Abstract
Susceptibility weighted imaging (SWI) is a well-established magnetic resonance technique, which is highly sensitive for blood, iron, and calcium depositions in the brain and has been implemented in the routine clinical use in both children and neonates. SWI in neonates might provide valuable additional diagnostic and prognostic information for a wide spectrum of neonatal neurological disorders. To date, there are few articles available on the application of SWI in neonatal neurological disorders. The purpose of this article is to illustrate and describe the characteristic SWI findings in various typical neonatal neurological disorders.
Introduction
Susceptibility weighted imaging (SWI) is an advanced magnetic resonance imaging (MRI) technique that renders high spatial resolution, three-dimensional (3D), fully velocity-compensated, gradient-echo MRI images that are exquisitely sensitive for the magnetic properties of blood, blood products, non-haem iron, and calcifications within the brain.1 These para- and diamagnetic substances disturb the magnetic field causing focal MR signal loss.
In addition, SWI is also very sensitive to the subtle differences of the paramagnetic susceptibility properties of oxygenated versus deoxygenated haemoglobin. Ultrafast functional MRI sequences take advantage of function-related differences in the local oxygenation of the various activated cortical brain regions to enable the investigation of brain function. This technique is also known as blood oxygenation-level dependent (BOLD) imaging. Similar to the ultrafast functional BOLD MRI sequences, SWI can indirectly visualize differences in the amount of oxygenated versus deoxygenated blood within vessels. Depending on the degree of oxygenation, SWI displays veins with varying degrees of signal loss (hypointensity), and consequently, SWI is also known as BOLD venography.
In SWI, both magnitude and phase images are reconstructed. Phase images allow to distinguish between blood and blood products (paramagnetic deoxyhaemoglobin) and calcifications (diamagnetic), which appear respectively hyper- and hypointense. To increase the signal intensity loss caused by susceptibility effects, additional post-processing is performed after the acquisition of both magnitude and phase images. The post-processing includes the creation of a phase mask enhancing the phase differences between substances with magnetic properties and surrounding tissues. Additionally, a minimal intensity projection (mIP) is commonly created by multiplying the phase with the magnitude images. Due to its high resolution, mIP may reduce the blooming artefacts and better visualize small vessels, haemorrhages, or foci of calcifications even in the periphery of the brain.
The aim of this review is to illustrate and describe the characteristic SWI findings in various typical neonatal neurological disorders.
SWI parameters in newborns
All presented SWI images were acquired on a 1.5 T clinical MRI system (Avanto, Siemens, Erlangen, Germany) using the following parameters: 48 ms TR (repetition time), 40 ms TE (echo time), 15° flip angle, 80 kHz bandwidth, 1.2 mm section thickness with 128 sections per slab, 146 × 180 mm field of view (FOV), 256 × 512 matrix size. The acquisition time is 4.57 min with the use of the iPAT factor 2. During post-processing, the neonatal mIP images are typically reconstructed with a lower effective mIP thickness than for most adult “factory settings” (e.g., 8 mm instead of 16 mm) to avoid partial volume effects with the skull base, which may mimic lesions.
Clinical application in neonatal diseases
SWI has been proven useful in the evaluation of various paediatric neurological disorders, such as traumatic brain injury (TBI), coagulopathic or other haemorrhagic disorders, vascular malformations, cerebral infarction, neoplasms, and neurodegenerative disorders associated with intracranial calcification or iron deposition.2–4
In neonates, SWI has the potential of providing additional, valuable information in perinatal asphyxia/hypoxic ischaemic encephalopathy (HIE) by identification of white and grey matter microhaemorhages, intraventricular haemorrhage, germinal matrix haemorrhage, and dilated/prominent intramedullary veins. In addition, SWI may potentially provide important information about cerebral haemodynamics and tissue viability in neonates affected with ischaemic stroke by the early recognition of brain tissue with critical perfusion.
SWI is a complementary sequence, used in combination with other conventional and functional MRI sequences such as T1, T2, fluid-attenuated inversion recovery (FLAIR), and diffusion-weighted/tensor imaging (DWI/DTI). SWI provides additional information that may not be readily apparent on conventional MRI sequences. However, detection and interpretation of abnormalities in the periphery of the brain, in particular at the air–tissue interface adjacent to, e.g., the skull or paranasal sinuses, may be problematic due to artefacts that results from local field inhomogeneity, or from artefacts caused by metallic implants such as ventriculo-peritoneal shunt reservoirs.
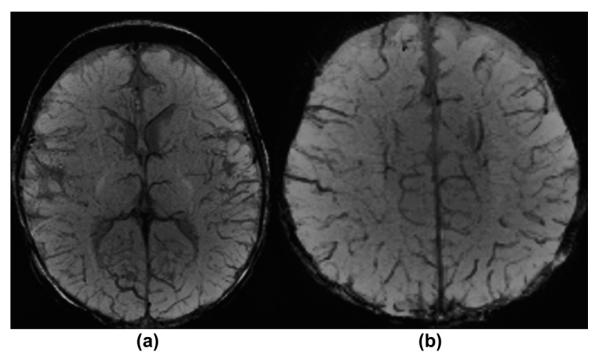
On normal mIP SWI images (Fig 1), the brain parenchyma is of intermediate signal intensity. The cerebrospinal fluid (CSF) signal is mildly hypointense relative to the brain. The sulcal and intramedullary veins are typically small and appear mildly hypointense. The venous SWI signal varies depending on the degree of blood oxygenation. The sulcal and intramedullary veins are usually faintly visible in neonates that are examined in “natural sleep”; in intubated/ventilated neonates the higher oxygenation typically increases the signal intensity of the veins, frequently making them almost invisible on SWI. In neonates with significant lung disease and low overall oxygenation, or global hypoperfusion of the brain due to, e.g., significantly increased intracranial pressure related to global brain oedema/injury, the veins become significantly more apparent/hypointense. The paediatric neuroradiologist should be familiar with these variations of the SWI signal intensity of the neonatal cerebral veins (Fig 2).
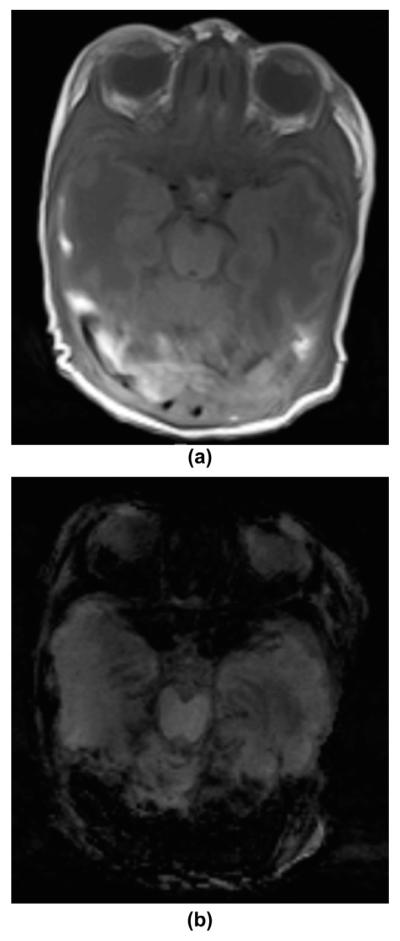
Figure 1.
A 2-month-old healthy infant in natural sleep. (a–b) Axial mIP SWI images at the level of the basal ganglia (a), and just above the lateral ventricles within the centrum semiovale (b) demonstrate normal SWI hypointense signal and calibre of sulcal and intramedullary veins as well as of the deep and superficial venous system. The CSF is mildly hypointense in comparison to the cerebral grey and white matter.
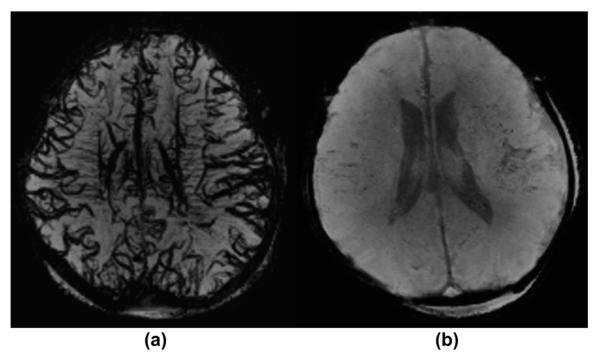
Figure 2.
A 4-day-old neonate affected with HIE under general anaesthesia. (a–b) Matching axial mIP SWI images show a clear difference in the SWI hypointensity of the sulcal and intramedullary veins. The initial MRI was acquired with 80% oxygenation (a) the veins are more prominent/hypointense compared to the follow-up MRI 2 days later performed with an effective oxygenation of 100% (b).
Intracranial haemorrhage
Intracranial haemorrhages are an important clinical problem in newborns with a relatively high frequency of occurrence and serious neurological complications including death.5 Several prenatal (e.g., maternal hypertension and obstetric disease), perinatal (e.g., traumatic delivery and perinatal asphyxia), and postnatal [e.g., haematological disorders, coagulopathies, sepsis, and extracorporeal membrane oxygenation (ECMO)] risk factors have been reported (Fig 3).5
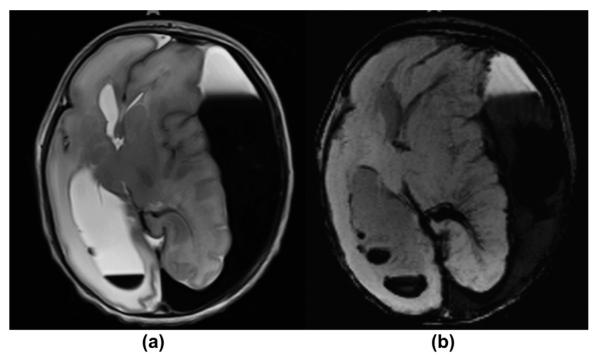
Figure 3.
A 1-day-old premature neonate with vitamin K-dependent coagulopathy presented with seizures and apnoea. (a) T2-weighted MRI image shows a large left convexity subdural haematoma with blood sedimentation and signal characteristics representing acute and subacute blood products. There is a significant left-to-right shift of the midline structures and high-grade compression of the left lateral ventricle. Associated right intraventricular layering haemorrhage is also noted. The right frontal sulci are effaced. Moderate oedema is seen within the cerebral white matter. (b) Axial mIP SWI image confirms the subdural and intraventricular blood collections. There are prominent intramedullary veins draining into the deep venous system adjacent to the left occipital horn, representing venous stasis due to mass effect. The intraventricular blood depositions are better seen on SWI compared to the T2-weighted image.
Based on their locations, intracranial haemorrhages may be classified as epidural, subdural, subarachnoid, intraventricular, and intraparenchymal (more frequent in full-term newborns).5 In addition, a separate group of haemorrhages: the germinal matrix/intraventricular haemorrhages (GMH/IVH grade 1–3) and periventricular haemorrhagic infarctions (PVHI) represent a distinct group of haemorrhages that typically occur in premature neonates.
Due to its high sensitivity for extravascular blood products, SWI is very useful in the sensitive and specific detection of these haemorrhagic lesions in the neonatal brain (Fig 4).6
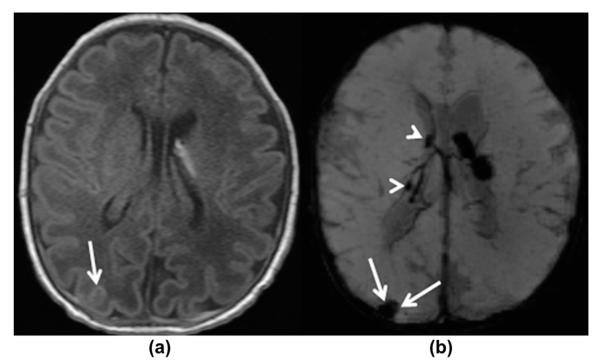
Figure 4.
A 6-day-old premature infant at 34 4/7 weeks of gestation with GMH grade I. (a) Axial, T1-weighted and (b) axial, mIP SWI images show a bilateral GMH, left greater than right. The small right GMH is only visible on the mIP SWI image (arrowheads on b). Additionally, small confluent haematomas are noted over the right occipital and posterior parietal convexities, most likely extra-axially located representing a subdural/subarachnoid haemorrhage (white arrows on a–b). They are easily visible on the mIP SWI image and could easily be missed on the T1-weighted images. The study was undertaken in an intubated child, hence the SWI signal intensity of the sulcal veins is increased due to the high oxygenation.
In preterm infants with GMH and venous infarction, SWI may show prominent hypointense intramedullary veins in the region of injury (Fig 5). This may result from the venous stasis as complication of PVHI or from the hydrocephalus caused by the IVH.
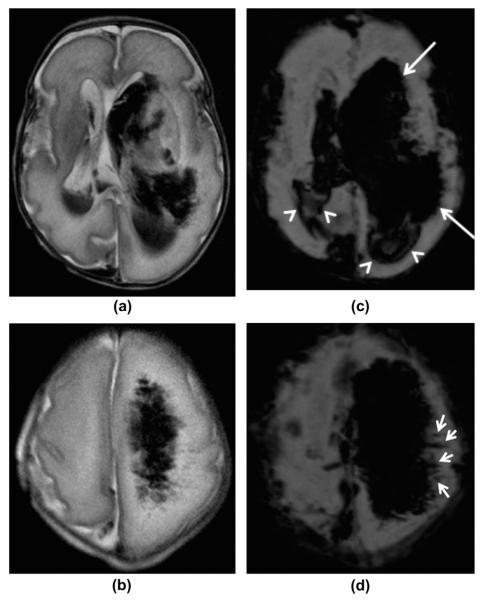
Figure 5.
A 4-day-old premature infant born at 30 weeks of gestation with left-sided PVHI. (a–b) Axial, T2-weighted and (c–d) axial, mIP SWI images at the level of the lateral ventricles and centrum semiovale, show bilateral intraventricular haemorrhages with left-sided periventricular haemorrhagic venous infarction and hydrocephalus. The mIP SWI images show the intraventricular blood products (white arrowheads on c), the subarachnoid/subdural blood along the Sylvian fissures, the intraparenchymal blood products (white arrows on c), and dilated/thrombosed SWI hypointense intramedullary veins in the left hemisphere (small arrows on d) more extensively than on the T2-weighted images. This is partially due to the higher sensitivity of SWI for the blood products and partially due to the blooming effect of the blood products on the SWI sequence. The dilated, obstructed intramedullary veins are much better seen on the SWI images compared to the T2-weighted images (small arrows). However, the white matter oedema is better seen on the T2-weighted images.
Finally, detecting blood products in the ventricles or subarachnoid space may reveal the cause of “unexplained” neonatal hydrocephalus. SWI hypointense haemorrhagic “staining” of the ventricles may persist for years after the initial haemorrhage.
Hypoxic–ischaemic encephalopathy
Hypoxic–ischaemic encephalopathy (HIE) represents a unique pattern of neonatal brain injury resulting from a combination of brain hypoxia and ischaemia. Depending on the degree of HIE, mild, moderate or severe long-term motor and cognitive impairments or even death may ensue.7 Depending on the degree and duration of the hypoxic–ischaemic injury and gestational age of the neonate, different neuroimaging patterns of injury have been reported.8 SWI may be especially helpful in the identification of SWI-hypointense intramedullary veins.9 In contrast to a focal ischaemic injury, the intramedullary veins are typically seen in both cerebral hemispheres (Fig 6).
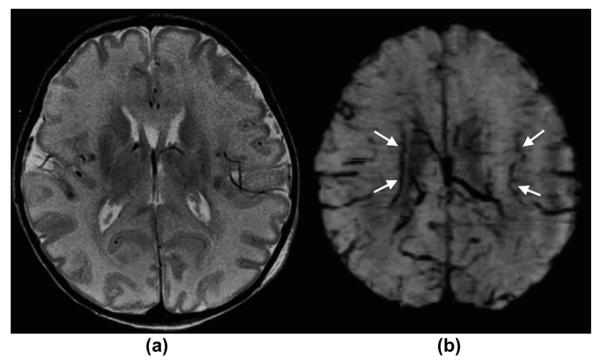
Figure 6.
A 5-day-old term newborn with severe hypoxic–ischaemic encephalopathy/injury. (a) Axial, T2-weighted and (b) mIP SWI images show moderate diffuse T2-hyperintense brain swelling, partially with a loss of the cortico-medullary differentiation in the occipital lobes and in the peri-rolandic region. mIP SWI image shows, in addition to findings seen on the T2-weighted image, prominent/dilated intramedullary and deep subependymal veins, which are typically seen in HIE/brain oedema. This “extra” information solidifies the diagnosis of HIE and may be helpful in predicting prognosis/outcome.
In addition, SWI may show petechial white matter haemorrhages as well as intracortical haemorrhagic lesions, which may occur as complication of HIE, in better detail.10
Finally, a SWI grading scale has been proposed to aid in predicting neurological outcomes after hypoxic–ischaemic injuries (Fig 7).11
Figure 7.
A 12-day-old term newborn with hypoxic–ischaemic encephalopathy after hypothermia therapy. (a) Axial, T2-weighted and (b) mIP SWI images show mild global T2-hyperintense white matter brain oedema as well as a large haemorrhagic lesion in the right occipital lobe and additional small petechial haemorrhages in the left occipital lobe and subependymal region along the optic radiation, which are faintly seen on the T2-weighetd image. The sulcal veins are nearly completely “invisible” in this ventilated neonate. All haemorrhagic findings are seen in better detail on the SWI image compared to the T2-weighted image.
Ischaemic stroke
Neonatal ischaemic stroke is rare and occurs most often in the term neonates.12 Affected newborns usually present with seizures in the first 48 h of life and additional nonspecific symptoms including irritability, lethargy, apnoea, and asymmetric weakness.
In ischaemic stroke, SWI may serve as an additional important diagnostic tool next to conventional T1 and T2-weighted MRI and DWI/DTI. SWI gives important and easy to collect haemodynamic information about critical brain perfusion by focusing on the venous drainage of ischaemic or critically perfused brain tissue.3,13 Widened, and increased SWI hypointense intramedullary and/or sulcal veins are typically seen draining the ischaemic brain region (Fig 8).
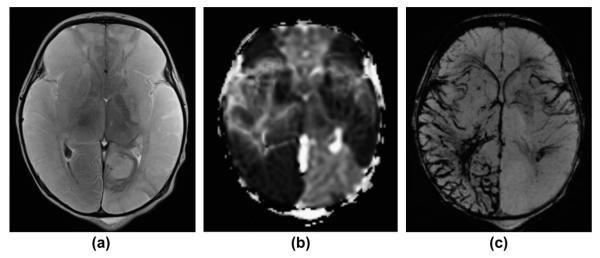
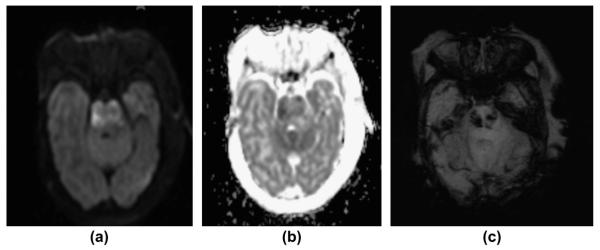
Figure 8.
A 2-day-old term newborn with extensive, multifocal thrombo-embolic strokes (risk factor: chorioamnionitis) with superimposed severe perinatal HIE. (a) Axial, T2-weighted image, (b) apparent diffusion coefficient (ADC) map, and (c) mIP SWI image show extensive bilateral, T2-hyperintense, ischaemic lesions with matching restricted diffusion (low ADC values) in the territories of the bilateral middle and the right posterior cerebral arteries. In the infarcted areas, mIP SWI shows widened/prominent, marked SWI-hypointense sulcal and intramedullary veins (slow venous flow/partial thrombosis and elevated concentration of deoxygenated haemoglobin). In the regions without restricted diffusion, the sulcal and intramedullary veins are “nearly invisible” (intubated/ventilated neonate). The only exception is the left insular region in which a mismatch between ADC and mIP SWI image is noted. This likely reflects an area with critical residual perfusion.
Moreover, SWI may occasionally show the SWI hypointense intravascular thrombus due to high concentrations of deoxyhaemoglobin within the thrombus and is also highly sensitive (compared to CT and T2*-weighted images) in detecting haemorrhages within the infarct.9
Sinovenous thrombosis
Neonatal sinovenous thrombosis has a significant risk for serious adverse neurological sequelae.14 Sepsis and/or meningitis, traumatic delivery, and maternal preeclampsia are common causes/risk factors.
In sinovenous thrombosis, SWI is typically characterized by marked prominence of the sulcal veins draining the brain region affected by the sinovenous thrombosis due to the higher concentration of deoxyhaemoglobin secondary to venous stasis and nearby obstruction (Fig 9).9 Additionally, SWI may easily show a haemorrhagic conversion of a venous infarction.9
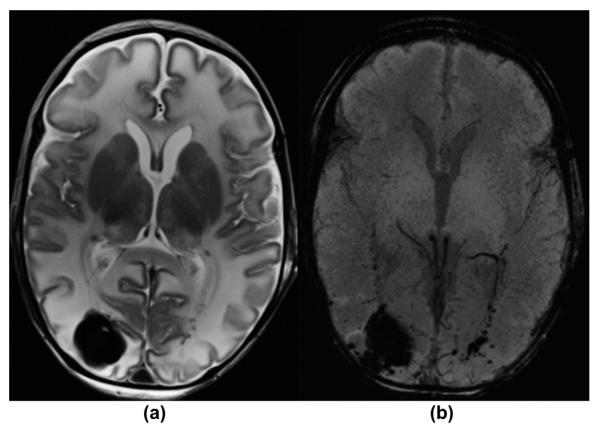
Figure 9.
An 11-day-old male infant with sinus vein thrombosis presented with perinatal depression, sepsis, and seizures. (a) T1-weighted MRI image demonstrates abnormal hyperintense signal within the transverse and sigmoid sinuses bilaterally, representing partial dural sinus thrombosis. (b) Axial mIP SWI image shows the typical matching blooming artefact of the intravascular venous thrombosis. No additional complications were noted.
Brain death
Independent of the cause, marked prominence of intramedullary and sulcal veins is present in severe diffuse neonatal cerebral oedema/brain death. The exact cause of the increased SWI hypointensity of the cerebral veins is likely multifactorial and includes increased venous concentration of deoxyhaemoglobin, venous stasis/thrombosis, and/or venous dilation due to adenosine release (Fig 10).15
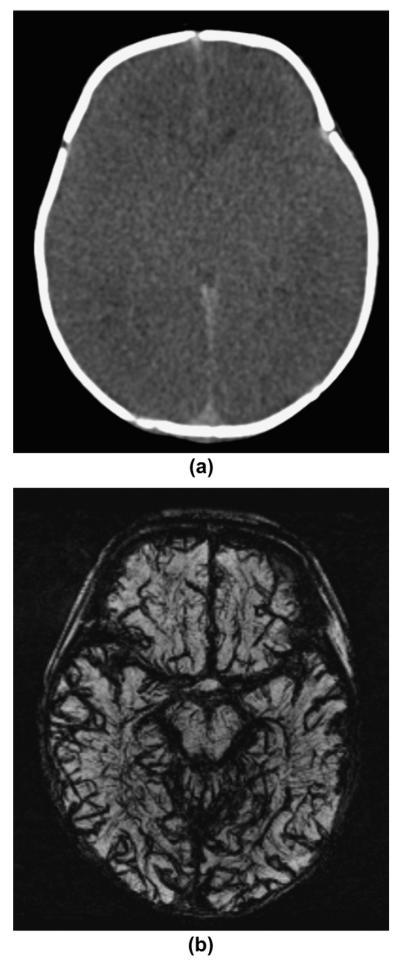
Figure 10.
A 1-day-old newborn with severe HIE and brain oedema presenting with seizures and subsequently matching clinical criteria for brain death. (a) Axial CT and (b) mIP SWI images show global hypodensity of the supratentorial brain with decreased grey–white differentiation and an effaced ventricular system. mIP SWI image shows a marked global prominence and hypointensity of the intramedullary and sulcal veins compatible with halted brain perfusion/brain death.
Infectious disorders
A wide spectrum of microbial organisms (e.g., viruses, bacteria, fungi) may cause infections during intrauterine development, in association with the birth process, or in the first postnatal days or weeks with devastating effects on brain structure and function. In neonatal infectious disorders, SWI is helpful to detect calcification and haemorrhage.
Congenital infections, e.g., by cytomegalovirus, are characterized by periventricular calcifications, which may be easily visualized by SWI.
Infarction is a prominent and serious complication in neonatal bacterial meningitis.16 The vascular lesions are most frequently related to sinovenous thrombosis and are often haemorrhagic, but the involvement of the cerebral arteries related to vasculitis may also be a significant causative factor (see above for the role of SWI in infarction and sinovenous thrombosis; Fig 11).
Figure 11.
A 24-day-old term neonate with confirmed bacterial meningitis. (a) Axial DWI image, (b) axial ADC map, and (c) axial mIP SWI image show DWI hyperintense signal abnormalities and restricted diffusion (low ADC values) affecting the pons representing ischaemic infarction as a complication of meningitis/vasculitis. mIP SWI image shows multiple hypointense foci within the pons representing haemorrhagic conversion/lesions within the infarcted region.
In neonatal herpes type 2 encephalitis, SWI may best visualize the full extent of the haemorrhagic lesions frequently affecting the temporal lobes.
Conclusions
SWI is an advanced MRI technique with high sensitivity for blood, non-haem iron, calcifications, and variations in venous oxygenation. SWI is known to increase the diagnostic sensitivity and specificity of MRI in a wide spectrum of neonatal neurological disorders. Familiarity with the various imaging findings is essential. We suggest that SWI should be included in the standard neonatal brain MRI protocol.
References
- 1.Haacke EM, Mittal S, Wu Z, et al. Susceptibility-weighted imaging: technical aspects and clinical applications, part 1. AJNR Am J Neuroradiol. 2009;30:19–30. doi: 10.3174/ajnr.A1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thomas B, Somasundaram S, Thamburaj K, et al. Clinical applications of susceptibility weighted MR imaging of the brain — a pictorial review. Neuroradiology. 2008;50:105–16. doi: 10.1007/s00234-007-0316-z. [DOI] [PubMed] [Google Scholar]
- 3.Tong KA, Ashwal S, Obenaus A, et al. Susceptibility-weighted MR imaging: a review of clinical applications in children. AJNR Am J Neuroradiol. 2008;29:9–17. doi: 10.3174/ajnr.A0786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mittal S, Wu Z, Neelavalli J, et al. Susceptibility-weighted imaging: technical aspects and clinical applications, part 2. AJNR Am J Neuroradiol. 2009;30:232–52. doi: 10.3174/ajnr.A1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gupta SN, Kechl AM, Kanamalla US. Intracranial hemorrhage in term newborns: management and outcomes. Pediatr Neurol. 2009;40:1–12. doi: 10.1016/j.pediatrneurol.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 6.Niwa T, Aida N, Takahara T, et al. Imaging and clinical characteristics of children with multiple foci of microsusceptibility changes in the brain on susceptibility-weighted MRI. Pediatr Radiol. 2010;40:1657–62. doi: 10.1007/s00247-010-1665-z. [DOI] [PubMed] [Google Scholar]
- 7.Fatemi A, Wilson MA, Johnston MV. Hypoxic–ischaemic encephalopathy in the term infant. Clin Perinatol. 2009;36:835–58. doi: 10.1016/j.clp.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rutherford M, Malamateniou C, McGuinness A, et al. Magnetic resonance imaging in hypoxic–ischaemic encephalopahty. Early Hum Dev. 2010;86:351–60. doi: 10.1016/j.earlhumdev.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 9.Lequin MH, Dudink J, Tong KA, et al. Magnetic resonance imaging in neonatal stroke. Semin Fetal Neonatal Med. 2009;14:299–310. doi: 10.1016/j.siny.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 10.Niwa T, Aida N, Shishikura A, et al. Susceptibility-weighted imaging findings of cortical laminar necrosis in pediatric patients. AJNR Am J Neuroradiol. 2008;29:1795–8. doi: 10.3174/ajnr.A1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kitamura G, Kido D, Wycliffe N, et al. Hypoxic–ischemic injury: utility of susceptibility-weighted imaging. Pediatr Neurol. 2011;45:220–4. doi: 10.1016/j.pediatrneurol.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 12.Govaert P, Ramenghi L, Taal R, et al. Diagnosis of perinatal stroke I: definitions, differential diagnosis and registration. Acta Paediatr. 2009;98:1556–67. doi: 10.1111/j.1651-2227.2009.01461.x. [DOI] [PubMed] [Google Scholar]
- 13.Chalian M, Tekes A, Meoded A, et al. Susceptibility-weighted imaging (SWI): a potential non-invasive imaging tool for characterizing ischemic brain injury? J Neuroradiol. 2011;38:187–90. doi: 10.1016/j.neurad.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 14.Berfelo FJ, Kersbergen KJ, van Ommen CH, et al. Neonatal cerebral sinovenous thrombosis from symptom to outcome. Stroke. 2010;41:1382–8. doi: 10.1161/STROKEAHA.110.583542. [DOI] [PubMed] [Google Scholar]
- 15.Bell MJ, Robertson CS, Kochanek PM, et al. Interstitial brain adenosine and xanthine increase during jugular venous oxygen desaturations in humans after traumatic brain injury. Crit Care Med. 2001;29:399–404. doi: 10.1097/00003246-200102000-00033. [DOI] [PubMed] [Google Scholar]
- 16.Hernandez MI, Sandoval CC, Tapia JL, et al. Stroke patterns in neonatal group B streptococcal meningitis. Pediatr Neurol. 2011;44:282–8. doi: 10.1016/j.pediatrneurol.2010.11.002. [DOI] [PubMed] [Google Scholar]