ABSTRACT
OBJECTIVES
Despite the known adverse effects of sleep deprivation on recovery from illness, studies have shown that sleep deprivation remains an incompletely addressed problem among acutely ill inpatients. Behavioral interventions are recommended as first-line therapy prior to using pharmacologic therapy due to the side effects of sedative hypnotics. The objective of this systematic review was to identify non-pharmacologic interventions that have been used to improve sleep quality and quantity of non-intensive care unit (ICU) inpatients.
DATA SOURCES
PubMed, Embase, Web of Science, CINAHL, and Cochrane Library through January 2013; manual searches of reference lists.
STUDY ELIGIBILITY CRITERIA, PARTICIPANTS, INTERVENTIONS
Any study in which a non-pharmacologic intervention was conducted in a general inpatient setting, and nighttime sleep quantity or quality was assessed.
STUDY APPRAISAL AND SYNTHESIS METHODS
Information on study design, populations, interventions, comparators, outcomes, time frame, and risk of bias were independently abstracted by two investigators.
RESULTS
13 intervention studies with 1,154 participants were included. Four studies were randomized controlled trials. Seven studies had a low to medium risk of bias, and there was significant heterogeneity in the interventions. Relaxation techniques improved sleep quality 0–38 %, interventions to improve sleep hygiene or reduce sleep interruptions improved sleep quantity 5 %, and daytime bright light exposure improved sleep quantity 7–18 %.
LIMITATIONS
The heterogeneity in the types and dose of interventions, outcome measures, length of follow-up, differences in patient populations, and dearth of randomized trials may dilute effects seen or make it more difficult to draw conclusions.
CONCLUSIONS AND IMPLICATIONS OF KEY FINDINGS
There is insufficient to low strength of evidence that any non-pharmacologic intervention improves sleep quality or quantity of general inpatients. Further studies are needed in this area to guide clinicians.
Electronic supplementary material
The online version of this article (doi:10.1007/s11606-013-2640-9) contains supplementary material, which is available to authorized users.
KEY WORDS: sleep deprivation, inpatient sleep, hospital sleep, hospital medicine, systematic review
INTRODUCTION
Adequate levels of sleep are needed in both health and illness. Sleep deprivation is known to have multiple harmful physiological effects, including a decline in immune function, memory, wound healing, vitality and strength, along with increased insulin resistance, pain perception and mortality. It increases our risk of illness and slows our recovery from illness. 1–9 There is also evidence that hospitalization is a risk factor for insomnia that remains for months or years after discharge.10,11
Despite these adverse effects on health and recovery, a number of studies have shown that sleep deprivation remains an incompletely addressed problem among acutely ill patients admitted to hospitals.12 A study of 100 hospitalized patients in a Canadian general or family practice ward showed that their level of sleep quality was not only worse than that of non-hospitalized US adults, but also comparable to non-hospitalized US insomniacs.13 A larger study performed at three British hospitals demonstrated that though 65 % of the patients reported sleeping well at home most of the time, 63 % of general medical ward patients, 79 % of surgical patients, and 57 % of acute psychiatric ward patients reported difficulty sleeping through the night at the hospital.14
Not only are patients deprived of sleep when hospitalized, but they also may suffer adverse health outcomes or impaired recovery as a result.9–21 In a prospective trial of elderly patients in a Japanese geriatric hospital, it was demonstrated that the 2-year survival for patients who suffered from nighttime insomnia and sleep onset delay was significantly decreased as compared to the survival of normal sleepers, even when age, gender, and their activity of daily living were accounted for.16
Sedative hypnotics are prescribed by a large majority of providers on an as needed basis, and utilized by 31–88 % of hospitalized patients.17 Yet, a recent meta-analysis and other studies show that adverse events, including cognitive events such as memory loss, confusion, and disorientation; psychomotor-type events, such as reports of dizziness, loss of balance, or falls; and reports of daytime fatigue were all more common with sedative use in elderly patients with insomnia as compared to placebo.22,23
Studies aiming to pinpoint the major causes of sleep deprivation among hospitalized patients have implicated both personal and external factors. Personal factors include pain, discomfort, needing to use the toilet, and anxiety.14,18,19 External factors include noise, medication timing, and lighting.14,18,24
It is unclear to what degree improving sleep among hospitalized patients improves outcomes. It is currently recommended that one first start with non-pharmacologic therapies for patients in whom insomnia is a concern before initiating pharmacologic therapy.20,21,25,26 The objective of this systematic review was to identify non-pharmacologic interventions that have been used to improve sleep in non-intensive care unit (ICU) inpatients, and their effects on sleep quantity and quality.
METHODS
Data Sources and Searches
Studies were identified through January 2013 using the following databases: PubMed, Embase, Web of Science, CINAHL, and Cochrane Library. The four main concepts searched within each library were sleep, insomnia, inpatient, and adult. Each of these concepts was expanded in a manner to ensure that the vocabulary was appropriate for the database and that the searches were as uniform as possible between databases (Figure A-1, available online). Relevant references were also checked manually.
Study Selection
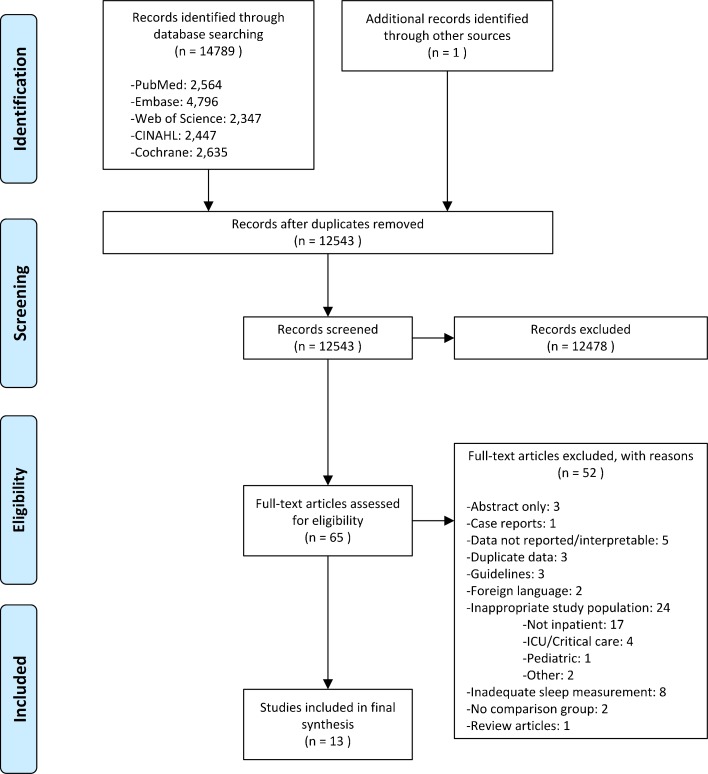
Two reviewers independently performed a title/abstract review. Studies were included if they were clinical trials that used a non-pharmacologic intervention to improve sleep in non-critically ill inpatients. If these criteria were unclear based on title and abstract review, they were included for full paper review. Any conflicts were discussed until a consensus was reached (Fig. 1).
Figure 1.
Summary of evidence search and selection.
Both reviewers independently performed a full paper review of all included studies. Studies were further screened at this stage and included if they met the aforementioned criteria, were in English, directly measured nighttime sleep quantity or quality, and used a study design that included a comparison group (Fig. 1). Measures of sleep quantity include polysomnography, actigraphy, self-report, or observer report. Measures of sleep quality include self-report scales.
Data Extraction and Quality Assessment
We created a detailed set of evidence tables containing all information abstracted from eligible studies by two independent reviewers. We assessed the risk of bias independently and in duplicate; conflicts were resolved by consensus. To assess the risk of bias, we used similar but separate criteria for randomized controlled trials and nonrandomized trials, based on guidance from the Agency for Healthcare Research and Quality (AHRQ) Methods Guide and Cochrane’s Effective Practice and Organization of Care (EPOC) reviews.27,28 For randomized controlled trials (RCTs), we emphasized four major and four minor criteria that we deemed important for a behavioral intervention (Table 1). To be rated as low risk of bias, a trial had to satisfy a minimum of three major and three minor criteria.
Table 1.
List of Major and Minor Criteria in Assessing Risk of Bias of Randomized Controlled Trials
| Major criteria* | Minor criteria* |
|---|---|
| • Was the control matched for time and attention by the instructors? • Was there a description of withdrawals and dropouts? • Was attrition < 20 % at the end of treatment? As several studies did not calculate attrition starting from the original number randomized, we recalculated the attrition from the original number randomized. • Were those who collected data on the participants blind to the allocation? |
• Was the method of randomization described in the paper? To answer “yes” for this question, the trials had to give some description of the randomization procedure. • Was allocation concealed? • Was intent-to-treat analysis used? To answer “yes” for this question, the trial must impute non-completer or other missing data, and it must do this from the original number randomized. • Did the trial evaluate the credibility, and if so, was it comparable? If the trial did not evaluate credibility, or if it evaluated credibility but did not find it comparable, then we did not give the trial a point. |
*We assigned two points each to the major criteria, weighting them more in assessing risk of bias. We assigned one point each to the minor criteria. Studies could therefore receive a total of 12 points. If studies met a minimum of three questions from major and three from minor (9–12 points), we assigned it a grade of “low risk of bias.” For studies ranging from six to eight points, we assigned a “medium risk of bias,” and for studies scoring five points or less, we assigned a “high risk of bias.”
For nonrandomized studies we created an algorithm adapted from the Cochrane EPOC reviews and AHRQ Methods Guide that assessed risk of bias in the following domains: representativeness of study population, selection of the comparison group, comparability of cohorts, blinded outcome assessment, completeness of follow-up, adequate description of intervention, reporting bias, and other potential sources of bias (Figure A-2, available online). The criteria were organized in a way to set a higher bar for nonrandomized studies to obtain a low risk of bias than for randomized trials. For example, if any of the seven major criteria, such as single blinding or description of withdrawals and dropouts, were not met, the study was rated as high risk of bias. If all seven major criteria were met, but any of the four minor criteria were not met, the study was rated as medium risk of bias. If all major and minor criteria were met, the study was rated as low risk of bias. Risk of bias was qualitatively averaged across studies to obtain an overall risk of bias for the group of studies within a domain of interventions.
Two reviewers graded the strength of evidence of each outcome for each of the intervention domains using the grading scheme recommended by AHRQ.29 In assigning evidence grades, we considered the four domains of risk of bias, directness of measures, consistency of results, and precision (Figure A-3, available online). We classified evidence into four categories: 1) “High” grade (indicating high confidence that the evidence reflects the true effect, and further research is very unlikely to change our confidence in the estimate of the effect); 2) “Moderate” grade (indicating moderate confidence that the evidence reflects the true effect, and further research may change our confidence in the estimate of the effect and may change the estimate); 3) “Low” grade (indicating low confidence that the evidence reflects the true effect, and further research is likely to change our confidence in the estimate of the effect and is likely to change the estimate); and 4) “Insufficient” grade (evidence is unavailable or inadequate to draw a higher grade).29
Consistency was assessed by comparing the general direction of effect. In assessing directness, self-report surveys were considered direct measures of sleep quality. Both actigraphy and polysomnography were considered direct measures of sleep quantity; all other outcomes were considered indirect. Due to the paucity of studies, we did not perform meta-analysis. We defined precision as at least 51 % of the studies in a group having significant results with the same overall direction of effect.
Data Synthesis
To estimate the direction and magnitude of difference between treatment and control groups, we calculated relative percent difference scores. This was calculated by dividing the end-line (post-intervention) differences between the intervention and control groups by the end-line value of the control group. We were unable to account for baseline differences, since many studies did not report baseline data. We also calculated standardized mean differences (effect sizes) using the Hedge’s g method for studies that provided enough information for the calculation.
RESULTS
Study Characteristics
Our search identified 13 studies (Table 2) utilizing four different study designs: randomized controlled trials, nonrandomized trials with a concurrent control group, nonrandomized trials with a prospectively obtained historical control group, and pre-intervention–post-intervention trials, in which outcomes were recorded prior to and after intervention implementation within the same group.
Table 2.
Characteristics and Results of Included Studies
| Study design* (Sample size) | Intervention | Comparator | Ward type (Mean age) | Sleep outcome† | Relative % change post-intervention (effect size)‡, §, ‖ | P value | ROB** | |
|---|---|---|---|---|---|---|---|---|
| Relaxation techniques | ||||||||
| Lareau 2008 | RCT (n = 70) | Quiet time, room temp changes, relaxation techniques each night of hospital stay | Usual care | Cardiology & medical (80) | RCSQ | 7 (.11) | 0.667 | M |
| Soden 2004 | RCT (n = 42) | Massage or aromatherapy + massage weekly × 4 weeks | Usual care | Palliative care (73) | VSH | NR | NR | M |
| Toth 2007 | RCT (n = 30) | Audiotape guided imagery for 20 min twice daily × 2 days | Reading, music, or other solitary activity | General medical (54) | RCSQ | 0 | 0.34¶ | H |
| Zimmerman 1996 | RCT (n = 96) | Music video for 30 min × 2 evenings | Scheduled 30 min rest period | Post-surgical (67) | RCSQ | 28 (.70) | < 0.05 | M |
| Williamson 1992 | NRC (n = 60) | White noise all night × 3 nights | Usual Care | Post-surgical (59) | RCSQ | 38 | 0.002* | M |
| Smith 2002 | NRHC (n = 41) | Massage for 15–20 min ×3 spread over 1 week | Nurse interaction for attention control | Oncology (62) | VSH | 20 (.63) | NR | H |
| Connell 2001 | Pre-post (n = 58) | Aromatherapy each night × 1 week | Baseline | Elderly care units NR | TST (OSS) | 10 | 0.004 | H |
| McDowell 1998 | Pre-post (n = 111) | Back rub, warm drink, relaxation tapes each night patient complained of insomnia | None | Medical (79) | PTSS | NR | < 0.001# | L |
| Sleep hygiene/Reduced sleep interruption | ||||||||
| Bartick 2010 | NRHC (n = 267) | Quiet Time 10 pm–6 am each night of hospital stay | Usual Care | Medical-surgical (61) | VSH | NR | NS | H |
| Edinger 1989 | NRHC (n = 321) | Stimulus Control × mean of 35 days | Usual Care | Psychiatric NR | TST (OSS) | 5 (.86) | NR | H |
| Daytime bright light | ||||||||
| Mishima 1994 | Pre-post (n = 24) | 2 h artificial light therapy daily × 4 weeks | Baseline | Psychiatric (75) | TST in demented patients (OSS) | 18 (.79) | 0.01 | H |
| Wakamura 2001 | Pre-post (n = 7) | 5 h artificial light therapy daily × 1 week | Baseline | Chest disease (67) | TST (WA) | 7 | 0.05 | L |
| Yamadera 2002 | Pre-post (n = 27) | 2 h artificial light therapy daily × 4 weeks | Baseline | Not specified (80) | % night spent sleeping (WA) | 10 (.31) | < 0.05 | M |
* RCT randomized controlled trial, NRC non-randomized trial with a control group, NRHC non-randomized trial with a historical control
† OSS an observer sleep scale in which a third party observer records the outcome; PTSS patient sleep scale in which outcome is patient-recorded; RCSQ Richards Campbell Sleep Questionnaire; TST total sleep time in minutes; VSH Verran and Snyder-Halpern Sleep scale; WA wrist actigraphy
‡This is a relative percent difference. For RCT, NRC, and NRHC, we use the end-line differences divided by the end-line value of the control group. This relative percent difference is calculated by: (interventionpost - controlpost)/(Controlpost). For pre–post studies, it is calculated by: (post-baseline)/(baseline). The trial by Edinger, which used a historical control, is an exception. It did not provide enough data to use the former equation, and was thus treated as a pre–post study for this calculation. Positive values indicate improvement
§ NR not reported or there was insufficient information to calculate a percent change; NS not significant (exact values not reported)
‖Hedge’s g effect sizes were calculated for studies that provided enough data to calculate it
¶Significance values are for a group by time test. For the remaining studies, the significance value is for the test between groups at the concluding follow-up time
#This paper evaluated significance by a correlation test
** ROB Risk of Bias; H High risk of bias; M Medium risk of bias; L low risk of bias
Three general types of interventions were used. Relaxation techniques included massage, some type of sound such as music or white noise, aromatherapy, warm drink, or some combination of these modalities. A second type attempted to reduce interruptions to sleep by creating a quiet time at night or helping an individual improve sleep hygiene. These two study types were largely conducted in the United States or Europe. A third type was the exposure to artificial bright light therapy during the day to improve sleep at night. These studies were all conducted in Japan. All of these interventions were conducted among medical, psychiatric, or surgical patients.
Three self-reported sleep quality scales were used (Table 3).
Table 3.
Description of Patient Self-Reported Sleep Quality Scales
| Scale | Description | Better sleep indicated by (Higher/Lower) score | Consistency/Reliability |
|---|---|---|---|
| Richards Campbell Sleep Questionnaire (RCSQ) | Visual analog scale ranging from 0 to 100* | Higher† | Crohnbach’s alpha of 0.90 |
| Verran Snyder Halpern sleep scale | Visual analog scale ranging from 0 to100 | Higher | Theta reliability of 0.82 |
| McDowell et al.35 patient sleep survey | Overall rating of the quality sleep on an ordinal scale: poor/fair/good sleep + details on any sleep problems. | N/A | NR |
*Lareau et al. utilized RCSQ on scale ranging from 0 to 500. Zimmerman et al. used scale ranging from 0 to 10
†Lareau et al. utilized RCSQ in opposite manner, with lower scores indicating better sleep
Relaxation Techniques
Four RCTs evaluated sleep quality using varying relaxation techniques, showed 0 to 28 % improvement in sleep quality overall. Lareau et al.30 randomized 70 patients to a usual care control versus nighttime intervention consisting of decreased noise and light, room temperature adjustment, minimizing unnecessary interruptions, clustering of nursing activities, relaxation techniques, and personal hygiene. In the intervention arm, sleep quality improved by 7 % (ns). The number of sleep medications used decreased from 2.2 in the control group to 1.6 in the intervention group (p = 0.04). Soden et al.31 randomized 42 cancer patients on hospice units to three arms: combined aromatherapy and massage once weekly for 4 weeks, massage only, or usual care. There were no differences between the groups over the study period; however, the massage groups slept better on the night of the massage (p = 0.02). The magnitude of improvement was not discernible. Toth et al.32 randomized 30 patients on a general medical ward to audiotape guided imagery for 20 min twice daily or a solitary activity of choice twice daily over a 2-day period. There were no differences between the groups. Zimmerman et al.33 randomized 96 post-coronary artery bypass grafting (CABG) patients to 30 min of music headphones, a soothing music video, or a 30-min rest period. The Richards Campbell Sleep Questionnaire (RCSQ) score was 28 % improved in the music video group compared to the control on the third postoperative day (p < 0.05).
Four nonrandomized studies found a 10–38 % improvement in sleep quality using relaxation techniques. Williamson et al.34 systematically assigned every other of 60 post-CABG surgery patients to usual care or a soft white noise, such as ocean sounds, that played throughout the night. They reported a 38 % improvement in RCSQ scores as compared to the control group (p = 0.002). McDowell et al.35 assessed 111 patients admitted to a medical ward who had difficulty sleeping during at least one night of their hospitalization. They were offered their choice of all or portions of a three-part intervention consisting of a five-min back massage, herbal tea or milk, and relaxation tapes that played classical music or nature sounds. Patients who declined all parts of the intervention were treated as the control group. Thus, this formed a usual care control group. Sleep quality was assessed by patients’ self-rating of their sleep as poor, fair, or good. The Spearman’s correlation of sleep quality with receipt of no parts of the protocol was 0.19 (p < 0.05), while that with receipt of two to three parts of the protocol was 0.64 (p < 0.001).
Smith et al.36 compared 20 patients admitted to an oncology ward for chemotherapy or radiation with 21 historical controls. Those who received a massage for 15–30 min three times during the week reported a 20 % improvement in the Verran Snyder Halpern (VSH) sleep score compared with attention control (p value not reported). Connell et al.37 identified patients on general wards who were having difficulty sleeping during the first week, and applied Roman Camomile oil on the patient’s pillow during the second week. Total sleep time increased by 10 % (36 min) per night during the intervention period (p = 0.004).
In summary, there is low strength of evidence that studies of relaxation techniques improve sleep quality or quantity. This is based on overall medium risk of bias, consistent results, directness of measures, and imprecise results (Table A-1, available online).
Sleep Hygiene or Reduced Sleep Interruption Programs
Bartick et al.38 compared a comprehensive reduced disturbance protocol among 106 patients on a general medical-surgical unit with 161 historical controls. Participants enrolled in the first 5 months of the study received usual care, while patients enrolled in the next 5 months of the study received the sleep intervention. The VSH sleep scale was administered each morning. However, 75 % of the patients felt too ill to use the modified VSH scale, making this data unusable. As-needed sedative use decreased by 49 % during the intervention period (p = 0.004).
Edinger et al.39 assigned 321 patients on three psychiatric wards to a sleep hygeine protocol or usual care. The intervention consisted of eliminating daytime napping and standardizing sleep and wake-up times. Total sleep time increased by 5 % (18 min) during the intervention as compared to usual care; significance testing was not reported.
In summary, there is insufficient strength of evidence that sleep hygiene or reduced sleep interruption programs improve sleep quantity or quality. This is based on high risk of bias, consistent results, directness of measures, and imprecise results (Table A-2, available online).
Daytime Bright Light Therapy
Mishima et al.40 exposed 24 elderly patients (14 with dementia) in a psychiatric hospital to 3,000–5,000 lux of artificial light from 9 am to 11 am daily for 4 weeks. Average total sleep time increased by 18 % (60 min) during the intervention in the demented patients (p < 0.01), and remained significantly increased in these patients post-intervention (p < 0.01). Wakamura et al.41 exposed seven patients on a chest disease ward to 3000 lux of artificial light between 10 am and 3 pm each day for 1 week. Average total sleep time was increased by 7 % (27 min;p = 0.05). Yamadera et al.42 exposed 27 Alzheimer’s disease patients to 3000 lux of bright light between 9 am and 11 am daily for 4 weeks. Wrist actigraphy was performed for 1 week prior to and during the last week of the intervention period. The percentage of nighttime spent sleeping increased by 5 % (p < 0.05), while the number of awakenings decreased (p < 0.01) during the intervention period.
For these studies, there is low strength of evidence that daytime bright light therapy improves the sleep quantity of hospitalized patients. This is based on overall high risk of bias, consistency of results, directness of measures, and precise results (Table A-3, available online).
DISCUSSION
Although non-pharmacologic therapies are recommended as first-line therapies,20,21,25,26 our review shows that very little work has been done in this area, and little evidence exists to guide clinicians on how to help hospitalized patients get restful sleep.
The relaxation techniques we reviewed used a variety of therapies including massage, music, and aromatherapy, and seemed to have a modest effect.33,34 Insomnia can be a consequence of acute stress, and is felt to be associated with sympathetic nervous system activation.43–45 The creation of a resting state that helps to deactivate it could explain why some of these relaxation techniques may have an effect.46–48 Additionally, it may be that hospital noise is a sleep disturbance and that some form of soothing noise, as implemented in several of these studies, masks these disruptive noises.
It would seem that reducing interruptions to sleep and improving one’s sleep hygiene should result in observably improved sleep quality and quantity. However, we did not see any evidence for this due to the paucity of studies, differences in the degree to which interruptions were reduced, as well as lack of adherence to the reduced interruption protocol, which was often not recorded or reported. The two studies in this domain were also rated as having high risk of bias.
The underlying rationale for bright light therapy appears to be regularization of one’s circadian rhythms.49 Since light can be a barrier to sleep, it may help one to avoid naps during the daytime, thereby facilitating sleep at night. Indeed, many patient rooms can be seen to have their blinds pulled down during the day, which may impair sleep at night. Therefore, further research regarding daytime bright light therapy, as well as variations involving keeping blinds open for certain time periods, may be important to helping patients sleep better.
Our review was limited in its ability to assess for publication bias due to the small number of studies. However, our conclusions are of insufficient to low strength of evidence, and unlikely to promote an intervention based on publication bias. Although it is fairly common to ignore baseline values in the calculation of effect sizes, the relative percent change estimates did not account for baseline differences, due to a number of studies not providing information on baseline values. For the studies that did provide baseline values, we calculated a relative percent difference in the difference-in-change estimate between the intervention and control groups, and compared them with just the relative percent change post-intervention. We did not find significant differences that would have changed our conclusions. The types and dose of interventions, length of follow-up, as well as populations studied, were all heterogeneous. This may make it more difficult to draw conclusions, or may dilute effect sizes. However, it provides a synthesis of the current state of knowledge on interventions to improve sleep in the inpatient setting. Some may have prioritized different areas in their assessment of risk of bias. However, we paid attention to many details that we feel are relevant for the assessment of risk of bias in a behavioral intervention, followed existing guidelines, and set a higher bar for non-randomized studies.
Future research could be helped by several considerations. First, to better study this, we need to create an optimal standardized inpatient sleep protocol that minimizes interruptions to sleep. During the entry of routine orders, physicians often do not consider whether an order may interrupt a patient at night. Even if the physician is cognizant of this issue, due to the nature of computerized order entry, it can be time-consuming to enter orders such that vital signs, medications, and other manipulations are not done during sleeping hours unless absolutely necessary. However, computerized order entry may also make it easier to protocolize reduced nighttime interruptions. Future research should develop such protocols. However, these protocols need to evaluate the degree to which they are successful in not disturbing patients without compromising patient care.
Second, circadian rhythms and homeostatic sleep drive are important regulators of sleep, and are heavily influenced by exposure to light. Understanding the degree to which we disturb these rhythms in the inpatient setting by alterations in exposure to light, noise, and activity can assist with designing appropriate interventions that facilitate sleep.
Third, various relaxation therapies have been explored individually and/or in combination with others. It is currently unclear what type of therapy is optimal, to what degree it helps sleep, and which patients it is most likely to help.
Fourth, studies have generally been categorized as medium to high risk of bias. Whenever possible, studies should use appropriate randomization, allocation concealment, and objective measures of sleep quality and quantity. Studies should blind those assessing outcomes and strive to minimize attrition.
Fifth, in what subgroups are non-pharmacologic therapies not effective, and how can we better define the threshold at which we should consider pharmacologic therapies? Answers to these questions would help guide clinicians to reduce the burden of sleep deprivation in their patients.
Electronic supplementary material
(DOCX 124 kb)
Acknowledgements
The authors thank Daniel J. Brotman, MD, Johns Hopkins University for his thoughtful comments during preparation of the manuscript. No compensation was given for his contributions. All authors had full access to the data in the study and take full responsibility for the integrity of the data and the accuracy of the analysis.
No funding was received for this work.
An earlier version of this work was presented at the Society of General Internal Medicine Annual Conference in Denver, CO in April 2013.
Author Contributions
All authors contributed to the collection, extraction, synthesis, and analysis of the data for this systematic review.
Conflict of Interest
The authors declare that they do not have a conflict of interest.
Footnotes
Author Contributions
All authors contributed to the collection, extraction, synthesis, and analysis of the data for this systematic review.
REFERENCES
- 1.Murphy WJ, Rui H, Longo DL. Effects of growth hormone and prolactin immune development and function. Life Sci. 1995;57(1):1–14. doi: 10.1016/0024-3205(95)00237-z. [DOI] [PubMed] [Google Scholar]
- 2.Spiegel K, Follenius M, Simon C, Saini J, Ehrhart J, Brandenberger G. Prolactin secretion and sleep. Sleep. 1994;17(1):20–27. doi: 10.1093/sleep/17.1.20. [DOI] [PubMed] [Google Scholar]
- 3.Redwine LHR, Gillin JC, Irwin M. Effects of sleep and sleep deprivation on interleukin-6, growth hormone, cortisol, and melatonin levels in humans. J Clin Endocrinol Metabol. 2000;85(10):6. doi: 10.1210/jcem.85.10.6871. [DOI] [PubMed] [Google Scholar]
- 4.Palmblad JPB, Wasserman J, Akerstedt T. Lymphocyte and Granulocyte Reactions during sleep deprivation. Psychosom Med. 1979;41(4):5. doi: 10.1097/00006842-197906000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Palmblad J, Cantell K, Strander H, et al. Stressor exposure and immunological response in man: interferon-producing capacity and phagocytosis. J Psychosom Res. 1976;20(3):193–199. doi: 10.1016/0022-3999(76)90020-9. [DOI] [PubMed] [Google Scholar]
- 6.Van Cauter E. Review Article—Sleep disturbances and insulin resistance. Diabet Med. 2011;28(12):1455–1462. doi: 10.1111/j.1464-5491.2011.03459.x. [DOI] [PubMed] [Google Scholar]
- 7.Broussard JL, Erhrmann DA, Cauter EV, Tasali E, Brady MJ. Impaired insulin signaling in human adipocytes after experimental sleep restriction: A randomized, crossover study. Ann Intern Med. 2012;157(8):549–557. doi: 10.7326/0003-4819-157-8-201210160-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cappuccio FP, Miller MA. A new challenge to widely held views on the role of sleep. Ann Intern Med. 2012;157:593–594. doi: 10.7326/0003-4819-157-8-201210160-00016. [DOI] [PubMed] [Google Scholar]
- 9.Arora VM, Fazal AZ, Zee PC, Knutson KL. Objective sleep duration and quality in hospitalized older adults: Associations with blood pressure and mood. J Am Geriatr Soc. 2011;59(11):2185–2186. doi: 10.1111/j.1532-5415.2011.03644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith MT, Klick B, Koachik S, et al. Sleep onset insomnia symptoms during hospitalization for major burn injury predict chronic pain. Pain. 2008;138(3):497–506. doi: 10.1016/j.pain.2008.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Griffiths M, Peerson A. Risk factors for chronic insomnia following hospitalization. J Adv Nurs. 2005;49(3):245–253. doi: 10.1111/j.1365-2648.2004.03283.x. [DOI] [PubMed] [Google Scholar]
- 12.Gay P. Sleep and sleep-disordered breathing in the hospitalized patient. Respir Care. 2010;55(9):1240–1254. [PubMed] [Google Scholar]
- 13.Frighetto L, Marra C, Bandali S, Wilbur K, Naumann T, Jewesson P. An assessment of quality of sleep and the use of drugs with sedating properties in hospitalized adult patients. Health Qual Life Outcome. 2004;2:17. doi: 10.1186/1477-7525-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Southwell MT, Wistow G. Sleep in hospitals at night: are patients’ needs being met? J Adv Nurs. 1995;21(6):1101–1109. doi: 10.1046/j.1365-2648.1995.21061101.x. [DOI] [PubMed] [Google Scholar]
- 15.Raymond I, Ancoli-Israel S, Choiniere M. Sleep disturbances, pain and analgesia in adults hospitalized for burn injuries. Sleep Med. 2004;5(6):551–559. doi: 10.1016/j.sleep.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 16.Manabe K, Matsui T, Yamaya M, et al. Sleep patterns and mortality among elderly patients in a geriatric hospital. Gerontology. 2000;46(6):318–322. doi: 10.1159/000022184. [DOI] [PubMed] [Google Scholar]
- 17.Flaherty JH. Insomnia among hospitalized older persons. Clin Geriatr Med. 2008;24(1):51–67. doi: 10.1016/j.cger.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 18.Tranmer JE, Minard J, Fox LA, Rebelo L. The sleep experience of medical and surgical patients. Clin Nurs Res. 2003;12(2):159–173. doi: 10.1177/1054773803012002004. [DOI] [PubMed] [Google Scholar]
- 19.Topf M, Thompson S. Interactive relationships between hospital patients’ noise-induced stress and other stress with sleep. Heart Lung. 2001;30(4):237–243. doi: 10.1067/mhl.2001.116592. [DOI] [PubMed] [Google Scholar]
- 20.Young JS, Bourgeois JA, Hilty DM, Hardin KA. Sleep in hospitalized medical patients, part 1: factors affecting sleep. J Hosp Med. 2008;3(6):473–482. doi: 10.1002/jhm.372. [DOI] [PubMed] [Google Scholar]
- 21.Young JS, Bourgeois JA, Hilty DM, Hardin KA. Sleep in hospitalized medical patients, part 2: behavioral and pharmacological management of sleep disturbances. J Hosp Med. 2009;4(1):50–59. doi: 10.1002/jhm.397. [DOI] [PubMed] [Google Scholar]
- 22.Glass J, Lactot KL, Herrmann N, Sproule BA, Busto UE. Sedative hypnotics in older people with insomnia: meta-analysis of risks and benefits. Br Med J (Clin Res Ed) 2005;331:1169–1175. doi: 10.1136/bmj.38623.768588.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kolla BP, Lovely JK, Mansukhani MP, Morgenthaler TI. Zolpidem is independently associated with increased risk of inpatient falls. J Hosp Med. 2013;8(1):1–6. doi: 10.1002/jhm.1985. [DOI] [PubMed] [Google Scholar]
- 24.Jarman H, Jacobs E, Walter R, Witney C, Zielinski V. Allowing the patients to sleep: flexible medication times in an acute hospital. Int J Nurs Pract. 2002;8(2):75–80. doi: 10.1046/j.1440-172x.2002.00346.x. [DOI] [PubMed] [Google Scholar]
- 25.Lenhart SE, Buysse DJ. Treatment of insomnia in hospitalized patients. Ann Pharmacother. 2001;35:1449–1457. doi: 10.1345/aph.1A040. [DOI] [PubMed] [Google Scholar]
- 26.Blair B, Martin J. Toward a good night’s sleep: An approach to insomnia in older patients. Consultant. 2012;52(12):795–803. [Google Scholar]
- 27.Suggested risk of bias criteria for EPOC reviews. Cochrane Effective Practice and Organization of Care Group. Available at http://epoc.cochrane.org/epoc-resources. Accessed September 4, 2013.
- 28.Methods Guide for Effectiveness and Comparative Effectiveness Reviews. Agency for Healthcare Research and Quality. Available at http://effectivehealthcare.ahrq.gov/index.cfm/search-for-guides-reviews-and-reports/?productid=318&pageaction=displayproduct. Accessed September 4, 2013. [PubMed]
- 29.Owens DK, Lohr KN, Atkins D, et al. AHRQ Series Paper 5: Grading the strength of a body of evidence when comparing medical interventions. J Clin Epidemiol. 2010;63:513–523. doi: 10.1016/j.jclinepi.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 30.Lareau R, Benson L, Watcharotone K, Manguba G. Examining the feasibility of implementing specific nursing interventions to promote sleep in hospitalized elderly patients. Geriatr Nurs. 2008;29(3):197–206. doi: 10.1016/j.gerinurse.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 31.Soden K, Vincent K, Craske S, Lucas C, Ashley S. A randomized controlled trial of aromatherapy massage in a hospice setting. Palliat Med. 2004;2:87–92. doi: 10.1191/0269216304pm874oa. [DOI] [PubMed] [Google Scholar]
- 32.Toth M, Wolsko PM, Foreman J, et al. A pilot study for a randomized, controlled trial on the effect of guided imagery in hospitalized medical patients [5] J Alternative Compl Med. 2007;13(2):194–197. doi: 10.1089/acm.2006.6117. [DOI] [PubMed] [Google Scholar]
- 33.Zimmerman L, Nieveen J, Barnason S, Schmaderer M. The effects of music interventions on postoperative pain and sleep in coronary artery bypass graft (CABG) patients… including commentary by Miaskowski C. Sch Inq Nurs Pract. 1996;10(2):153–174. [PubMed] [Google Scholar]
- 34.Williamson JW. The effects of ocean sounds on sleep after coronary artery bypass graft surgery. Am J Crit Care. 1992;1(1):91–97. [PubMed] [Google Scholar]
- 35.McDowell JA, Mion LC, Lydon TJ, Inouye SK. A nonpharmacologic sleep protocol for hospitalized older patients. J Am Geriatr Soc. 1998;46(6):700–705. doi: 10.1111/j.1532-5415.1998.tb03803.x. [DOI] [PubMed] [Google Scholar]
- 36.Smith MC, Kemp J, Hemphill L, Vojir CP. Outcomes of therapeutic massage for hospitalized cancer patients. J Nurs Scholarsh. 2002;34(3):257–262. doi: 10.1111/j.1547-5069.2002.00257.x. [DOI] [PubMed] [Google Scholar]
- 37.Connell FEA, Tan G, Gupta I, Gompertz P, Bennett GCJ, Herzberg JL. Can aromatherapy promote sleep in elderly hospitalized patients? Geriatr Today. 2001;4(4):191–195. [Google Scholar]
- 38.Bartick MC, Thai X, Schmidt T, Altaye A, Solet JM. Decrease in as-needed sedative use by limiting nighttime sleep disruptions from hospital staff. J Hosp Med. 2010;5(3):E20–24. doi: 10.1002/jhm.549. [DOI] [PubMed] [Google Scholar]
- 39.Edinger JD, Lipper S, Wheeler B. Hospital ward policy and patients’ sleep patterns: a multiple baseline study. Rehabil Psychol. 1989;34(1):43–50. [Google Scholar]
- 40.Mishima K, Okawa M, Hishikawa Y, Hozumi S, Hori H, Takahashi K. Morning bright light therapy for sleep and behavior disorders in elderly patients with dementia. Acta Psychiatr Scand. 1994;89:1–7. doi: 10.1111/j.1600-0447.1994.tb01477.x. [DOI] [PubMed] [Google Scholar]
- 41.Wakamura T, Tokura H. Influence of bright light during daytime on sleep parameters in hospitalized elderly patients. J Physiol Anthropol Appl Human Sci. 2001;20(6):345–351. doi: 10.2114/jpa.20.345. [DOI] [PubMed] [Google Scholar]
- 42.Yamadera H, Ito T, Suzuki H, Asayama K, Ito R, Endo S. Effects of bright light on cognitive and sleep-wake (circadian) rhythm disturbances in Alzheimer-type dementia. Psychiatry Clin Neurosci. 2000;54:352–353. doi: 10.1046/j.1440-1819.2000.00711.x. [DOI] [PubMed] [Google Scholar]
- 43.Bonnett MHAD. Heart rate variability in insomniacs and matched normal sleepers. Psychosom Med. 1998;60(5):610–615. doi: 10.1097/00006842-199809000-00017. [DOI] [PubMed] [Google Scholar]
- 44.Brown THMT. The Influence of PTSD, Sleep Fears, and Neighborhood Stress on Insomnia and Short Sleep Duration in Urban, Young Adult African Americans. Behav Sleep Med. 2013;11:1–9. doi: 10.1080/15402002.2013.784704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kompier MATT, van Veldhoven M. Tossing and turning–insomnia in relation to occupational stress, rumination, fatigue, and well-being. Scan J Work Environ Health. 2012;38(3):238–246. doi: 10.5271/sjweh.3263. [DOI] [PubMed] [Google Scholar]
- 46.Gross CRKM, Reilly-Spong M, Wall M, Winbush NY, Patterson R, Mahowald M, Cramer-Bornemann M. Mindfulness-based stress reduction versus pharmacotherapy for chronic primary insomnia: a randomized controlled clinical trial. Explore. 2011;7(2):76–87. doi: 10.1016/j.explore.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hoge EAML, Metcalf CA, Morris LK, Robinaugh DJ, Worthington JJ, Pollack MH, Simon NM. Randomized controlled trial of mindfulness meditation for generalized anxiety disorder: effects on anxiety and stress reactivity. J Clin Psychiatry. 2013;74. [DOI] [PMC free article] [PubMed]
- 48.Nakamura YLD, Kuhn R, Donaldson GW. Investigating efficacy of two brief mind-body intervention programs for managing sleep disturbance in cancer survivors: a pilot randomized controlled trial. J Cancer Surviv. 2013;7(2):165–182. doi: 10.1007/s11764-012-0252-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Czeisler CA, Richardson GS, Zimmerman JC, Moore-Ede MC, Weitzman ED. Entrainment of human circadian rhythms by light–dark cycles: a reassessment. Photochem Photobiol. 1981;34(2):239–247. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 124 kb)