Abstract
Perinatal hypoxic-ischemic encephalopathy (HIE) is a significant cause of mortality and morbidity in infants and young children. Therapeutic opportunities are very limited for neonatal and pediatric HIE. Specific neural systems and populations of cells are selectively vulnerable in HIE; however, the mechanisms of degeneration are unresolved. These mechanisms involve oxidative stress, excitotoxicity, inflammation, and the activation of several different cell death pathways. Decades ago the structural and mechanistic basis of the cellular degeneration in HIE was thought to be necrosis. Subsequently, largely due to advances in cell biology and to experimental animal studies, emphasis has been switched to apoptosis or autophagy mediated by programmed cell death (PCD) mechanisms as important forms of degeneration in HIE. We have conceptualized based on morphological and biochemical data that this degeneration is better classified according to an apoptosis-necrosis cell death continuum and that programmed cell necrosis has prominent contribution in the neurodegeneration of HIE in animal models. It is likely that neonatal HIE evolves through many cell death chreodes influenced by the dynamic injury landscape. The relevant injury mechanisms remain to be determined in human neonatal HIE, though preliminary work suggests a complexity in the cell death mechanisms greater than that anticipated from experimental animal models. The accurate identification of the various cell death chreodes and their mechanisms unfolding within the immature brain matrix could provide fresh insight for developing meaningful therapies for neonatal and pediatric HIE.
The implementation of effective therapies for human brain damage after perinatal hypoxia-ischemia (HI) is an unmet need. The problem of HI encephalopathy (HIE) is extremely difficult to understand deeply, and perhaps to model accurately. The failure to translate from bench to bedside may be in part because recent pharmacological attempts in experimental settings have primarily been directed at specific forms of neurodegeneration, particularly apoptosis or autophagy. In neuropathological descriptions of HI brain injury in human newborns1 and in neonatal experimental animal models, neuronal necrosis is identified as the major cellular pathology.2–5 This work has been largely ignored or forgotten because of the acute, unexpected onset and assumed uncontrolled unpredictability and the lack of information about regulated molecular signaling pathways that could be used to occlude necrotic cell death. Instead, much attention has been devoted to the study of apoptosis and its signaling cascades following experimental neonatal brain injury because the rediscovered process represents a “new” avenue for intervention, and biochemical assays and pharmacologic tools are available for its detection and manipulation. Apoptosis cascades are activated following neonatal HI in animal models,6–8 but the weight of neuropathologic evidence in most models supports the conclusion that variants of cellular necrosis acutely contribute most robustly to HI-induced neurodegeneration.5,9,10 The lack of concordance of the biochemical signaling data showing activation of apoptosis cascades with observable primarily necrotic neuropathology is an important clue in understanding experimental HIE, and possibly human HIE. This discrepancy suggests an alternative explanation to the standard “either apoptosis or necrosis” interpretation of cell death following neonatal HI.
An important feature of neonatal HI in rodents is that cell death manifests along a continuum from apoptosis to necrosis with activation of signaling pathways resulting in cell death phenotypes with hybrid structural and biochemical features.5,7 Simultaneously, regulated cell signaling programs resulting in a primarily necrotic phenotype have been recognized recently, and small-molecule drugs have been designed that modulate this “programmed necrosis.”11,12 Despite this progress, it is not yet known which different forms of cell death and their associated molecular mechanisms seen in experimental settings of neonatal HI brain injury are relevant to HIE in human newborns because the latter is understudied and has not been examined with modern ideas and approaches. In this review, we will highlight findings on basic neuronal cell death mechanisms in the term experimental animal brain that is still immature (rodent) or relatively mature (piglet) compared to the human term brain. We include a brief preview of work on the molecular neuropathology of human pediatric HIE. With this needed information it may be possible to redirect efforts to developing more effective global or mechanism-based therapies relevant to human neonatal HIE.
Forms of Cell Death
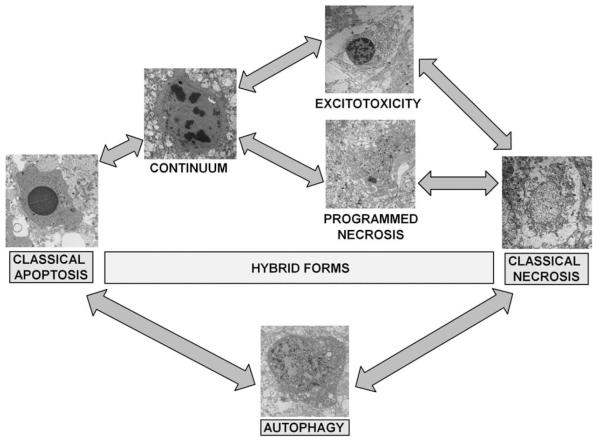
Cell death processes have been generally classified into distinct categories, most commonly, necrosis, apoptosis, and autophagy. These forms of cellular degeneration were originally classified as different because they appeared different morphologically under a microscope; however, these distinctions are now being replaced with a much more nuanced understanding of the overlap and interaction of common mechanisms shared by various forms of cell death (Fig 1).
FIGURE 1.
Cell death phenotypes in experimental neonatal HI brain injury. After its initial description by Portera-Cailliau and colleagues,31 the continuum concept, in its original form organized cell death as a linear spectrum with apoptosis and necrosis at the extremes and different syncretic hybrid forms in between. Subsequently, we have found that this concept is fully realized in the neonatal rodent brain following HI, with classically apoptotic and classically necrotic cells found intermixed with various hybrid forms. Cells at the extremes have the well-described structures of necrosis (swelling and vacuolation of organelles, loss of cell membrane integrity, maintenance of nuclear membrane integrity, and random digestion of chromatin) and apoptosis (condensation and darkening of cytoplasm within intact cell membrane, intact organelles until late phases of apoptosis, compaction of chromatin into few uniformly dense and rounded aggregates, and loss of nuclear membrane). Cells with irregular chromatin condensation organized in a “clockface” pattern around an intact nuclear membrane may or may not have preservation of the cytoplasmic membrane and their cytoplasmic organelles are disrupted. This structure has commonly been reported in models of excitotoxicity. Increasing organization of chromatin into regular crescentic or rounded aggregates, partial dissolution of the nuclear membrane and preservation or the cytoplasmic membrane with or without swelling of cytoplasmic organelles within a condensed cytoplasm is the rule, as cell death forms more closely mimic apoptosis and is most commonly referred to as the continuum cell death phenotype. A more recently described cell death phenotype with structural similarity to classical necrosis is referred to as “programmed necrosis” and occurs with random chromatin dissolution, swelling and vacuolation of cytoplasmic organelles including the endoplasmic reticulum and mitochondria as in this example from the neonatal murine model of HI. Various relationships between the classical and hybrid forms of cell death are suggested by their appearance on electron microscopy as represented by the arrows in the figure. Autophagocytic-appearing neurons with large numbers of cytoplasmic vacuoles, partially condensed nuclear chromatin, and preservation of cellular integrity are also found after neonatal HI. Autophagocytic cell death and apoptosis and possibly necrosis also exist on a continuum. Drugs known to inhibit or promote autophagy clearly modulate cell death along the apoptosis-necrosis continuum. HI = hypoxia-ischemia.
Necrosis
Necrosis is a lytic destruction of individual or groups of cells. Necrosis is the major cell death phenotype in brain following acute neonatal HI injury in rat pup2,5 and we have found this to also be true in the neonatal rat, mouse, and piglet9,10,13 (see Fig 1). Classical necrosis is generally defined by cytoplasmic swelling, nuclear dissolution (karyolysis), and lysis,14 and is thought to be caused by rapid and severe failure to sustain cellular homeostasis, notably cell volume control.15 The process of necrosis involves damage to the structural and functional integrity of the cell plasma membrane and associated enzymes, (eg, Na+,K+ adenosine triphosphatase [ATPase]), abrupt influx and overload of ions (eg, Na+ and Ca2+) and H2O, and rapid mitochondrial damage and energetic collapse.13,16–18 Metabolic inhibition and oxidative stress from reactive oxygen species (ROS) are major culprits in triggering necrosis. Recently, it has been shown that cell necrosis might not be as chaotic or random as envisioned originally but can involve the activation of specific signaling pathways or programs to eventuate in cell death.19,20
Apoptosis
Apoptosis is an orderly and compartmentalized dismantling of single cells or groups of cells into consumable components for nearby intact cells. Classical apoptosis has a distinctive structural appearance21,22 (see Fig 1). It is 1 example of programmed cell death (PCD) that is ATP-driven and sometimes a gene transcription-requiring and caspase-dependent process.23 It is classified as Type 1 PCD.24 Apoptosis is only 1 example of PCD; importantly, other nonapoptotic and apoptotic, caspase-independent, forms of PCD exist.20,25–28 Variants of classical apoptosis or nonclassical apoptosis occur during nervous system development24,29 and frequently in pathophysiological settings of nervous system injury and disease,30–32 notably following neonatal HI.5,8,9,33 Axonal damage (axotomy) and target deprivation in the mature nervous system induces apoptosis in neurons that is similar structurally, but not identical, to developmental PCD.32 Excitotoxins induce readily and robustly nonclassical forms of apoptosis in neurons.30,31 Types of cell death similar to those seen with excitotoxicity occur frequently in pathological cell death resulting from neonatal HI (see Fig 1).7–9,13
Autophagy
Autophagy allows a cell to degrade and recycle its own cytoplasm and organelles.34 The degradation of organelles and long-lived proteins is carried out by the lysosomal system as a homeostatic nonlethal stress response mechanism to protect cells from low supplies of nutrients. Autophagy is classified as Type II PCD.24 A hallmark of autophagic cell death is accumulation of autophagic vacuoles of lysosomal origin. Autophagy is seen in developmental and pathological conditions35–37 including degeneration of Purkinje neurons in the Lucher mutant mouse, thus possibly linking excitotoxic and autophagic cell deaths.38
The molecular controls of autophagy appear common in eukaryotic cells.39,40 Double-membrane autophagosomes for sequestration of cytoplasmic components are derived from the endoplasmic reticulum (ER) or the plasma membrane. Target of rapamycin (TOR) kinase, phosphatidylinositol 3 (PI3)-kinase, a family of cysteine proteases called autophagins, and death-associated proteins function in autophagy.41,42
Autophagy may have a significant role in neurodegeneration after neonatal HI that is insult-severity, time, and region-specific.5,35,43,44 Genetic deletion of the atg7 gene results in a near complete protection from HI in adult mice44 and pharmacologic inhibition of autophagy with 3-methyladenine up to 4 hours after focal ischemia is neuroprotective in p12 rats.45 Conversely, induction of autophagy immediately following neonatal global HI in mice may be an endogenous neuroprotective mechanism.46 Preinsult blockade of autophagy with methyladenine inhibits expression of autophagocytic proteins and switches cell death from apoptotic to necrotic; conversely, enhancing early autophagy with preinsult administration of rapamycin provides neuroprotection in this model.46 Interestingly, known neuroprotective preconditioning strategies also increase markers of autophagy46 while providing neuroprotection.
Excitotoxic Cell Death
Neuronal death can be induced by excitotoxicity. Because of studies showing that glutamate receptors predispose the neonatal brain to excitotoxic injury, this concept has become fundamental to the understanding of how cerebral HI injures the newborn brain in experimental settings.47–49 Excitotoxic neurodegeneration is mediated by excessive activation of glutamate-gated ion channel receptors and voltage-dependent ion channels. Increased cytosolic free Ca2+ causes activation of Ca2+-sensitive proteases, protein kinases/phosphatases, phospholipases, and nitric oxide synthase (NOS) when glutamate receptors are stimulated. The excessive interaction of ligand with subtypes of glutamate receptors causes pathophysiological changes in intracellular ion concentrations, pH, protein phosphorylation, and energy metabolism.50,51
Excitotoxic degeneration in vivo includes somatodendritic swelling, mitochondrial damage, and chromatin condensation into irregular clumps.30,31,52 features that are thought to be typical of cellular necrosis; however, excitotoxicity can also trigger cytological features more like apoptosis.30,31,53 The morphological and molecular regulatory distinctions between the different forms of cell death became blurred and uncertain due to observations made on degenerating neurons in vivo and to a concept developed in 199730,31 that attempts to accommodate these observations. This concept posits that cell death exists as a continuum with necrosis and apoptosis at different ends of a spectrum with hybrid forms of degeneration manifesting in between (see Fig 1).30–32,54 Specifically, degeneration of neurons in diseased or damaged human and animal nervous systems is not always strictly necrosis or apoptosis, according to the usual binary classification of cell death. It also occurs as intermediate or hybrid forms with coexisting morphological and biochemical characteristics that lie in a structural continuum with necrosis and apoptosis at the two extremes.30,31 Thus, neuronal cell death can be probabilistic and syncretic manifesting along numerous possible cell death chreodes (the pathways taken by a cell in response to injury, derived from C.H. Waddington). These chreodes can be influenced by cell autonomous and cell nonautonomous agencies. Moreover the time-space landscape of the injured brain is likely to exert canalization influences.
Molecular Mechanisms of Cell Death in the Immature Experimental Animal Brain
The bcl-2 Family of Survival and Death Proteins
The bcl-2 protooncogene family is a large group of apoptosis regulatory genes encoding about 25 different proteins, defined by at least 1 conserved B-cell lymphoma (Bcl) homology domain (BH1-BH4) that functions in protein-protein interactions with other family members.55,56 Some of the protein products of these genes (eg, Bcl-2, Bcl-xL, and Mcl-1) have all 4 BH1-BH4 domains and are antiapoptotic. Other, proapoptotic gene products, are multidomain proteins possessing BH1-BH3 sequences (eg, Bax and Bak) or proteins with only the critical BH3 death domain (eg, Bad, Bid, Bim, Bik, Noxa, and Puma). Bcl-xL and Bax have α-helices resembling the pore-forming subunit of diphtheria toxin57 that function by conformation-induced insertion into the outer mitochondrial membrane to form channels or pores that regulate release of apoptogenic factors. This mechanism is likely operative in apoptosis signaling following neonatal HI.58
In neonatal rats, HI enhances the already proapoptosis balance of Bcl-2 family proteins. Marked increases in mitochondrial Bax occur within 24 hours of HI in neonatal rats, during which time there is no change in the relative amount of Bcl-xL33 and changes in the subcellular distribution of Bax occurs rapidly, prior to the activation of downstream apoptosis-effector mechanisms59 following excitotoxic neonatal brain injury. In agreement with these data, pretreatment with Bax-inhibitory peptide has been shown to be selectively neuroprotective for neonatal but not adult HI.60 Genetic modulation of these proteins, overexpression of Bcl-xL, and knockout of Bax, Bad, and Bim render neonatal mice resistant to HI, but Bid deficient mice are not protected from neonatal HI.61–63
Caspases: Cell Demolition Proteases
Caspases (cysteinyl aspartate-specific proteinases) are cysteine proteases; 14 members have been identified64 and their basic biology and mechanisms of activation have been reviewed.64–68 So far 3 caspase-related signaling pathways have been identified that can lead to apoptosis67,69–71; 2 and possibly all 3 are likely involved in neurodegeneration following neonatal HI.7,8,33,72–74 The intrinsic mitochondria-mediated pathway is controlled by Bcl-2 family proteins. It is regulated by cytochrome c release from mitochondria, promoting the activation of caspase-9 through Apaf-1 and then caspase-3 activation. The extrinsic death receptor pathway involves the activation of cell-surface death receptors, including Fas and tumor necrosis factor receptor, leading to the formation of the death-inducible signaling complex (DISC) and caspase-8 activation that in turn cleaves and activates downstream caspases such as caspase-3, caspase-6, and caspase-7. Caspase-8 can also cleave Bid, leading to the translocation, oligomerization, and insertion of Bax or Bak into the mitochondrial membrane. Another pathway involves the activation of caspase-2 by DNA damage or ER stress as a premitochondrial signal.75 In a related model of neonatal brain injury, erythropoietin is neuroprotective against hyperoxia-induced cell death and was found to inhibit caspase 2, 3, and 8 activity following exposure to 24 hours of FiO2 = 0.80.76
Caspases are undoubtedly involved in the evolution of experimental neonatal brain injury caused by HI, although their importance varies between animal models. Caspase-3 cleavage and activation occur in brain after HI in neonatal rodents33,72,77–79 and after hypoxia in neonatal piglet.80 The extent of caspase-3 cleavage and activation following brain injury is greater in developing rodents compared to adults.72,81 This principle is replicated in immature and mature neuronal culture systems.82 Cerebroventricular injection of a pan-caspase inhibitor or intraperitoneal injection of a serine protease inhibitor 3 hours after neonatal HI in rat has neuroprotective effects.83,79 Subsequent studies find a 30% to 50% decrease in HI-induced tissue loss in neonatal rat brain 15 days after the insult and treatment with nonselective inhibitors of caspase-8 and caspase-9.84,85 However, the lack of enzyme-specificity of caspase inhibitor drugs prevents unambiguous identification of caspases in mediating brain injury in most studies. The class of irreversible tetrapeptide caspase inhibitors covalently coupled to chloromethylketone, fluoromethylketone, or aldehydes are nonspecific and efficiently inhibit other classes of cysteine proteases including calpains.86 Calpains, Ca2+-activated, neutral, cytosolic cysteine proteases, are activated highly following neonatal HI in rats,77,87 and MDL28170, a drug that inhibits calpains and caspase-3, exerts neuroprotective actions in the neonatal rat brain by decreasing necrosis and apoptosis.88 Drugs similar to MDL28170 may be valuable tools for the treatment of neonatal HI. Cathepsins, cysteine proteases concentrated in the lysosomal compartment, are also activated following neonatal HI in piglets.13 More potent, selective, and reversible nonpeptide caspase-3 inhibitors have been developed89 and have been used to protect against brain injury following neonatal HI in rat.90 The protective effects of these nonpeptide caspase-3 inhibitors is modest compared to that reported with nonselective pan-caspase inhibition79 and the drug had to be given prior to the insult for neuroprotection.90 Genetic deletion of caspase-3 worsens neonatal HI brain injury by upregulation of non-caspase-dependent cell death pathways.91 These findings have important implications for design of caspase-active therapeutics.
Not all forms of apoptotic cell death are caspase-dependent92,93 and not all caspase functions are death related. Caspases are also critical regulators of nondeath functions in cells, notably some maturation processes. These nonapoptotic functions include modulation of synaptic plasticity via involvement in long-term potentiation94 and cleavage of alpha-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid (AMPA) receptor subunits,95 and normal differentiation and migration of neurons to the olfactory bulb.96 Because of the potential importance of the nonapoptotic functions of caspase-3, it may be more appropriate to block the formation of “stress-induced” cleaved caspase-3 following injury with selective caspase-8 and 9 inhibitors, which would not interfere with basal levels of caspase-3 activity. The nonapoptotic functions of caspase-8 and 9 are unknown and the effects of acute caspase-8 and 9 inhibition on the developing brain other than for neuroprotection are similarly unknown.
Cell Surface Death Receptors
Cell death can be initiated at the cell membrane by surface death receptors of the tumor necrosis factor (TNF) receptor superfamily. The signal for cell death is initiated at the cell surface by aggregation (trimerization) of the death domain containing members of this receptor family by their specific ligand. Clustering of the ligand on the target cell recruits Fas-associated death domain (FADD), a cytoplasmic adapter molecule that functions in the activation of the caspase 8-Bid pathway, thus forming the DISC71 (Fig 2). Signaling for apoptosis then proceeds via the extrinsic or intrinsic pathway. A much more complex and nuanced understanding of death receptor signaling in apoptotic and necrotic cell death has emerged from work in vitro97 and is discussed below.
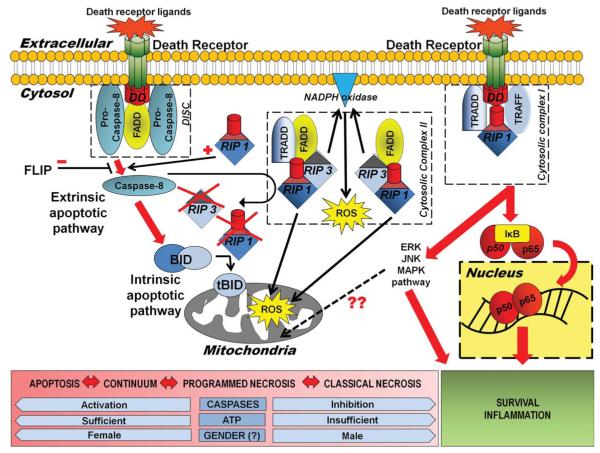
FIGURE 2.
Death receptor signaling and programmed necrosis. This diagram summarizes the possible pleiotropic outcomes following death receptor activation and the signaling pathways leading to these outcomes. Ligand binding to and trimerization of death domain containing members of the TNF receptor superfamily, recruits FADD, a death effector domain containing adaptor protein and procaspase 8, forming the DISC. Signaling for apoptosis then proceeds via the extrinsic or intrinsic pathway. In the extrinsic pathway, active caspase-8 directly cleaves caspase-3. Activation of the mitochondrial or intrinsic pathway proceeds via caspase-8 mediated cleavage of cytosolic Bid. The truncated form of Bid then translocates to mitochondria, thereby functioning as a BH3-only transducer of the death receptor signal at the cell plasma membrane to mitochondria. Simultaneously, cleaved caspase-8 may inactivate death receptor signaling via RIP1 kinase, by cleaving and inactivating both RIP1 and RIP3. Conversely, RIP1 may act to stimulate TNF apoptosis signaling by promoting activation of caspase-8. In the setting of caspase inhibition or severe energy failure, death receptor signaling may preferentially proceed via RIP1 kinase, a death domain containing protein, that binds the death receptor signaling complex containing the adaptor proteins TRADD and TRAF2 at the cell membrane and then TRADD and FADD and RIP3 as cytosolic complex II. Along with RIP3, RIP 1 kinase, death receptor–mediated cell death occurs with ROS production and a cell death morphology resembling necrosis. Both mitochondrial and NADPH oxidase may be the source of enhanced free radical production in this signaling paradigm. When ubiquitinated and bound to the membrane cytosolic complex I, RIP1 kinase activates downstream kinase pathways, which may also contribute to ROS production (possibly via JNK) and also cleave the IKK subunit from the NF-jB complex, initiating NF-κB proinflammatory/prosurvival signaling. Gender likely also has a determinant effect on cell death phenotype following HI. DISC = “death-induced signaling complex”; FADD = Fas-associated death domain; HI = hypoxia-ischemia; IKK = IkappaB kinase complex; JNK = c-Jun N-terminal kinase; NADPH = nicotinamide adenine dinucleotide phosphate; NF-κB = nuclear factor kappa B; RIP1 = receptor interacting protein 1; RIP3 = receptor interacting protein 3; ROS = reactive oxygen species; TNF = tumor necrosis factor; TRADD = TNF receptor-associated death domain; TRAF2 = TNF receptor-associated factor 2.
Evidence for the importance of death receptor signaling pathways in experimental neonatal brain injury is growing. Activation of multiple components of the Fas death receptor signaling pathway have been found in neonatal rat and mouse models of HI and hyperoxic brain injury33,98,99 and is associated with apoptosis.33,78 This thalamic neuron apoptosis may be in part the result of target deprivation via a Fas-dependent pathway. Blocking Fas death receptor signaling by either pharmacologic or genetic means affords protection in these models.85,98,99
p53 Tumor Suppressor
Cell death by apoptosis can be triggered by DNA damage and p53 is involved.100 p53 functions in apoptosis or growth arrest and repair. Activated p53 binds the promoters of several genes encoding proteins associated with growth control and cell cycle checkpoints and apoptosis (eg, Bax, Bcl-2, Bcl-xL, and Fas).101–104 p53 deficiency protects against neuronal apoptosis induced by axotomy and target deprivation in vivo105,106 and thus may be important in remote, delayed neurodegeneration following neonatal HI. The role of p53 in acute neurodegeneration following neonatal HI has been studied. p53 levels increase after HI.107 Acute inhibition of nuclear factor kappa B (NF-jB) following neonatal HI prevents both nuclear and mitochondrial accumulation of p53 while providing significant sustained neuroprotection.108
Emerging Concepts in Cell Death Following Neonatal Brain Injury
The Cell Death Continuum
In mammalian animal models of neurodegeneration cell death exists as a continuum of necrosis and apoptosis at opposite ends of a degenerative spectrum with intermediate hybrid form manifesting in between (see Fig 1).30–32 The age or maturity of the brain, and the subtype of excitatory glutamate receptor that is activated influence the mode and speed of neuronal cell death.30,31,109,110 This structural and temporal diversity of neuronal cell death is seen with a variety of brain injuries including excitotoxicity, HI, target deprivation, and axonal trauma. Biochemical evidence for the existence of an intermediate “continuum” form of cell death has been verified by the coexpression of markers for both apoptosis and necrosis in neurons in the injured forebrain at 3 hours following HI in neonatal rat. Diversity of cell death is 1 of the fundamental differences in injury-associated neuronal death in immature and mature central nervous system (CNS), manifesting much more often in the injured immature brain.7 Additionally, cell death is clearly pleiomorphic in neurons within the same brain.6,7,111 To help explain these data we formulated the concept of the cell death continuum (see Fig 1).
Different cell populations seem to follow different cell death chreodes after injury. In the brain, this predilection is age-dependent. Forebrain cortical and striatal neurons in immature rat and mouse elegantly exhibit the full spectrum of the cell death continuum after excitotoxic and HI insults. In cortex of postnatal day 4 (p4) rat, layer-specific cell death chreodes, including apoptotic, necrotic, and hybrid, are witnessed in pyramidal and nonpyramidal neurons.31 Injury-induced death chreodes in forebrain tend to narrow to necrotic and hybrid forms with increased maturity.30 This developmental maturity-related aspect of the cell death continuum is a likely reason for the more necrotic-like cell death chreodes followed in newborn piglet forebrain neurons after HI.13 The neonatal piglet is at a distinctly different stage of brain development (more mature) when compared to neonatal rats and mice, and more similar to term human newborns.112 In contrast to the varied cell death fates of forebrain neurons, thalamic neurons seem to have limited death chreodes and usually die apoptotically, independent of brain maturation, but the time course is collapsed in the immature brain compared to the mature brain.33,105,109 Nonneuronal cells in forebrain also appear to exhibit restricted cell death chreodes as compared to forebrain neurons.113 N-methyl-D-aspartate (NMDA) receptor excitotoxicity114 induces apoptosis in O4 oligodendrocyte precursors in neonatal rodent, while HI in newborn piglet115 and excitotoxicity in neonatal rat31 induces necrosis in astrocytes.
Recent data suggests that under certain conditions and perhaps in specific brain regions, autophagy is also part of the complex continuum of neurodegeneration following neonatal HI in mice.43 Pharmacologic inhibition of autophagy, prior to neonatal HI worsens injury and shifts the cell death continuum from apoptosis to necrosis in P7 rats.46 One possible explanation for these results is that autophagy may play an important role in maintaining cellular energy stores at adequate levels early on following a severe HI insult and thus allowing for successful completion of apoptosis. Otherwise, without the energy savings provided by induction of autophagic mechanisms, ATP levels may fall below the critical threshold required for completion of apoptosis and forms of necrosis ensue by default. Conversely, inhibition of autophagy is neuroprotective at later time points and in less severe insults.45 In these settings, maintenance of cellular energy may not be as critical and autophagic mechanisms may be activated in stressed but possibly still viable neurons. Crosstalk between autophagic and apoptotic pathways may result in the cells ultimately being eliminated by apoptotic mechanisms.116 It remains to be seen whether inhibiting delayed autophagy provides functional benefits. Once again, it is clearly evident that timing and energy state are critical variables in any attempt to modulate autophagy or any other form of cell death following neonatal HI.117
Crosstalk Between Cell Death Pathways
Syncretic engagement of different cell death signaling mechanisms responsible for the morphological cell death continuum anticipates certain molecular crosstalk among apoptotic and necrotic networks. Ca2+ deregulation and activation of Ca2+-dependent enzymes is a dominant theme for both necrosis and apoptosis.118 Indeed, calpains are activated in ischemic necrosis,119 and m-calpain can activate caspase-3 in vitro,77 and after neonatal rat HI, m-calpain may trigger reduced Bcl-2 levels.58 Evidence for crosstalk between calpain and caspase pathways as a fundamental mechanism contributing to “continuum” cell death is found.77 The significance of this finding is evident from the finding that highly specific caspase-3 inhibition following HI provides complete blockade of caspase activation but either only partial,90 or no neuroprotection120 and genetic knockdown of caspase 3 actually worsens injury following neonatal HI.91 In the pharmacologic studies, specific caspase inhibitors failed to prevent the necrotic mode of cell death induced by HI as revealed by the presence of necrosis markers.90
m-Calpain can also activate ER-resident caspase-12 in vitro and in cultured rodent cells,121 although the relevance of this finding to human HIE is nebulous due to mutations of the caspase-12 gene acquired in human evolution that inactivate the functional protease.122 However, as first delineated by the Korsemeyer laboratory,123 the ER, which regulates intracellular Ca2+ levels, participates in a loop with mitochondria involving Ca2+ transfer to modulate mitochondrial permeability transition and cytochrome c release through the actions of Bcl-2 protein family members. This ER-mitochondria connection now seems to be critical crosstalk mechanism for regulated and deliberate programmed necrosis involving the BH3-only-like protein Nix/BNip3L.124 Nix/BNip3L has dual, but distinct, actions at the mitochondria and ER. At mitochondria, Nix/BNip3L induces canonical Bax/Bak-dependent outer mitochondrial membrane (OMM) permeabilization, cytochrome c release, caspase activation and apoptosis, while, at the ER, Nix/BNip3L induces acute release of luminal Ca2+ that triggers cyclophilin D–dependent opening of the mitochondrial permeability transition pore and cellular necrosis.124
Receptor interacting protein kinase-1 (RIP1) is also an important crosstalk molecule for apoptotic-necrotic pathways. During apoptosis induced by TNF receptor and Fas, activated caspase-8 negatively regulates necrotic signaling by cleaving RIP1.125 TNF-induced and FasL-induced cell death is converted from apoptosis to necrosis in the presence of broad-spectrum caspase inhibitors and depends on the presence of RIP1,26,126 RIP1 also translocates to mitochondria and suppresses adenosine diphosphate (ADP)/ATP exchange.127 This mitochondrial RIP1 relocation may influence the mitochondrial permeability transition pore because the adenine nucleotide translocator appears to be a component of this complex.128
Autophagic and apoptotic cell death pathways also crosstalk. The product of the tumor suppressor gene beclin1 interacts with the prosurvival regulator Bcl-2.129 Autophagy can block apoptosis by sequestration of mitochondria. If the capacity for autophagy is reduced, stressed cells die by apoptosis, whereas inhibition or blockade of caspases can convert the cell death process into autophagy.130,131 This transition to autophagy is dependent on RIP1 and c-Jun N-terminal kinase (JNK).132 In summary, crosstalk among multiple signaling pathways supports the concept of a cell death continuum.31,133 A combination of computational analysis along with experimental biology is now being utilized to model and understand the complex pathways leading to programs of cell death.134 This systems biology approach provides for quantitative analysis of the interconnectivity among various cell death programs and seems ideally suited for the study of HI neurodegeneration in the newborn.
Possible Mechanisms Driving the Cell Death Continuum
A fundamental cornerstone of the continuum is thought to be gradations in the responses of cells to stress. Some general cellular mechanisms thought to be driving the continuum are the developmental expression of different subtypes of glutamate receptors, mitochondrial energetics, the propinquity of developing neurons to the cell cycle, neurotrophin requirements, DNA damage vulnerability, and the degree of axonal collateralization.110,135 Although the molecular mechanisms that drive this cell death continuum in the brain are currently uncertain, cell culture data hint that ATP levels,17 intracellular Ca2+ levels,14 level of caspase activity,125 mitochondrial permeability transition,136 and gender137 could be involved (see Fig 2). The mechanisms discussed for “crosstalk” between autophagy, apoptosis, and necrosis are highly relevant to those driving the “continuum.” In the newborn brain, with its proclivity to apoptotic cell death, the concept that energy failure interrupts and prevents successful completion of apoptosis with a resultant increase in necrotic cell death forms is an appealing explanation for the abundant expression of biochemical markers of apoptosis and paucity of evidence for complete execution of apoptosis.5,7
Identifying the specific mechanisms driving the cell death continuum in vivo is important to rational therapeutic design. Two studies of motor neurons demonstrate that the levels of oxidative stress101 and activation of the mitochondrial permeability transition pore138 drive the cell death continuum. First, peripheral nerve injury by avulsion induces motor neurons to undergo unequivocal and uniform apoptosis139 that is triggered in part by oxidative stress and is Bax-dependent and p53-dependent.106 However, in the presence of human mutant SOD1 in motor neurons, which greatly enhances the level of oxidative stress in spinal cord,138 avulsion-induced apoptosis is converted to a rapid necrotic-like process.101 The second study,138 also using human mutant SOD1 transgenic mice, involved genetic ablation of cyclophilin D, a pepidyl-prolyl cis-trans isomerase located in the mitochondrial matrix, which regulates the mitochondrial permeability transition pore and cellular necrosis.140 These mutant mice develop severe age-related motor neuron degeneration over a period of 12–18 weeks that uniformly resembles a slow necrotic process with marked inflammation.141 However, in mutant SOD1 mice without cyclophilin D, the necrotic process in motor neurons is occluded and converted to an autophagic-like process resulting in a remarkable extension of lifespan.138 In a recent study of neonatal mouse HIE, necrostatin treatment decreased the cellular neurosis and increased the apoptotic cell death, demonstrating a possible role for RIP1 in emergence of cell death phenotype.10 Although many other possible mechanisms regulating cell death can be derived from studies on cultured cells, including intracellular Ca2+, ATP, and glutathione levels142 and the severities and types of DNA damage accumulation,143,144 our in vivo work validates the reality of the cell death continuum and provides the first insights into the mechanisms driving the cell death continuum.
Programmed Cell Necrosis
Forms of neurodegeneration in the developing brain similar to “continuum” cell death have been previously described and termed “pathological apoptosis”6 and excitotoxic neurodegeneration.145 The important contribution, of these regulated but morphologically hybrid forms of cell death, to adult neurodegeneration have been reviewed20,39,146,147 but their importance in neonatal brain injury is just now emerging.6,7 These regulated forms of cell death are good examples of molecular switching between apoptotic and necrotic modes of cell death.146 An additional regulated form of cell death, programmed necrosis, is increasingly recognized as a key form of neurodegeneration and also lies along the apoptosis necrosis continuum10,148 (see Fig 1). The death domain containing kinase, RIP1, is a key signaling intermediate in programmed necrosis and is inhibited by a small-molecule drug called necrostatin.11,126 Necrostatin has shown promise as a neuroprotectant in adult animal models of myocardial ischemia and traumatic and ischemic brain injury12,149,150 and we show that it provides robust sustained neuroprotection following neonatal HI in mice.10 Necrostatin has also been utilized in vitro to demonstrate distinct signaling pathways to morphologic necrosis.151 In vitro, it appears that varying forms of necrosis proceed with different kinetics, are not all RIP1 kinase dependent, and require different therapeutic approaches.151 This is relevant to neonatal HI because following HI on P7 and immediate posttreatment with necrostatin, no demonstrable neuroprotection is seen on P8, but robust and lasting neuroprotection can be demonstrated at 4 days and 3 weeks after HI,10 also suggesting that not all forms of HI induced necrosis are RIP1 kinase-dependent.
Necrostatin’s primary mechanism of action is allosteric inhibition of RIP1 kinase11 with possible downstream anti-inflammatory effects149 and perhaps other mechanisms. Depending on the experimental conditions, RIP1 functions as the crucial adaptor kinase at the crossroads of a death receptor stimulated cell’s decision to live or die, and also at a critical juncture determining whether programmed cell apoptosis or necrosis is predominant after death receptor activation (see Fig 2).125,126 RIP1-mediated activation of the regulated necrosis signaling pathway preferentially occurs in the presence of caspase inhibition.126,152 Inhibition of endogenous caspase-8 mediated-cleavage of RIP1 is essential for maximal execution of death receptor–mediated programmed necrosis. Caspase inhibition can also occur in the setting of significant energy failure,17,153–155 such as that which occurs following neonatal HI (see Fig 2). Others and we have hypothesized that this energy failure interrupts the neonatal brain’s proclivity to apoptosis,6,7,154,155 resulting in the hybrid, continuum cell, or programmed necrosis death morphology, possibly via activation of RIP1-mediated necrosis.10
In the setting of energy sufficiency, RIP1 kinase primarily responds to stimulation by a TNF receptor with NF-κB activation and cell survival (see Fig 2).126 In settings of partially limited cellular energy, RIP1 kinase cleaves and activates caspase 8, initiating the apoptotic cascade.126,156 If energy levels are further compromised or caspases are inhibited, RIP1 (with RIP3) signaling proceeds to necrosis.157–159 This multifunctional signaling capacity makes RIP kinases ideal candidates to sense the cell’s energy sufficiency and to modulate the cell death continuum (see Fig 2).
Necrostatin is not an antioxidant and it does not prevent cell death in vitro caused by hydrogen peroxide,151 nevertheless, necrostatin clearly modulates redox mechanisms in experimental systems.159 Necrostatin also blocks nitric oxide (NO)-mediated mitochondrial dysfunction caused by lipopolysaccharide (LPS) stimulation of macrophages.158 Necrostatin inhibition of glutamate excitotoxicity occurs with an increase in glutathione levels and a decrease in ROS production.159 It remains controversial whether necrostatin delays opening of mitochondrial permeability transition pore,150,160 blocks the reduction in mitochondrial membrane potential caused by excitotoxic stimuli,161 and blocks NO-mediated nitration of the NDUFB8 complex 1 subunit, preventing complex 1 dysfunction and mitochondrial depolarization.158 Furthermore, RIP1 is an essential component of the TNF receptor-associated death domain (TRADD)-RIP1-Rac1 complex formed upon death receptor activation.162 This complex activates membrane bound NOX1 nicotinamide adenine dinucleotide phosphate (NADPH) oxidase to produce O2− (see Fig 2).163 Superoxide radical formation is critical to TNF and Fas induced necrotic cell death in L929 (fibrosarcoma cells) and is potentiated in the presence of pan-caspase inhibition, the exact setting in which necrostatin has maximal effect to block cell death.152,164
Neurodegeneration in Newborn Human HIE
Assessment of the cellular and molecular pathology seen in newborn human HIE is needed to identify the standard against which experimental observations in animal models should be compared; however, few detailed neuropathological and molecular mechanism-based studies of cell death have been done on pediatric human HIE autopsy brains.165 Magnetic resonance spectroscopy studies of infants at 24 hours of life after perinatal asphyxia reveals abnormalities of neuronal integrity in basal ganglia.166 Most available postmortem studies of human HIE have focused on “pontosubicular necrosis.”167 In asphyxic term humans on day 1, neuronal necrosis in striatum is suggested.168 We have now begun to evaluate (Fig 3) brain samples from full-term human infants (n = 6) that suffered from complications during delivery resulting in HIE and death ranging from 3 days to months after the insult. Cerebral cortex, striatum, and cerebellum were evaluated for cytopathology (cell death phenotypes) and molecular markers for cell death pathways (p53 and cleaved caspase-3). Degeneration occurs in selective populations of neurons throughout forebrain and cerebellar cortex with no evidence of infarct or major gliomesodermal changes. The neurodegeneration was divisible on a single-cell basis and occurs as 2 dominant forms: lytic, necrotic-like, or condensed, traditional ischemic-like (see Fig 3). The necrotic-like neurons were swollen or erupted with residual cytoplasm around the nucleus. The ischemic-like neurons displayed homogenization and vacuolation of the cytoplasm, cell shrinkage (but no apparent frank lysis), and nuclear collapse (pyknosis) or chromatin clumping into irregular clumps (see Fig 3C) rather than nuclear condensation into round or crescentic masses. The nuclear degeneration seen is consistent with a cell death process that is not strictly apoptosis. There were no classically apoptotic or closely apoptotic-like neurons seen. Nevertheless, subsets of degenerating cortical neurons in human HIE were positive for cleaved caspase-3 (see Fig 3), but many degenerating neurons were not positive for cleaved caspase-3. Some caspase-3–positive apparent pyramidal cortical neurons showed large irregular clumping of chromatin consistent with continuum cell death (see Fig 3C). One recent study found cells (of unknown identity) positive for cleaved caspase-3 in the cerebral cortex of a human neonate with HIE.169 Many degenerating cortical neurons, surprisingly some cells with a necrotic-like morphology, were also positive for active (phosphorylated) p53 (see Fig 3D). This type of neurodegeneration is a form we have not seen before in animal models of HI. In white matter, there was no morphological evidence for oligodendrocyte apoptosis, although appreciable white matter damage was present, as evidenced by the rarefaction and vacuolation. Notwithstanding the limitations inherent in study of human postmortem tissue, these findings show that classic apoptosis has little contribution to the evolving neuropathology in the newborn human brain with HIE. They also suggest that caspase-3-regulated and p53-regulated cell death mechanisms could be operative in driving a syncretic cell death continuum variant phenotype in addition to cellular necrosis.
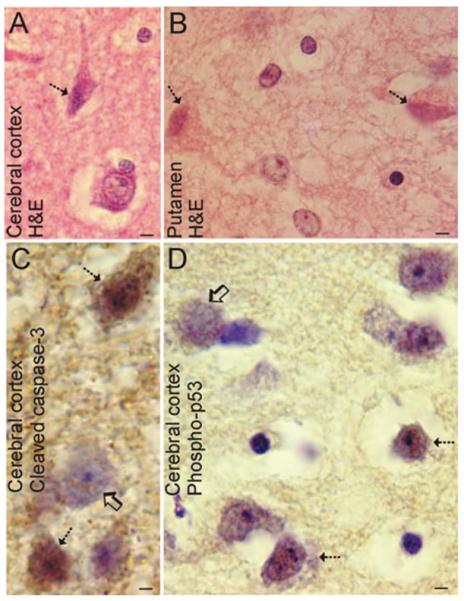
FIGURE 3.
Neuronal cell death in human newborn HIE. (A,B) Hematoxylin staining of neocortex from an infant that survived 3 days after HI due to delivery complications reveals selective degeneration of neurons (hatched arrows) in the form of typical ischemic neuronal death with eosinophilic cytoplasm, shrunken cell body, and condensed nucleus. Other damaged neurons are swollen with a vacuolated cytoplasm (hatched arrow in B). This pattern of neurodegeneration is much less phenotypically heterogeneous than that seen in neonatal rodent models of HI but similar to that seen in newborn piglet HI. (A) Bars = 33μm; (B) Bars = 7μm. (C) Subsets of neocortical neurons (hatched arrows) in human infants with HIE display cleaved caspase-3 throughout the cell. Other cells in the microscopic field visualized by the cresyl violet counterstaining have no labeling for cleaved caspase-3 (open arrow). Bar = 15μm. (D) Subsets of neocortical neurons (hatched arrows) in human infants with HIE display active p53 within the nucleus. Other cells (open arrow) in the field have no labeling for active p53. Bar = 15μm. HI = hypoxia-ischemia; HIE = hypoxic-ischemic encephalopathy.
Modeling Newborn Human HIE in Animals
Animal models are critical for identifying relevant injury-related mechanisms of HIE and for testing preclinical efficacy of therapeutics. However, the relevance of the animal model must be appreciated in the context of human pathobiology by understanding the relative brain-maturity of the model compared to that of the human term newborn and by recognizing that the pathobiology observed in the model should be similar to that seen in human neonatal HIE. Fundamental physiological, neurobiological, and pathobiological issues are very important when considering the relevance of experimental animals as models for brain injury in human newborns. The brain of a human newborn is nearly 2,000-fold larger than the brain of a newborn mouse (L.J.M., personal observations). In contrast to the newborn rat/mouse, the percentage of adult brain weight at birth in piglets is much closer to human.112 Additionally, the body size of the piglet and chest and cranial geometries, anatomy and physiology of the cardiovascular and pulmonary systems,170 as well as the cortical and basal ganglia topology171 are much more similar to human infants. The pig and human brain have similarities in material properties, growth patterns, and the extent of peak growth at the time of birth.112,172,173 The superficial and deep brain anatomy of piglet also more closely resembles the human brain. Important similarities between the pig and human brain have been found in hippocampus, basal ganglia, and brainstem.173 Gray and white matter patterns and distributions are similar in human and piglet brains and the maturation of the postnatal pig brain is comparable to human with respect to myelination and electrical activity. Thus, we anticipated that piglets would be useful for modeling brain injury in human newborns.
We used a 4–7-day-old piglet model of HI that simulates the brain damage and some of the clinical deficits found in human newborns that are victims of asphyxia.171,174–176 This injury model is most relevant to asphyxia in the full-term neonate.177 Importantly, the basal ganglia and somatosensory systems, including primary somatosensory cortex and ventrobasal thalamus, are selectively vulnerable in this model.171 The putamen is the most vulnerable. The death of striatal neurons after HI in piglets appears uniformly necrotic-like,13 contrasting with findings in neonatal rat striatum after HI.7–9 Nevertheless, despite the necrotic-like phenotype, this neurodegeneration in piglets evolves temporally with a specific pattern of subcellular organelle damage and biochemical defects within the basal ganglia.13 Glutathione is depleted by 3 hours after the insult. Peroxynitrite-mediated oxidative damage to membrane proteins occurs at 3–12 hours after HI, and the Golgi apparatus and cytoskeleton are early targets for extensive tyrosine nitration. Striatal neurons sustain hydroxyl radical damage to DNA and RNA within 6 hours after HI. The early emergence of this biochemical injury coincides with elevated NMDA receptor phosphorylation, a biochemical surrogate marker for activation.175,178 Ultrastructural damage to the Golgi apparatus and rough ER occurs at 3–12 hours, while most mitochondria appear intact until 12 hours. Mitochondria undergo an early suppression of metabolic activity, then a transient burst of activity at 6 hours after the insult, followed by mitochondrial failure. Cytochrome c is depleted at 6 hours after HI, does not accumulate in the cytosol compartment, and is not restored thereafter. Lysosomal destabilization occurs within 3–6 hours after HI, consistent with the lack of evidence for autophagy. Damage in newborn piglet striatum after cerebral HI induced by asphyxic cardiac arrest thus evolves rapidly over 24 hours, at which time ~80% of the neurons in the putamen are dead, and closely resembles excitotoxic neuronal damage caused by NMDA receptor activation.13,31,175,176 Although this injury needs to be evaluated in the context of programmed necrosis molecular mechanisms, it is noteworthy that the morphology of the degeneration seen in the piglet forebrain mirrors more faithfully the pattern seen in human newborns (Fig 3) than the patterns of neurodegeneration seen in rodents,7–9 and is skewed more to the cell necrosis part of the cell death continuum.
Conclusion
When studying mechanisms of cell death in human brain injury and in animal/cell models of brain injury, it is helpful to embrace the idea that apoptosis, necrosis, autophagy, or non-apoptotic PCD are not distinctly independent cell death chreodes but are instead entities of a cell death continuum. For the nervous system, one must overlay this complexity with cell death mechanisms that are influenced by brain maturity, capacities for protein/RNA synthesis and DNA repair, antioxidant status, neurotrophin requirements, location in brain and location relative to the primary sites of injury, timing of therapeutic interventions, as well as intensity of the insult. These factors that influence nervous system damage can make the pathobiology of perinatal HIE seem to abandon strict certainty and causality, and yield a neuropathology that is probabilistic and highly uncertain. The cell death continuum predicts that apoptosis inhibitor drugs administered at acute time points in the face of energy failure or perhaps delayed time points in the setting of significant cytokine production may push cell degeneration from apoptosis to apoptosis-variant or necrotic cell death, or exacerbate HI injury. This is seen in vitro with caspase inhibitors applied following chemical hypoxia179 and in vivo in the setting of genetic knockdown of caspase-3.91 Single therapies targeted at inhibition of apoptosis alone early after neonatal HI are unlikely to ameliorate most of the early necrotic neurodegeneration following neonatal HI,9,13 and are ineffective as posttreatments for neonatal HI in some studies.120 When given as a pretreatment, inhibitors of autophagy may also push neurodegeneration along the cell death continuum in a deleterious manner.46 Conversely, inhibitors of programmed necrosis may push neurodegeneration from necrosis to apoptosis.10 In settings where post-injury inflammation contributes significantly to delayed neurodegeneration, this may be particularly beneficial. Necrostatin may be a useful tool to direct therapy at “programmed necrosis” and other hybrid forms of neurodegeneration and may allow improved understanding of some of the complex interactions and ordering of key components of death receptor–mediated injury mechanisms that result from hypoxic-ischemic injury to the immature brain. Importantly, necrostatin-like drugs may additionally clarify the contribution of hybrid or continuum forms of neurodegeneration to HI neonatal brain injury. This task is likely to be simplified by the recent identification of the genes most important to the cellular signaling network that regulates programmed necrosis and the molecular bifurcation that controls the chreode of apoptotic vs hybrid and necrotic forms of cell death.180
It is especially important to prepare for the possibility that pharmacological interventions directed against a single mechanism of injury might only delay, convert, or worsen the evolving brain damage associated with HIE in newborns. For this reason, hypothermia appears to be an ideal strategy because it protects against necrosis and apoptosis181 and may be working along both the cell death continuum and along the space/time matrix that strongly influences cell death in the neonatal brain.
Acknowledgments
This research was supported by grants from the March of Dimes Foundation (6-08-275); NIH (NINDS NS 059529 to F.J.N., NIA AG 016282 to L.J.M., NINDS NS 060703 to L.J.M., NINDS NS 052098 to L.J.M.), and by a grant from the Broccoli Foundation (to F.J.N.).
Potential Conflict of Interest F.J.N. has received grants from the March of Dimes and the NIH; was a member of the board and a question writer for the Neonatal Subboard of American Board of Pediatrics; has grant(s) pending from the NIH; and received a lecture honorarium from the American Academy of Pediatrics. L.J.M. received grants from the NIH NIA and NINDS.
References
- 1.Folkerth RD. Neuropathologic substrate of cerebral palsy. J Child Neurol. 2005;20:940–949. doi: 10.1177/08830738050200120301. [DOI] [PubMed] [Google Scholar]
- 2.Towfighi J, Zec N, Yager J, et al. Temporal evolution of neuropathologic changes in an immature rat model of cerebral hypoxia: a light microscopic study. Acta Neuropathol. 1995;90:375–386. doi: 10.1007/BF00315011. [DOI] [PubMed] [Google Scholar]
- 3.Myers RE. Four patterns of perinatal brain damage and their conditions of occurrence in primates. Adv Neurol. 1975;10:223–234. [PubMed] [Google Scholar]
- 4.Adamsons K, Myers RE. Perinatal asphyxia, causes, detection and neurologic sequelae. Pediatr Clin North Am. 1973;20:465–480. doi: 10.1016/s0031-3955(16)32855-3. [DOI] [PubMed] [Google Scholar]
- 5.Carloni S, Carnevali A, Cimino M, Balduini W. Extended role of necrotic cell death after hypoxia-ischemia-induced neurodegeneration in the neonatal rat. Neurobiol Dis. 2007;27:354–361. doi: 10.1016/j.nbd.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 6.Blomgren K, Leist M, Groc L. Pathological apoptosis in the developing brain. Apoptosis. 2007;12:993–1010. doi: 10.1007/s10495-007-0754-4. [DOI] [PubMed] [Google Scholar]
- 7.Northington FJ, Zelaya ME, O’Riordan DP, et al. Failure to complete apoptosis following neonatal hypoxia-ischemia manifests as “continuum” phenotype of cell death and occurs with multiple manifestations of mitochondrial dysfunction in rodent forebrain. Neuroscience. 2007;149:822–833. doi: 10.1016/j.neuroscience.2007.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakajima W, Ishida A, Lange MS, et al. Apoptosis has a prolonged role in the neurodegeneration after hypoxic ischemia in the newborn rat. J Neurosci. 2000;20:7994–8004. doi: 10.1523/JNEUROSCI.20-21-07994.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Northington FJ, Ferriero DM, Graham EM, et al. Early neurodegeneration after hypoxia-ischemia in neonatal rat is necrosis while delayed neuronal death is apoptosis. Neurobiol Dis. 2001;8:207–219. doi: 10.1006/nbdi.2000.0371. [DOI] [PubMed] [Google Scholar]
- 10.Northington FJ, Chavez-Valdez R, Graham EM, et al. Necrostatin decreases oxidative damage, inflammation, and injury after neonatal HI. J Cereb Blood Flow Metab. 2011;31:178–189. doi: 10.1038/jcbfm.2010.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Degterev A, Hitomi J, Germscheid M, et al. Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat Chem Biol. 2008;4:313–321. doi: 10.1038/nchembio.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Degterev A, Huang Z, Boyce M, et al. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol. 2005;1:112–119. doi: 10.1038/nchembio711. [DOI] [PubMed] [Google Scholar]
- 13.Martin LJ, Brambrink AM, Price AC, et al. Neuronal death in newborn striatum after hypoxia-ischemia is necrosis and evolves with oxidative stress. Neurobiol Dis. 2000;7:169–191. doi: 10.1006/nbdi.2000.0282. [DOI] [PubMed] [Google Scholar]
- 14.Trump BF, Berezesky IK. The role of altered [Ca2+]i regulation in apoptosis, oncosis, and necrosis. Biochim Biophys Acta. 1996;1313:173–178. doi: 10.1016/0167-4889(96)00086-9. [DOI] [PubMed] [Google Scholar]
- 15.Trump BF, Goldblatt PJ, Stowell RE. Studies on necrosis of mouse liver in vitro. ultrastructural alterations in the mitochondria of hepatic parenchymal cells. Lab Invest. 1965;14:343–371. [PubMed] [Google Scholar]
- 16.Bonfoco E, Krainc D, Ankarcrona M, et al. Apoptosis and necrosis: two distinct events induced, respectively, by mild and intense insults with N-methyl-D-aspartate or nitric oxide/superoxide in cortical cell cultures. Proc Natl Acad Sci U S A. 1995;92:7162–7166. doi: 10.1073/pnas.92.16.7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leist M, Single B, Castoldi AF, et al. Intracellular adenosine tri-phosphate (ATP) concentration: a switch in the decision between apoptosis and necrosis. J Exp Med. 1997;185:1481–1486. doi: 10.1084/jem.185.8.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Golden WC, Brambrink AM, Traystman RJ, Martin LJ. Failure to sustain recovery of Na,K-ATPase function is a possible mechanism for striatal neurodegeneration in hypoxic-ischemic newborn piglets. Brain Res Mol Brain Res. 2001;88:94–102. doi: 10.1016/s0169-328x(01)00032-8. [DOI] [PubMed] [Google Scholar]
- 19.Proskuryakov SY, Konoplyannikov AG, Gabai VL. Necrosis: a specific form of programmed cell death? Exp Cell Res. 2003;283:1–16. doi: 10.1016/s0014-4827(02)00027-7. [DOI] [PubMed] [Google Scholar]
- 20.Festjens N, Vanden Berghe T, Vandenabeele P. Necrosis, a well-orchestrated form of cell demise: signalling cascades, important mediators and concomitant immune response. Biochim Biophys Acta. 2006;1757:1371–1387. doi: 10.1016/j.bbabio.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 21.Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kerr JF, Gobe GC, Winterford CM, Harmon BV. Anatomical methods in cell death. Methods Cell Biol. 1995;46:1–27. doi: 10.1016/s0091-679x(08)61921-4. [DOI] [PubMed] [Google Scholar]
- 23.Tata JR. Requirement for RNA and protein synthesis for induced regression of the tadpole tail in organ culture. Dev Biol. 1966;13:77–94. doi: 10.1016/0012-1606(66)90050-9. [DOI] [PubMed] [Google Scholar]
- 24.Clarke PG. Developmental cell death: morphological diversity and multiple mechanisms. Anat Embryol (Berl) 1990;181:195–213. doi: 10.1007/BF00174615. [DOI] [PubMed] [Google Scholar]
- 25.Lockshin RA, Zakeri Z. Caspase-independent cell deaths. Curr Opin Cell Biol. 2002;14:727–733. doi: 10.1016/s0955-0674(02)00383-6. [DOI] [PubMed] [Google Scholar]
- 26.Holler N, Zaru R, Micheau O, et al. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat Immunol. 2000;1:489–495. doi: 10.1038/82732. [DOI] [PubMed] [Google Scholar]
- 27.Schwartz LM, Smith SW, Jones ME, Osborne BA. Do all programmed cell deaths occur via apoptosis? Proc Natl Acad Sci U S A. 1993;90:980–984. doi: 10.1073/pnas.90.3.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Amin F, Bowen ID, Szegedi Z, et al. Apoptotic and non-apoptotic modes of programmed cell death in MCF-7 human breast carcinoma cells. Cell Biol Int. 2000;24:253–260. doi: 10.1006/cbir.2000.0495. [DOI] [PubMed] [Google Scholar]
- 29.Pilar G, Landmesser L. Ultrastructural differences during embryonic cell death in normal and peripherally deprived ciliary ganglia. J Cell Biol. 1976;68:339–356. doi: 10.1083/jcb.68.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Portera-Cailliau C, Price DL, Martin LJ. Non-NMDA and NMDA receptor-mediated excitotoxic neuronal deaths in adult brain are morphologically distinct: further evidence for an apoptosis-necrosis continuum. J Comp Neurol. 1997;378:88–104. [PubMed] [Google Scholar]
- 31.Portera-Cailliau C, Price DL, Martin LJ. Excitotoxic neuronal death in the immature brain is an apoptosis-necrosis morphological continuum. J Comp Neurol. 1997;378:70–87. [PubMed] [Google Scholar]
- 32.Martin LJ, Al-Abdulla NA, Brambrink AM, et al. Neurodegeneration in excitotoxicity, global cerebral ischemia, and target deprivation: a perspective on the contributions of apoptosis and necrosis. Brain Res Bull. 1998;46:281–309. doi: 10.1016/s0361-9230(98)00024-0. [DOI] [PubMed] [Google Scholar]
- 33.Northington FJ, Ferriero DM, Flock DL, Martin LJ. Delayed neurodegeneration in neonatal rat thalamus after hypoxia-ischemia is apoptosis. J Neurosci. 2001;21:1931–1938. doi: 10.1523/JNEUROSCI.21-06-01931.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klionsky DJ, Emr SD. Autophagy as a regulated pathway of cellular degradation. Science. 2000;290:1717–1721. doi: 10.1126/science.290.5497.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lockshin RA, Zakeri Z. Programmed cell death: early changes in metamorphosing cells. Biochem Cell Biol. 1994;72:589–596. doi: 10.1139/o94-078. [DOI] [PubMed] [Google Scholar]
- 36.Schweichel JU, Merker HJ. The morphology of various types of cell death in prenatal tissues. Teratology. 1973;7:253–266. doi: 10.1002/tera.1420070306. [DOI] [PubMed] [Google Scholar]
- 37.Xue L, Fletcher GC, Tolkovsky AM. Autophagy is activated by apoptotic signalling in sympathetic neurons: an alternative mechanism of death execution. Mol Cell Neurosci. 1999;14:180–198. doi: 10.1006/mcne.1999.0780. [DOI] [PubMed] [Google Scholar]
- 38.Yue Z, Horton A, Bravin M, et al. A novel protein complex linking the delta 2 glutamate receptor and autophagy: implications for neurodegeneration in lurcher mice. Neuron. 2002;35:921–933. doi: 10.1016/s0896-6273(02)00861-9. [DOI] [PubMed] [Google Scholar]
- 39.Yuan J, Lipinski M, Degterev A. Diversity in the mechanisms of neuronal cell death. Neuron. 2003;40:401–413. doi: 10.1016/s0896-6273(03)00601-9. [DOI] [PubMed] [Google Scholar]
- 40.Mizushima N, Ohsumi Y, Yoshimori T. Autophagosome formation in mammalian cells. Cell Struct Funct. 2002;27:421–429. doi: 10.1247/csf.27.421. [DOI] [PubMed] [Google Scholar]
- 41.Bursch W. The autophagosomal-lysosomal compartment in programmed cell death. Cell Death Differ. 2001;8:569–581. doi: 10.1038/sj.cdd.4400852. [DOI] [PubMed] [Google Scholar]
- 42.Inbal B, Bialik S, Sabanay I, et al. DAP kinase and DRP-1 mediate membrane blebbing and the formation of autophagic vesicles during programmed cell death. J Cell Biol. 2002;157:455–468. doi: 10.1083/jcb.200109094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ginet V, Puyal J, Clarke PG, Truttmann AC. Enhancement of autophagic flux after neonatal cerebral hypoxia-ischemia and its region-specific relationship to apoptotic mechanisms. Am J Pathol. 2009;175:1962–1974. doi: 10.2353/ajpath.2009.090463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Koike M, Shibata M, Tadakoshi M, et al. Inhibition of autophagy prevents hippocampal pyramidal neuron death after hypoxicischemic injury. Am J Pathol. 2008;172:454–469. doi: 10.2353/ajpath.2008.070876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Puyal J, Vaslin A, Mottier V, Clarke PG. Postischemic treatment of neonatal cerebral ischemia should target autophagy. Ann Neurol. 2009;66:378–389. doi: 10.1002/ana.21714. [DOI] [PubMed] [Google Scholar]
- 46.Carloni S, Buonocore G, Balduini W. Protective role of autophagy in neonatal hypoxia-ischemia induced brain injury. Neurobiol Dis. 2008;32:329–339. doi: 10.1016/j.nbd.2008.07.022. [DOI] [PubMed] [Google Scholar]
- 47.Johnston MV. Neurotransmitter alterations in a model of perinatal hypoxic-ischemic brain injury. Ann Neurol. 1983;13:511–518. doi: 10.1002/ana.410130507. [DOI] [PubMed] [Google Scholar]
- 48.McDonald JW, Silverstein FS, Johnston MV. Neurotoxicity of N-methyl-D-aspartate is markedly enhanced in developing rat central nervous system. Brain Res. 1988;459:200–203. doi: 10.1016/0006-8993(88)90306-x. [DOI] [PubMed] [Google Scholar]
- 49.McDonald JW, Trescher WH, Johnston MV. Susceptibility of brain to AMPA induced excitotoxicity transiently peaks during early postnatal development. Brain Res. 1992;583:54–70. doi: 10.1016/s0006-8993(10)80009-5. [DOI] [PubMed] [Google Scholar]
- 50.Choi DW. Excitotoxic cell death. J Neurobiol. 1992;23:1261–1276. doi: 10.1002/neu.480230915. [DOI] [PubMed] [Google Scholar]
- 51.Lipton SA, Rosenberg PA. Excitatory amino acids as a final common pathway for neurologic disorders. N Engl J Med. 1994;330:613–622. doi: 10.1056/NEJM199403033300907. [DOI] [PubMed] [Google Scholar]
- 52.Olney JW. Glutamate-induced neuronal necrosis in the infant mouse hypothalamus. An electron microscopic study. J Neuropathol Exp Neurol. 1971;30:75–90. doi: 10.1097/00005072-197101000-00008. [DOI] [PubMed] [Google Scholar]
- 53.van Lookeren Campagne M, Lucassen PJ, Vermeulen JP, Balazs R. NMDA and kainate induce internucleosomal DNA cleavage associated with both apoptotic and necrotic cell death in the neonatal rat brain. Eur J Neurosci. 1995;7:1627–1640. doi: 10.1111/j.1460-9568.1995.tb01158.x. [DOI] [PubMed] [Google Scholar]
- 54.Martin LJ. Neuronal death in amyotrophic lateral sclerosis is apoptosis: possible contribution of a programmed cell death mechanism. J Neuropathol Exp Neurol. 1999;58:459–471. doi: 10.1097/00005072-199905000-00005. [DOI] [PubMed] [Google Scholar]
- 55.Metzstein MM, Stanfield GM, Horvitz HR. Genetics of programmed cell death in C. elegans: past, present and future. Trends Genet. 1998;14:410–416. doi: 10.1016/s0168-9525(98)01573-x. [DOI] [PubMed] [Google Scholar]
- 56.Cory S, Adams JM. The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer. 2002;2:647–656. doi: 10.1038/nrc883. [DOI] [PubMed] [Google Scholar]
- 57.Muchmore SW, Sattler M, Liang H, et al. X-ray and NMR structure of human Bcl-xL, an inhibitor of programmed cell death. Nature. 1996;381:335–341. doi: 10.1038/381335a0. [DOI] [PubMed] [Google Scholar]
- 58.Zhu C, Hallin U, Ozaki Y, et al. Nuclear translocation and calpain-dependent reduction of Bcl-2 after neonatal cerebral hypoxiaischemia. Brain Behav Immun. 2010;24:822–830. doi: 10.1016/j.bbi.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 59.Lok J, Martin LJ. Rapid subcellular redistribution of Bax precedes caspase-3 and endonuclease activation during excitotoxic neuronal apoptosis in rat brain. J Neurotrauma. 2002;19:815–828. doi: 10.1089/08977150260190410. [DOI] [PubMed] [Google Scholar]
- 60.Wang X, Carlsson Y, Basso E, et al. Developmental shift of cyclophilin D contribution to hypoxic-ischemic brain injury. J Neurosci. 2009;29:2588–2596. doi: 10.1523/JNEUROSCI.5832-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Parsadanian AS, Cheng Y, Keller-Peck CR, et al. Bcl-xL is an anti-apoptotic regulator for postnatal CNS neurons. J Neurosci. 1998;18:1009–1019. doi: 10.1523/JNEUROSCI.18-03-01009.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gibson ME, Han BH, Choi J, et al. BAX contributes to apoptotic-like death following neonatal hypoxia-ischemia: evidence for distinct apoptosis pathways. Mol Med. 2001;7:644–655. [PMC free article] [PubMed] [Google Scholar]
- 63.Ness JM, Harvey CA, Strasser A, et al. Selective involvement of BH3-only Bcl-2 family members Bim and Bad in neonatal hypoxia-ischemia. Brain Res. 2006;1099:150–159. doi: 10.1016/j.brainres.2006.04.132. [DOI] [PubMed] [Google Scholar]
- 64.Wolf BB, Green DR. Suicidal tendencies: apoptotic cell death by caspase family proteinases. J Biol Chem. 1999;274:20049–20052. doi: 10.1074/jbc.274.29.20049. [DOI] [PubMed] [Google Scholar]
- 65.Stennicke HR, Deveraux QL, Humke EW, et al. Caspase-9 can be activated without proteolytic processing. J Biol Chem. 1999;274:8359–8362. doi: 10.1074/jbc.274.13.8359. [DOI] [PubMed] [Google Scholar]
- 66.Schwartz LM, Milligan CE. Cold thoughts of death: the role of ICE proteases in neuronal cell death. Trends Neurosci. 1996;19:555–562. doi: 10.1016/s0166-2236(96)10067-9. [DOI] [PubMed] [Google Scholar]
- 67.Li P, Nijhawan D, Budihardjo I, et al. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 68.Zou H, Li Y, Liu X, Wang X. An APAF-1.cytochrome c multimeric complex is a functional apoptosome that activates procaspase-9. J Biol Chem. 1999;274:11549–11556. doi: 10.1074/jbc.274.17.11549. [DOI] [PubMed] [Google Scholar]
- 69.Liu X, Zou H, Slaughter C, Wang X. DFF, a heterodimeric protein that functions downstream of caspase-3 to trigger DNA fragmentation during apoptosis. Cell. 1997;89:175–184. doi: 10.1016/s0092-8674(00)80197-x. [DOI] [PubMed] [Google Scholar]
- 70.Liu X, Kim CN, Yang J, et al. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell. 1996;86:147–157. doi: 10.1016/s0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- 71.Li H, Zhu H, Xu CJ, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94:491–501. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- 72.Hu BR, Liu CL, Ouyang Y, et al. Involvement of caspase-3 in cell death after hypoxia-ischemia declines during brain maturation. J Cereb Blood Flow Metab. 2000;20:1294–1300. doi: 10.1097/00004647-200009000-00003. [DOI] [PubMed] [Google Scholar]
- 73.Wang X, Karlsson JO, Zhu C, et al. Caspase-3 activation after neonatal rat cerebral hypoxia-ischemia. Biol Neonate. 2001;79:172–179. doi: 10.1159/000047087. [DOI] [PubMed] [Google Scholar]
- 74.Zhu C, Qiu L, Wang X, et al. Involvement of apoptosis-inducing factor in neuronal death after hypoxia-ischemia in the neonatal rat brain. J Neurochem. 2003;86:306–317. doi: 10.1046/j.1471-4159.2003.01832.x. [DOI] [PubMed] [Google Scholar]
- 75.Robertson JD, Enoksson M, Suomela M, et al. Caspase-2 acts upstream of mitochondria to promote cytochrome c release during etoposide-induced apoptosis. J Biol Chem. 2002;277:29803–29809. doi: 10.1074/jbc.M204185200. [DOI] [PubMed] [Google Scholar]
- 76.Kaindl AM, Sifringer M, Koppelstaetter A, et al. Erythropoietin protects the developing brain from hyperoxia-induced cell death and proteome changes. Ann Neurol. 2008;64:523–534. doi: 10.1002/ana.21471. [DOI] [PubMed] [Google Scholar]
- 77.Blomgren K, Zhu C, Wang X, et al. Synergistic activation of caspase-3 by m-calpain after neonatal hypoxia- ischemia: a mechanism of “pathological apoptosis”? J Biol Chem. 2001;276:10191–10198. doi: 10.1074/jbc.M007807200. [DOI] [PubMed] [Google Scholar]
- 78.Felderhoff-Mueser U, Sifringer M, Pesditschek S, et al. Pathways leading to apoptotic neurodegeneration following trauma to the developing rat brain. Neurobiol Dis. 2002;11:231–245. doi: 10.1006/nbdi.2002.0521. [DOI] [PubMed] [Google Scholar]
- 79.Cheng Y, Deshmukh M, D’Costa A, et al. Caspase inhibitor affords neuroprotection with delayed administration in a rat model of neonatal hypoxic-ischemic brain injury. J Clin Invest. 1998;101:1992–1999. doi: 10.1172/JCI2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Delivoria-Papadopoulos M, Ashraf QM, Ara J, Mishra OP. Nuclear mechanisms of hypoxic cerebral injury in the newborn: the role of caspases. Semin Perinatol. 2008;32:334–343. doi: 10.1053/j.semperi.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 81.Zhu C, Wang X, Xu F, et al. The influence of age on apoptotic and other mechanisms of cell death after cerebral hypoxia-ischemia. Cell Death Differ. 2005;12:162–176. doi: 10.1038/sj.cdd.4401545. [DOI] [PubMed] [Google Scholar]
- 82.Lesuisse C, Martin LJ. Immature and mature cortical neurons engage different apoptotic mechanisms involving caspase-3 and the mitogen-activated protein kinase pathway. J Cereb Blood Flow Metab. 2002;22:935–950. doi: 10.1097/00004647-200208000-00005. [DOI] [PubMed] [Google Scholar]
- 83.Feng Y, LeBlanc MH. Treatment of hypoxic-ischemic brain injury in newborn rats with TPCK 3 h after hypoxia decreases caspase-9 activation and improves neuropathologic outcome. Dev Neurosci. 2003;25:34–40. doi: 10.1159/000071466. [DOI] [PubMed] [Google Scholar]
- 84.Feng Y, Fratkin JD, LeBlanc MH. Inhibiting caspase-9 after injury reduces hypoxic ischemic neuronal injury in the cortex in the newborn rat. Neurosci Lett. 2003;344:201–204. doi: 10.1016/s0304-3940(03)00466-x. [DOI] [PubMed] [Google Scholar]
- 85.Feng Y, Fratkin JD, LeBlanc MH. Inhibiting caspase-8 after injury reduces hypoxic-ischemic brain injury in the newborn rat. Eur J Pharmacol. 2003;481:169–173. doi: 10.1016/j.ejphar.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 86.Rozman-Pungercar J, Kopitar-Jerala N, Bogyo M, et al. Inhibition of papain-like cysteine proteases and legumain by caspase-specific inhibitors: when reaction mechanism is more important than specificity. Cell Death Differ. 2003;10:881–888. doi: 10.1038/sj.cdd.4401247. [DOI] [PubMed] [Google Scholar]
- 87.Ostwald K, Hagberg H, Andine P, Karlsson JO. Upregulation of calpain activity in neonatal rat brain after hypoxic-ischemia. Brain Res. 1993;630:289–294. doi: 10.1016/0006-8993(93)90668-d. [DOI] [PubMed] [Google Scholar]
- 88.Kawamura M, Nakajima W, Ishida A, et al. Calpain inhibitor MDL 28170 protects hypoxic-ischemic brain injury in neonatal rats by inhibition of both apoptosis and necrosis. Brain Res. 2005;1037:59–69. doi: 10.1016/j.brainres.2004.12.050. [DOI] [PubMed] [Google Scholar]
- 89.Han Y, Giroux A, Colucci J, et al. Novel pyrazinone mono-amides as potent and reversible caspase-3 inhibitors. Bioorg Med Chem Lett. 2005;15:1173–1180. doi: 10.1016/j.bmcl.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 90.Han BH, Xu D, Choi J, et al. Selective, reversible caspase-3 inhibitor is neuroprotective and reveals distinct pathways of cell death after neonatal hypoxic-ischemic brain injury. J Biol Chem. 2002;277:30128–30136. doi: 10.1074/jbc.M202931200. [DOI] [PubMed] [Google Scholar]
- 91.West T, Atzeva M, Holtzman DM. Caspase-3 deficiency during development increases vulnerability to hypoxic-ischemic injury through caspase-3-independent pathways. Neurobiol Dis. 2006;22:523–537. doi: 10.1016/j.nbd.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 92.Beresford PJ, Zhang D, Oh DY, et al. Granzyme A activates an endoplasmic reticulum-associated caspase-independent nuclease to induce single-stranded DNA nicks. J Biol Chem. 2001;276:43285–43293. doi: 10.1074/jbc.M108137200. [DOI] [PubMed] [Google Scholar]
- 93.Fan Z, Beresford PJ, Oh DY, et al. Tumor suppressor NM23-H1 is a granzyme A-activated DNase during CTL-mediated apoptosis, and the nucleosome assembly protein SET is its inhibitor. Cell. 2003;112:659–672. doi: 10.1016/s0092-8674(03)00150-8. [DOI] [PubMed] [Google Scholar]
- 94.Gulyaeva NV. Non-apoptotic functions of caspase-3 in nervous tissue. Biochemistry (Mosc) 2003;68:1171–1180. doi: 10.1023/b:biry.0000009130.62944.35. [DOI] [PubMed] [Google Scholar]
- 95.Lu C, Fu W, Salvesen GS, Mattson MP. Direct cleavage of AMPA receptor subunit GluR1 and suppression of AMPA currents by caspase-3: implications for synaptic plasticity and excitotoxic neuronal death. Neuromolecular Med. 2002;1:69–79. doi: 10.1385/NMM:1:1:69. [DOI] [PubMed] [Google Scholar]
- 96.Yan XX, Najbauer J, Woo CC, et al. Expression of active caspase-3 in mitotic and postmitotic cells of the rat forebrain. J Comp Neurol. 2001;433:4–22. doi: 10.1002/cne.1121. [DOI] [PubMed] [Google Scholar]
- 97.Vandenabeele P, Declercq W, Van Herreweghe F, Vanden Berghe T. The role of the kinases RIP1 and RIP3 in TNF-induced necrosis. Sci Signal. 2010;3:re4. doi: 10.1126/scisignal.3115re4. [DOI] [PubMed] [Google Scholar]
- 98.Graham EM, Sheldon RA, Flock DL, et al. Neonatal mice lacking functional Fas death receptors are resistant to hypoxic-ischemic brain injury. Neurobiol Dis. 2004;17:89–98. doi: 10.1016/j.nbd.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 99.Dzietko M, Boos V, Sifringer M, et al. A critical role for Fas/CD-95 dependent signaling pathways in the pathogenesis of hyper-oxia-induced brain injury. Ann Neurol. 2008;64:664–673. doi: 10.1002/ana.21516. [DOI] [PubMed] [Google Scholar]
- 100.Levrero M, De Laurenzi V, Costanzo A, et al. The p53/p63/p73 family of transcription factors: overlapping and distinct functions. J Cell Sci. 2000;113(Pt 10):1661–1670. doi: 10.1242/jcs.113.10.1661. [DOI] [PubMed] [Google Scholar]
- 101.Martin LJ, Chen K, Liu Z. Adult motor neuron apoptosis is mediated by nitric oxide and Fas death receptor linked by DNA damage and p53 activation. J Neurosci. 2005;25:6449–6459. doi: 10.1523/JNEUROSCI.0911-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tan Z, Levid J, Schreiber SS. Increased expression of Fas (CD95/APO-1) in adult rat brain after kainate-induced seizures. Neuroreport. 2001;12:1979–1982. doi: 10.1097/00001756-200107030-00040. [DOI] [PubMed] [Google Scholar]
- 103.van Lookeren C, Gill R. Tumor-suppressor p53 is expressed in proliferating and newly formed neurons of the embryonic and postnatal rat brain: comparison with expression of the cell cycle regulators p21Waf1/Cip1, p27Kip1, p57Kip2, p16Ink4a, cyclin G1, and the proto-oncogene Bax. J Comp Neurol. 1998;397:181–198. doi: 10.1002/(sici)1096-9861(19980727)397:2<181::aid-cne3>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 104.Nijboer CH, Heijnen CJ, Groenendaal F, et al. A dual role of the NF-kappaB pathway in neonatal hypoxic-ischemic brain damage. Stroke. 2008;39:2578–2586. doi: 10.1161/STROKEAHA.108.516401. [DOI] [PubMed] [Google Scholar]
- 105.Martin LJ, Kaiser A, Yu JW, et al. Injury-induced apoptosis of neurons in adult brain is mediated by p53-dependent and p53-independent pathways and requires Bax. J Comp Neurol. 2001;433:299–311. doi: 10.1002/cne.1141. [DOI] [PubMed] [Google Scholar]
- 106.Martin LJ, Liu Z. Injury-induced spinal motor neuron apoptosis is preceded by DNA single-strand breaks and is p53- and Bax-dependent. J Neurobiol. 2002;50:181–197. doi: 10.1002/neu.10026. [DOI] [PubMed] [Google Scholar]
- 107.Calvert JW, Cahill J, Yamaguchi-Okada M, Zhang JH. Oxygen treatment after experimental hypoxia-ischemia in neonatal rats alters the expression of HIF-1alpha and its downstream target genes. J Appl Physiol. 2006;101:853–865. doi: 10.1152/japplphysiol.00268.2006. [DOI] [PubMed] [Google Scholar]
- 108.Nijboer CH, Heijnen CJ, Groenendaal F, et al. Strong neuroprotection by inhibition of NF-kappaB after neonatal hypoxia-ischemia involves apoptotic mechanisms but is independent of cytokines. Stroke. 2008;39:2129–2137. doi: 10.1161/STROKEAHA.107.504175. [DOI] [PubMed] [Google Scholar]
- 109.Natale JE, Cheng Y, Martin LJ. Thalamic neuron apoptosis emerges rapidly after cortical damage in immature mice. Neuroscience. 2002;112:665–676. doi: 10.1016/s0306-4522(02)00098-2. [DOI] [PubMed] [Google Scholar]
- 110.Martin LJ. Neuronal cell death in nervous system development, disease, and injury (Review) Int J Mol Med. 2001;7:455–478. [PubMed] [Google Scholar]
- 111.Sheldon RA, Hall JJ, Noble LJ, Ferriero DM. Delayed cell death in neonatal mouse hippocampus from hypoxia-ischemia is neither apoptotic nor necrotic. Neurosci Lett. 2001;304:165–168. doi: 10.1016/s0304-3940(01)01788-8. [DOI] [PubMed] [Google Scholar]
- 112.Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Hum Dev. 1979;3:79–83. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- 113.Rothstein RP, Levison SW. Gray matter oligodendrocyte progenitors and neurons die caspase-3 mediated deaths subsequent to mild perinatal hypoxic/ischemic insults. Dev Neurosci. 2005;27:149–159. doi: 10.1159/000085987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mueller D, Shamblott MJ, Fox HE, et al. Transplanted human embryonic germ cell-derived neural stem cells replace neurons and oligodendrocytes in the forebrain of neonatal mice with excitotoxic brain damage. J Neurosci Res. 2005;82:592–608. doi: 10.1002/jnr.20673. [DOI] [PubMed] [Google Scholar]
- 115.Martin LJ, Brambrink AM, Lehmann C, et al. Hypoxia-ischemia causes abnormalities in glutamate transporters and death of astroglia and neurons in newborn striatum. Ann Neurol. 1997;42:335–348. doi: 10.1002/ana.410420310. [DOI] [PubMed] [Google Scholar]
- 116.Maiuri MC, Zalckvar E, Kimchi A, Kroemer G. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol. 2007;8:741–752. doi: 10.1038/nrm2239. [DOI] [PubMed] [Google Scholar]
- 117.Ferriero DM. Timing is everything—delaying therapy for delayed cell death. Dev Neurosci. 2002;24:349–351. doi: 10.1159/000069048. [DOI] [PubMed] [Google Scholar]
- 118.Orrenius S, Zhivotovsky B, Nicotera P. Regulation of cell death: the calcium-apoptosis link. Nat Rev Mol Cell Biol. 2003;4:552–565. doi: 10.1038/nrm1150. [DOI] [PubMed] [Google Scholar]
- 119.Yamashima T. Implication of cysteine proteases calpain, cathepsin and caspase in ischemic neuronal death of primates. Prog Neurobiol. 2000;62:273–295. doi: 10.1016/s0301-0082(00)00006-x. [DOI] [PubMed] [Google Scholar]
- 120.Joly LM, Mucignat V, Mariani J, et al. Caspase inhibition after neonatal ischemia in the rat brain. J Cereb Blood Flow Metab. 2004;24:124–131. doi: 10.1097/01.WCB.0000100061.36077.5F. [DOI] [PubMed] [Google Scholar]
- 121.Nakagawa T, Yuan J. Cross-talk between two cysteine protease families. Activation of caspase-12 by calpain in apoptosis. J Cell Biol. 2000;150:887–894. doi: 10.1083/jcb.150.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Fischer H, Koenig U, Eckhart L, Tschachler E. Human caspase 12 has acquired deleterious mutations. Biochem Biophys Res Commun. 2002;293:722–726. doi: 10.1016/S0006-291X(02)00289-9. [DOI] [PubMed] [Google Scholar]
- 123.Scorrano L, Oakes SA, Opferman JT, et al. BAX and BAK regulation of endoplasmic reticulum Ca2+: a control point for apoptosis. Science. 2003;300:135–139. doi: 10.1126/science.1081208. [DOI] [PubMed] [Google Scholar]
- 124.Chen Y, Lewis W, Diwan A, et al. Dual autonomous mitochondrial cell death pathways are activated by Nix/BNip3L and induce cardiomyopathy. Proc Natl Acad Sci U S A. 2010;107:9035–9042. doi: 10.1073/pnas.0914013107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lin Y, Devin A, Rodriguez Y, Liu ZG. Cleavage of the death domain kinase RIP by caspase-8 prompts TNF-induced apoptosis. Genes Dev. 1999;13:2514–2526. doi: 10.1101/gad.13.19.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Festjens N, Vanden Berghe T, Cornelis S, Vandenabeele P. RIP1, a kinase on the crossroads of a cell’s decision to live or die. Cell Death Differ. 2007;14:400–410. doi: 10.1038/sj.cdd.4402085. [DOI] [PubMed] [Google Scholar]
- 127.Temkin V, Huang Q, Liu H, et al. Inhibition of ADP/ATP exchange in receptor-interacting protein-mediated necrosis. Mol Cell Biol. 2006;26:2215–2225. doi: 10.1128/MCB.26.6.2215-2225.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Martin LJ. The mitochondrial permeability transition pore: a molecular target for amyotrophic lateral sclerosis therapy. Biochim Biophys Acta. 2010;1802:186–197. doi: 10.1016/j.bbadis.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lian J, Wu X, He F, et al. A natural BH3 mimetic induces autophagy in apoptosis-resistant prostate cancer via modulating Bcl-2-Beclin1 interaction at endoplasmic reticulum. Cell Death Differ. 2011;18:60–71. doi: 10.1038/cdd.2010.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ogier-Denis E, Codogno P. Autophagy: a barrier or an adaptive response to cancer. Biochim Biophys Acta. 2003;1603:113–128. doi: 10.1016/s0304-419x(03)00004-0. [DOI] [PubMed] [Google Scholar]
- 131.Yu L, Lenardo MJ, Baehrecke EH. Autophagy and caspases: a new cell death program. Cell Cycle. 2004;3:1124–1126. [PubMed] [Google Scholar]
- 132.Yu L, Alva A, Su H, et al. Regulation of an ATG7-beclin 1 program of autophagic cell death by caspase-8. Science. 2004;304:1500–1502. doi: 10.1126/science.1096645. [DOI] [PubMed] [Google Scholar]
- 133.Martin LJ, Portera-Cailliau C, Ginsberg SD, Al-Abdulla NA. Animal models and degenerative disorders of the human brain. Lab Animal. 1998;27:18–25. [Google Scholar]
- 134.Bialik S, Zalckvar E, Ber Y, et al. Systems biology analysis of programmed cell death. Trends Biochem Sci. 2010;35:556–564. doi: 10.1016/j.tibs.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 135.Martin LJ. The apoptosis-necrosis cell death continuum in CNS development, injury and disease: contributions and mechanisms. In: Lo EH, Marwah J, editors. Neuroprotection. Prominent Press; Boston: 2002. pp. 379–412. [Google Scholar]
- 136.Crompton M. The mitochondrial permeability transition pore and its role in cell death. Biochem J. 1999;341(Pt 2):233–249. [PMC free article] [PubMed] [Google Scholar]
- 137.Zhu C, Xu F, Wang X, et al. Different apoptotic mechanisms are activated in male and female brains after neonatal hypoxiaischaemia. J Neurochem. 2006;96:1016–1027. doi: 10.1111/j.1471-4159.2005.03639.x. [DOI] [PubMed] [Google Scholar]
- 138.Martin LJ, Gertz B, Pan Y, et al. The mitochondrial permeability transition pore in motor neurons: involvement in the pathobiology of ALS mice. Exp Neurol. 2009;218:333–346. doi: 10.1016/j.expneurol.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Martin LJ, Kaiser A, Price AC. Motor neuron degeneration after sciatic nerve avulsion in adult rat evolves with oxidative stress and is apoptosis. J Neurobiol. 1999;40:185–201. [PubMed] [Google Scholar]
- 140.Baines CP, Kaiser RA, Purcell NH, et al. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–662. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- 141.Martin LJ, Liu Z, Chen K, et al. Motor neuron degeneration in amyotrophic lateral sclerosis mutant superoxide dismutase-1 transgenic mice: mechanisms of mitochondriopathy and cell death. J Comp Neurol. 2007;500:20–46. doi: 10.1002/cne.21160. [DOI] [PubMed] [Google Scholar]
- 142.Zhang DW, Shao J, Lin J, et al. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science. 2009;325:332–336. doi: 10.1126/science.1172308. [DOI] [PubMed] [Google Scholar]
- 143.Liu Z, Martin LJ. Isolation of mature spinal motor neurons and single-cell analysis using the comet assay of early low-level DNA damage induced in vitro and in vivo. J Histochem Cytochem. 2001;49:957–972. doi: 10.1177/002215540104900804. [DOI] [PubMed] [Google Scholar]
- 144.Strozyk E, Poppelmann B, Schwarz T, Kulms D. Differential effects of NF-kappaB on apoptosis induced by DNA-damaging agents: the type of DNA damage determines the final outcome. Oncogene. 2006;25:6239–6251. doi: 10.1038/sj.onc.1209655. [DOI] [PubMed] [Google Scholar]
- 145.Ishimaru MJ, Ikonomidou C, Tenkova TI, et al. Distinguishing excitotoxic from apoptotic neurodegeneration in the developing rat brain. J Comp Neurol. 1999;408:461–476. [PubMed] [Google Scholar]
- 146.Henriquez M, Armisen R, Stutzin A, Quest AF. Cell death by necrosis, a regulated way to go. Curr Mol Med. 2008;8:187–206. doi: 10.2174/156652408784221289. [DOI] [PubMed] [Google Scholar]
- 147.Bredesen DE. Programmed cell death mechanisms in neurological disease. Curr Mol Med. 2008;8:173–186. doi: 10.2174/156652408784221315. [DOI] [PubMed] [Google Scholar]
- 148.Nagley P, Higgins GC, Atkin JD, Beart PM. Multifaceted deaths orchestrated by mitochondria in neurones. Biochim Biophys Acta. 2010;1802:167–185. doi: 10.1016/j.bbadis.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 149.You Z, Savitz SI, Yang J, et al. Necrostatin-1 reduces histopathology and improves functional outcome after controlled cortical impact in mice. J Cereb Blood Flow Metab. 2008;28:1564–1573. doi: 10.1038/jcbfm.2008.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lim SY, Davidson SM, Mocanu MM, et al. The cardioprotective effect of necrostatin requires the cyclophilin-D component of the mitochondrial permeability transition pore. Cardiovasc Drugs Ther. 2007;21:467–469. doi: 10.1007/s10557-007-6067-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Berghe TV, Vanlangenakker N, Parthoens E, et al. Necroptosis, necrosis and secondary necrosis converge on similar cellular dis-integration features. Cell Death Differ. 2010;17:922–930. doi: 10.1038/cdd.2009.184. [DOI] [PubMed] [Google Scholar]
- 152.Shen HM, Pervaiz S. TNF receptor superfamily-induced cell death: redox-dependent execution. FASEB J. 2006;20:1589–1598. doi: 10.1096/fj.05-5603rev. [DOI] [PubMed] [Google Scholar]
- 153.Eguchi Y, Shimizu S, Tsujimoto Y. Intracellular ATP levels determine cell death fate by apoptosis or necrosis. Cancer Res. 1997;57:1835–1840. [PubMed] [Google Scholar]
- 154.Leist M, Jaattela M. Four deaths and a funeral: from caspases to alternative mechanisms. Nat Rev Mol Cell Biol. 2001;2:589–598. doi: 10.1038/35085008. [DOI] [PubMed] [Google Scholar]
- 155.Leist M, Single B, Naumann H, et al. Inhibition of mitochondrial ATP generation by nitric oxide switches apoptosis to necrosis. Exp Cell Res. 1999;249:396–403. doi: 10.1006/excr.1999.4514. [DOI] [PubMed] [Google Scholar]
- 156.Micheau O, Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 2003;114:181–190. doi: 10.1016/s0092-8674(03)00521-x. [DOI] [PubMed] [Google Scholar]
- 157.Vandenabeele P, Vanden Berghe T, Festjens N. Caspase inhibitors promote alternative cell death pathways. Sci STKE. 2006;2006:pe44. doi: 10.1126/stke.3582006pe44. [DOI] [PubMed] [Google Scholar]
- 158.Davis CW, Hawkins BJ, Ramasamy S, et al. Nitration of the mitochondrial complex I subunit NDUFB8 elicits RIP1- and RIP3-mediated necrosis. Free Radic Biol Med. 2010;48:306–317. doi: 10.1016/j.freeradbiomed.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Xu X, Chua CC, Kong J, et al. Necrostatin-1 protects against glutamate-induced glutathione depletion and caspase-independent cell death in HT-22 cells. J Neurochem. 2007;103:2004–2014. doi: 10.1111/j.1471-4159.2007.04884.x. [DOI] [PubMed] [Google Scholar]
- 160.Smith CC, Davidson SM, Lim SY, et al. Necrostatin: a potentially novel cardioprotective agent? Cardiovasc Drugs Ther. 2007;21:227–233. doi: 10.1007/s10557-007-6035-1. [DOI] [PubMed] [Google Scholar]
- 161.Hsu TS, Yang PM, Tsai JS, Lin LY. Attenuation of cadmium-induced necrotic cell death by necrostatin-1: potential necrostatin-1 acting sites. Toxicol Appl Pharmacol. 2009;235:153–162. doi: 10.1016/j.taap.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 162.Shen HM, Lin Y, Choksi S, et al. Essential roles of receptor-interacting protein and TRAF2 in oxidative stress-induced cell death. Mol Cell Biol. 2004;24:5914–5922. doi: 10.1128/MCB.24.13.5914-5922.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kim YS, Morgan MJ, Choksi S, Liu ZG. TNF-induced activation of the Nox1 NADPH oxidase and its role in the induction of necrotic cell death. Mol Cell. 2007;26:675–687. doi: 10.1016/j.molcel.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 164.Vercammen D, Brouckaert G, Denecker G, et al. Dual signaling of the Fas receptor: initiation of both apoptotic and necrotic cell death pathways. J Exp Med. 1998;188:919–930. doi: 10.1084/jem.188.5.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Elder DE, Zuccollo JM, Stanley TV. Neonatal death after hypoxic ischaemic encephalopathy: does a postmortem add to the final diagnoses? BJOG. 2005;112:935–940. doi: 10.1111/j.1471-0528.2005.00608.x. [DOI] [PubMed] [Google Scholar]
- 166.Barkovich AJ, Westmark KD, Bedi HS, et al. Proton spectroscopy and diffusion imaging on the first day of life after perinatal asphyxia: preliminary report. AJNR Am J Neuroradiol. 2001;22:1786–1794. [PMC free article] [PubMed] [Google Scholar]
- 167.Takizawa Y, Takashima S, Itoh M. A histopathological study of premature and mature infants with pontosubicular neuron necrosis: neuronal cell death in perinatal brain damage. Brain Res. 2006;1095:200–206. doi: 10.1016/j.brainres.2006.04.027. [DOI] [PubMed] [Google Scholar]
- 168.Meng SZ, Ohyu J, Takashima S. Changes in AMPA glutamate and dopamine D2 receptors in hypoxic-ischemic basal ganglia necrosis. Pediatr Neurol. 1997;17:139–143. doi: 10.1016/s0887-8994(97)00087-8. [DOI] [PubMed] [Google Scholar]
- 169.Taniguchi H, Mohri I, Okabe-Arahori H, et al. Early induction of neuronal lipocalin-type prostaglandin D synthase after hypoxic-ischemic injury in developing brains. Neurosci Lett. 2007;420:39–44. doi: 10.1016/j.neulet.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 170.Zhengyu Z, Zhimou X, Yubin W, et al. Defibrillation and resuscitation in a piglet model of pediatric ventricular fibrillation following AHA 2005 Guidelines. Indian J Pediatr. 2010;Aug:26. doi: 10.1007/s12098-010-0128-8. Epub ahead of print. DOI 10.1007/s12098-010-0128-8. [DOI] [PubMed] [Google Scholar]
- 171.Martin LJ, Brambrink AM, Koehler RC, Traystman RJ. Primary sensory and forebrain motor systems in the newborn brain are preferentially damaged by hypoxia-ischemia. J Comp Neurol. 1997;377:262–285. doi: 10.1002/(sici)1096-9861(19970113)377:2<262::aid-cne8>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 172.Glauser EM. Advantages of piglets as experimental animals in pediatric research. Exp Med Surg. 1966;24:181–190. [PubMed] [Google Scholar]
- 173.Lind NM, Moustgaard A, Jelsing J, et al. The use of pigs in neuroscience: modeling brain disorders. Neurosci Biobehav Rev. 2007;31:728–751. doi: 10.1016/j.neubiorev.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 174.Brambrink AM, Ichord RN, Martin LJ, et al. Poor outcome after hypoxia-ischemia in newborns is associated with physiological abnormalities during early recovery. Possible relevance to secondary brain injury after head trauma in infants. Exp Toxicol Pathol. 1999;51:151–162. doi: 10.1016/S0940-2993(99)80089-X. [DOI] [PubMed] [Google Scholar]
- 175.Mueller-Burke D, Koehler RC, Martin LJ. Rapid NMDA receptor phosphorylation and oxidative stress precede striatal neurode-generation after hypoxic ischemia in newborn piglets and are attenuated with hypothermia. Int J Dev Neurosci. 2008;26:67–76. doi: 10.1016/j.ijdevneu.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Yang ZJ, Carter EL, Torbey MT, et al. Sigma receptor ligand 4-phenyl-1-(4-phenylbutyl)-piperidine modulates neuronal nitric oxide synthase/postsynaptic density-95 coupling mechanisms and protects against neonatal ischemic degeneration of striatal neurons. Exp Neurol. 2010;221:166–174. doi: 10.1016/j.expneurol.2009.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Johnston MV. Selective vulnerability in the neonatal brain. Ann Neurol. 1998;44:155–156. doi: 10.1002/ana.410440202. [DOI] [PubMed] [Google Scholar]
- 178.Yang ZJ, Torbey M, Li X, et al. Dopamine receptor modulation of hypoxic-ischemic neuronal injury in striatum of newborn piglets. J Cereb Blood Flow Metab. 2007;27:1339–1351. doi: 10.1038/sj.jcbfm.9600440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Formigli L, Papucci L, Tani A, et al. Aponecrosis: morphological and biochemical exploration of a syncretic process of cell death sharing apoptosis and necrosis. J Cell Physiol. 2000;182:41–49. doi: 10.1002/(SICI)1097-4652(200001)182:1<41::AID-JCP5>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 180.Hitomi J, Christofferson DE, Ng A, et al. Identification of a molecular signaling network that regulates a cellular necrotic cell death pathway. Cell. 2008;135:1311–1323. doi: 10.1016/j.cell.2008.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Ohmura A, Nakajima W, Ishida A, et al. Prolonged hypothermia protects neonatal rat brain against hypoxic-ischemia by reducing both apoptosis and necrosis. Brain Dev. 2005;27:517–526. doi: 10.1016/j.braindev.2005.01.004. [DOI] [PubMed] [Google Scholar]