Abstract
On the Indian sub continent, dromedarian camel —‘the ship of the desert’ is an important constituent of the socio economic life style of nomadic owners in the semi arid to arid ecosystems. The animal suffers from a few parasitic diseases viz. surra, coccidiosis, sarcocystis, gastro intestinal concurrent metazoan infections, mange, nasal bots and ticks infestations. However, anaplasmosis in camel has not been reported so far from the Indian subcontinent. Systematic investigations of a 7 year male Jaisalmeri camel, with a clinical history of dullness, progressive loss of condition and stamina revealed subclinical Anaplasma marginale infection. The animal had depressed haematological indices, dry and constipated bowels, pale and icteric conjunctiva suggestive of anaemia. The animal positively responded to the specific integrated therapy. Reexamination of the animal on day 21 post-therapy revealed depressed haematological indices restored to normal levels and the erythrocytes were free from the pathogen. Neglected attention, poor and/or underreporting of camel diseases vis-a-vis economic significance of the versatile animal has been discussed. This appears to be the pioneer documentation of anaplasmosis in camels from Indian subcontinent.
Keywords: Anaplasma marginale, Dromedarian camel, Pioneer documentation, Subclinical anaplasmosis
Introduction
Ever since the dawn of the human civilization on the Indian sub continent (2,750 BC), the one humped camel (Camelus dromedarius) has been serving multipurpose and versatile role ranging from ploughing to irrigation of agricultural land to the marketing of the arid crop yields in the urban market. The large ruminant has been popularly known as ‘ship of the desert’, being important constituent of the socio economic life style of nomadic camel owners in the semi arid to arid ecosystems (Viswanath and Kumar 2008). Camel outshines the other domestic livestock in the region for its unique physiological capabilities to combat harsh climate, thriving well upon scare poor quality biomass as feed resource and paucity of drinking water for major part of the year (Gahlot and Chabbra 2009). An overview of literature on parasitic diseases of camel evidenced a few diseases countable on the finger tips viz. surra, coccidiosis, sarcocystis, gastro intestinal concurrent metazoan infections, mange, nasal bots, ticks infestations, etc. besides isolated publication on anaplasmosis (Chabbra and Sangwan 2006; Alsaad 2009; Gahlot and Chabbra 2009). This communication addresses the occurence of subclinical anaplasmosis and its successful therapeutic management in dromedarian camel, bred and brought up under extensive system of management on the Indian subcontinent.
Materials and methods
A 7 year old, male Jaisalmeri camel was brought to Veterinary Clinical Complex, Apollo College of Veterinary Medicine (ACVM), Jaipur, with the clinical history of dullness, partial inappetence, progressive loss of physical condition and stamina. The camel was used for transportation of goods in the outskirts of Jaipur city. Routine system-wise clinical examination was conducted on the spot, peripheral venous thin blood smears were prepared. Body hair coat, ears and interdigital space were searched for presence of blood feeding surface parasites and rectal coprological sample was collected, processed in laboratory and pathogens were identified using standard techniques (Bowman et al. 2003; Soulsby 2005; Taylor et al. 2007). Subsequently, the camel was therapeutically managed with synchronous integrated specific therapy based on the identified pathogen(s) and clinical findings, including a single dose of albendazole at 40 mg/kg orally and parenteral administration of oxytetracycline at 20 mg/kg deep intramuscular, safe acaricide-Betacyfluthrin 2.45 % spray (0.01 % aqueous solution) into the ear canal and interdigital space of the four limbs besides antianaemic and supportive therapy comprised of injectible B- complex liver extract at 10 ml, biweekly six intramuscular injections and Feritas at 1 ml/50 kg body weight.
Results and discussion
Detailed clinical examination of the camel revealed dull, dry and lustreless hair coat, a few Boophilus microplus engorged ticks in the ear canal and interdigital space. The general condition of the animal was poor in look, depressed, progressive loss of appetite, reluctant to carry load and slightly enlarged superficial lymph nodes. The pulse and the respiration rates were normal with rectal temperature 101.4 °F. The faecal pellets were dry and constipated. The conjunctiva was pale and icteric, suggestive of anaemia. Laboratory coproscopic examination revealed a moderate infection of Trichostrongyle spp. eggs. The pretreatment examination of the blood confirmed lower value of haematological indices (Haemoglobin 10.4 g/dl, TEC 5.5 × 106/mm3) besides, neutrophilia and eosinophilia (Neutrophils 65 %, Lymphocytes 28 %, Monocytes 2 % and Eosinophils 5 %). The Giemsa stained thin blood smear revealed intraerythrocytic small, polymorphic (oval to spherical) rickettsial bodies near to the periphery of erythrocyte cell membrane (Fig. 1). The morphological characteristics with moderate parasitaemia (30 %), clinical observations and subnormal haematological indices reported herein, confirmed that the camel was suffering from the subclinical Anaplasma marginale infection. The clinical manifestations reported here in can hardly be attributed to mild Trichostrongylosis. The animal positively responded to the integrated therapy by way of restoring normal haematological indices, and erythrocytes were free from A. marginale, when the camel was re-examined. However, the camel was administered with another shot of prophylactic deep intramuscular dose of Oxytetracycline LA, prior to discharge from college clinics.
Fig. 1.
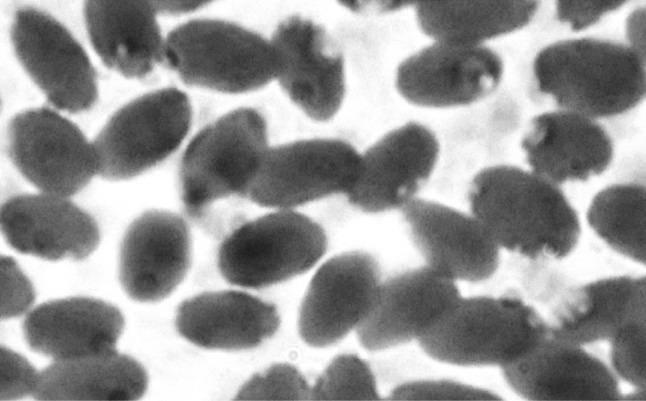
Giemsa stained blood smears showing A. marginale
Anaplasmosis (Gall sickness) mainly prevalent in the tropics and subtropics, has a peracute to chronic course in the large ruminants. It is a non contagious, insect bite/tick borne haemoparasitic disease of domesticated and wild ruminants including camel (Aiello and Mays 1998). Until 1957, Anaplasma species were originally considered a protozoan parasite (Ristic 1968), however, subsequently, the parasite has been assigned to order Reckettsiales under family Anaplasmataceae. It was based on a combination of 16S ribosomal RNA, groESL and surface protein gene sequence analysis (Dumler et al. 2001). As on today the family includes five genera (Ehrlichia, Anaplasma, Cowdria, Wolbehia and Neorickettsia). Four species of Anaplasma (A. maeginale, A. cemtrale, A.caudatum and A. ovis) have been documented infecting domestic ruminants especially, cattle, sheep, goat and deer (Smith 1990; Soulsby 2005; Bhatia et al. 2008). Of these, A. marginale has been claimed as most pathogenic. The severity and course of disease depends upon age, population density of heamatophagous ticks and flies, respectively on the body and/or in the surroundings of the host. The disease recognizes no sex preferences (Urquhart et al. 2003; Taylor et al. 2007).
The clinical picture, haematological profile presented herein and positive response to treatment confirmed that the 7-year-old dromedarian camel suffered from subclinical anaplasmosis. This seems to be the first documentation of Anaplasmosis in dromedarian camel on the Indian subcontinent. The disease has been under-reported in camel. Perusal to literature revealed isolated reports on occurrence of disease from Saudi Arabia (Alsaad 2009; Al-Khalifa et al. 2009; Gahlot and Chabbra 2009). This is possibly attributed to neglected attention of researchers and field veterinarians despite the established fact that camel ethno medicine had been in practice ever since domestication of camel and multipurpose utility of the animal in the sub tropics and tropics (Kohler-Rollefson 1994). The disease so far neglected and under reported needs adequate attention.
Acknowledgments
The authors gratefully acknowledged the facilities provided by the Dean, ACVM, Jaipur.
References
- Aiello SE, Mays A. The merck veterinary manual. 8. New Jersey: Merck & Co; 1998. [Google Scholar]
- Al-Khalifa MS, Hussein HS, Diab FM, Khalid GM. Blood parasites of livestock in certain regions in Saudi Arabia. Saudi J Bio Sci. 2009;16:63–67. doi: 10.1016/j.sjbs.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsaad KM. Clinical, hematological and biochemical studies of anaplasmosis in Arabian one humped camels (Camelus dromedaries) J Animal Vet Adv. 2009;8:1794–1797. [Google Scholar]
- Bhatia BB, Pathak KML, Banerjee DP. Textbook of veterinary parasitology. Ludhiana: Kalyani Publishers; 2008. pp. 395–396. [Google Scholar]
- Bowman DD, Lynn RC, Eberhard ML, Alcaraz A. Georgi’s parasitology for veterinarians. 8. USA: Saunders-an imprint of Elsevier; 2003. pp. 1–422. [Google Scholar]
- Chabbra MB, Sangwan AK. Parasitic diseases of camel––an update 1. protozoal diseases. J Camel Pract Res. 2006;13(1):7–14. [Google Scholar]
- Dumler JS, Barbet AF, Bekker CPJ, Dasch GA, Palmer GH, Ray SC, Rikihisa Y, Rurangirwa FR. Reorganization of the genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six new species combinations and designation of Ehrlichia equi and “HE agent” as subjective synonyms of Ehrlichia phagocytophila. Int J Syst Evol Microbiol. 2001;51:2145–2165. doi: 10.1099/00207713-51-6-2145. [DOI] [PubMed] [Google Scholar]
- Gahlot TK, Chabbra MB. Selected research on camelid parasitology. Bikaner: Camel Publishing House; 2009. [Google Scholar]
- Kohler-Rollefson I. Ethanoveterinary practices of camel pastoralists in North Africa and Indian. J Camel Pract Res. 1994;1(2):75–82. [Google Scholar]
- Ristic M. Anaplasmosis. In: Weinman D, Ristic M, editors. Infectious blood diseases of man and animals. New York: Academic Press; 1968. pp. 473–542. [Google Scholar]
- Smith BP. Large animal internal medicine. St. Louis: The C.V. Mosby Company; 1990. pp. 1085–1088. [Google Scholar]
- Soulsby EJL. Helminths, arthropods and protozoa of domesticated animals. 7. London: ELBS and Bailliere Tindal; 2005. pp. 752–754. [Google Scholar]
- Taylor MA, Coop RL, Wall RL. Veterinary parasitology. 3. UK: Blackwell Publishing Ltd; 2007. [Google Scholar]
- Urquhart GM, Armour J, Duncan JL, Dunn AM, Jennings FW. Veterinary parasitology. UK: Blackwell Science Ltd; 2003. pp. 250–251. [Google Scholar]
- Viswanath CS, Kumar AT. Handbook of animal husbandry. New Delhi: ICAR publication; 2008. pp. 293–325. [Google Scholar]


