Abstract
Dysfunction in central glucocorticoid signaling is implicated in HPA axis dysregulation and major depression. In comparison with men, women are twice as likely to suffer from depression and have heightened HPA axis responses to stress. We hypothesized that this striking increase in stress vulnerability in females may be due to sex differences in central glucocorticoid signaling. The current study tests the role of the forebrain Type II glucocorticoid receptor (GR) on HPA axis function in female mice and depression-like behavior in both female and male mice. This was accomplished by using mice with selective deletion of GR in forebrain cortico-limbic sites including the prefrontal cortex, hippocampus and basolateral amygdala (FBGRKO). In order to examine HPA axis function in female FBGRKO, we measured nadir, peak circadian and restraint-induced corticosterone concentrations in female FBGRKO. The data indicate that unlike male FBGRKO, basal and stress-induced corticosterone concentrations are not increased in female FBGRKO. Given the pronounced effect of central glucocorticoid signaling on mood, we also examined the necessity of corticolimbic GR on depression-like behavior with the sucrose preference and forced swim tests (FST) in male and female FBGRKO mice. Consistent with previous studies, male FBGRKO displayed increased depression-like behavior as indicated by greater immobility in the FST and decreased sucrose preference compared with littermate controls, effects that were not observed in females. Overall the findings indicate a marked sex difference in the function of forebrain GR on HPA axis regulation and depression-like behaviors, and may have implications for therapeutic approaches using GR-modulating drugs.
Keywords: affective disorders, estrogen, androgens, glucocorticoid receptors, stress, sex differences
Depression is a serious and complex neuropsychiatric disorder affecting nearly 10 percent of the population. While many factors are associated with the onset or exacerbation of depression symptoms, the role of the hypothalamic-pituitary adrenocortical (HPA) axis is one of the most well-studied. Glucocorticoids, the end product of HPA axis, exert a negative feedback to constrain this system. Glucocorticoids regulate the HPA axis via binding glucocorticoid receptors (GR) in numerous target areas, including the pituitary, and several forebrain regions (e.g., hippocampus, prefrontal cortex and amygdala) (Herman, Ostrander, Mueller, and Figueiredo, 2005). Upon ligand binding, the GR translocates to the cell nucleus, where it can directly modulate gene transcription by binding to glucocorticoid response elements on target genes or interact with transcription factors to repress or augment transcription of target genes (Anacker, Zunszain, Carvalho, and Pariante).
Impairment in the glucocorticoid signaling system is associated with HPA axis dysregulation and depression (Modell, Yassouridis, Huber, and Holsboer, 1997; Pariante and Miller, 2001). For example, some depressed individuals fail to suppress cortisol following dexamethasone administration, a synthetic glucocorticoid (Carroll, 1984; Vreeburg, Hoogendijk, van Pelt, Derijk, Verhagen, van Dyck, Smit, Zitman, and Penninx, 2009), suggesting decreased GR signaling in either peripheral (pituitary) or central (brain) areas involved in negative feedback. Consistent with this notion of decreased GR signaling, in some instances, depressed individuals are hypercortisolemic relative to their non-depressed counterparts (Musselman and Nemeroff, 1996; Vreeburg et al., 2009). Overall clinical studies suggest a role for GR in normal HPA axis function and mood.
In order to more definitively study how central GR regulates HPA axis function and depression-like behavior, (Boyle, Brewer, Funatsu, Wozniak, Tsien, Izumi, and Muglia, 2005) developed a forebrain glucocorticoid receptor knockout mouse FBGRKO. In FBGRKO mice, GR is selectively decreased in forebrain regions including the hippocampus, medial prefrontal cortex and basolateral amygdala. Collectively, their findings, as well work from our laboratory (Furay, Bruestle, and Herman, 2008) reveal that deletion of forebrain GR in male mice induces HPA axis activity mirroring melancholic depression. The FBGRKO mice also display increased depression-like and anxiety-like behaviors (Boyle et al., 2005; Boyle, Kolber, Vogt, Wozniak, and Muglia, 2006).
While the advent of the FBGRKO mouse has furthered our understanding regarding the role of forebrain GR on HPA axis function and depression-like behavior, it offers few clues to one of the biggest unanswered questions regarding depression: why are women more likely to suffer from this condition compared with men (Earls, 1987; Kessler, McGonagle, Swartz, Blazer, and Nelson, 1993)? In addition, female rodents have elevated HPA axis activity under basal and stressed induced conditions relative to males (Critchlow, Liebelt, Bar-Sela, Mountcastle, and Lipscomb, 1963; Handa, Burgess, Kerr, and O'Keefe, 1994; Kitay, 1961). These marked sex differences in HPA axis function and mood may be due to fundamental sex differences in central glucocorticoid signaling. To test this possibility, we used FBGRKO mice to assess the impact of GR deletion in limbic forebrain structures on HPA axis function and depression-like behavior in male and female mice. Here, we report that the deletion of limbic forebrain GR induces marked sex differences in HPA axis regulation and mood-like behaviors, with females being relatively protected against HPA axis hyperactivity and depression-like behaviors caused by absence of the GR.
Experimental procedures
Subjects
Male and female forebrain-specific GR knock-out mice (FBGRKO) were generated by breeding knock-in mice engineered with loxP sites flanking exons 1C and 2 of the mouse GR gene (GRloxP) with mice expressing Cre Recombinase driven by the CamKinaseIIα promoter (CamK-Cre) (Boyle, Brewer, Vogt, Wozniak, and Muglia, 2004; Brewer, Khor, Vogt, Muglia, Fujiwara, Haegele, Sleckman, and Muglia, 2003). Heterozygous mice were bred to produce GRloxP Cre- and GRloxP Cre+ offspring, of which only the latter have the GR gene deletion. Mice used in this study were on a mixed C57B6 X S129 X CBA background. In all cases GRloxP homozygous Cre- and GRloxP homozygous Cre+ littermates were used. The FBGRKO have an acquired deletion of GR, such that by 6 months of age, there is a 90% reduction of GR immunoreactivity in a majority of the forebrain (prefrontal cortex, hippocampus, and basolateral amygdala), sparing GR expression in the hypothalamus and central amygdala (Boyle et al., 2005). Animals used in these studies were adult (6–12 months) male and female Cre+ (FBGRKO) and littermate Cre- (control) mice.
Animals were housed in a temperature controlled vivarium, with a12:12 light/dark cycle, with lights-on at 6:00 AM. Ad libitum access to food and water was provided throughout the study. All protocols were reviewed and approved by the Institutional Animal Care and Use Committees and were in accordance to the National Institutes of Health Council on Animal Care guidelines involving vertebrate animals in biomedical research.
HPA axis Testing
Basal and restraint-stress regulation of HPA axis in males and females
The acute stress component was completed using male and female littermate controls and FBGRKO mice that were tested at the same time, using the same procedures. Although, the male portion of this study was previously published (Furay et al., 2008); here, we are reprinting (with permission) the male portion to permit a side by side comparison of these data. Female controls (n=12) and FBGRKO (n=11) were included in these studies. Blood samples were collected on three occasions: 1) basal AM (between 6:30 and 8:00am); 2) basal PM (between 4:30 and 5:30pm) 3) 30 minutes and 120 minutes following restraint stress onset. Based on previous data in our laboratory, 120 minutes was determined to be an optimal timepoint to assess GR-mediated feedback responses. As such, at 120 minutes corticosterone concentrations should return back to pre-stress levels. During the restraint testing, mice were placed in well-ventilated plastic tubes. Approximately 15–20μl of blood was collected at each timepoint. All samples were collected within 2 minutes from the initial handling of the animal’s cage. After the final blood collection at 120 minutes, mice were perfused as detailed below.
Behavioral Tests
A separate set of female littermate control (n=8) and FBGRKO (n=8), and male littermate control (n=8) and FBGRKO(n=8) mice (6–12 months of age) were used for behavioral analyses. Two weeks intervened between the forced swim test (FST) and sucrose consumption tests.
Forced Swim Test
Female and male mice were placed in a 2-liter beaker half-filled with water (23 ± 2°C). This level of water prevents the mice from reaching the bottom of the container and from escaping. Each session was videotaped and all behaviors were scored in 5s intervals by two independent observers who were blind to the genotype or sex of each mouse. The total counts of each behavior during the 10 min testing session was summed for each animal and averaged based on the group. The behaviors scored are defined as follows: (i) climbing – rapid movement of limbs in and out of the water with the body parallel to the apparatus with, (ii) diving, (iii) swimming – moving limbs in an active manner and making circular movements around the apparatus; and immobility – no active movements or floating in the water without struggling. Although the traditional forced swim test includes two days of testing, here we utilize a modified version of the forced swim test which includes only one day. This was done to assess depression-like behavior in a novel environment. Note, the modified version of this task has been utilized by our laboratory (Jankord, Solomon, Albertz, Flak, Zhang, and Herman, 2011; Wulsin, Herman, and Solomon, 2010) and others (Boyle et al., 2005; Jankord et al., 2011; Walf and Frye, 2005; Wulsin et al., 2010) to assess depression-like behavior. The FST and sucrose testing procedures and data analyses were done in such a way to be consistent with the seminal study which reported depression-like behavior in male FBGRKO mice (Boyle et al., 2005; Wulsin, Herman, and Solomon). However, based on pilot studies conducted in our laboratory with FST and mice, the water temperature was increased from the 18–20°C as previously published in (Boyle et al., 2005) to 23°C ±2.
Sucrose Preference
According to the procedure previously published in (Boyle et al., 2005), mice were continuously exposed to two bottles containing 1% sucrose solution or tap water for 10 days. Bottles were weighed and switched daily to obviate side preferences. Sucrose preference was determined by dividing the total amount of sucrose consumed by the total fluid averaged (sucrose + water) over 10 days.
Radioimmunoassay
Plasma was separated via centrifugation at 3000 × g for 15 minutes and stored at −20°C until radioimmunoassay. Plasma CORT was measured using an 125I kit from MP Biomedicals (Orangeburg, NY). All samples were run in duplicate and within the same assay. Intra-assay coefficient of variation was less than 10%.
Glucocorticoid-Receptor Immunohistochemistry
Approximately 120 min after restraint onset, animals were sacrificed by overdose with Fatal-Plus Solution (Vortech Pharmaceuticals, LTD), transcardially flushed with 0.9% saline, and perfused with 3.7% formaldehyde in .1M phosphate buffered saline (PBS). Brains were extracted, post-fixed in 3.7% formaldehyde solution for 24 hours, and then stored in 30% sucrose in DEPC treated potassium phosphate buffered saline (KPBS) at 4°C. Brains were sectioned at 25μm on a sliding microtome. Slices were washed in KPBS, endogenous peroxidases quenched with 1% H2O2, washed in KPBS, blocked in incubation solution for one hour at room temperature (1% triton X-100 and 2% bovine serum albumin in KPBS), and incubated with primary antibody in blocking solution (GR-M-20) 1:1000; Santa Cruz Biotechnology) at 4°C overnight. Slices were then rinsed with KPBS, incubated for one hour with biotinylated anti-rabbit (1:500; Vector Laboratories), rinsed with KPBS, incubated with Vectastain ABC (1:1000; Vector Laboratories), rinsed with KPBS, incubated with 0.4 mg/ml Diaminobenzidine in 0.05% H202 in KPBS, rinsed with KPBS and mounted onto Fisher Super stick glass slides. Slides were cover slipped with DPX mountant (BioChemika).
In situ hybridization
Adjacent brain sections were used to assess restraint stress-induced corticotropin releasing hormone (CRH) and arginine vasopressin (AVP) mRNA in male and female FBGRKO mice and littermate controls, using previously described procedures (Choi, Furay, Evanson, Ostrander, Ulrich-Lai, and Herman, 2007). Briefly, riboprobes complementary to AVP and CRH mRNA were generated by in vitro transcription using 35S-labeled UTP. The AVP (exon C) DNA construct is a 161 bp insert in a pCR4 TOPO vector, which was linearized with NotI restriction enzyme, and transcribed using T3 RNA polymerase. The CRH DNA construct is a 765 bp fragment cloned into pGEM3 vector, which was linearized with HindIII and transcribed using T7 RNA polymerase. Each 15 μl riboprobe transcription reaction was made from 1.0–2.5 μg of linearized DNA fragment, 62.5 μCi of [35S]UTP, 330 μM ATP, 330 μM GTP, 330 μM CTP, 10 μM cold UTP, 1× transcription buffer, 66.6 mM dithiothreitol (DTT), 40 U of RNase Inhibitor, and 20 U of the appropriate RNA polymerase. Before hybridization, slides were washed in potassium PBS, acetylated, delipidated in chloroform, and dehydrated through a graded ethanol series. Each riboprobe was diluted (1.0 × 106 cpm/50 μl buffer) in hybridization buffer (50% formamide, 1× Denhardt's solution, 10% dextran sulfate, 200 μg/ml fish sperm single-stranded DNA, 100 μg/ml yeast tRNA, and 20 mM DTT). Coverslipped slides were placed in hybridization chambers over blotting paper soaked in 50% formamide and incubated overnight at 55°C. The next day, coverslips were removed, and slides were washed in 2× saline sodium citrate (SSC). Slides were subsequently incubated in 100 μg/ml RNase A for 30 min at 37°C, washed numerous times in 0.2× SSC, once in 0.2× SSC for 1 h at 65°C, and finally dehydrated through a graded ethanol series.
Image analysis
Hybridized slides were exposed on Eastman Kodak (Rochester, NY) Biomax MR film (24 h for AVP and 7 d for CRH). Film images of brain sections were captured by a digital video camera. Anatomical brain regions were identified using Paxinos and Franklin mouse brain atlas (2nd edition). Semi-quantitative analyses of autoradiograph images were performed using Scion (Frederick, MD) Image software, and hybridization signal was expressed as gray level units. The gray level signal of a hybridized tissue region of interest was corrected by subtracting the gray level signal over a non hybridized area of tissue (white matter) and expressed as integrated gray level (IGL). 14C standards were also measured using Scion Image and transferred to Assay Zap [Biosoft (Cambridge, UK) and P. L. Taylor (West Markham, East Lothian, Scotland)] to generate a standard curve to verify that all measured gray levels were on the linear range of the film.
Statistical Analysis
Data were analyzed using independent sample t-test, ANOVA and repeated measures ANOVA where appropriate. When the data were not homogeneously distributed, log or square root transformations were performed. All data are reported as mean ±SEM. Fisher’s Least Protected Differences was used for post-hoc testing when necessary. Significance was taken at p ≤0.05. Outliers were defined by standardized scores greater than 1.96 of the standard deviation and when necessary, data were reanalyzed following outlier removal. All behavioral data are analyzed to evaluate the relative effect of the loss forebrain GR on depression-like behavior within the same sex (i.e., FBGRKO female vs. littermate control female).
Results
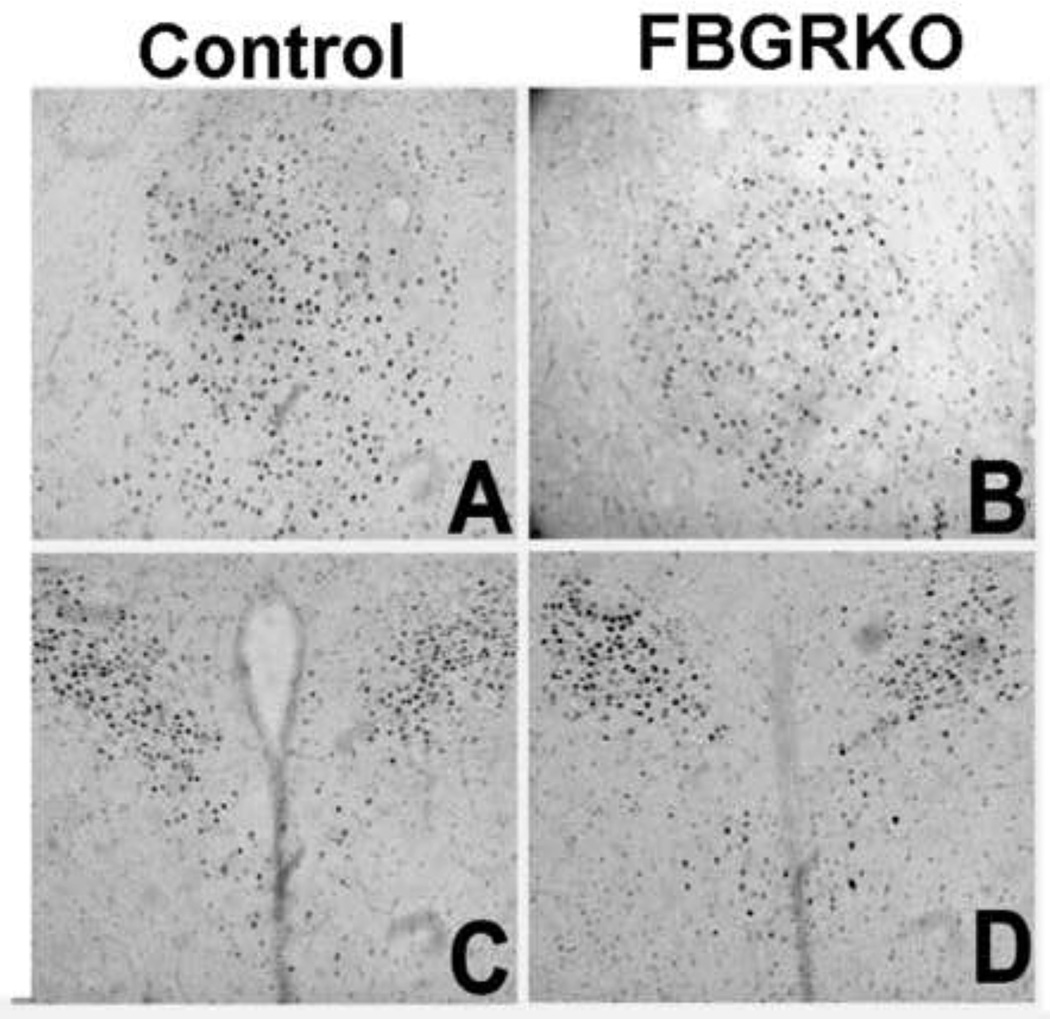
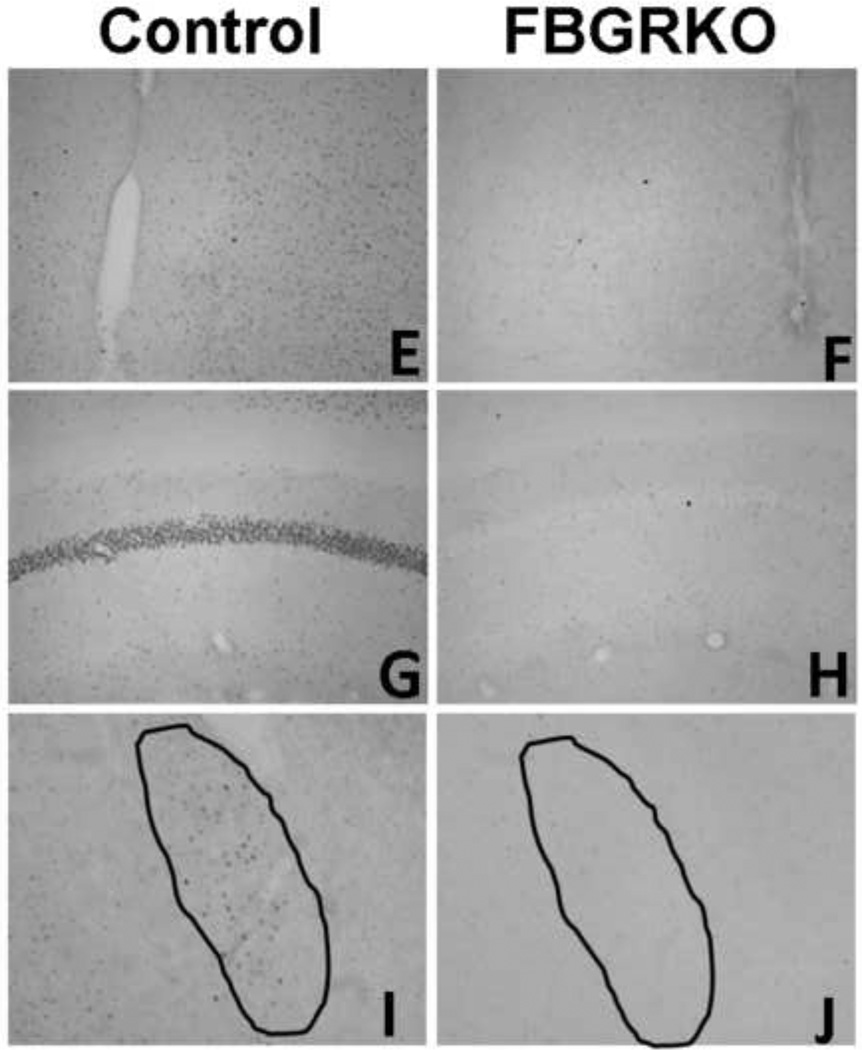
Brain sections were processed for GR immunohistochemistry (Fig. 1) to verify genotyping and ascertain that the knockout was limited to forebrain regions. GR immunoreactivity is comparable in the central amygdala (CeA) and paraventricular nucleus of the hypothalamus (PVN) in control and FBGRKO mice (A-D). (E, G, and I) illustrate the presence of GR immunostained nuclei in the medial prefrontal cortex, hippocampus, and basolateral amygdala of control mice, respectively. In contrast, GR immunoreactivity is greatly attenuated in these same brain areas in FBGRKO mice (F, H, and J). This distribution of GR in female FBGRKO is similar to previously published reports in male FBGRKO(Boyle et al., 2005; Furay et al., 2008). Thus, sex differences in HPA axis regulation and behavior are unlikely due to differential GR deletion in forebrain regions.
Figure 1.
Expression of GR in female mice. GR immunoreactivity in the CeA (A and B), PVN (C and D), medial prefrontal cortex (E and F) hippocampus (G and H), and basolateral amygdala of female littermate control (A, C, E, G and I) and FBGRKO (B, D, F, H and J) mice.
Circadian HPA axis activity and restraint stress-responses
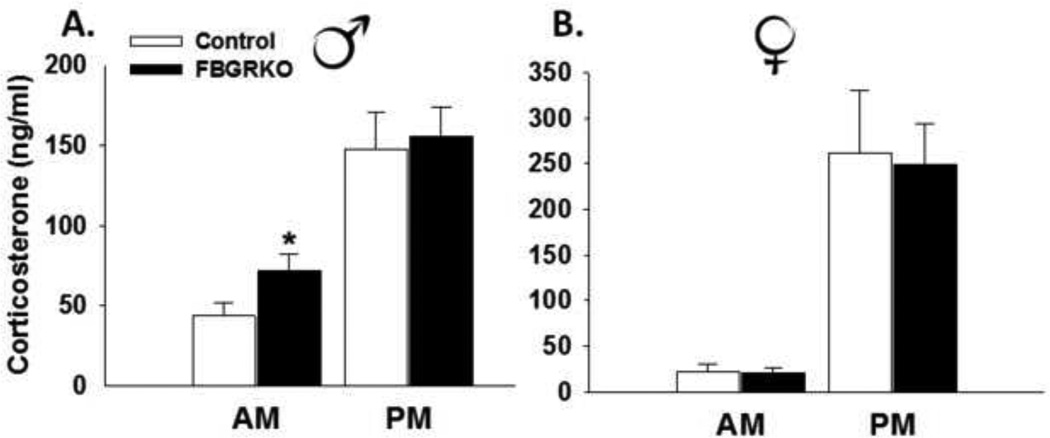
Male FBGRKO have increased basal AM corticosterone concentrations relative to control mice. There is no difference in peak corticosterone concentrations between male FBGRKO and control mice (Fig 2A, previously published from (Furay et al., 2008). Consistent with the males, both female FBGRKO and control mice have intact diurnal corticosterone rhythmicity (Fig. 2B) with both groups having significantly higher afternoon corticosterone concentrations (F(1,20)=26.46, p<0.05) relative to morning corticosterone levels. However, unlike males, there was no genotype effect nor was there a genotype×time interaction on nadir or peak corticosterone concentrations (p >0.05).
Figure 2.
(A) Nadir and peak corticosterone concentrations in male FBGRKO and littermate controls previously published in (Furay et al., 2008). Male FBGRKO have significantly elevated nadir corticosterone concentrations (p <0.05) relative to male littermate controls. There are no differences in peak corticosterone concentrations between male FBGRKO and littermate controls (p >0.05). (B) There is no difference in nadir or peak corticosterone concentrations between female FBGRKO and littermate controls (ps > 0.05) (n=11–12). **Although the basal neuroendocrine data from the males was previously published, the female and males were from the same study. As such, the data for the males is included for a side by side comparison.
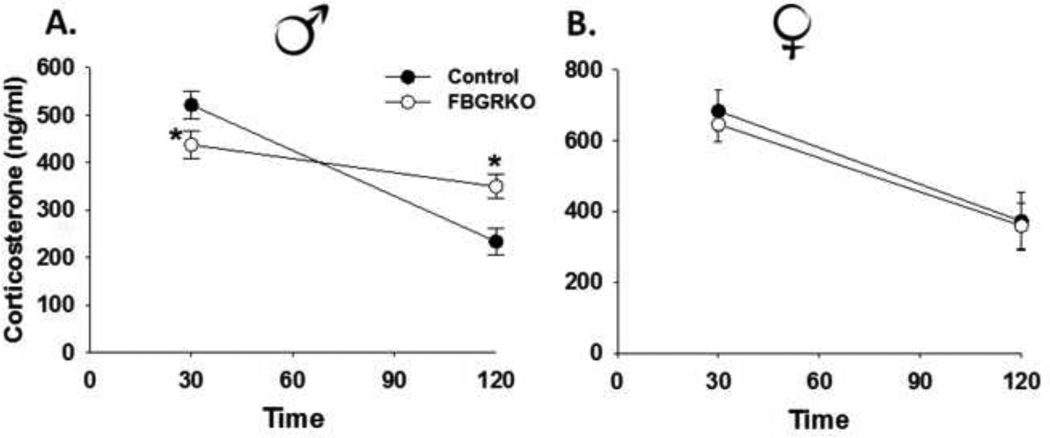
Male FBGRKO have significantly lower corticosterone concentrations 30 min post restraint stress relative to control mice. However, they exhibit a higher corticosterone response 120 min post restraint (Fig. 3A, previously published from (Furay et al., 2008)), suggesting an impairment in GR mediated feedback responses to stress in male FBGRKO. In contrast, there was no significant main effect of genotype or genotype by time interaction on restraint-stress induced corticosterone in females (p>0.05). However, there was a significant main effect of time (F(1,20)=10.68, p <0.05), with 30 min post restraint corticosterone concentrations being significantly higher than were the 120 min concentrations (Fig. 3B).
Figure 3.
(A) Plasma corticosterone concentrations in response to a 30-min restraint in male FBGRKO and littermate controls previously published (Furay et al., 2008). Male FBGRKO have significantly lower corticosterone at 30 min and significantly higher corticosterone concentrations at 120 min time points relative to male littermate controls (ps<0.05) and (B) female control and FBGRKO mice. Unlike male FBGRKO, there is no difference in restraint-induced corticosterone concentrations at the 30 or 120 min time points between FBGRKO female mice (p >0.05). All analyses were conducted such that the relative effect of the loss of forebrain GR was only examined within the same sex (n=11–12). **Although the stress-induced neuroendocrine data from the males was previously published, the female and males were from the same study. As such, the data for the males are included for a side by side comparison.
CRH and AVP In situ hybridization analyses
In order to examine the effect of forebrain GR loss on stress-related neuropeptide expression in male and female FBGRKO mice, we used adjacent brain sections from (Furay et al., 2008). Unlike previous findings which report increased CRH mRNA expression following restraint stress in male FBGRKO mice (Boyle et al., 2005; Boyle et al., 2006), we did not observe a difference in CRH or AVP mRNA expression between male FBGRKO and controls (p > 0.05) There was no effect of genotype of CRH mRNA expression in the PVN or CeA in female mice (p >0.05). Likewise, there was no genotype effect on AVP mRNA expression in the PVN. There was however a significant increase in AVP mRNA expression in the supraoptic nucleus (SON) in female FBGRKO mice compared with controls (t(14)=-3.42, p<0.01). The in situ hybridization data are presented in (Table 1).
Table 1.
In situ hybridization analyses for CRH and AVP expression (corrected gray level) in the paraventricular nucleus of hypothalamus (PVN), central amygdala (CeA) and supraoptic nucleus (SON) of male and female FBGRKO and littermate controls.
| Male | Female | |||
|---|---|---|---|---|
| Control | FBGRKO | Control | FBGRKO | |
| CRH | ||||
| PVN | 44.55±4.88 | 33.22±3.81 | 43.07±3.73 | 48.17±2.76 |
| CeA | 30.77±2.58 | 27.44±2.43 | 41.15±3.34 | 44.29±3.99 |
| AVP | ||||
| PVN | 75.63±8.82 | 72.39±8.86 | 92.33±8.57 | 100.74±4.89 |
| SON | 102.14±5.59 | 90.47±11.9 | 103.99±6.25 | 129.97±3.71* |
All data are expressed as mean ± SEM.
indicates statistical significance, p< 0.05.
Forced swim test and sucrose consumption/preference tests
Despair-like and anhedonia-like behaviors in male
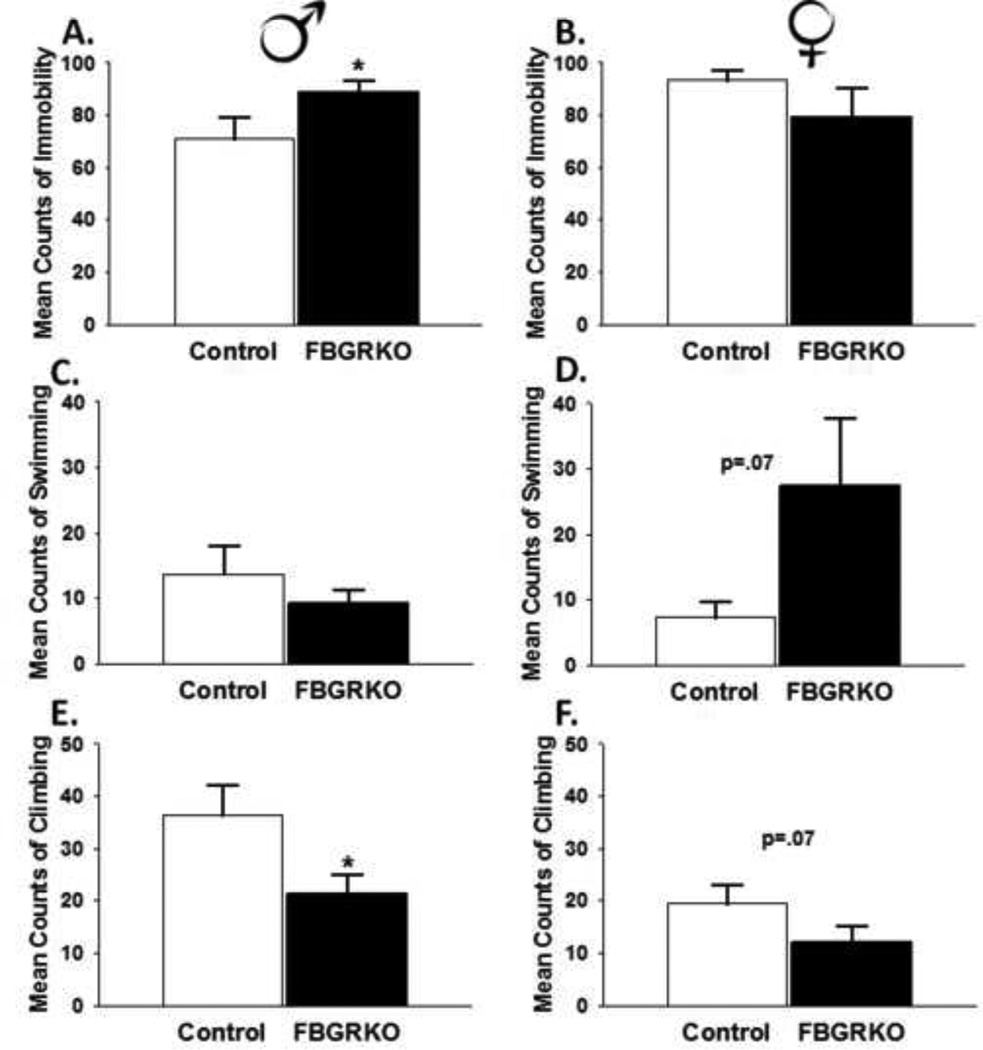
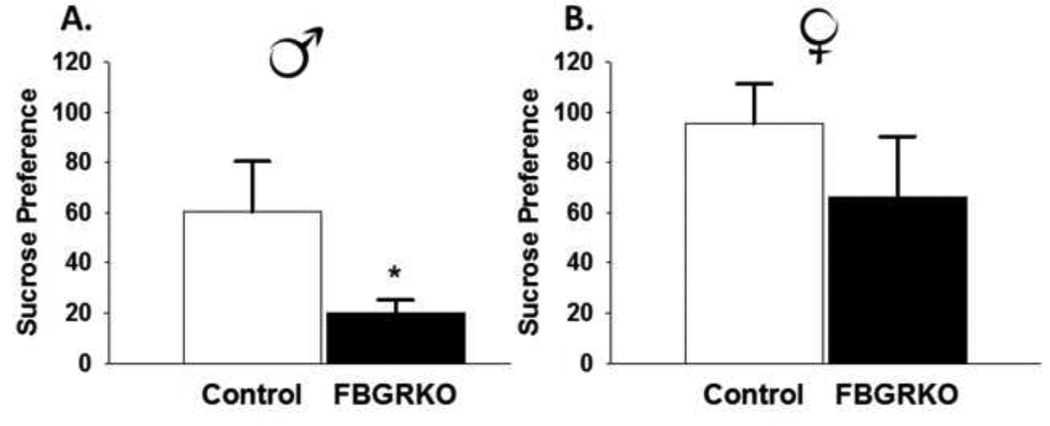
We used the FST to assess despair-like behavior in FBGRKO mice. Within this task, increased immobility and decreased activity are interpreted as increases in helplessness behavior(Porsolt, Le Pichon, and Jalfre, 1977). Male FBGRKO mice spent more time immobile (t(14)=2.02, p <0.05) and significantly less time climbing (t(14)=−2.18, p <0.05) (Fig. 4A and 3E, respectively) relative to controls. There was no significant difference in swimming time between male FBGRKO and controls (p >0.05) (Fig. 4C). To assess anhedonia, we used the sucrose preference test. Male FBGRKO consumed significantly less sucrose (t(13)=1.8, p=.05) compared with their littermate controls (Fig. 5A).
Figure 4.
FST Behavioral Analyses. (A) Male FBGRKO mice are significantly more immobile compared to male controls (*p <0.05). (B) In contrast, female FBGRKO and control female mice do not differ in their display of immobility (p >0.05). (C) There was no significant difference in swimming between male FBGRKO and their respective controls (p >0.05). (D) However, there was a trend for female FBGRKO mice to swim more compared with their respective controls (p=0.07). (E) Male FBGRKO displayed less climbing than male controls (*p<0.05). (F) There was a trend for female FBGRKO to display less climbing compared with their respective controls (p =0.07). All analyses were conducted such that the relative effect of the loss of forebrain GR was only examined within the same sex (n=7–8).
Figure 5.
Sucrose Consumption and Preference. (A) Male FBGRKO had decreased sucrose preference compared with male controls (p <0.05). (B) Female FBGRKO do not differ from their respective controls in with regards to sucrose preference (p >0.05). All analyses were conducted such that the relative effect of the loss of forebrain GR was only examined within the same sex (n=7–8).
Despair-like behavior and anhedonia in females
Unlike male FBGRKO, female FBGRKO do not differ from controls in FST immobility time (p >0.05) (Fig. 4B). There was no significant effect of genotype on swimming (Fig. 4D) or climbing (Fig. 4F) in female mice. Consistent with a lack of despair-like behavior in female FBGRKO, we did not observe decreased sucrose preference (p>0.05; Fig 5B).
Discussion
Our results demonstrate a sex difference in the role of forebrain GR in regulating HPA axis activity and depression-like behavior. Previous data from our laboratory (Furay et al., 2008) and others, (Boyle et al., 2005; Boyle et al., 2006) report elevated basal corticosterone secretion and/or enhanced stress responsiveness in male FBGKRO mice relative to controls. In contrast, here, we report no differences in HPA function in female FBGRKOs. In addition, enhanced depression-like behaviors (helplessness, anhedonia) were observed in males, but not females. Immunohistochemical analyses revealed that both female and male FBGRKO mice have a similar degree of knockdown of GR in the same neural regions including the hippocampus, medial prefrontal cortex and basolateral amygdala, indicating no sex differences in the loss of GR expression. These data indicate that forebrain GR modulates HPA axis function and mood in a sex dependent manner.
In order to determine the underlying molecular mechanisms that may account for the sex difference in HPA axis function and behavior in FBGRKO mice, we examined central stress peptide expression. Based on previous data, (Boyle et al., 2006) as well as the neuroendocrine and behavioral profiles in male FBGRKO mice, we expected to see increased CRH mRNA expression in the PVN. However, this was not the case, as there were no significant differences between FBGKRO and control mice of either sex. This lack of a genotype effect of PVN CRH mRNA following restraint stress might reflect differences in when the animals were sacrificed post restraint (i.e., 60 min vs 120 min). Consistent with previous findings (Boyle et al., 2006), we also did not see a significant effect of stress on AVP mRNA expression in the PVN or amygdalar CRH mRNA expression in FBGRKO mice versus controls. For reasons that we can not explain, we did however observe increased AVP mRNA expression in the SON in female FBGRKO mice relative to female control mice.
Female FBGRKO mice do not exhibit basal HPA axis dysregulation or exaggerated stress responses. The data suggest that the presence of ovarian hormones and/ or lack of androgens in females confer protection from the loss of GR signaling on HPA axis activity in these critical regions. Importantly, GR signaling is modulated by ovarian hormones (Burgess and Handa, 1992). For example, estrogens can either enhance or dampen HPA axis activity via binding to ERά or ERβ, respectively (for review; see (Weiser, Foradori, and Handa, 2008)). Our results suggest that in females, GR regulation of HPA axis function and behavior is subserved by brain regions other than those targeted in the FBGRKO mouse. Along these same lines, a potential explanation for a lack of an effect on HPA axis function and mood-like behavior in female FBGRKO may be due to increased ER signaling in females. While the role of estrogens and activation of their cognate receptors with regards to HPA axis activation and mood are controversial at best, recent studies suggest that activation of ERβ prevents the GR dependent effects of the central amygdala on anxiety-like behaviors and increased stress responsiveness (Weiser, Foradori, and Handa, 2010). Consistent with this fact, studies using a viral vector expressing a chimeric estrogen-glucocorticoid receptor suggests that increased estrogen signaling protects against the glucocorticoid -induced impairments in spatial and fear memory formation (Ferguson, Lin, and Sapolsky, 2008; Rodrigues and Sapolsky, 2009). This virus strategy is accomplished by combining the ligand binding domain of GR and the DNA binding domain of the ER such that the damaging effects of glucocorticoid hyperactivity are transferred into beneficial estrogenic signaling. In addition, chimeric estrogen-glucocorticoid expression in the amygdala prevents glucocorticoid mediated increases in anxiety-like behavior and alterations in dendritic morphology of basoloateral amygdala neurons (Anacker, Zunszain, Carvalho, and Pariante, 2010; Mitra and Sapolsky, 2010) These studies provide evidence suggesting opposing actions of the two steroids in corticolimbic regions, and suggests that estrogen may protect against the potentially damaging effects from the loss GR on HPA axis regulation and depression-like behavior. Clearly, more studies are warranted to further determine the actions of central ERs in the absence of GR signaling in forebrain areas.
Alternatively, the sensitivity of males and females to limbic forebrain glucocorticoid signals may be gated subcortically. For example, in male rats, androgens are important inhibitors of the HPA axis in the medial preoptic area (Viau and Meaney, 1996), and may make the male more sensitive to forebrain afferent control than females. The combination of HPA axis and behavioral dysfunction in male FBGRKO mice suggests that the deleterious actions on depression behavior in males may be due to elevated glucocorticoids produced by the inability to effectively terminate the stress response. Thus, it is possible that differences in central feedback signaling in females may be serially linked to the lack of a behavioral phenotype, in that the female FBGRKO are not behaving in the context of chronically or episodically elevated glucocorticoid levels. This interpretation assumes that the effects of behavioral effects of GR in females are mediated by other GR-sensitive sites not targeted by the FBGRKO. At this point, a gonadal hormone dependent mechanism is purely speculative because we didn’t specifically evaluate the effects of gonadal hormones on HPA axis function or depression-like behavior in female (i.e., estrous cycle, ovariectomy) or male (i.e., castration) FBGRKO.
Conclusions
Decreased GR signaling in critical forebrain regions is implicated in affective disorders in humans. Consistent with this notion, knockdown of GR in the medial prefrontal cortex, hippocampus and basolateral amygdala induces depression-like behaviors in male mice (Boyle et al., 2005). Our data replicate these findings in males, but not females, suggesting that this mechanism for development of mood disorders only holds in males. These data suggest that the development of HPA axis and mood symptoms of depression is mediated by an alternative brain circuitry and/or different molecular substrate in females. One potential shortcoming of the present study is that we investigated HPA axis dysregulation and depression-like behavior in relatively mildly stressed animals (i.e., restraint and swim stress). Given that depression is preceded or exacerbated by chronic stress and that females are suggested to be more stress sensitive, future studies will examine whether a history of chronic stress interacts with the loss of forebrain GR to induce a neuroendocrine or behavioral depression-like phenotype.
Another potential limitation of the present study is that females were randomly cycling during both the HPA axis and behavioral tests. As with many knockout lines, we did not have enough females of either genotype to carefully stage a thorough estrous-cycle dependent study. We acknowledge that gonadal hormones may have influenced both HPA axis responses on the day of the restraint challenge and mood-like behavior during both the FST and sucrose preference tests. However, we do not believe that this lessens the overall significance of the findings, particularly because the sucrose preference test was done over the course of 10 days. Future studies will investigate the role of gonadal hormones with gonadectomized FBGRKO mice. Overall, the data suggest a different organization of glucocorticoid signaling in stress circuitry of males and females. Although we admit that the interpretation of the findings are somewhat counter-intuitive, given the female-biased increase in mood disorders, these findings may be an important first step in furthering our understanding regarding sex differences in the molecular neurochemistry and/or neural regulation of stress responsiveness and emotional behavior.
Highlights.
Forebrain glucocorticoid receptors modulate acute stress responses in males, but not females
Forebrain glucocorticoid receptors regulate depression-like behavior in males, but not females
Females utilize different neurocircuitry and/or receptor system to modulate stress responses
Acknowledgements
This work was supported by NIH MH 049698 to JPH and MH 069727 to JPH and MBS. The authors would like to thank Dr. Louis J. Muglia (Vanderbilt University) for the original breeding pairs of the FBGRKO mice.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anacker C, Zunszain PA, Carvalho LA, Pariante CM. The glucocorticoid receptor: Pivot of depression and of antidepressant treatment. Psychoneuroendocrinology. doi: 10.1016/j.psyneuen.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anacker C, Zunszain PA, Carvalho LA, Pariante CM. The glucocorticoid receptor: pivot of depression and of antidepressant treatment. Psychoneuroendocrinology. 2010;36(3):415–425. doi: 10.1016/j.psyneuen.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle MP, Brewer JA, Funatsu M, Wozniak DF, Tsien JZ, Izumi Y, Muglia LJ. Acquired deficit of forebrain glucocorticoid receptor produces depression-like changes in adrenal axis regulation and behavior. Proc Natl Acad Sci U S A. 2005;102(2):473–478. doi: 10.1073/pnas.0406458102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle MP, Brewer JA, Vogt SK, Wozniak DF, Muglia LJ. Genetic dissection of stress response pathways in vivo. Endocr Res. 2004;30(4):859–863. doi: 10.1081/erc-200044120. [DOI] [PubMed] [Google Scholar]
- Boyle MP, Kolber BJ, Vogt SK, Wozniak DF, Muglia LJ. Forebrain glucocorticoid receptors modulate anxiety-associated locomotor activation and adrenal responsiveness. J Neurosci. 2006;26(7):1971–1978. doi: 10.1523/JNEUROSCI.2173-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer JA, Khor B, Vogt SK, Muglia LM, Fujiwara H, Haegele KE, Sleckman BP, Muglia LJ. T-cell glucocorticoid receptor is required to suppress COX-2-mediated lethal immune activation. Nat Med. 2003;9(10):1318–1322. doi: 10.1038/nm895. [DOI] [PubMed] [Google Scholar]
- Burgess LH, Handa RJ. Chronic estrogen-induced alterations in adrenocorticotropin and corticosterone secretion, and glucocorticoid receptormediated functions in female rats. Endocrinology. 1992;131(3):1261–1269. doi: 10.1210/endo.131.3.1324155. [DOI] [PubMed] [Google Scholar]
- Carroll BJ. Dexamethasone suppression test for depression. Adv Biochem Psychopharmacol. 1984;39:179–188. [PubMed] [Google Scholar]
- Choi DC, Furay AR, Evanson NK, Ostrander MM, Ulrich-Lai YM, Herman JP. Bed nucleus of the stria terminalis subregions differentially regulate hypothalamic-pituitary-adrenal axis activity: implications for the integration of limbic inputs. J Neurosci. 2007;27(8):2025–2034. doi: 10.1523/JNEUROSCI.4301-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchlow V, Liebelt RA, Bar-Sela M, Mountcastle W, Lipscomb HS. Sex difference in resting pituitary-adrenal function in the rat. Am J Physiol. 1963;205(5):807–815. doi: 10.1152/ajplegacy.1963.205.5.807. [DOI] [PubMed] [Google Scholar]
- Earls F. Sex differences in psychiatric disorders: origins and developmental influences. Psychiatr Dev. 1987;5(1):1–23. [PubMed] [Google Scholar]
- Ferguson D, Lin S, Sapolsky R. Viral vector-mediated blockade of the endocrine stress-response modulates non-spatial memory. Neurosci Lett. 2008;437(1):1–4. doi: 10.1016/j.neulet.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furay AR, Bruestle AE, Herman JP. The role of the forebrain glucocorticoid receptor in acute and chronic stress. Endocrinology. 2008;149(11):5482–5490. doi: 10.1210/en.2008-0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handa RJ, Burgess LH, Kerr JE, O'Keefe JA. Gonadal steroid hormone receptors and sex differences in the hypothalamo-pituitary-adrenal axis. Horm Behav. 1994;28(4):464–476. doi: 10.1006/hbeh.1994.1044. [DOI] [PubMed] [Google Scholar]
- Herman JP, Ostrander MM, Mueller NK, Figueiredo H. Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29(8):1201–1213. doi: 10.1016/j.pnpbp.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Jankord R, Solomon MB, Albertz J, Flak JN, Zhang R, Herman JP. Stress vulnerability during adolescent development in rats. Endocrinology. 2011;152(2):629–638. doi: 10.1210/en.2010-0658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Swartz M, Blazer DG, Nelson CB. Sex and depression in the National Comorbidity Survey. I: Lifetime prevalence, chronicity and recurrence. J Affect Disord. 1993;29(2-3):85–96. doi: 10.1016/0165-0327(93)90026-g. [DOI] [PubMed] [Google Scholar]
- Kitay JI. Sex differences in adrenal cortical secretion in the rat. Endocrinology. 1961;68:818–824. doi: 10.1210/endo-68-5-818. [DOI] [PubMed] [Google Scholar]
- Mitra R, Sapolsky RM. Expression of chimeric estrogen-glucocorticoidreceptor in the amygdala reduces anxiety. Brain Res. 2010;1342:33–38. doi: 10.1016/j.brainres.2010.03.092. [DOI] [PubMed] [Google Scholar]
- Modell S, Yassouridis A, Huber J, Holsboer F. Corticosteroid receptor function is decreased in depressed patients. Neuroendocrinology. 1997;65(3):216–222. doi: 10.1159/000127275. [DOI] [PubMed] [Google Scholar]
- Musselman DL, Nemeroff CB. Depression and endocrine disorders: focus on the thyroid and adrenal system. Br J Psychiatry Suppl. 1996;(30):123–128. [PubMed] [Google Scholar]
- Pariante CM, Miller AH. Glucocorticoid receptors in major depression: relevance to pathophysiology and treatment. Biol Psychiatry. 2001;49(5):391–404. doi: 10.1016/s0006-3223(00)01088-x. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;26(5604):730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- Rodrigues SM, Sapolsky RM. Disruption of fear memory through dualhormone gene therapy. Biol Psychiatry. 2009;65(5):441–444. doi: 10.1016/j.biopsych.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viau V, Meaney MJ. The inhibitory effect of testosterone on hypothalamic-pituitary-adrenal responses to stress is mediated by the medial preoptic area. J Neurosci. 1996;16(5):1866–1876. doi: 10.1523/JNEUROSCI.16-05-01866.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vreeburg SA, Hoogendijk WJ, van Pelt J, Derijk RH, Verhagen JC, van Dyck R, Smit JH, Zitman FG, Penninx BW. Major depressive disorder and hypothalamic-pituitary-adrenal axis activity: results from a large cohort study. Arch Gen Psychiatry. 2009;66(6):617–626. doi: 10.1001/archgenpsychiatry.2009.50. [DOI] [PubMed] [Google Scholar]
- Walf AA, Frye CA. Antianxiety and antidepressive behavior produced by physiological estradiol regimen may be modulated by hypothalamic-pituitaryadrenal axis activity. Neuropsychopharmacology. 2005;30(7):1288–1301. doi: 10.1038/sj.npp.1300708. [DOI] [PubMed] [Google Scholar]
- Weiser MJ, Foradori CD, Handa RJ. Estrogen receptor beta in the brain: from form to function. Brain Res Rev. 2008;57(2):309–320. doi: 10.1016/j.brainresrev.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser MJ, Foradori CD, Handa RJ. Estrogen receptor beta activation prevents glucocorticoid receptor-dependent effects of the central nucleus of the amygdala on behavior and neuroendocrine function. Brain Res. 2010;1336:78–88. doi: 10.1016/j.brainres.2010.03.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulsin AC, Herman JP, Solomon MB. Mifepristone decreases depression-like behavior and modulates neuroendocrine and central hypothalamic-pituitaryadrenocortical axis responsiveness to stress. Psychoneuroendocrinology. 35(7):1100–1112. doi: 10.1016/j.psyneuen.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulsin AC, Herman JP, Solomon MB. Mifepristone decreases depression-like behavior and modulates neuroendocrine and central hypothalamic-pituitary-adrenocortical axis responsiveness to stress. Psychoneuroendocrinology. 2010;35(7):1100–1112. doi: 10.1016/j.psyneuen.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]