Abstract
Background
Pauses during chest compressions are thought to have a detrimental effect on resuscitation outcome. The Guidelines 2005 have recently eliminated the post-defibrillation pause. Previous animal studies have shown that multiple pauses of increasing duration decrease resuscitation success. We investigated the effect of varying the characteristics of a single pause near defibrillation on resuscitation outcome.
Methods
Part A: 48 swine were anesthetized, fibrillated for 7 min and randomized. Chest compressions were initiated for 90 s followed by defibrillation and then resumption of chest compressions. Four groups were studied—G2000: 40 s pause beginning 20 s before, and ending 20 s after defibrillation, A1: a 20 s pause just before defibrillation, A2: a 20 s pause ending 30 s prior to defibrillation, and group A3: a 10 s pause ending 30 s prior to defibrillation. Part B: 12 swine (Group B) were studied with a protocol identical to Part A but with no pause in chest compressions. Primary endpoint was survival to 4 h.
Results
The survival rate was significantly higher for groups A1, A2, A3, and B (5/12, 7/12, 5/12, and 5/12 survived) than for the G2000 group (0/12, p < 0.05). Survival did not differ significantly among groups A1, A2, A3, and B.
Conclusions
These results suggest that the Guidelines 2005 recommendation to omit the post-shock pulse check and immediately resume chest compressions may be an important resuscitation protocol change. However, these results also suggest that clinical maneuvers further altering a single pre-shock chest compression pause provide no additional benefit.
Keywords: Resuscitation, Defibrillation, Sudden cardiac death, Pause
1. Introduction
For sudden cardiac arrest victims with ventricular fibrillation, the two primary treatments available today are rapid defibrillation and performance of chest compressions until the patient’s heart can maintain perfusion without help.1 Automatic external defibrillators (AEDs) have been introduced to extend the use of defibrillators from ambulances into less conventional settings including firefighters, policeman, and lay people.2 Yet even with their introduction, cardiac arrest survival rates remain very low,2 possibly because the defibrillator’s use has not been optimally coordinated with the delivery of chest compressions.
The new 2005 AHA/ILCOR Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiac Care recommend several protocol changes aimed at reducing hands-off pauses in chest compressions, including elimination of stacked defibrillation shocks and post-shock pulse and rhythm checks.1 Preliminary clinical data indicate that implementing protocol changes such as these can decrease the AED-prompted hands-off time substantially. This study showed further that these changes decreased the actual amount of hands-off time and were associated with improved resuscitation outcomes.3 In light of these encouraging findings, it is of interest to determine whether alterations to other chest compression pauses inherent in current protocols, such as not removing hands for shock delivery,4 might provide further improvement.
There are at least three characteristics of pauses in chest compressions that may be important to a resuscitation effort: (1) the timing of a particular pause with respect to other resuscitation events, (2) the duration of any particular pause, and (3) the total duration of all pauses during a resuscitation. Pauses during resuscitation have been evaluated in several animal studies.5–8 These studies have not systematically varied these three characteristics to determine their relative importance.
Understanding whether some chest compression pauses are truly more deleterious than others, based on their location in the resuscitation sequence, is important for further refinement of resuscitation protocols. It is presently unknown whether alteration of the timing or duration of a pre-shock pause meaningfully impacts resuscitation outcomes within a protocol when chest compressions are resumed immediately after delivery of a single shock.
In this study, we used a swine model of prolonged unsupported VF to better elucidate the relationship between resuscitation outcome and the timing and duration of a single chest compression pause early in the resuscitation attempt. Specifically, we hypothesized (1) that removal of the pause associated with post-shock pulse and rhythm checks would improve outcome, and (2) that changes in the length and timing of a single pre-shock pause could further improve outcome.
2. Methods
2.1. Animal preparation
All procedures were approved by the Institutional Animal Care and Use Committee at the University of Alabama at Birmingham. Further, all pre-operative and operative care for animals complied with Section 6 of the Animal Welfare Act of 1989 and adhered to the principles outlined in the “Guide for the care and use of animals,” National Institutes of Health publication No. 85-23.
Forty-eight domestic swine, 25–40 kg, were studied in Part A and an additional 12 animals were studied in Part B. Animals were pre-anesthetized with telazol/xylazine (4.4 mg/kg of each) and atropine (0.04 mg/kg), then intubated, anesthetized with isoflurane (1.2–3%) and supported on a pressure-controlled mechanical ventilator (Ohmeda Modulus II, BOC Healthcare, NJ) with a minute ventilation of 10–15 ml/kg/min. Normal saline was administered IV at a rate of 5–10 ml/kg/h. Blood gases and electrolytes were measured every half hour and respiratory parameters and infusion fluid composition were adjusted accordingly. ECG lead II was monitored throughout the study.
The animal was placed in dorsal recumbency. The left and right chest walls were shaved. Self-adhesive defibrillation electrodes were placed on the anterior left and right chest walls. The right jugular vein was isolated and a high fidelity pressure catheter (Millar Microtip, Houston, TX) advanced under fluoroscopy to the junction of the right atrium and superior vena cava. A quadripolar EP catheter was inserted into the left jugular vein and advanced into the apex of the right ventricle for ventricular fibrillation induction. The left carotid artery was isolated and a high fidelity pressure catheter inserted and advanced into the left ventricular cavity. The left femoral artery was isolated and a high fidelity pressure catheter advanced into the descending thoracic aorta. After induction of anesthesia, ventilator oxygen fraction was decreased until the animals’ pO2 was less than 150 mmHg.
2.2. Experimental procedure
All times are referenced to the beginning of ventricular fibrillation. After recording 30 s of baseline data, ventricular fibrillation was induced by applying 60 Hz alternating current to the endocardium of the right ventricle. Fibrillation was allowed to persist unsupported for 7min, after which ventilation and chest compressions were initiated. Ventilation was performed by restarting the ventilator at the same rate and tidal volume as before ventricular fibrillation induction. Chest compressions were performed using a model 1005 ‘Thumper’ device (Michigan Instruments, Grand Rapids, MI) adjusted to the maximum chest compression depth possible without causing left ventricular pressure to exceed aortic pressure by >10 mmHg. Compression depth was consistent with AHA guidelines for adult chest compressions. Ninety seconds after chest compressions were initiated, they were paused briefly for delivery of a single 200J biphasic defibrillation shock (LIFEPAK® 12 defibrillator/monitor, Medtronic ERS, Redmond, WA).
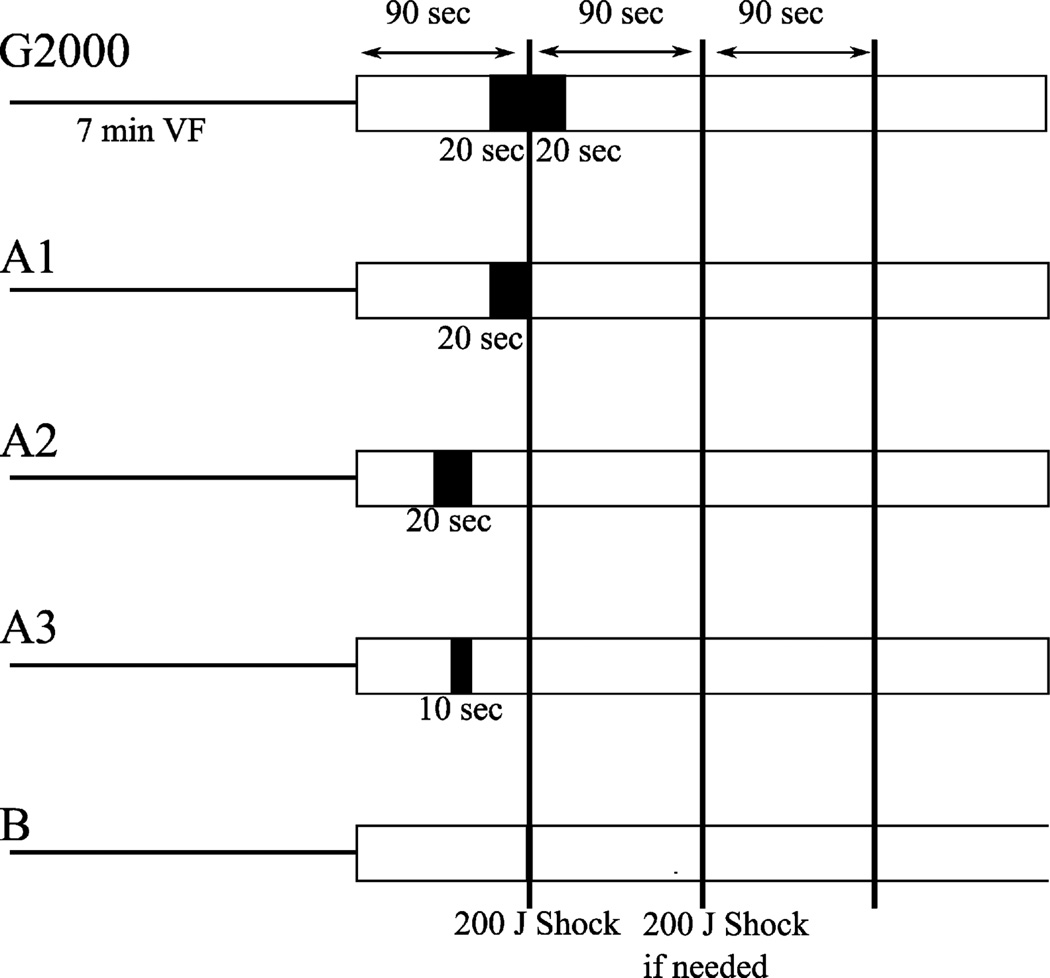
By design, the timing and duration of the pause in chest compressions differed between groups (Fig. 1). In Part A, animals were randomized to four groups. The G2000 group had a pause starting 20 s before and ending 20 s after the first defibrillation shock. This timing was chosen to approximate pause durations required for pre-shock AED rhythm analysis and preparation to administer a shock, plus a post-shock pulse check and preparation to resume chest compressions, typical of well-performed Guidelines 2000-driven care. Groups A1, A2, and A3 each had a pause prior to the first defibrillation shock but the timing and duration of that pause varied with group. These groups were included to evaluate the impact of the characteristics of a pre-shock pause, in the absence of a post-shock pause. In Part B, an additional group of animals (Group B) was studied with an identical protocol, except that the pause in chest compressions was omitted. In all groups, after the first shock, CPR was stopped every 90 s for 3 s to assess heart rhythm, determine whether return of spontaneous circulation (ROSC) had occurred (aortic systolic pressure >50 mmHg), and deliver a single 200J shock if necessary (Fig. 1).
Fig. 1.
Timing diagram of resuscitation protocol. Part A: G2000 Group: a 40 s pause starting 20 s before and ending 20 s after attempted defibrillation. Group A1: a 20 s pause just before attempted defibrillation. Group A2: a 20 s pause ending 30 s before attempted defibrillation. Group A3: a 10 s pause ending 30 s before attempted defibrillation. Part B: Group B: no pauses other than the 3 s pause at the defibrillation attempt. Once CPR was resumed after the first defibrillation attempt, the resuscitation protocol was identical for all 5 groups and included a pause for 3 s every 90 s to determine rhythm and perfusion and deliver a defibrillation shock if necessary.
Chest compressions were continued until ROSC occurred or 30 min of resuscitation had elapsed. Following 7.5 min of resuscitation, epinephrine, 0.01 mg/kg, was given every 3 min if ROSC had not occurred, or if aortic systolic blood pressure was less than 50 mmHg. After 1 h, if systolic aortic blood pressure fell below 50 mmHg, dobutamine was started at 5 µg/(kg min) and titrated to the lowest dose that would maintain a systolic aortic blood pressure >100 mmHg. Three hours after fibrillation induction, dobutamine was stopped and the animal was monitored for 1 h. Survival was assessed at 4 h.
Defibrillation success was defined as conversion of a shockable rhythm to a non-shockable rhythm for at least 5 s.9 Refibrillation was defined as fibrillation recurring more than 5 s after a successful defibrillation shock. Timing of CPR, pauses, and defibrillation was orchestrated via prompts from the automatic slide advance feature of a PowerPoint presentation (Microsoft, Redmond, WA).
2.3. Data collection and analysis
Surface ECG lead II, an intracardiac electrogram, left ventricular, aortic, and right atrial pressures, end-tidal CO2, and ‘Thumper’ pressure waveform were collected on a PC-based data acquisition system (Dataq, Akron, OH) at a sampling rate of 250 samples/s. Data was analyzed off-line using Matlab (Mathworks, Natick, MA). Cardiac function was evaluated by examining ± dp/dt of the left ventricular pressure. The derivative of left ventricular pressure was calculated using a 5-point parabolic fit. Maxima and minima were determined for beats at baseline and after 4 h. Coronary perfusion pressure, defined as aortic minus right atrial pressure measured just before the initiation of a chest compression, was measured for the last two compressions before and the first two compressions after the first two pauses.
Survival data was compared using Fisher’s exact test. Continuous variables were compared using two-level analysis of variance. One level was baseline versus post-resuscitation and the second level was the group to which the animal was assigned. Significance was defined as p < 0.05.
3. Results
Forty-eight animals were studied in Part A and 12 animals were studied in Part B. Average animal weight was 38 ± 5 kg. Twenty-two animals were female and thirty-eight were male. Baseline physiologic characteristic are shown in Table 1.
Table 1.
Baseline physiologic characteristics for the animals in each study group.
| Group | Weight (kg) mean ± s.d. | Gender M/F | HR (bpm) mean ± s.d. | Arterial blood pressure (mmHg) |
|---|---|---|---|---|
| G2000 | 38 ± 6 | 4/8 | 97 ± 10 | 93 ± 11/68 ± 9 |
| A1 | 36 ± 5 | 6/6 | 95 ± 14 | 98 ± 12/74 ± 10 |
| A2 | 37 ± 4 | 10/2 | 102 ± 18 | 97 ± 7/74 ± 6 |
| A3 | 37 ± 6 | 8/4 | 96 ± 13 | 96 ± 13/70 ± 10 |
| B | 32 ± 4 | 10/2 | 110 ± 12 | 106 ± 12/80 ± 13 |
Compared to the G2000 group (40 s pause straddling the shock), the survival rate of the other four treatment groups was significantly higher (Table 2). Survival rates did not differ significantly among the four groups with variously timed pre-shock pauses of 0–20 s: groups A1, A2, A3 and B.
Table 2.
Outcome variables for animals in each study group.
| Group | n | 4 h survival | Number of refibrillations, mean ± s.d. | Time to ROSC (min), mean ± s.d. (n) | Dobutamine dosage, mean±s.d. (µg/(kg min)) | ||
|---|---|---|---|---|---|---|---|
| Hour 1 | Hour 2 | Hour 3 | |||||
| G2000 | 12 | 0/12 | 2.6 ± 3.2 | 16.8 ± 6.7 (2) | 5.0 ± 0 | 5.0 ± 0 | 3.0 ± 1.4 |
| A1 | 12 | 5/12 | 2.5 ± 1.7 | 11.7 ± 3.6 (5) | 3.8 ± 1.2 | 3.0 ± 0.7 | 1.0 ± 0 |
| A2 | 12 | 7/12 | 0.6 ± 1.2 | 9.8 ± 3.5 (7) | 5.0 ± 0 | 3.4 ± 1.5 | 1.0 ± 0.6 |
| A3 | 12 | 5/12 | 2.5 ± 4.0 | 10.5 ± 3.1 (5) | 5.0 ± 0 | 3.0 ± 0.7 | 1.4 ± 0.9 |
| B | 12 | 5/12 | 2.1 ± 4.0 | 10.3 ± 4.5 (5) | 3.0 ± 2.7 | 1.2 ± 1.3 | 0.4 ± 0.5 |
Among those animals that achieved ROSC, time to ROSC was similar across all groups (Table 2). ROSC never occurred immediately following defibrillation, or by the pause in compressions 90 s after the defibrillation; the shortest interval observed from defibrillation to ROSC was 3 min.
All animals that had ROSC in Part A, and three of five that had ROSC in Group B, required dobutamine support to maintain arterial blood pressure. For animals that had ROSC, there was no significant difference between groups in dobutamine dosage requirements over time (Table 2). All that achieved ROSC within 30 min, except for the two in the G2000 group, survived for a full hour after dobutamine support was withdrawn at 3 h.
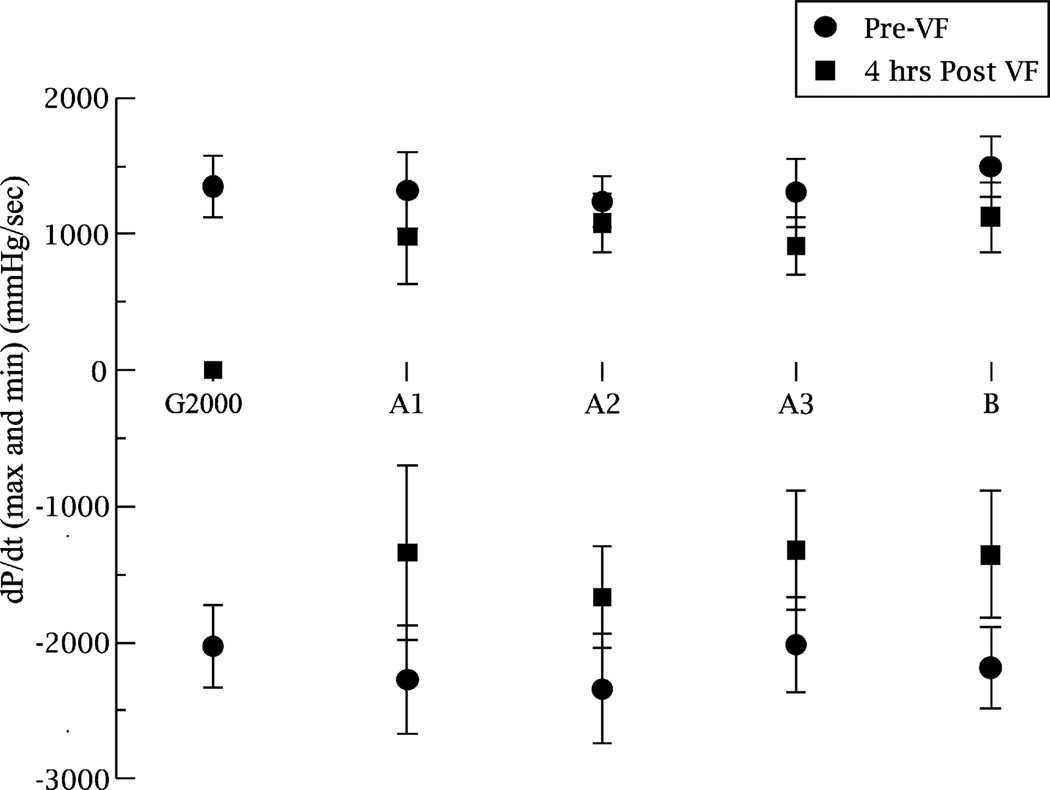
Neither positive dp/dt nor negative dp/dt was significantly different between groups at baseline. For 4 h survivors, there was a significant difference in both positive and negative LV dp/dt between baseline and 4 h across all groups. There was no significant difference between groups in either the positive or negative LV dp/dt measured at 4 h (Fig. 2).
Fig. 2.
Cardiac function at baseline and in 4 h survivors as assessed by left ventricular dp/dt. The X-axis shows the five groups. Y-values are + and − left ventricular dp/dt (mm Hg/s), mean ± s.d.: circles are measurements at baseline; squares are measurements after 4 h of survival.
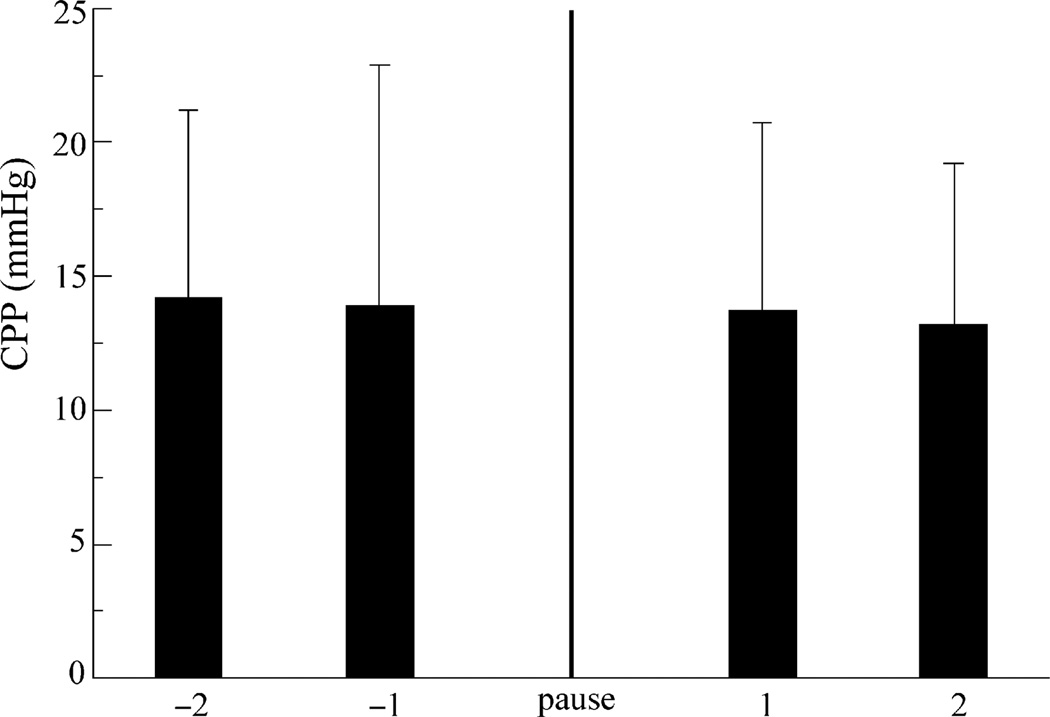
Coronary perfusion pressure for the last two chest compressions prior to the first pause did not differ from that of the first two compressions after that pause, regardless of the timing or duration of the pause (Fig. 3).
Fig. 3.
Coronary perfusion pressure (CPP) for groups in Part A for the compressions around the first pause (n = 48). Shown are mean ± s.d for the last two compressions before the first pause and the first two compressions immediately following the first pause. There was no significant difference between CPP values before and after the pause.
There was no significant difference between groups in the success of the first shock, or the first three shocks administered to treat the initial VF episode (Fishers exact test) (Fig. 4). There was no significant difference between groups in the number of refibrillation occurrences (Table 2).
Fig. 4.
Results of the first three defibrillation shocks, delivered at 7, 8.5, and 10 min as necessary, to halt the initial episode of induced VF. Y-axis shows percent success. There we no significant differences in VF termination rate between groups.
4. Discussion
The major findings of the study are: (1) removing the post-shock pause associated with pulse or rhythm checks improved outcome and (2) in the absence of a post-shock pause, altering, shortening or eliminating pre-shock pauses in chest compressions did not further improve outcome.
Although the post-shock pause in chest compressions previously used to assess heart rhythm and pulse was eliminated by the 2005 Guidelines, an initial pause in compressions is still needed to determine whether a shock is indicated.1 For future Guidelines, an important question is whether changing the timing or duration of that initial pause, or eliminating it entirely, would be beneficial to the patient. The main finding of this study is that, in a setting where eliminating the post-shock component of a single early pause in chest compressions significantly improved survival, further improvements in survival were not obtained by altering the timing or shortening the duration of a pre-shock pause, or by eliminating it entirely.
Animal studies have strongly suggested that pauses during chest compressions have a detrimental effect on survival.6–8 However, it has not been determined whether some pauses are more deleterious than others depending on their location in the resuscitation sequence. While several studies have suggested that a pause immediately prior to a shock is particularly harmful, these studies did not specifically evaluate the impact of pause timing independent from total pause duration, and they featured other protocol characteristics that may have influenced the observed results. For example, in two prior studies,6,7 resuscitation efforts were stopped immediately after the first defibrillation attempt, making it unknown if the observed survival differences might have been altered if chest compressions had been resumed after defibrillation as occurs in the field. Note that in our study, circulation never returned after defibrillation without administering additional chest compressions. In two other studies,5,8 the repeated application of pre-shock chest compression pauses of varying durations, either for rescue breathing or rhythm analysis, also resulted in differences in cumulative “hands-off” time over the entire resuscitation attempt, which might have independently impacted outcomes.
In our study, 4 h survival was significantly improved by eliminating the 20 s component of the chest compression pause that followed the first shock. Importantly, this improvement came from shortening only a single pause in compressions, which occurred early in the resuscitation. The finding that this had such a dramatic effect on survival suggests that the Guidelines 2005 recommendation to omit the post-shock pulse check and immediately resume chest compressions may be a particularly important resuscitation protocol change related to chest compression pauses. On the other hand, no further improvement in survival was obtained by shifting the pre-shock pause earlier in time and adding a period of chest compressions before shock delivery, or by shortening the pre-shock pause, or even by eliminating it entirely. The insensitivity of survival to these changes suggests that clinical maneuvers aimed solely at altering, further shortening or eliminating these pauses, such as shifting the timing of the rhythm analysis or analyzing the rhythm during ongoing chest compressions, may not on their own provide meaningful clinical benefit, especially when only one shock is required during resuscitation.
While multiple theories have been proposed to explain why inserting a relatively short hands-off interval might have a disproportionately negative effect on resuscitation outcomes, the exact explanation remains unclear. One explanation that has been suggested is that coronary perfusion pressure drops during the pause and takes a number of compressions to recover after the pause. Berg et al. showed this effect for pauses for ventilation.8 However, our data did not show the same effect of the pause; the coronary perfusion pressure recovered to its pre-pause level on the first compression after that pause, even for the 40 s pauses in the control group.
There are two possible explanations for this difference in results. One, our study used a mechanical chest compression device while the Berg study used manual chest compressions. It is possible that, unlike a mechanical chest compression device, the person performing chest compressions required a few compressions to re-establish the correct depth of compression. Second, the pauses in the Berg study were associated with two breaths while the pauses in this study were not. The increased intrathoracic pressure associated with positive pressure ventilation may force blood retrogradely out of the chest and it may take some time for this blood to return to the chest. Since our study did not necessarily provide breaths during the pause, blood would not be forced retrogradely and so the coronary perfusion pressure following chest compressions remained the same as before the pause. Whatever the basis for these differing observations of coronary perfusion pressure behavior across a chest compression pause, it is clear that the deleterious effect of the prolonged pause in the G2000 group of our study was not due to a precipitous drop and slow recovery in perfusion pressure after the pause.
4.1. Limitations
There are several limitations for our study. First, the animals are anesthetized prior to and during cardiac arrest and have no underlying cardiac disease that might impact survival. Further, the number of animals studied and the statistical power of the study are limited. In this study, we found a significant difference in survival across groups, but the number of survivors limited our ability to detect a difference in post-resuscitation cardiac function. These are limitations of most resuscitation studies involving animal models and should be considered whenever one is interpreted. Second, our group with no pauses (Group B) was studied with the same experimental protocol, but it was not randomized with the other four groups. Its inclusion in this manuscript puts the survival rates of the other groups into context but does not change the main finding of our study. Third, the group with markedly worse outcome was both the only group with a pause longer than 20 s, and the only group with a post-shock pause. The study is therefore unable to determine whether it was elimination of the post-shock pause, or shortening the pause duration from 40 to 20 s, or both, that improved outcome. Evaluation of that question will require further study.
5. Conclusions
The findings of this study suggest that the Guidelines 2005 recommendation to omit the pause associated with post-shock rhythm and pulse checks and immediately resume chest compressions may be a particularly important resuscitation protocol change. The findings also suggest that clinical maneuvers aimed solely at altering, shortening or eliminating pre-shock pauses in chest compressions provide either no additional benefit, or a benefit too small to be detected in this study. We make this conclusion with the caveat that it is likely to be important to keep cumulative pause time less than a particular length, potentially less than 40 s, though we did not test this specific hypothesis in this study. Further research is needed to confirm these experimental results in a clinical setting, and to better define how outcome is affected by combining multiple 20 s pauses over the duration of the resuscitation effort.
Acknowledgments
This work was supported in part by grants from Medtronic Emergency Response Systems and NIH HL-42760. Dr. Walcott and Dr. Ideker are Principle Investigators on a grant from Medtronic Physio-Control. Ms. Melnick is paid in part by Medtronic Physio-Control. Dr. Banville, Dr. Chapman, and Mr. Walker are employees of Medtronic Physio-Control.
Footnotes
A Spanish translated version of the summary of this article appears as Appendix in the final online version at doi:10.1016/j.resuscitation.2008.11.012.
Conflict of interest statement Dr. Killings worth has no conflict of interest associated with this study.
References
- 1.American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2005;112:IV1–IV203. doi: 10.1161/CIRCULATIONAHA.105.166550. [DOI] [PubMed] [Google Scholar]
- 2.Hallstrom AP, Ornato JP, Weisfeldt M, et al. Public-access defibrillation and survival after out-of-hospital cardiac arrest. N Engl J Med. 2004;351:637–646. doi: 10.1056/NEJMoa040566. [DOI] [PubMed] [Google Scholar]
- 3.Rea TD, Helbock M, Perry S, et al. Increasing use of cardiopulmonary resuscitation during out-of-hospital ventricular fibrillation arrest: survival implications of guideline changes. Circulation. 2006;114:2760–2765. doi: 10.1161/CIRCULATIONAHA.106.654715. [DOI] [PubMed] [Google Scholar]
- 4.Lloyd MS, Heeke B, Walter PF, Langberg JJ. Hands-on defibrillation: an analysis of electrical current flow through rescuers in direct contact with patients during biphasic external defibrillation. Circulation. 2008;117:2510–2514. doi: 10.1161/CIRCULATIONAHA.107.763011. [DOI] [PubMed] [Google Scholar]
- 5.Yu T, Weil MH, Tang W, et al. Adverse outcomes of interrupted precordial compression during automated defibrillation. Circulation. 2002;106:368–372. doi: 10.1161/01.cir.0000021429.22005.2e. [DOI] [PubMed] [Google Scholar]
- 6.Steen S, Liao Q, Pierre L, Paskevicius A, Sjöberg T. The critical importance of minimal delay between chest compressions and subsequent defibrillation: a haemodynamic explanation. Resuscitation. 2003;58:249–258. doi: 10.1016/s0300-9572(03)00265-x. [DOI] [PubMed] [Google Scholar]
- 7.Sato Y, Weil MH, Sun S, et al. Adverse effects of interrupting precordial compression during cardiopulmonary resuscitation. Crit Care Med. 1997;25:733–736. doi: 10.1097/00003246-199705000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Berg RA, Sanders AB, Kern KB, et al. Adverse hemodynamic effects of interrupting chest compressions for rescue breathing during cardiopulmonary resuscitation for ventricular fibrillation cardiac arrest. Circulation. 2001;104:2465–2470. doi: 10.1161/hc4501.098926. [DOI] [PubMed] [Google Scholar]
- 9.Gliner BE, White RD. Electrocardiographic evaluation of defibrillation shocks delivered to out-of-hospital sudden cardiac arrest patients. Resuscitation. 1999;41:133–144. doi: 10.1016/s0300-9572(99)00040-4. [DOI] [PubMed] [Google Scholar]