Abstract
Context:
Articular cartilage injuries are common in patients presenting to surgeons with primary complaints of knee pain or mechanical symptoms. Treatment options include comprehensive nonoperative management, palliative surgery, joint preservation operations, and arthroplasty.
Evidence Acquisition:
A MEDLINE search on articular cartilage restoration techniques of the knee was conducted to identify outcome studies published from 1993 to 2013. Special emphasis was given to Level 1 and 2 published studies.
Study Design:
Clinical review.
Level of Evidence:
Level 3.
Results:
Current surgical options with documented outcomes in treating chondral injuries in the knee include the following: microfracture, osteochondral autograft transfer, osteochondral allograft transplant, and autologous chondrocyte transplantation. Generally, results are favorable regarding patient satisfaction and return to sport when proper treatment algorithms and surgical techniques are followed, with 52% to 96% of patients demonstrating good to excellent clinical outcomes and 66% to 91% returning to sport at preinjury levels.
Conclusion:
Clinical, functional, and radiographic outcomes may be improved in the majority of patients with articular cartilage restoration surgery; however, some patients may not fully return to their preinjury activity levels postoperatively. In active and athletic patient populations, biological techniques that restore the articular surface may be options that provide symptom relief and return patients to their prior levels of function.
Keywords: cartilage injuries, knee, microfracture, osteochondral autograft transfer, osteochondral allograft transplant, autologous chondrocyte implantation
Advances in arthroscopy and magnetic resonance imaging (MRI) have resulted in increased recognition of articular cartilage defects of the knee. These injuries are ubiquitous, with numerous studies reporting articular defects in 60% to 66% of knees undergoing arthroscopy for pain.1,10,26,58 Lesions manifest in a variety of sizes, depths, and locations and present across a spectrum of severity, ranging from isolated, small, shallow lesions in low-demand patients that are quiescent on MRI to large, multifocal, full-thickness chondral defects with MRI evidence of significant bone marrow edema in higher activity patients. These defects have limited healing potential secondary to the poor regenerative capacity and avascular nature of cartilage.37 As a result, chondral lesions can be a source of pain and mechanical symptoms as well as a risk factor for posttraumatic arthritis.7,55 Nonoperative treatment options include activity modification, nonsteroidal anti-inflammatory medications, unloader bracing for unicondylar lesions with malalignment through the affected compartment, muscle strengthening, and intra-articular corticosteroid and viscosupplementation injections.
Failure of nonoperative management may be an indication for surgical procedures ranging from palliative therapies to more advanced restorative techniques.8 The simplest surgical option is arthroscopic lavage and debridement, which may improve pain and mechanical symptoms in the short term but does not address the potential for progressive joint degeneration in the long term.8,20 Other surgical options include microfracture, autologous chondrocyte implantation (ACI), osteochondral autograft transfer (OAT), and osteochondral allograft transplantation (OCA). New technologies are emerging, such as implantation of particulate autograft cartilage (Cartilage Autograft Implantation System; Depuy Mitek, Raynham, Massachusetts) or particulate juvenile allograft cartilage (DeNovo Natural Tissue; Zimmer, Warsaw, Indiana), but clinical outcome data are limited for these techniques.15
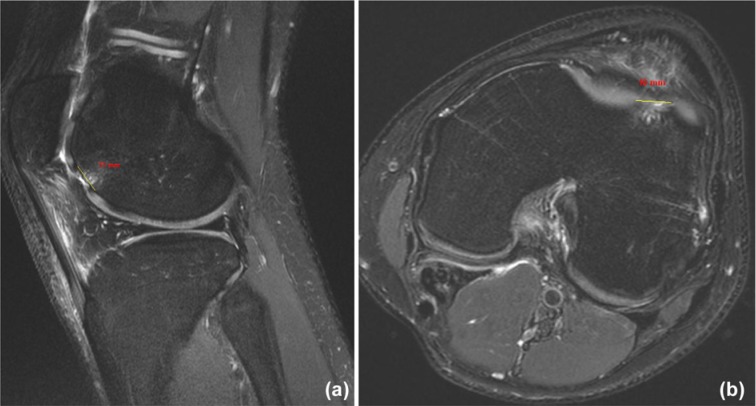
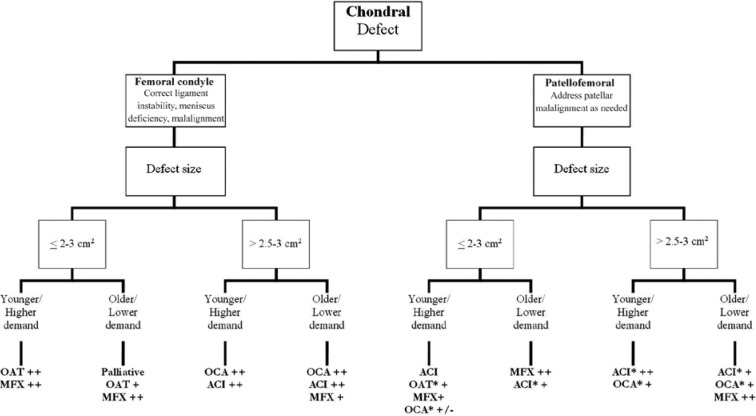
A great deal of research has been dedicated to articular cartilage injuries of the knee, and treatment algorithms continue to evolve as our understanding of the pathology advances and follow-up of patients treated with specific techniques increases.3,36 A number of factors should be taken into consideration when making treatment decisions, such as the location of the lesion(s) (patellofemoral vs femoral condyle), size of the defect (area), involvement of subchondral bone, and the age and activity level of the patient. Defect location is readily discernible on the basis of history, physical examination, and advanced imaging techniques such as MRI. Area is measured on MRI by determining the largest diameter in 2 orthogonal planes (sagittal, coronal, or axial), then multiplying these values to estimate the square area (Figure 1). In terms of age and performance level, patients are generally classified as either (1) younger (<40 years old) or high-demand patients or (2) older (40-50 years old) or low-demand patients. With these 3 variables (size, location, and patient age/demand) along with the patient’s goals and commitment to rehabilitation, treatment can be based on an algorithm (Figure 2).9
Figure 1.
(a) Sagittal and (b) axial MRI of chondral defect measuring 11 × 10 mm.
Figure 2.
Treatment algorithm. Younger, <40 years old; older, 40-50 years old; MFX, microfracture; OAT, osteochondral autograft transfer; OCA, osteochondral allograft transplantation; ACI, autologous chondrocyte implantation; *, consider anteromedialization tibial tubercle osteotomy; ++, based on level 1/2 recommendations; +, based on level 3/4 recommendations; +/-, consider option depending on individual patient characteristics. Adapted from Cole et al.9
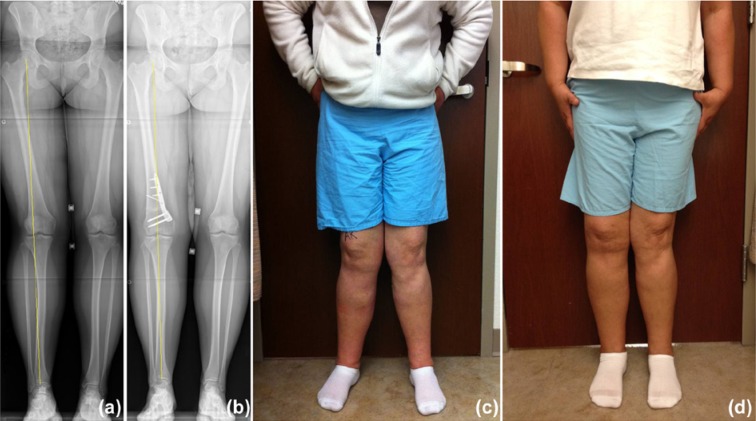
Regardless of the chondral defect treatment strategy, the importance of addressing concomitant knee pathology and limb alignment cannot be understated. Meniscus pathology is treated with resection, repair, or transplantation. Ligamentous instability is addressed with reconstruction to reduce the risk of additional injury to native and restored cartilage tissue. Limb malalignment is corrected with a varus- or valgus-producing osteotomy to prevent overload of the diseased compartment (Figure 3). In patients with patellofemoral chondral lesions, special attention should be directed toward correcting patellar instability, tilt, malalignment, or compressive overload with medial patellofemoral ligament reconstruction, selective lateral retinacular release, and tibial tubercle osteotomy to off-load and stabilize the patella as needed. At the time of surgery, all patients should undergo examination under anesthesia with diagnostic arthroscopy with detailed articular cartilage mapping; concomitant knee pathology should be addressed before treatment of the chondral injury.
Figure 3.
Example of a right distal femoral osteotomy to correct valgus malalignment and off-load the lateral compartment of the knee. (a) Preoperative x-ray, (b) postoperative x-ray, (c) preoperative clinical photograph, and (d) postoperative clinical photograph of patients undergoing distal femoral osteotomy.
Microfracture
Indications
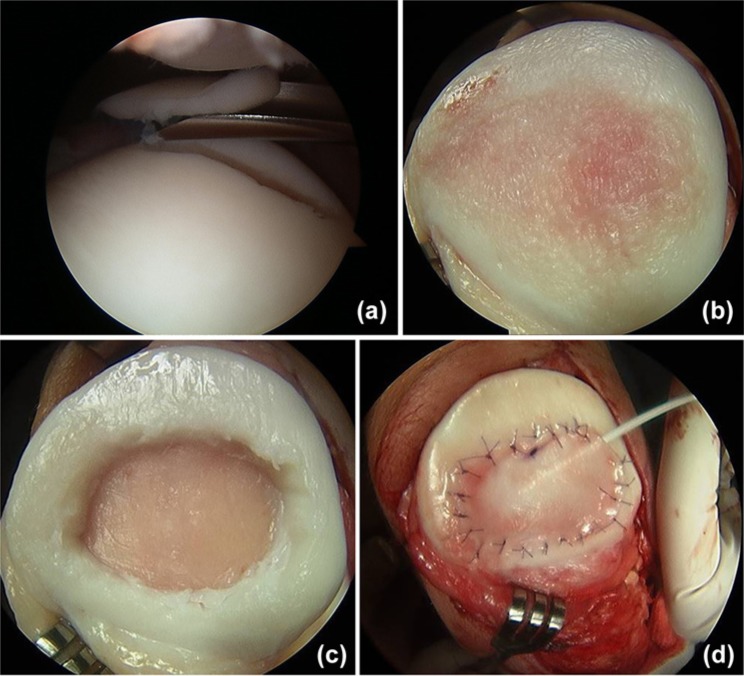
Microfracture is the most commonly performed method of cartilage restoration by marrow stimulation.40 This technique relies on the influx of marrow products (stem cells, growth factors, and platelets) to form a fibrin clot, which is slowly remodeled into fibrocartilage rather than normal hyaline articular cartilage (Figure 4). Mature fibrocartilage is predominantly type I collagen with minimal amounts of type II collagen, resulting in a less durable construct over time.46,49
Figure 4.
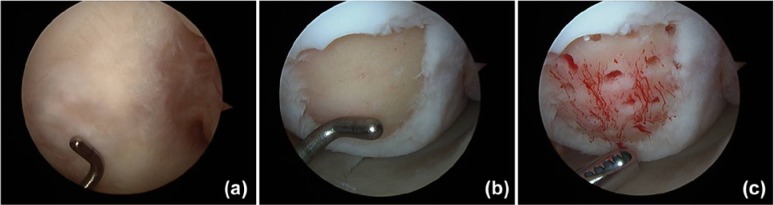
Microfracture case demonstrating (a) a condylar cartilage defect that is (b) debrided to a clean base with a healthy stable rim of supporting chondral tissue. (c) Following microfracture, the inflow is turned off to demonstrate initial influx of marrow products.
Microfracture may be indicated for the treatment of small (≤ 2-3 cm2), full-thickness chondral lesions31,46 and, occasionally, for treating large lesions (≥ 2.5-3 cm2) in older and low-demand patients.56 Optimal indications include young patients with small, contained, full-thickness defects less than 1 year from injury. Relative contraindications include age >50 years; concomitant knee pathology that cannot easily be addressed, such as significant trauma, infection, or neoplasm; inability to follow postoperative rehabilitation protocols; diffuse joint degeneration; and underlying avascular necrosis.57
Results
Overall, short-term clinical results after microfracture have been favorable, demonstrating good to excellent ratings in 67% to 80% of patients, with a similar percentage of athletes returning to preinjury sport levels.46,56 Although initial short-term results are encouraging, sports participation declines over time, with up to 80% demonstrating decreased Tegner scores (from a mean of 6 to 5) at a mean follow-up of 5 years.16 More predictable return to sports was observed in patients under 40 years of age with lesions less than 2 cm2, symptoms less than 12 months, and no history of surgery.48 As with most articular cartilage restoration techniques, condylar lesions tend to demonstrate superior outcomes compared with patellofemoral injuries.45 A recent systematic review (level of evidence, 3) of 12 published studies analyzing patients undergoing microfracture revealed a mean return to sport rate of 66% at a mean of 8 months (range, 2-16 months).45
A prospective randomized controlled trial of competitive athletes comparing microfracture to OAT revealed improved clinical outcomes in both groups; however, the OAT group demonstrated good to excellent results in 96% of patients, compared with only 52% for microfracture.21 Only 52% of microfracture patients were able to return to sport at preinjury levels after surgery.21 A randomized controlled trial of 80 patients treated with ACI or microfracture demonstrated improved clinical outcomes in both groups at 5 years of follow-up, with no significant differences in clinical or radiographic outcomes.31
Potential complications of microfracture include fracture of subchondral bridges, bony overgrowth, incomplete microfracture with limited influx of marrow products, hypertrophic overgrowth, and fragmentation or delamination of the fibrocartilage-subchondral bone junction. An additional disadvantage is a potentially compromised result following subsequent ACI after failed microfracture due to inadequate remaining bone stock.44
In summary, microfracture is a fibrocartilage repair technique that is relatively simple, inexpensive, and associated with minor morbidity. The bone marrow products filling the defect are remodeled into a fibrocartilage tissue that is histologically different and biomechanically inferior to native hyaline cartilage. Short-term results have been favorable in well-selected patients, but less successful outcomes occur with longer follow-up in athletic, high-demand patients.16,45,48
Osteochondral Autograft Transfer
Indications
OAT replaces chondral defects with normal hyaline articular cartilage, a distinct advantage over microfracture. A plug of the patient’s own healthy cartilage and bone is harvested from a nonweightbearing portion of the joint. One or multiple strategically arranged plugs can be transferred to fill the defect. Mosaicplasty, or multiple plug transfer, was originally reported in the early 1990s and further developed and refined in the later 1990s.5,24,38 Although OAT was originally confined to small cartilaginous defects of the knee, the technique is now used in multiple joints, such as the hip, ankle, shoulder, and elbow.
Since both cartilage and bone are harvested in the donor plug, OAT has the advantage of filling osteochondral defects, making OAT an option in treating smaller osteochondritis dissecans lesions not amendable to primary fixation.25 OAT utilizes the patient’s own tissue, which eliminates the risk of infectious disease transmission possible with allografts. The main limitation of OAT is defect size.28 The technique is best suited for defects 1 to 4 cm2.25 Much larger lesions, up to 8 to 9 cm2, can be filled with multiple plugs with a risk of significant donor site morbidity.22 Because of a high failure rate and bone-cartilage depth mismatch between the trochlear donor site and the patellar recipient site, some have recommended against the use of OAT in patellar lesions.4 The ideal candidate for OAT is a young, high-demand patient with unifocal femoral condyle or trochlear full-thickness cartilage loss with an area ≤ 2 to 3 cm2 (Figure 5).
Figure 5.
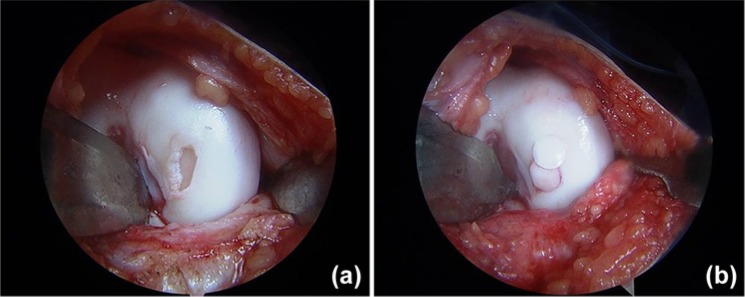
Osteochondral autograft transfer (OAT) highlighting (a) a lesion of the medial femoral condyle after debridement and (b) subsequent OAT utilizing a mosaicplasty technique.
Results
Histologic analysis demonstrates a high rate of survivorship of the transferred hyaline cartilage.23 Good to excellent clinical results have been obtained in up to 92% of femoral condyle lesions, 87% of tibial lesions, and 79% of trochlear or patellar lesions.22 This has been replicated in multiple studies with significant improvements in both pain and activity levels in 85% of patients with osteochondral defects of the knee.13,30 A level 3 systematic review of 5 published studies of patients undergoing OAT revealed a mean return to sport rate of 91% at a mean time of 7 months (range, 4-11 months).45
Krych et al compared OAT to microfracture in a study of 96 patients (48 OAT mosaicplasties and 48 microfractures) and found equally improved Short Form–36 physical component, Knee Outcome Survey activities of daily living, and International Knee Documentation Committee scores at 5 years of follow-up.32 However, the OAT group was able to maintain a higher level of athletic activity compared with the microfracture group, with significantly improved Marx Activity Rating Scale scores at 2-, 3-, and 5-year follow-up.32
Complications of OAT and mosaicplasty include overfilling or overcoverage of the donor site with fibrocartilage-type tissue, potentially resulting in pain or mechanical symptoms.22 A report on 1097 OAT mosaicplasty procedures demonstrated a relatively low complication rate, including 56 (8%) hemarthroses, 4 (0.4%) infections, and 4 (0.4%) deep venous thromboses.25
In summary, OAT is a well-studied surgical option for treating full-thickness, small chondral, or osteochondral injuries in the knee. The defect is filled with native hyaline cartilage that is more durable than fibrocartilage while avoiding the use of allograft tissue. It is most reliable when used in young, high-demand patients with isolated lesions ≤ 2 to 3 cm2. Although results tend to be slightly better for isolated femoral condyle lesions, OAT remains a viable option for treating trochlear defects as well.22
Osteochondral Allograft Transplant
Indications
OCA has demonstrated consistent clinical results and can be used to treat a variety of articular defects in the knee using size-matched cadaveric donor plugs that permit immediate structural restoration of the joint articular surface.41 The cadaveric graft eliminates donor site morbidity and allows for treatment of larger lesions (> 2-3 cm2). Although a microscopic immunogenic response to transplanted allograft chondral tissues is possible at the bone-to-bone interface, a clinically significant response within the joint is unlikely because the intact cartilage matrix prevents contact between the donor chondrocytes and host antibodies.34
The composition of osteochondral allografts consists of viable hyaline cartilage supported by 5 to 8 mm of subchondral bone.11,51 The cartilage receives its nutrition from synovial fluid by means of diffusion, and basic science research has demonstrated chondrocyte viability in fresh allograft implants.11,51 The most common method for graft storage is refrigeration for a 14-day period while microbiologic and serologic analyses are conducted. Although chondrocytes may remain viable up to 42 days after harvest,39 implantation is recommended at 14 to 28 days after procurement for optimal cell viability.35 Retrieval studies at 25 years following transplantation have demonstrated survival of the allograft chondral tissue.18
OCA is an appealing option for large chondral and osteochondral defects with an area > 2 to 3 cm2 (Figure 6). The technique can be used to treat articular defects of the femoral condyle, trochlea, or patella in young, high-demand patients as well as older, low-demand patients. Prior failure of microfracture or ACI is not a contraindication, and bony defects can be addressed with the use of OCA.12 Preoperatively, donor tissues must be size matched to individual patients based on x-ray, computed tomography, or MRI measurements. Given the limited window of chondrocyte viability in fresh specimens, the timing and logistics of surgery are often challenging for both the surgeon and patient.
Figure 6.
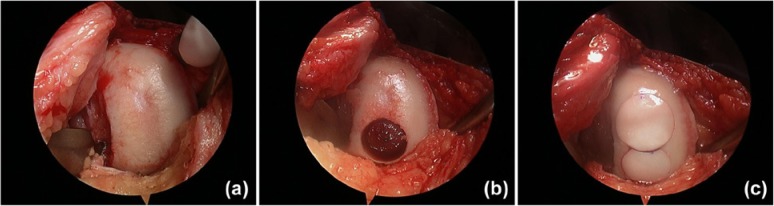
(a) Example of osteochondral allograft transplantation used to treat a large chondral lesion of the medial femoral condyle. (b) The site was prepared to receive the first plug in the posterior position. After this was placed, it was determined that an additional plug was needed anteriorly. (c) The site was prepared for a second plug, which was subsequently placed anterior to the first utilizing the mosaicplasty technique.
Results
In appropriately selected patients (see Figure 2), OCA has demonstrated 5- and 10-year Kaplan-Meier survival rates of 95% and 85%, respectively, for femoral condyle lesions and 80% 10-year survival rates for tibial plateau grafts.19 This level 2 evidence has been replicated in a number of level 4 studies. Longer term follow-up (mean, 7.8 years) of very large condylar defects with a mean area of 7.5 cm2 has revealed good to excellent results in 72% of patients, with a 15% rate of reoperation.14 Patellofemoral lesions tend to have less favorable outcomes, with only 60% good to excellent results in 20 patellofemoral defects followed for 8 years.29
Krych et al reported rates of return to sport for 43 competitive athletes after OCA33 (23% of whom had failed prior cartilage surgery); the mean defect size was 7.25 cm2. Seventy-nine percent were able to return at the preinjury level, and 88% returned in a limited fashion at a mean 2.5 years of follow-up. Factors negatively influencing return to sport included symptoms for greater than 12 months before surgery and patient age >25 years.33
In summary, OCA is a viable option for large, full-thickness chondral defects of the knee with an area of 2 to 8 cm2 or greater. Benefits include lack of donor site morbidity, immediate articular surface restoration with hyaline cartilage, proven cell viability, and replacement of deficient subchondral bone. Drawbacks of the procedure include graft availability, theoretical risk of viral and bacterial transmission,12 and logistical considerations of scheduling surgery within a narrow window of time to optimize cell viability.
Autologuos Chondrocyte Implantation
Indications
Initially described in 1994,6 ACI is a 2-stage process in which a biopsy of the patient’s articular cartilage is obtained in the first stage, and following ex vivo expansion, cells are implanted into the defect during the second stage (Figure 7). ACI has the advantage of treating large lesions (up to 10 cm2) by restoring hyaline-like cartilage.31 It is useful in treating injuries that have failed debridement or other reparative techniques. Limitations of ACI include 2-staged procedures, ex vivo cell expansion, and increased expense, costing approximately $66,000 per case.53 ACI requires well-preserved bone stock at the base of the chondral defect. Bone loss greater than 6 to 8 mm (commonly seen in osteochondritis dissecans) may require bone grafting as a separate procedure or can be performed concomitantly as a single-stage procedure; however, abnormal subchondral bone has been implicated in the increased rate of failure of ACI as a salvage procedure for failed microfracture.44
Figure 7.
Example of a full-thickness patellar defect treated with autologous chondrocyte implantation. (a) The initial biopsy obtained during the first stage was taken from the superolateral trochlea. (b) During the second stage of the procedure, the patella was completely everted though an arthrotomy and the lesion readily identified. (c) This was thoroughly debrided, and (d) a collagen patch was sewn into place leaving a small opening in the superior portion for cell injection.
The ideal ACI candidate is a young active patient with a full-thickness chondral defect between 2 and 10 cm2 that is surrounded by stable and healthy cartilage. ACI is best suited for unifocal femoral condyle lesions but may be used for multifocal disease.43 It is important to counsel patients on the comprehensive postoperative course required for the 2-stage procedure. Competitive athletes should understand that unrestricted return to play is not permitted for 12 to 18 months.47
Results
Early reports demonstrated good to excellent results in 84% to 90% of patients at 3 years following femoral condyle ACI.6 Longer term studies have been encouraging, with good to excellent results reported in 82% of patients at 5 to 11 years after surgery.52 Similar results have been demonstrated in a multicenter study of 32 pediatric patients (ages, 11-17) with a minimum 2-year follow-up.42 A level 3 systematic review of patients undergoing ACI has revealed a mean return to sport rate of 67% at a mean 18 months (range, 12-36 months) across 7 studies.45
Comparative studies of ACI to other cartilage treatment methods have demonstrated equivalent or superior outcomes, and ACI has demonstrated improved clinical outcomes for patellar and trochlear lesions compared with other restoration techniques.4 In a level 1 study comparing ACI with microfracture in 80 patients (40 microfractures and 40 ACI), no significant differences in clinical or radiographic results were found at a mean follow-up of 5 years.31 Limitations of this study include lack of patient matching based on defect size or location. When compared with OAT, ACI was associated with a higher Lysholm score at a mean follow-up of 2 years.27 ACI was better than OAT for large defects (mean, 4.7 cm2), with good to excellent results in 88% compared with 69%.4 In the comparison of advanced generation ACI technology, such as matrix-induced chondrocyte implantation (MACI) and characterized chondrocyte implantation (CCI), to microfracture, both techniques resulted in improved clinical outcomes.2,54 These newer techniques are gaining popularity in Europe as an attempt to improve the biologic quality of the chondrocytes (CCI) and refine the implantation process (MACI). CCI improves the yield of the cell proliferation by reducing chondrocyte cell de-differentiation that occurs during ex vivo cell expansion with traditional ACI. MACI preloads cultured chondrocytes onto a scaffold that improves implantation and fixation of the grafts.2,54
Despite these encouraging reports, ACI is associated with potential complications, including periosteal graft hypertrophy, disturbed fusion, and graft failure.50 Periosteal graft hypertrophy is common, occurring in 15% to 36% of patients.17,50,59 Autograft periosteal hypertrophy and donor site morbidity have been eliminated with advanced graft coverage techniques, such as the collagen-covered ACI procedure.17
ACI is a reliable technique that can be used to treat large, unifocal or multifocal cartilage injuries in high-demand patients. It has the benefit of filling defects with hyaline-like cartilage and is not limited by lesional geometry. The 2-stage surgical procedure requires meticulous planning, ex vivo cell expansion by commercial companies, and considerable cost.
Suggested Postoperative Care
Postoperative rehabilitation9,57 is critical for optimal functional outcomes regardless of the technique used. Patients unable or unwilling to follow therapy guidelines are not surgical candidates. Generally, postoperative care is similar for all techniques, with the goals of obtaining full range of knee motion, gradually increasing weightbearing, and returning to preoperative activity/athletic levels. The 3 phases of rehabilitation include healing (weeks 0-6), transition (weeks 6-12), and remodeling (weeks 13+). The focus of the healing phase is range of motion, edema reduction, and low-resistance strengthening of the hips, quadriceps, hamstrings, gluteal musculature, and core. The transitional phase focuses on return to full weightbearing, gait training, continued range of motion, and progressive strengthening. The final phase, remodeling, focuses on gradual return to impact loading and athletics as determined by the treating surgeons, therapists, and trainers. Depending on the individual case, full return to sport can take 9 to 18 months. The most important factor influencing the postoperative rehabilitation protocol is the location of the treated lesions: patellofemoral or condylar (ie, weightbearing or nonweightbearing). Concomitant surgical procedures, such as ligamentous reconstruction, realignment osteotomy, or meniscal treatment, should be factored into the postoperative care program.
Summary
Articular cartilage injuries of the knee are a common finding in patients with knee pain. Select patients who fail a comprehensive nonoperative treatment program and are willing to comply with demanding postoperative rehabilitation may be candidates for chondral restoration techniques. The best technique for an individual patient depends on age, activity level, lesion location, and lesion size. Each technique shares the common goals of filling articular defects with either fibrocartilage (microfracture), hyaline-like cartilage (ACI), or hyaline cartilage (OAT, OCA) in an attempt to relieve symptoms, improve activity level, and possibly delay the onset of posttraumatic arthritis. These surgical procedures have been extensively evaluated in a multitude of clinical studies with levels of evidence ranging from 1 to 4. Careful patient selection, precise surgical technique, and strict adherence to postoperative rehabilitation are essential for optimal clinical and functional outcomes in patients with full-thickness cartilage defects of the knee.

Footnotes
The following author declared potential conflicts of interest: Michael J. Stuart, MD, is a consultant and received royalties from Arthrex, Inc., and also received research support from Stryker and USA Hockey Foundation.
References
- 1. Aroen A, Loken S, Heir S, et al. Articular cartilage lesions in 993 consecutive knee arthroscopies. Am J Sports Med. 2004;32:211-215 [DOI] [PubMed] [Google Scholar]
- 2. Basad E, Ishaque B, Bachmann G, Stürz H, Steinmeyer J. Matrix-induced autologous chondrocyte implantation versus microfracture in the treatment of cartilage defects of the knee: a 2-year randomised study. Knee Surg Sports Traumatol Arthrosc. 2010;18:519-527 [DOI] [PubMed] [Google Scholar]
- 3. Bekkers JEJ, Inklaar M, Saris DB. Treatment selection in articular cartilage lesions of the knee: a systematic review. Am J Sports Med. 2009;37(suppl 1):148S-155S [DOI] [PubMed] [Google Scholar]
- 4. Bentley G, Biant LC, Carrington RWJ, et al. A prospective, randomised comparison of autologous chondrocyte implantation versus mosaicplasty for osteochondral defects in the knee. J Bone Joint Surg Br. 2003;85:223-230 [DOI] [PubMed] [Google Scholar]
- 5. Bobicć V. Arthroscopic osteochondral autograft transplantation in anterior cruciate ligament reconstruction: a preliminary clinical study. Knee Surg Sports Traumatol Arthrosc. 1996;3:262-264 [DOI] [PubMed] [Google Scholar]
- 6. Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889-895 [DOI] [PubMed] [Google Scholar]
- 7. Buckwalter JA, Mankin HJ. Articular cartilage: degeneration and osteoarthritis, repair, regeneration, and transplantation. Instr Course Lect. 1998;47:487-504 [PubMed] [Google Scholar]
- 8. Cole BJ, Lee SJ. Complex knee reconstruction: articular cartilage treatment options. Arthroscopy. 2003;19(suppl 1):1-10 [DOI] [PubMed] [Google Scholar]
- 9. Cole BJ, Pascual-Garrido C, Grumet RC. Surgical management of articular cartilage defects in the knee. J Bone Joint Surg Am. 2009;91:1778-1790 [PubMed] [Google Scholar]
- 10. Curl WW, Krome J, Gordon ES, Rushing J, Smith BP, Poehling GG. Cartilage injuries: a review of 31,516 knee arthroscopies. Arthroscopy. 1997;13:456-460 [DOI] [PubMed] [Google Scholar]
- 11. Czitrom AA, Keating S, Gross AE. The viability of articular cartilage in fresh osteochondral allografts after clinical transplantation. J Bone Joint Surg Am. 1990;72:574-581 [PubMed] [Google Scholar]
- 12. Demange M, Gomoll AH. The use of osteochondral allografts in the management of cartilage defects. Current Rev Musculoskelet Med. 2012;5:229-235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dozin BP, Malpeli MP, Cancedda RMD, et al. Comparative evaluation of autologous chondrocyte implantation and mosaicplasty: a multicentered randomized clinical trial. Clin J Sport Med. 2005;15:220-226 [DOI] [PubMed] [Google Scholar]
- 14. Emmerson BC, Gortz S, Jamali AA, Chung C, Amiel D, Bugbee WD. Fresh osteochondral allografting in the treatment of osteochondritis dissecans of the femoral condyle. Am J Sports Med. 2007;35:907-914 [DOI] [PubMed] [Google Scholar]
- 15. Farr J, Cole BJ, Sherman S, Karas V. Particulated articular cartilage: CAIS and DeNovo NT. J Knee Surg.2012;25:23-29 [DOI] [PubMed] [Google Scholar]
- 16. Gobbi A, Nunag P, Malinowski K. Treatment of full thickness chondral lesions of the knee with microfracture in a group of athletes. Knee Surg Sports Traumatol Arthrosc. 2005;13:213-221 [DOI] [PubMed] [Google Scholar]
- 17. Gooding CR, Bartlett W, Bentley G, Skinner JA, Carrington R, Flanagan A. A prospective, randomised study comparing two techniques of autologous chondrocyte implantation for osteochondral defects in the knee: periosteum covered versus type I/III collagen covered. Knee. 2006;13:203-210 [DOI] [PubMed] [Google Scholar]
- 18. Gross A, Kim W, Las Heras F, Backstein D, Safir O, Pritzker K. Fresh osteochondral allografts for posttraumatic knee defects: long-term followup. Clin Orthop Relat Res. 2008;466:1863-1870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gross AE, Shasha N, Aubin P. Long-term followup of the use of fresh osteochondral allografts for posttraumatic knee defects. Clin Orthop Relat Res. 2005;(435):79-87 [DOI] [PubMed] [Google Scholar]
- 20. Gudas R, Gudaite A, Mickevicius T, et al. Comparison of osteochondral autologous transplantation, microfracture, or debridement techniques in articular cartilage lesions associated with anterior cruciate ligament injury: a prospective study with a 3-year follow-up. Arthroscopy. 2013;29:89-97 [DOI] [PubMed] [Google Scholar]
- 21. Gudas R, Kalesinskas RJ, Kimtys V, et al. A prospective randomized clinical study of mosaic osteochondral autologous transplantation versus microfracture for the treatment of osteochondral defects in the knee joint in young athletes. Arthroscopy. 2005;21:1066-1075 [DOI] [PubMed] [Google Scholar]
- 22. Hangody L, Fules P. Autologous osteochondral mosaicplasty for the treatment of full-thickness defects of weight-bearing joints: ten years of experimental and clinical experience. J Bone Joint Surg Am. 2003;85(suppl 2):25-32 [DOI] [PubMed] [Google Scholar]
- 23. Hangody L, Kish G, Kárpáti Z, Szerb I, Udvarhelyi I. Arthroscopic autogenous osteochondral mosaicplasty for the treatment of femoral condylar articular defects: a preliminary report. Knee Surg Sports Traumatol Arthrosc. 1997;5:262-267 [DOI] [PubMed] [Google Scholar]
- 24. Hangody L, Sukosd L, Szigeti I, Karpati Z. Arthroscopic autogenous osteochondral mosaicplasty. Hung J Orthop Trauma. 1996;39:49-54 [Google Scholar]
- 25. Hangody L, Vásárhelyi G, Hangody LR, et al. Autologous osteochondral grafting: technique and long-term results. Injury. 2008;39(suppl 1):32-39 [DOI] [PubMed] [Google Scholar]
- 26. Hjelle K, Solheim E, Strand T, Muri R, Brittberg M. Articular cartilage defects in 1,000 knee arthroscopies. Arthroscopy. 2002;18:730-734 [DOI] [PubMed] [Google Scholar]
- 27. Horas U, Pelinkovic D, Herr G, Aigner T, Schnettler R. Autologous chondrocyte implantation and osteochondral cylinder transplantation in cartilage repair of the knee joint: a prospective, comparative trial. J Bone Joint Surg Am. 2003;85:185-192 [DOI] [PubMed] [Google Scholar]
- 28. Jakob RP, Franz T, Gautier E, Mainil-Varlet P. Autologous osteochondral grafting in the knee: indication, results, and reflections. Clin Orthop Relat Res. 2002;401:170-184 [DOI] [PubMed] [Google Scholar]
- 29. Jamali AA, Emmerson BC, Chung C, Convery FR, Bugbee WD. Fresh osteochondral allografts. Clin Orthop Relat Res. 2005;(437):176-185 [PubMed] [Google Scholar]
- 30. Karataglis D, Green MA, Learmonth DJA. Autologous osteochondral transplantation for the treatment of chondral defects of the knee. Knee. 2006;13:32-35 [DOI] [PubMed] [Google Scholar]
- 31. Knutsen G, Drogset JO, Engebretsen L, et al. A randomized trial comparing autologous chondrocyte implantation with microfracture: findings at five years. J Bone Joint Surg Am. 2007;89:2105-2112 [DOI] [PubMed] [Google Scholar]
- 32. Krych AJ, Harnly HW, Rodeo SA, Williams RJ, 3rd. Activity levels are higher after osteochondral autograft transfer mosaicplasty than after microfracture for articular cartilage defects of the knee: a retrospective comparative study. J Bone Joint Surg Am. 2012;94:971-978 [DOI] [PubMed] [Google Scholar]
- 33. Krych AJ, Robertson CM, Williams RJ, 3rd. Return to athletic activity after osteochondral allograft transplantation in the knee. Am J Sports Med. 2012;40:1053-1059 [DOI] [PubMed] [Google Scholar]
- 34. Langer F, Gross AE. Immunogenicity of allograft articular cartilage. J Bone Joint Surg Am. 1974;56:297-304 [PubMed] [Google Scholar]
- 35. LaPrade RF, Botker J, Herzog M, Agel J. Refrigerated osteoarticular allografts to treat articular cartilage defects of the femoral condyles: a prospective outcomes study. J Bone Joint Surg Am. 2009;91:805-811 [DOI] [PubMed] [Google Scholar]
- 36. Magnussen RA, Dunn WR, Carey JL, Spindler KP. Treatment of focal articular cartilage defects in the knee: a systematic review. Clin Orthop Relat Res. 2008;466:952-962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mankin HJ. The response of articular cartilage to mechanical injury. J Bone Joint Surg Am. 1982;64:460-466 [PubMed] [Google Scholar]
- 38. Matsusue Y, Yamamuro T, Hama H. Arthroscopic multiple osteochondral transplantation to the chondral defect in the knee associated with anterior cruciate ligament disruption. Arthroscopy. 1993;9:318-321 [DOI] [PubMed] [Google Scholar]
- 39. McCulloch PC, Kang RW, Sobhy MH, Hayden JK, Cole BJ. Prospective evaluation of prolonged fresh osteochondral allograft transplantation of the femoral condyle: mimimum 2-year follow-up. Am J Sports Med. 2007;35:411-420 [DOI] [PubMed] [Google Scholar]
- 40. McNickle AG, Provencher MT, Cole BJ. Overview of existing cartilage repair technology. Sports Med Arthrosc. 2008;16:196-201 [DOI] [PubMed] [Google Scholar]
- 41. Meyers MH, Akeson W, Convery FR. Resurfacing of the knee with fresh osteochondral allograft. J Bone Joint Surg Am. 1989;71:704-713 [PubMed] [Google Scholar]
- 42. Micheli LJ, Moseley JB, Anderson AF, et al. Articular cartilage defects of the distal femur in children and adolescents: treatment with autologous chondrocyte implantation. J Pediatr Orthop. 2006;26:455-460 [DOI] [PubMed] [Google Scholar]
- 43. Minas T. Autologous chondrocyte implantation for focal chondral defects of the knee. Clin Orthop Relat Res. 2001;(391):S349-S361 [DOI] [PubMed] [Google Scholar]
- 44. Minas T, Gomoll AH, Rosenberger R, Royce RO, Bryant T. Increased failure rate of autologous chondrocyte implantation after previous treatment with marrow stimulation techniques. Am J Sports Med. 2009;37:902-908 [DOI] [PubMed] [Google Scholar]
- 45. Mithoefer K, Hambly K, Della Villa S, Silvers H, Mandelbaum BR. Return to sports participation after articular cartilage repair in the knee: scientific evidence. Am J Sports Med. 2009;37(suppl 1):167S-176S [DOI] [PubMed] [Google Scholar]
- 46. Mithoefer K, McAdams T, Williams RJ, Kreuz PC, Mandelbaum BR. Clinical efficacy of the microfracture technique for articular cartilage repair in the knee: an evidence-based systematic analysis. Am J Sports Med. 2009;37:2053-2063 [DOI] [PubMed] [Google Scholar]
- 47. Mithofer K, Peterson L, Mandelbaum BR, Minas T. Articular cartilage repair in soccer players with autologous chondrocyte transplantation: functional outcome and return to competition. Am J Sports Med. 2005;33:1639-1646 [DOI] [PubMed] [Google Scholar]
- 48. Mithoefer K, Williams RJ, Warren RF, Wickiewicz TL, Marx RG. High-impact athletics after knee articular cartilage repair: a prospective evaluation of the microfracture technique. Am J Sports Med. 2006;34:1413-1418 [DOI] [PubMed] [Google Scholar]
- 49. Nehrer S, Spector M, Minas T. Histologic analysis of tissue after failed cartilage repair procedures. Clin Orthop Relat Res. 1999;365:149-162 [DOI] [PubMed] [Google Scholar]
- 50. Niemeyer P, Pestka JM, Kreuz PC, et al. Characteristic complications after autologous chondrocyte implantation for cartilage defects of the knee joint. Am J Sports Med. 2008;36:2091-2099 [DOI] [PubMed] [Google Scholar]
- 51. Pearsall AW, 4th, Tucker JA, Hester RB, Heitman RJ. Chondrocyte viability in refrigerated osteochondral allografts used for transplantation within the knee. Am J Sports Med. 2004;32:125-131 [DOI] [PubMed] [Google Scholar]
- 52. Peterson L, Brittberg M, Kiviranta I, Akerlund EL, Lindahl A. Autologous chondrocyte transplantation: biomechanics and long-term durability. Am J Sports Med. 2002;30:2-12 [DOI] [PubMed] [Google Scholar]
- 53. Samuelson EM, Brown DE. Cost-effectiveness analysis of autologous chondrocyte implantation: a comparison of periosteal patch versus type I/III collagen membrane. Am J Sports Med. 2012;40:1252-1258 [DOI] [PubMed] [Google Scholar]
- 54. Saris DBF, Vanlauwe J, Victor J, et al. Treatment of symptomatic cartilage defects of the knee: characterized chondrocyte implantation results in better clinical outcome at 36 months in a randomized trial compared to microfracture. Am J Sports Med. 2009;37:10S-19S [DOI] [PubMed] [Google Scholar]
- 55. Shelbourne KD, Jari S, Gray T. Outcome of untreated traumatic articular cartilage defects of the knee: a natural history study. J Bone Joint Surg Am. 2003;85(suppl 2):8-16 [DOI] [PubMed] [Google Scholar]
- 56. Steadman JR, Briggs KK, Rodrigo JJ, Kocher MS, Gill TJ, Rodkey WG. Outcomes of microfracture for traumatic chondral defects of the knee: average 11-year follow-up. Arthroscopy. 2003;19:477-484 [DOI] [PubMed] [Google Scholar]
- 57. Steadman JR, Rodkey WG, Rodrigo JJ. Microfracture: surgical technique and rehabilitation to treat chondral defects. Clinical Orthop Relat Res. 2001;391:S362-S369 [DOI] [PubMed] [Google Scholar]
- 58. Widuchowski W, Widuchowski J, Trzaska T. Articular cartilage defects: study of 25,124 knee arthroscopies. Knee. 2007;14:177-182 [DOI] [PubMed] [Google Scholar]
- 59. Wood JJ, Malek MA, Frassica FJ, et al. Autologous cultured chondrocytes: adverse events reproted to the United States Food and Drug Administration. J Bone Joint Surg Am. 2006;88:503-507 [DOI] [PubMed] [Google Scholar]