Abstract
Introduction
Xeroderma pigmentosum (XP) is a rare autosomal recessive disorder of DNA repair, with a prevalence of 1 in 1 million. It may also be a cause of neurological symptoms including sensorineural hearing loss, peripheral neuropathy, ataxia, and chorea. Severe neurological symptoms including mental retardation, short stature, and hypogonadism invoke De Sanctis-Cacchione syndrome (DCS).
Case Report
The patient was a 55-year-old woman with a history of mental retardation who developed chorea at age 32 and ataxia at age 37. She had numerous facial scars from 10 prior basal cell carcinoma excisions as well as diminished deep tendon reflexes, bilateral hearing loss, dysphagia, and skin freckling. Brain MRI revealed severe cortical, cerebellar, and brainstem atrophy. Supportive treatment and prevention of further damage from UV light is the mainstay of treatment in XP and DCS.
Conclusion
XP and related disorders should be considered in the setting of neurological disorder and multiple cutaneous cancers.
Key Words: Xeroderma pigmentosum, De Sanctis-Cacchione syndrome, Cutaneous cancer, Mental retardation
Introduction
Xeroderma pigmentosum (XP) is a rare autosomal recessive disorder of DNA repair, with a prevalence of 1 in 1 million [1]. Affected patients are at 1,000 times higher risk to develop basal cell carcinoma, squamous cell carcinoma, and malignant melanoma [2]. XP may also be a cause of neurological symptoms including sensorineural hearing loss, peripheral neuropathy, ataxia, and chorea [3]. Severe neurological symptoms including mental retardation, short stature, and hypogonadism invoke De Sanctis-Cacchione syndrome (DCS) [4].
Case Report
The patient was a 55-year-old female who presented for evaluation of incoordination and gait disturbance. There were no issues during birth or early development. She was able to speak at 10 months and walked at 12 months. She had some learning disabilities, especially concerning visuospatial tasks, but was able to finish high school and earn a college degree with special education accommodations.
At age 32, she developed ‘head-bobbing’ stereotypies and mild facial chorea. Ataxic gait developed at age 37, at which time she was noted to walk as if she were intoxicated. She began to use a rolling walker at age 50 and required a wheelchair at age 52. Family history is significant for a second cousin (i.e. with shared great-grandparents) with ‘amyotrophic lateral sclerosis’, which in retrospect could have been DCS progressing to quadriparesis [5].
On initial examination, she was microcephalic and exhibited dwarfism at less than 150 cm tall. She weighed about 40 kg. There were numerous facial scars from her 10 basal cell carcinoma excision surgeries. She had diffuse skin freckling. On mental status examination, she was alert and oriented to person and doctor's office, but not to date. There was significant cognitive impairment with ability to follow only one-step commands. Her small stature and overall demeanor were particularly childlike. She was very anxious and perseverated on statements such as being afraid of falling out of her wheelchair or requesting to go to the bathroom. She was able to perform the Luria sequence only by directly copying the examiner, and even those attempts were partially complicated by motor perseveration.
On eye movement examination, saccade initiation and velocity were diminished. There was no facial chorea; in fact, she had hypomimia. There was bilateral hearing loss. There were intermittent side-to-side and up-and-down head movements which were most likely titubation secondary to cerebellar dysfunction. In addition, there was moderate finger-to-nose dysmetria and bilateral upper extremity dysdiadochokinesis as well as involuntary movements likely due to a combination of ataxia and possible proprioceptive sensory loss (for online suppl. video 1, see www.karger.com/doi/10.1159/000362115).
Unfortunately, she could not cooperate enough for a detailed sensory examination. Sensation was grossly intact to light touch. She was able to protrude her tongue for 10 s, demonstrating a lack of motor impersistence. Deep tendon reflexes were diminished throughout. There was severe postural instability; hence, she was able to stand up only with assistance. Bilateral Achilles tendon shortening was present and contributed to difficulty in standing.
Commercially available laboratory testing was negative for the Huntington's disease gene as a cause of chorea. A paraneoplastic antibody panel for anti-Ri, Yo, cancer-associated retinopathy, Zic4, amphiphysin, CV2, Hu, Ma, Ta, voltage-gated potassium channel, P/Q type voltage-gated calcium channel, glutamic acid decarboxylase, NMDA receptor (NR1), and ganglionic nicotinic acetylcholine receptor (Athena Diagnostics, Worcester, Mass., USA) was also negative. Additional commercially available genetic testing was negative for spinocerebellar ataxia (SCA1,SCA2,SCA3,SCA6,SCA7,SCA8,SCA10, and SCA17), dentatorubral-pallidoluysian atrophy (DRPLA) as well as ataxia with oculomotor apraxia type 1 (aprataxin/APTX) and type 2 (senataxin/SETX), Marinesco-Sjögren syndrome (SIL1), sensory ataxic neuropathy, dysarthria, and ophthalmoparesis (SANDO/POLG1), ataxia with isolated vitamin E deficiency (AVED/TTPA), and Friedreich's ataxia (FRDA) (Athena Diagnostics). Neuroacanthocytosis is a concern in the setting of mental retardation, possible peripheral neuropathy, and chorea, but a blood smear was negative for acanthocytes.
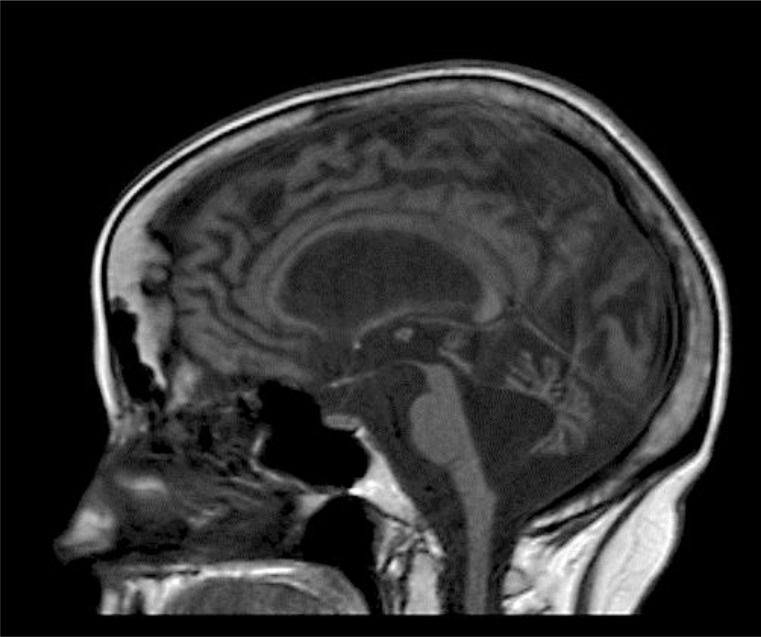
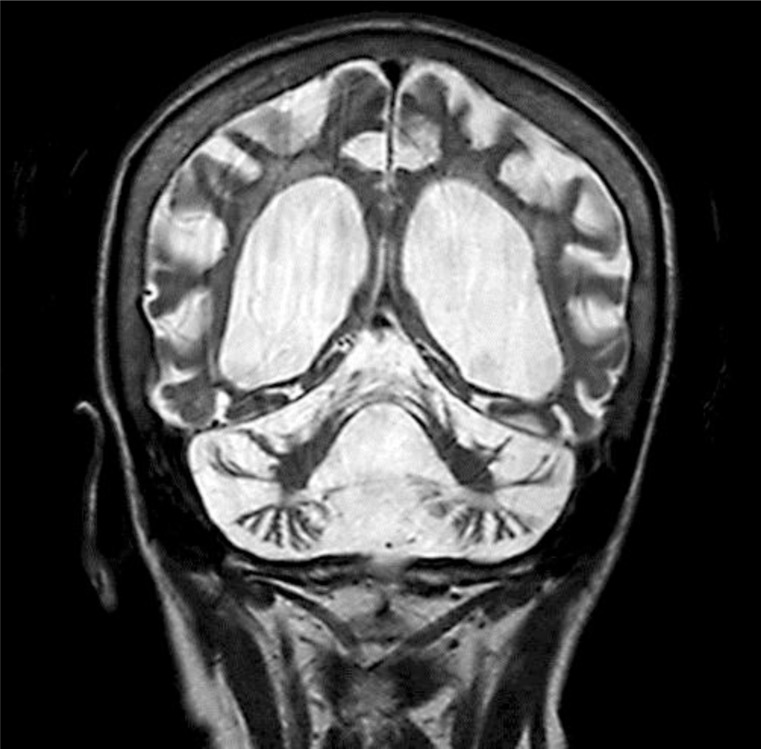
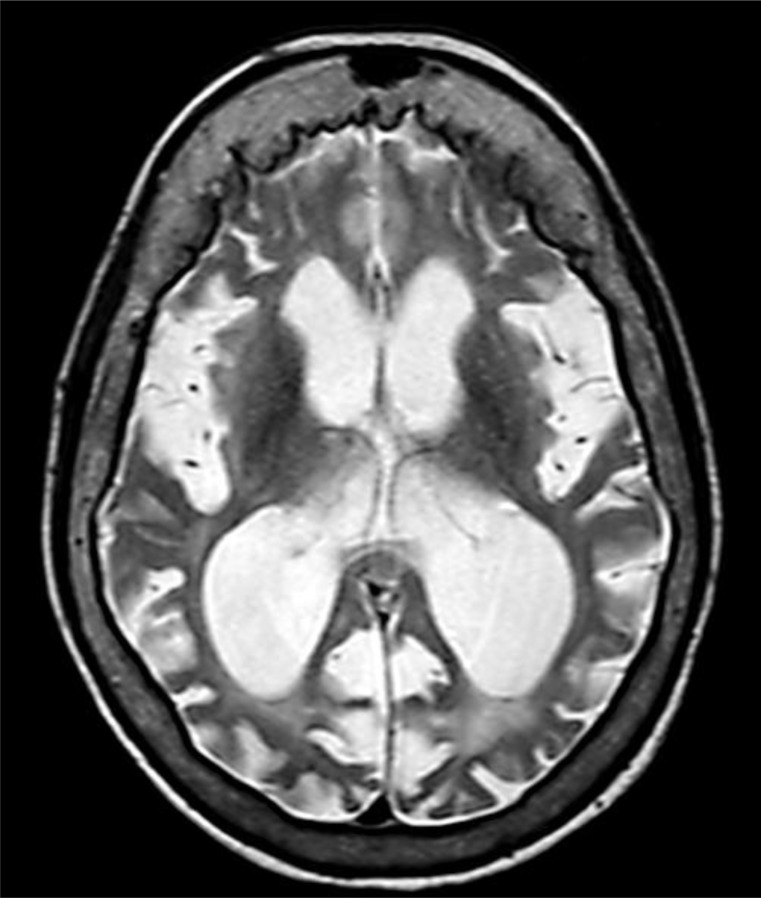
There were no intracranial calcifications on computed tomography of the head, arguing against Cockayne syndrome (CS). MRI at age 54 revealed severe generalized atrophy (fig. 1, fig. 2, fig. 3). Clinical diagnosis of XP was made by a dermatology consultant. Genetic testing for causative genes has not been performed because commercial testing is not available in the USA.
Fig. 1.
Sagittal T1-weighted MRI brain image demonstrating severe atrophy affecting the cortex, brainstem, and cerebellum. There is resulting enlargement of the third and fourth ventricles.
Fig. 2.
Coronal T2-weighted MRI brain image showing lateral and third ventricle enlargement secondary to global atrophy.
Fig. 3.
Axial T2-weighted MRI brain image displaying cortical atrophy and consequent lateral ventricular enlargement as well as sulcal enlargement. Hyperostosis frontalis interna is also present.
At age 55, the patient developed dysphagia to solids, resulting in aspiration pneumonia. She recovered after appropriate antibiotic therapy and now receives nutrition via a gastrostomy tube. Anxiety improved after starting escitalopram, but this medication had to be stopped after an episode of liver enzyme elevation. Given that an attempt to install a coating on her home windows to block UV light was unsuccessful for technical reasons, she was instructed to spend as much time as possible in the interior rooms of the house which do not have direct sunlight.
Discussion
DNA repair problems were identified as a cause of XP by Cleaver [6] in 1969. There is a defect in nucleotide excision repair – removing pyrimidine dimers produced by UV light [7]. About 20% of XP cases may have associated neurological symptoms [3, 8]. Decreased deep tendon reflexes, hearing loss, ataxia, and chorea can occur as in our case. XP complementation groups A, B, C, D, E, F, G, and V exist.
Small stature and childlike demeanor suggesting sexual immaturity makes DCS likely [4]. DCS may be considered a severe subtype of XP complementation groups A or D, with mutations in XPA or ERCC2/XPD [8]. Older descriptions of DCS also include features of microcephaly, choreoathetosis, ataxia, sensorineural deafness, and progression to quadriparesis with shortening of the Achilles tendons [5]. These features have drifted into the modern definition of XP. The current definition of DCS includes ‘cutaneous photosensitivity, microcephaly, mental retardation, short stature, hypogonadism, spasticity, peripheral neuropathy, and sensorineural deafness’ [3]. Our patient appears to fit both the modern and classic definitions of DCS.
This case does not have CS phenotypic features. In contrast to XP and DCS, classic clinical CS phenotype includes intracranial calcification, normal to increased deep tendon reflexes, and an absence of cutaneous cancer. The CSB gene was initially discovered in CS, but identical CSB mutations can, peculiarly, also cause XP and DCS [8]. Hence, there is considerable phenotypic heterogeneity. In addition, there is a genetic and clinical overlap between XP, trichothiodystrophy (TTD) and CS. TTD is in the differential diagnosis, but less likely given the absence of brittle hair and ichthyosis. Other TTD symptoms include photosensitivity, intellectual impairment, short stature, microcephaly, brain demyelination, protruding ears, and micrognathia [9].
Global cortical, brainstem, and cerebellar atrophy similar to this case were demonstrated in a case report by Mittal et al. [3]. While hyperostosis frontalis interna may be a benign incidental finding, interestingly it was also present on images in that case report [3].
Supportive treatment and prevention of further damage from UV light is the mainstay of treatment for dermatological manifestations of XP and DCS. Effective treatment for neurological manifestations of these disorders is not available.
Conclusion
XP and related syndromes should be considered in the setting of neurological disorder and multiple skin cancers.
Supplementary Material
Video 1: Bilateral upper extremity ataxia and involuntary movements are demonstrated. Notice facial disfiguration secondary to basal cell carcinoma excision procedures.
References
- 1.Webb S. Xeroderma pigmentosum. BMJ. 2008;336:444–446. doi: 10.1136/bmj.39485.698356.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rapin I, Lindenbaum Y, Dickson DW, Kraemer KH, Robbins JH. Cockayne syndrome and xeroderma pigmentosum: DNA repair disorders with overlaps and paradoxes. Neurology. 2000;55:1442–1449. doi: 10.1212/wnl.55.10.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mittal H, Mehndiratta S, Kaushik JS, Godbole T. De Sanctis-Cacchione syndrome. Indian J Dermatol Venereol Leprol. 2013;79:849. doi: 10.4103/0378-6323.120760. [DOI] [PubMed] [Google Scholar]
- 4.De Sanctis C, Cacchione A. L'idiozia xerodermica. Riv Sper Freniatr Med Leg Alien Ment. 1932;56:269–292. [Google Scholar]
- 5.Reed WB, Sugarman GI, Mathis RI. De Sanctis-Cacchione syndrome. Arch Dermatol. 1977;113:1561–1563. doi: 10.1001/archderm.113.11.1561. [DOI] [PubMed] [Google Scholar]
- 6.Cleaver JE. Xeroderma pigmentosum: a human disease in which an initial stage of DNA repair is defective. Proc Natl Acad Sci USA. 1969;63:428–435. doi: 10.1073/pnas.63.2.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wood RD, Robins P, Lindahl T. Complementation of the xeroderma pigmentosum DNA repair defect in cell-free extracts. Cell. 1988;53:97–106. doi: 10.1016/0092-8674(88)90491-6. [DOI] [PubMed] [Google Scholar]
- 8.Colella S, Nardo T, Botta E, Lehmann AR, Stefanini M. Identical mutations in the CSB gene associated with either Cockayne syndrome or the DeSanctis-Cacchione variant of xeroderma pigmentosum. Hum Mol Genet. 2000;9:1171–1175. doi: 10.1093/hmg/9.8.1171. [DOI] [PubMed] [Google Scholar]
- 9.Faghri S, Tamura D, Kraemer KH, Digiovanna JJ. Trichothiodystrophy: a systematic review of 112 published cases characterises a wide spectrum of clinical manifestations. J Med Genet. 2008;45:609–621. doi: 10.1136/jmg.2008.058743. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video 1: Bilateral upper extremity ataxia and involuntary movements are demonstrated. Notice facial disfiguration secondary to basal cell carcinoma excision procedures.