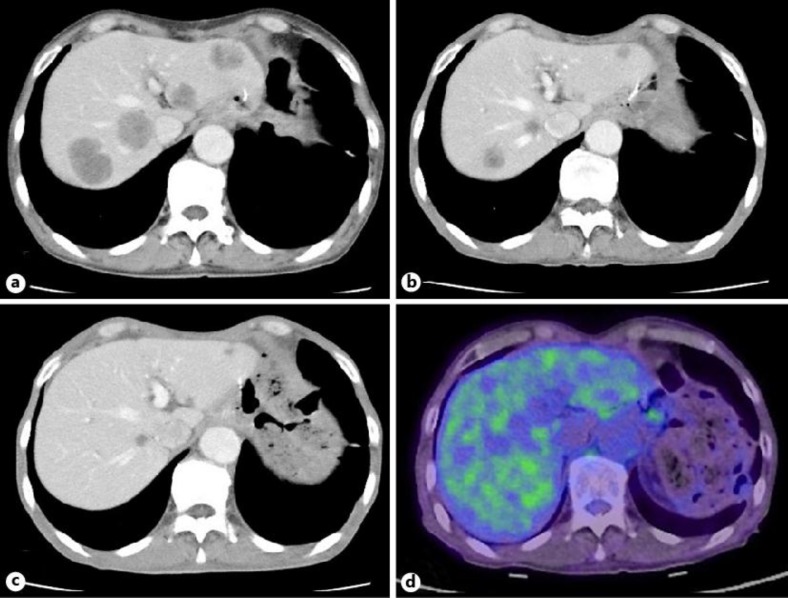
About 2.5 months after curative surgery, an abdominal CT scan showed multiple metastatic lesions in the bilateral lobes of the liver (fig. 3a). The serum concentrations of CEA and CA19–9 increased to 669.6 ng/ml and 7,380 U/ml, respectively. Systemic treatment with capecitabine (2,000 mg/m2, days 1–14), cisplatin (80 mg/m2, day 1), and trastuzumab (8 mg/kg dose-loading followed by 6 mg/kg, day 1) was initiated every 21 days. After 3 treatment cycles, all metastatic liver lesions had regressed (fig. 3b) and a partial response (PR) was confirmed after 6 cycles (fig. 3c). After confirmation of a PR, the patient was maintained on combination chemotherapy with capecitabine and trastuzumab. The levels of all tumor markers were within normal limits 9 months after chemotherapy initiation. PET and CT scans performed 13 months after chemotherapy initiation revealed no uptake in the metastatic liver lesions (fig. 3d). Thereafter, maintenance therapy with trastuzumab alone was started and the PR was maintained for 16 months after initiation of chemotherapy.
Fig. 3.
a An abdominal CT scan revealed multiple liver metastases. b After 3 cycles of chemotherapy with capecitabine, cisplatin, and trastuzumab, a PR was observed. c After 6 treatment cycles, a PR was confirmed with no evidence of new lesions. d Thirteen months after initial chemotherapy, a PET-CT scan showed no increase in the metastatic hepatic lesions.
Discussion
To our knowledge, this is the first report of HER2-positive ASC successfully treated with trastuzumab-based combination chemotherapy. Several hypotheses for the origin of a squamous cell carcinoma component in ASC have been proposed: (1) squamous metaplasia of a pre-existing adenocarcinoma [1, 2, 5, 10]; (2) oncogenic transformation of metaplastic or ectopic squamous cells [4], and (3) stem cell differentiation into both adenocarcinoma and squamous cell carcinoma [11, 12]. Accumulating evidence supports the first hypothesis. First, although the occurrence of metaplastic or ectopic squamous epithelium is extremely rare, neither can explain the coexistence of an adenocarcinoma component. Second, ASC is most often diagnosed at an advanced stage of disease [10], and the squamous cell carcinoma component is often located in a deeper layer than the adenocarcinoma component [2]. In a study by Mori et al. [10], ASC was exclusively found in 9 (0.9%) of 976 patients with advanced disease, whereas it was not found among 1,028 patients with early-stage disease. Third, independent ultrastructural studies revealed the existence of a single malignant cell containing both secretory vesicles and tonofibrils in ASC [10, 13]. Moreover, independent immunohistochemical studies demonstrated that both components expressed identical levels of the p53 tumor suppressor gene [1, 2]. In our patient, there was no normal squamous epithelium in the surrounding mucosa and both components positively stained for HER2, supporting this hypothesis.
Previous studies indicated that both components can metastasize into a variety of patterns. Lymph node metastasis frequently occurs with an adenocarcinoma pattern [1, 7] and occasionally with both components [1, 10, 13, 14]. Lee et al. [1] reported that metastatic lymph nodes were composed of only the adenocarcinoma components in 9 (64.3%) of 14 cases, whereas both components and only the squamous cell carcinoma component were found in 3 and only 1 patient, respectively [1]. Consistent with this observation, metastatic lymph nodes in our patient consisted of only the adenocarcinoma component. Recently, Saito et al. [2] reported that the Ki-67 index in the adenocarcinoma component was higher than that in the squamous cell carcinoma component in all 8 patients examined, further supporting these findings. Regarding other metastatic sites, some previous studies reported that the adenocarcinoma component was predominant [6, 7]; however, others found both components [5, 14, 15]. Mori et al. [5] found that both components existed in almost all metastatic lesions at autopsy in 9 patients. In the present case, the tumor was strongly positive for HER2 in both components; therefore, it was not of therapeutic significance to examine which component type had metastasized to the liver.
ASC is often diagnosed as advanced or recurrent disease; therefore, the majority of patients may be candidates for systemic chemotherapy. In several case reports, systemic chemotherapy, such as S-1 [16], paclitaxel [15, 17], or irinotecan plus cisplatin [18], has been reported as an effective treatment for advanced ASC; however, no standard chemotherapy has been established for ASC because of its rarity. We selected trastuzumab-based chemotherapy due to the fact that this tumor was clinicopathologically similar to classical adenocarcinoma, even though the clinical prognosis is different between these two types of tumors [5]. The durable response achieved in our patient can be attributed to the strong immunoreactivity of HER2 and high-level HER2 amplification in both the adenocarcinoma and squamous cell carcinoma components. Considering that the differentiated type is more common in the adenocarcinoma component [1, 2, 5], and that HER2 overexpression is more frequently observed in the intestinal type of gastric adenocarcinoma [19], further studies to evaluate the rate of HER2-positivity and its clinical significance in ASC are warranted.
In summary, we reported a patient with HER2-positive ASC who achieved a durable PR after administration of trastuzumab-based combination chemotherapy. Our results indicate that trastuzumab is an effective treatment for ASC as well as gastric adenocarcinoma; in addition, they indicate that HER2 status should be examined in ASC.
Disclosure Statement
The authors declare that they have no potential conflicts of interest.
References
- 1.Lee WA, Woo DK, Kim YI, Kim WH. p53, p16 and RB expression in adenosquamous and squamous cell carcinomas of the stomach. Pathol Res Pract. 1999;195:747–752. doi: 10.1016/S0344-0338(99)80116-2. [DOI] [PubMed] [Google Scholar]
- 2.Saito S, Hosoya Y, Morishima K, Ui T, Haruta H, Kurashina K, Meguro Y, Zuiki T, Sata N, Fujii H, Matsubara D, Niki T, Lefor AT, Yasuda Y. A clinicopathological and immunohistochemical study of gastric cancer with squamous cell carcinoma components: a clinically aggressive tumor. J Dig Dis. 2012;13:407–413. doi: 10.1111/j.1751-2980.2012.00610.x. [DOI] [PubMed] [Google Scholar]
- 3.Quan J, Zhang R, Liang H, Li F, Liu H. The clinicopathologic and prognostic analysis of adenosquamous and squamous cell carcinoma of the stomach. Am Surg. 2013;79:E206–E208. [PubMed] [Google Scholar]
- 4.Boswell JT, Helwig EB. Squamous cell carcinoma and adenoacanthoma of the stomach. A clinicopathologic study. Cancer. 1965;18:181–192. doi: 10.1002/1097-0142(196502)18:2<181::aid-cncr2820180209>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 5.Mori M, Iwashita A, Enjoji M. Adenosquamous carcinoma of the stomach. A clinicopathologic analysis of 28 cases. Cancer. 1986;57:333–339. doi: 10.1002/1097-0142(19860115)57:2<333::aid-cncr2820570224>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 6.Mori E, Watanabe A, Maekawa S, Itasaka H, Maeda T, Yao T. Adenosquamous carcinoma of the remnant stomach: report of a case. Surg Today. 2000;30:643–646. doi: 10.1007/s005950070105. [DOI] [PubMed] [Google Scholar]
- 7.Yoshida K, Manabe T, Tsunoda T, Kimoto M, Tadaoka Y, Shimizu M. Early gastric cancer of adenosquamous carcinoma type: report of a case and review of literature. Jpn J Clin Oncol. 1996;26:252–257. doi: 10.1093/oxfordjournals.jjco.a023224. [DOI] [PubMed] [Google Scholar]
- 8.Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, Aprile G, Kulikov E, Hill J, Lehle M, Ruschoff J, Kang YK, ToGA Trial Investigators Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 9.Japanese Gastric Cancer Association Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101–112. doi: 10.1007/s10120-011-0041-5. [DOI] [PubMed] [Google Scholar]
- 10.Mori M, Fukuda T, Enjoji M. Adenosquamous carcinoma of the stomach. Histogenetic and ultrastructural studies. Gastroenterology. 1987;92:1078–1082. doi: 10.1016/0016-5085(87)90986-3. [DOI] [PubMed] [Google Scholar]
- 11.Straus R, Heschel S, Fortmann DJ. Primary adenosquamous carcinoma of the stomach. A case report and review. Cancer. 1969;24:985–995. doi: 10.1002/1097-0142(196911)24:5<985::aid-cncr2820240518>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 12.Mingazzini PL, Barsotti P, Malchiodi Albedi F. Adenosquamous carcinoma of the stomach: histological, histochemical and ultrastructural observations. Histopathology. 1983;7:433–443. doi: 10.1111/j.1365-2559.1983.tb02256.x. [DOI] [PubMed] [Google Scholar]
- 13.Grigolato PG, Tardanico R, Benetti A, Berenzi A, Villanacci V. Adenosquamous carcinoma of the stomach. Histochemical and ultrastructural study. Arch Anat Cytol Pathol. 1987;35:87–94. [PubMed] [Google Scholar]
- 14.Faria GR, Eloy C, Preto JR, Costa EL, Almeida T, Barbosa J, Paiva ME, Sousa-Rodrigues J, Pimenta A. Primary gastric adenosquamous carcinoma in a Caucasian woman: a case report. J Med Case Rep. 2010;4:351. doi: 10.1186/1752-1947-4-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tohma T, Yamamoto Y, Seki Y, Takaishi S, Sakuma Y, Funami Y, Tobita K. Weekly paclitaxel therapy is effective for gastric adenosquamous carcinoma: a case report. Hepatogastroenterology. 2009;56:568–570. [PubMed] [Google Scholar]
- 16.Ebi M, Shimura T, Yamada S, Hirata Y, Tsukamoto H, Okamoto Y, Mizoshita T, Tanida S, Kataoka H, Kamiya T, Inagaki H, Joh T. A patient with gastric adenosquamous carcinoma with intraperitoneal free cancer cells who remained recurrence-free with postoperative s-1 chemotherapy. Intern Med. 2012;51:3125–3129. doi: 10.2169/internalmedicine.51.8402. [DOI] [PubMed] [Google Scholar]
- 17.Endo K, Kohnoe S, Okamura T, Haraguchi M, Adachi E, Toh Y, Baba H, Maehara Y. Gastric adenosquamous carcinoma producing granulocyte-colony stimulating factor. Gastric Cancer. 2005;8:173–177. doi: 10.1007/s10120-005-0330-y. [DOI] [PubMed] [Google Scholar]
- 18.Ishiguro A, Takahata T, Hirose K, Matsumoto Y, Tanaka S, Suzuki K, Hanada N, Itoh J, Kawasaki H, Kijima H, Fukuda S, Saijo Y. A case of gastric adenosquamous carcinoma successfully treated with second-line chemotherapy (CPT-11 and CDDP) (in Japanese) Gan To Kagaku Ryoho. 2010;37:1579–1582. [PubMed] [Google Scholar]
- 19.Hofmann M, Stoss O, Shi D, Buttner R, van de Vijver M, Kim W, Ochiai A, Ruschoff J, Henkel T. Assessment of a HER2 scoring system for gastric cancer: results from a validation study. Histopathology. 2008;52:797–805. doi: 10.1111/j.1365-2559.2008.03028.x. [DOI] [PubMed] [Google Scholar]