Abstract
Understanding how sensory pathways transmit information under natural conditions remains a major goal in neuroscience. The vestibular system plays a vital role in everyday life, contributing to a wide range of functions from reflexes to the highest levels of voluntary behavior. Recent experiments establishing that vestibular (self-motion) processing is inherently multimodal also provide insight into a set of interrelated questions: What neural code is used to represent sensory information in vestibular pathways? How does the organism’s interaction with the environment shape encoding? How is self-motion information processing adjusted to meet the needs of specific tasks? This review highlights progress that has recently been made towards understanding how the brain encodes and processes self-motion to ensure accurate motor control.
Keywords: vestibular system, self-motion, variability, multisensory, corollary discharge
Introduction
The vestibular system encodes self-motion information by detecting the motion of the head-in-space. In turn, it provides us with our subjective sense of self-motion and orientation thereby playing a vital role in the stabilization of gaze, control of balance and posture. Neurophysiological and clinical studies have provided important insights into how, even at the earliest stages of processing, vestibular pathways integrate information from other modalities to generate appropriate and accurate behaviors (for a review see [1]). The present review will first consider the encoding of self-motion information at the earliest stages of vestibular processing, and next highlight the strategies of multimodal integration that are used within vestibular pathways. It will then consider the vestibular system’s role in ensuring the accuracy of three specific classes of behaviors: 1) The control of gaze to ensure clear vision during everyday activities, 2) The production of the compensatory neck and limb movements required to ensure postural equilibrium during both self generated and externally applied movements, and 3) More complex voluntary motion tasks such as navigation and reaching. Taken together, the findings of recent behavioral, single unit recording, and lesion studies emphasize the essential role of the multimodal integration of vestibular with extra-vestibular signals to ensure accurate motor control.
Overview of the Vestibular System
The vestibular system is phylogenetically the oldest part of the inner ear, yet it was only recognized as an entity distinct from the cochlea in the middle of the 19th century. This is because when the system is functioning normally, we are usually unaware of a distinct sensation arising from vestibular activity; it is integrated with visual, proprioceptive and other extra-vestibular information such that combined experience leads to a sense of motion. For this reason, the significance of this sensory system is best appreciated by the study of patients for whom the daily activities that we take for granted become a significant challenge. For example, following complete vestibular loss, even the smallest head movements are accompanied by gaze instability and postural imbalance, which produce frequent and debilitating episodes of vertigo.
Early Vestibular Processing and the Sensory Coding of Self-Motion: The Sensory Periphery
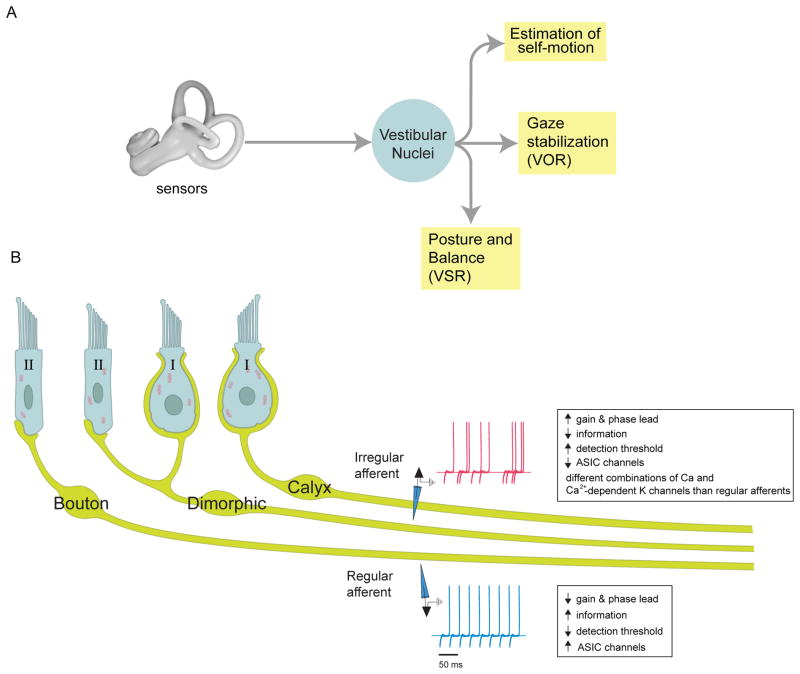
To address the first major question - What neural code is used to represent vestibular sensory information? - recent studies have focused on the afferent fibers which innervate the vestibular sensory organs of the inner ear. The sensory organs comprise two types of sensors: the three semicircular canals, which sense angular acceleration in all three dimensions, and the two otolith organs (the saccule and utricle), which sense linear acceleration (i.e., gravity and translational movements) in all three dimensions. The afferent fibers of the vestibular component of the VIII nerve carry signals from the receptor cells of these sensory organs to the vestibular nuclei. In turn, the central neurons of the vestibular nuclei project to the neural structures that control eye movements, posture, and balance, as well as to upstream structures involved in the computation of self-motion (Figure 1A).
Figure 1. Early Vestibular Processing and the Sensory Coding of Self-Motion: The Sensory Periphery.
a) Vestibular signals from the labyrinth of the inner ear are transferred to the vestibular nuclei (VN) via the vestibular afferents of the VIII nerve. In turn, the VN projects to other brain areas to i) stabilize the visual axis of gaze via the vestibulo-ocular reflex, ii) control posture and balance, and iii) produce estimates of self-motion. b) Drawing of a regular afferent’s bouton ending contacting a type II hair cell (cell B), an irregular afferent’s calyx ending around a type I hair cell (cell C), and an irregular afferent contacting both types of hair cells [i.e. a dimorphic hair cell (cell D) also termed a D-irregular)]. Insets show example extracellular traces highlighting the difference in the resting discharge variability of regular (blue) and irregular (red) afferents. Abbreviations: acid-sensing (ASIC) conductances, ACIS.
Individual afferent fibers innervating the sensory neuroepithelium of either the canals or otoliths display diversity in discharge regularity in the absence of stimulation (Figure 1B). This regularity is typically quantified using a normalized coefficient of variation (CV*) and corresponds to distinct morphological as well as physiological properties [2]. The larger diameter irregular afferent fibers can carry information from either the type I hair cells located at the center of neuroepithelium (C-irregulars) or both type I hair cells and type II hair cells (dimorphic or D-irregulars). In contrast, more regular afferent fibers preferentially carry information from type II hair cells in the peripheral neuroepithelium and have relatively small axon diameters. Spiking regularity of afferent fibers is associated with differences in ion channel distribution [3–4].
Over the range of frequencies typically experienced during everyday behaviors (i.e., up to 20 Hz) [5–6], canal afferents encode head velocity, while otolith afferents encode linear acceleration [1–2]. Quantification of individual afferent responses to sinusoidal motion stimuli reveals important differences in the dynamics of regular versus irregular afferent activity. Notably, irregular afferents have gains and phases that are greater than those of regular afferents over the physiological frequency range of natural head movements [2, 7–12]. For example, irregular afferents are two times more sensitive to head motion at 15 Hz than are regular afferents [8–12]. Consequently it is logical to ask: Why do we have regular vestibular afferents?
The results of recent experiments using information theoretic measures [11, 13] have provided an answer to this question. On average, regular afferents transmit two times more information about head motion than do irregular afferents over the physiological frequency range. Consistent with this finding, regular afferents are also twice as sensitive for detecting head motion as irregular afferents (detection thresholds approximately 4 vs 8 degrees/s). Thus, regular and irregular afferents effectively comprise two parallel information channels (Figure 1B); one which encodes high frequency stimuli with higher gains (i.e., irregular afferents), and the other which transmits information about the detailed time course of the stimulus over the behaviorally significant frequency range (i.e., regular afferents).
The importance of precise spike timing in sensory coding has been demonstrated in other systems including the visual [14–16], auditory [17–19], tactile [20–22], and olfactory [23–24] systems. Interestingly, all but one of these studies [20] focused on higher stages of processing. This latter study reported that peripheral sensory neurons encode information in their spike timings rather than using a rate code. The strategy used by vestibular system afferents differs since spike timing and rate codes coexist at the sensory periphery. As discussed below, current work is now focused on understanding the mechanisms by which vestibular nuclei neurons integrate inputs from these two information channels.
Early Central Vestibular Processing and the Sensory Coding of Self-Motion: Central Neurons
The responses of the vestibular nucleus neurons, to which the afferent fibers directly project, have been well characterized in head-restrained alert monkeys (see [25] for a review). Traditionally, these neurons have been grouped according to differences in their sensitivity to eye motion and passive head motion, as well as differences in their connectivity. Here, for the purpose of simplicity, we will consider two main categories 1) VOR neurons and 2) posture/self-motion neurons (Figure 2A)
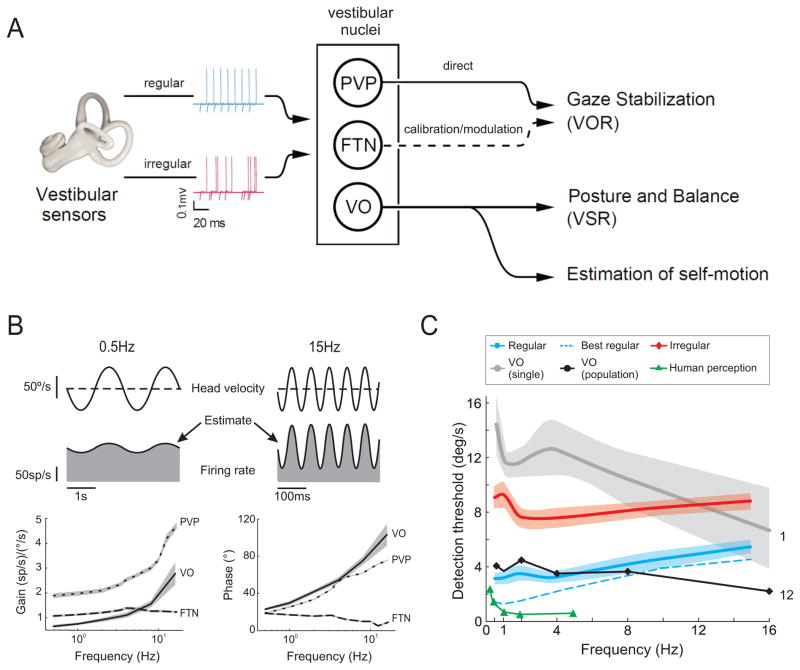
Figure 2. Early Vestibular Processing and the Sensory Coding of Self-Motion: Central Neurons.
a) Neurons in the vestibular nuclei that receive direct input from the vestibular afferents can be categorized into two main categories i) neurons that control and modulate the vestibulo-ocular reflex to ensure gaze stability during everyday life (i.e., PVPs and FTNs), and ii) neurons that control posture and balance, and also project to higher order structures involved in the estimation of self-motion (i.e., VO neurons). b) Firing rate response of an example VO neuron in the vestibular nucleus recorded in alert monkeys during sinusoidal head rotation at 0.5 and 15 Hz. Plots below show response gains averaged for populations of VO, PVP, and FTN neurons recorded over a wide range of frequencies of head rotation. Note, PVP neurons have relatively higher gains which increase more dramatically at higher frequencies. Side bands show +/-1 SEM. Data replotted from [32]. c) Average detection threshold values for regular (blue) and irregular (red) afferents, and VO neurons (gray) at different frequencies of sinusoidal head rotation in alert monkeys. Estimates of the information transmitted by a pooled population of 12 VO neurons (black) as well as human behavioral thresholds [37] are superimposed (green) for comparison. Side bands show +/- SEM. Data replotted from [13].
The most direct pathway that mediates the VOR comprises a three neuron arc: vestibular nerve afferents project to central neurons in the vestibular nuclei (i.e., VOR neurons), which in turn project to extraocular motoneurons. The majority of VOR neurons are the so-called position-vestibular-pause (PVP) neurons; a distinct group of neurons which derive their name from the signals they carry during passive head rotations and eye movements. In addition, a second class of neuron, termed floccular target neurons (FTN), also contribute to the direct VOR pathway. Notably, FTNs receive input from the flocculus of the cerebellum as well as from the vestibular nerve. The responses of FTNs complement those of PVP neurons during our daily activities, and play a vital role in calibrating the VOR to maintain excellent performance in response to the effects of aging as well as changes in environmental requirements, such as those brought about by the corrective lens worn to correct myopia or during the motor learning required during prism adaptation (for review, see [26]).
The second category of vestibular nuclei neurons are the Vestibular-Only (VO - alternatively called non-eye movement) neurons. Like VOR neurons, VO neurons receive direct inputs from the vestibular nerve. However, these neurons do not project to oculomotor structures, and thus do not mediate to the VOR. Instead, many of these neurons project to the spinal cord and are thought to mediate, at least in part, the vestibular spinal reflexes (see review [27]). In addition, VO neurons are reciprocally interconnected with the nodulus/uvula of the cerebellum [28] and appear to be the source of vestibular input to vestibular-sensitive neurons in thalamus and cortex [29–30]. Thus, while PVPs mediate the vestibulo-ocular reflex (VOR), stabilize gaze and ensure clear vision during daily activities, VO neurons are the substrate by which the vestibular system plays a critical role in ensuring postural equilibrium as well as the higher-order vestibular processing required for stable spatial orientation.
Computational Analyses of Vestibular Processing: Linear Control System Approach
The common view that early vestibular processing is fundamentally linear has long made it an attractive model for the study of sensorimotor integration. During the past decade, investigations using a linear systems approach have been directed towards understanding early vestibular processing over the physiologically relevant frequency range of motion [13, 31–33]. Interestingly, the response dynamics of both VO neurons [13] and PVP neurons [32, 34] are nearly comparable to those of irregular and regular afferents, respectively. In contrast, FTN neurons appear to be a notable exception; they show remarkably flat gain (and phase) tuning [32]. The functional implications of these differences, summarized in Figure 2B for the gain response of each of these three central neuron classes, are not yet fully understood.
Computational Analyses of Vestibular System: Information transmission, Detection thresholds, and Spike Timing
As reviewed above, neural variability plays an important role determining the strategy used by vestibular afferent fibers to encode behaviorally relevant stimuli (i.e. Figure 1B). While regular afferents transmit information about sensory input in a spike timing code, irregular afferents use a rate code. However, there is no evidence that different afferent classes preferentially contribute to different vestibular pathways (e.g. oculomotor versus vestibulo-spinal) [35–36].
How then is the information encoded by these two streams of afferent input combined at the next stage of processing? Recent experiments on VO neurons provide insight into this question [13]. First, while VO neuron response gains are generally greater than those of individual afferents, VO neurons transmit less information and have significantly greater velocity detection thresholds than even the relatively ‘noisy’ irregular afferents (Figure 2C). Second, while combining the responses of many VO neurons (i.e., >20) lowers velocity detection thresholds, values remains higher than those measured during behavioral experiments (~2.5 vs. 0.5–1°/s; [37]). Thus, there is an apparent discrepancy between the precision of coding at sequential stages of vestibular processing and the brain’s ability to estimate self-motion.
The higher variability displayed by vestibular central neurons could potentially serve to prevent phase locking or entrainment [38–39]. For example, in the visual system thalamic relay cells transmit detailed information in their spike trains [14–15], while cortical neurons display large variability in their responses [40]. However, in response to more naturalistic stimuli, network interactions among visual cortical neurons can sharpen timing reliability [16]. A critical assumption of prior analyses of vestibular processing (Figure 2C) is that a neuron’s ability to reconstruct the stimulus (i.e. coding fraction) can be measured by computing the coding fraction [41–42]. However given that i) coding fractions compute the quality of the linear reconstruction of the stimulus, and ii) coding fractions are low for central vestibular neurons (i.e. VO cells) it is important to consider whether this assumption is valid. Experiments directed towards understanding the implication of non-linear behaviors such as phase-locking are likely to provide new insights into how self-motion information is encoded for the subsequent computation of self-motion as well as gaze and posture control.
Multimodal integration within vestibular pathways
Vestibular inputs are not our only source of self-motion information. As an organism interacts with its environment, somatosensory, proprioceptive, and visual inputs as well as motor-related signals also provide self-motion cues. A distinguishing aspect of early vestibular processing is that it combines multimodal sensory information at the first stage of central processing. Thus a second major question is: How does the organism’s interaction with its environment shape and alter vestibular encoding? Figure 3A illustrates the sources of the extra-vestibular sensory inputs as well as premotor signals related to the generation of eye and head movements that are relayed to the vestibular nuclei.
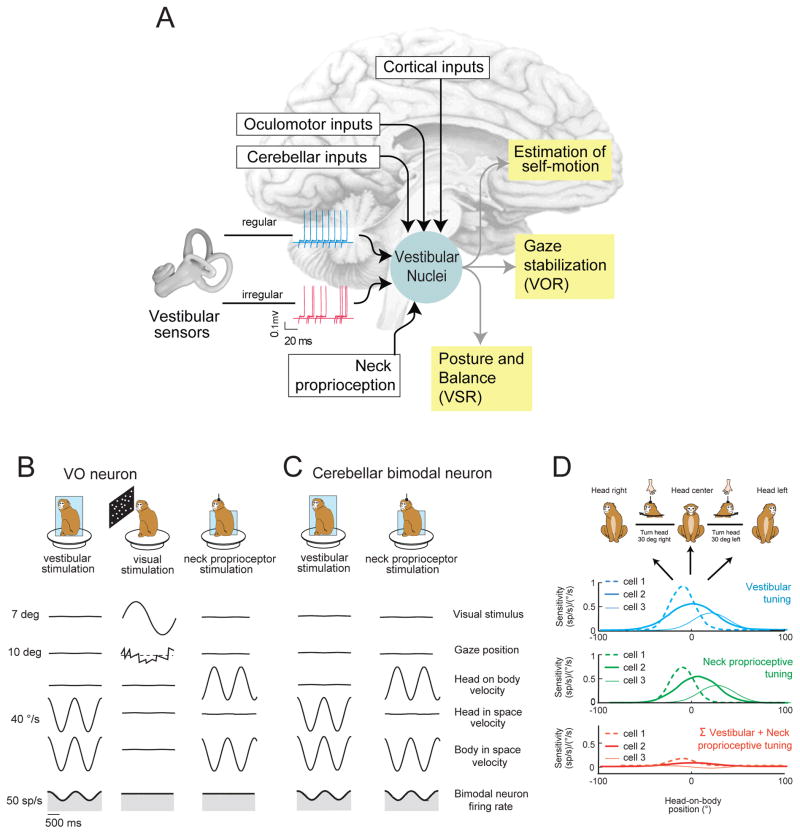
Figure 3. Multimodal integration within vestibular pathways.
a) The vestibular nuclei (VN) receive direct input from multiple brain areas including: i) the vestibular afferents of the VIII nerve, ii) oculomotor areas of the brainstem, iii) the vestibular cerebellum, and iv) several areas of cortex [e.g., parietoinsular vestibular cortex (PIVC)], premotor areas 6, 6pa, somatosensory area 3a, and superior temporal cortex. b) VO neurons in the vestibular nuclei of the rhesus monkey are sensitive to vestibular stimulation, but are not well modulated by full field visual or neck proprioceptive stimulation [25, 44, 45, 55]. c,d) Neurons in the rostral fastigial nucleus of the vestibular cerebellum receive input from VO neurons. c. 50% of rostral fastigial neurons respond to neck proprioceptive (center) as well as vestibular (left) stimulation (i.e., bimodal neurons) [58]. d. When the head moves relative to body (as it would during a voluntary orienting head turn) the vestibular and dynamic neck proprioceptive inputs sum to produce complete response cancellation, consistent with these neurons’ encoding body motion. Vestibular (blue) and neck (green) turning curves are shown for 3 example neurons: cell 1 (dashed curve), cell 2 (solid thick curve), and cell 3 (solid thin curve). Note, for each cell responses to each modality sum linearly during combined stimulation such that bimodal neurons are not modulated during head-on-body motion (red curves). Thus, by combining their vestibular and neck related inputs, these neurons effectively encode body-in-space motion, rather than head-in-space motion. Data in (d) replotted with permission from [58].
Integration of vestibular and visual inputs
Optic flow information provides an important sensory cue for self-motion, capable of generating powerful sensations even when a subject is stationary. Optic flow information also induces the generation of optokinetic eye movements that complement the VOR to ensure stable gaze during self-motion at lower frequencies. It was initially thought that all neurons in the vestibular nuclei were driven by large-field visual as well as vestibular stimulation [28, 43]. This idea was theoretically very attractive since it provided a physiological substrate by which the brain could combine visual and vestibular signals to estimate self-motion. However, visual-vestibular convergence is not as prevalent as was initially believed. Notably, while eye-movement sensitive neurons show clear eye-movement related modulation during large-field visual (i.e. optokinetic) as well as vestibular stimulation, VO neurons do not show robust modulation in response to optokinetic stimulation [44–45]. Thus while VOR neurons (PVPs and FTNs) integrate visual-vestibular input to generate the premotor commands required by the extraocular motoneurons to drive optokinetic eye movements [46–47], this is not the case for VO neurons (Figure 3B).
How then does the brain integrate full-field visual and vestibular inputs for higher level functions such as the computation and perception of self-motion? The results of recent studies by Angelaki, DeAngelis and colleagues (see [48] for a review) suggest that neurons in higher level structures such as extrastriate visual cortex, most notably dorsal medial superior temporal extrastriate cortex (area MSTd) as well as in ventral intraparietal cortex (area VIP) respond both to motion in darkness as well as to optic flow stimuli. Responses to motion in the dark are eliminated following bilateral labyrinthectomy [49–50] consistent with the proposal that neurons integrate vestibular and visual signals to compute self-motion.
Integration of vestibular and somatosensory/proprioceptive inputs
Somatosensory/proprioceptive inputs reach the vestibular nuclei by means of dorsal-root axons as well as second-order neurons [reviewed in 25]. Additionally, cerebellar and cortical areas sensitive to such inputs send direct projections to the vestibular nuclei (reviewed in [51–53]) making this area a likely candidate for encoding body motion. In decerebrate or anesthetized preparations, passive neck proprioceptive stimulation influences the activity of vestibular nuclei neurons (see [27, 54] for reviews). However, passive activation of proprioceptors does not directly affect neuronal responses in alert rhesus monkey (Figure 3C; [55]). In contrast, the same stimulation can affect the responses of both VO neurons and VOR neurons in other species of primate (i.e., squirrel monkey [56] and cynomolgus monkey) [57]. One possible explanation for this species difference is that neck-related inputs to vestibular pathways are particularly critical for postural stabilization in those primates that make their home in a challenging three-dimensional arboreal environment.
Proprioceptive-vestibular integration is typically antagonistic in species where both inputs drive vestibular nuclei neurons. As a result, when the head moves relative to body (for example, during a voluntary orienting head turn) neurons fire less robustly than for comparable head motion produced by whole-body motion (i.e. a condition in which only the vestibular system is stimulated). Strikingly, recent studies have reported complete cancellation of vestibular modulation by proprioceptive inputs within the rostral fastigial nucleus of the cerebellum (Figure 3C,D; [58]); a nucleus which is reciprocally connected to the vestibular nucleus [59–60]). Approximately half of the neurons in this region are sensitive to proprioceptive as well as vestibular inputs (Figure 3C; bimodal neurons), while the other half are only sensitive to vestibular input (unimodal neurons). When delivered in isolation the vestibular- and proprioceptive- related responses of bimodal neurons have comparable tuning (e.g., strength and location of maximal response) that varies as a function of head-on-body position (Figure 3D). Accordingly, although their processing of each sensory modality is intrinsically nonlinear, responses sum linearly during combined stimulation such that bimodal neurons robustly encode body-in-space motion. Unimodal neurons, in contrast, encode head-in-space motion much like the VO neurons of the vestibular nuclei.
The integration of vestibular and proprioceptive information in the rostral fastigial nucleus of the vestibular cerebellum is vital for the accurate control of posture and balance as well as higher-order functions such as self-motion perception. For example, the corrective movements produced by vestibulospinal reflexes must account for changes in the position of the head relative to the body [61–63]. However, patients with lesions to this cerebellar region do not exhibit the required changes in body sway that normally occur when head-on-body position is altered during galvanic stimulation [64]. In addition, the convergence of vestibular and neck proprioceptive inputs is required to perceive body motion independently of head motion [65]. A prediction would be that body motion perception is also impaired in these patients.
Vestibular Pathways and the Control of Motor Behavior
The final question to be addressed in this review is: How is the processing of self-motion information adjusted to meet the needs of specific tasks? Below I consider how, by combining vestibular with extra-vestibular signals, the brain effectively shapes behaviors. I first consider the vestibulo-ocular reflex (VOR), whose relative simplicity has made it an excellent model system for bridging the gap between neuronal circuits and behavior. I then consider more complex voluntary behaviors including voluntary orienting movements, reaching, and navigation.
The Vestibulo-ocular Reflex: Complementary response dynamics ensure stable gaze
In our daily lives, we move through the world and, at the same time, maintain stable gaze. This is because the vestibulo-ocular reflex (VOR) produces compensatory eye movements of equal and opposite magnitude to head rotations to stabilize the visual axis (i.e., gaze) relative to space. The VOR is arguably our fastest behavior; in response to head movement, eye movements are generated with a latency of only 5–6 ms [6]. This short latency is consistent with the minimal synaptic and axonal delays of the three neuron pathway (e.g., Figure 2A). Thus, the VOR reflex stabilizes gaze considerably faster (by an order of magnitude) than would be possible via the most rapid visually evoked eye movements (reviewed in [66]).
During natural behaviors such as active head turns, walking, and running, the motion of the head in space can have frequency content approaching 20 Hz. [6, 67]. The VOR shows remarkably compensatory gain (i.e. eye velocity/head velocity = 1) as well as minimal phase lag over the physiological relevant range of head movements (Figure 4A; [5–6]). The latter observation is particularly impressive considering that the VOR has a 5–6 ms latency, and thus the evoked eye movements would lag head movements by >30° at 15 Hz if not appropriately compensated. However, as reviewed above, linear control system analysis has shown that both vestibular afferents and the PVP neurons (Figure 2B) to which they project are characterized by the requisite phase leads.
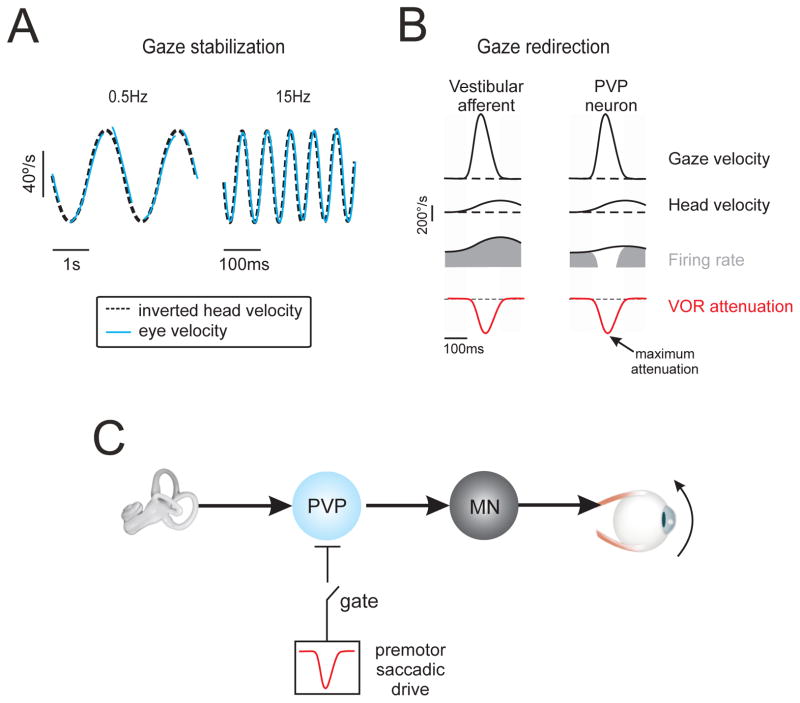
Figure 4. The vestibulo-ocular reflex (VOR): compensatory response dynamics ensure stable gaze.
a) The VOR is compensatory over a wide frequency range. Example eye and head velocity traces, during sinusoidal rotations of the head-on-body in the dark at 0.5 and 15 Hz. b) Single unit recording experiments in monkeys show that vestibular afferents encode the active head movements made during gaze shifts. However, neurons at the next stage of processing in the VOR pathways (i.e. PVP neurons) and resultant VOR are attenuated (red trace). The time course of the neuronal [34, 74] and VOR suppression [69] are comparable; response attenuation is maximal early in the gaze shift and progressively recovers to reach normal (i.e. compensatory) values near gaze-shift end. c) Mechanism underlying VOR suppression during gaze shifts. In addition to their input from the vestibular nerve, PVP neurons receive a strong inhibitory input from the premotor saccadic pathway, which effectively suppresses their activity during gaze shifts. In this way, VOR suppression is mediated by behaviorally-dependent gating of an inhibitory gaze command signal. Accordingly, during gaze shifts PVP neuron responses can be explained by the linear summation of their i) head velocity input and ii) this inhibitory saccadic drive.
The results of single unit recordings have provided insight into how the VOR effectively stabilizes gaze across a wide range of head velocities. The VOR is compensatory for head velocities as large as 300–500°/s [6, 10]. Yet, the responses of a typical PVP neuron or FTNs i) are silenced for off-direction rotations at velocities of 100–200 deg/s and also ii) demonstrate substantial non linearities (i.e., firing rate saturation) for on-direction rotations at velocities >200 deg/s [12]. The apparent discrepancy between neuronal and behavioral VOR responses can be reconciled by considering the next stage of neural processing. Specifically, recordings from extraocular motoneurons have shown that the oculomotor plant itself has complementary dynamics [68]. Accordingly, the VOR remains compensatory for head movements spanning the range of frequencies and velocities encountered in daily life.
The Vestibulo-ocular Reflex: Multimodal Integration reduces reflex efficacy during gaze redirection
Head motion is often purposefully made to voluntarily redirect our visual axis (i.e., gaze) to a target of interest. These voluntary gaze movements can be rapid (gaze shifts) or slow (gaze pursuit), and comprise a coordinated sequence of eye and head movements made towards the target of interest. Importantly, if the VOR were intact during these voluntary gaze movements, it would command an eye movement in the opposite direction to the intended change in gaze and thus be counterproductive. Instead, VOR efficacy depends on the current behavioral goal: while it is compensatory when the goal is to stabilize gaze (i.e., Figure 4A), it is suppressed when the behavioral goal is to redirect gaze [69].
The results of single unit studies have established that the integration of extra-vestibular information in early vestibular processing underlies VOR suppression during gaze redirection. While vestibular afferents robustly encode head motion during gaze shifts and pursuit [12, 70–71], PVP neurons show response suppression that mirrors the time course of behavioral VOR suppression (Figure 4B; [34, 72–74]). A well characterized inhibitory projection from the brainstem saccade generator (paramedian pontine reticular formation (PPRF)) to the vestibular nuclei is presumed to be the neurophysiological basis of this suppressive input (Figure 4C; for a discussion see [73, 75]). PVP neurons also show response suppression when gaze is more slowly redirected using combined eye-head motion during gaze pursuit [34]. Thus VOR pathways combine vestibular afferent input with premotor saccadic (or pursuit) command signals, such that PVP neurons encode head motion in a manner that critically depends on current gaze strategy. Surprisingly, it has been recently shown that gaze motion is comparable during ocular-only and eye-head pursuit [76], indicating that head motion does not influence gaze redirection even when VOR pathways are suppressed. More work is required to fully understand how the brain coordinates the premotor control of eye-head motion to ensure accurate gaze redirection.
The analysis of multimodal integration in early vestibular processing reveals an elegant solution to the problem of adjusting VOR reflex efficacy as a function of behavioral goals. The VOR is robust and compensatory over a wide range of velocities and frequencies when the goal is to stabilize gaze during head motion. However, when the goal is to redirect gaze, reflex efficacy is suppressed by an efferent copy of the command to voluntarily redirect gaze. Similarly, other features of gaze strategy (e.g. fixation distance and gaze eccentricity) have been shown to modulate VOR pathway responses [77–79] and in turn modulate VOR gain [80]. Understanding the distributed nature of the premotor circuitry responsible for these computations remains a challenge for ongoing and future investigations.
Balance and the computation of self-motion: Mechanisms for the differential processing of activelygenerated versus passive head movement
The vestibular system is often described as the balance system since it plays a vital role in ensuring stable body posture as well as gaze. Vestibulo-spinal reflexes (VSR) play an important role by coordinating head and neck movement with the trunk and body to maintain the head in an upright position. Like the VOR, the most direct pathways mediating the VSR comprise three neurons: vestibular afferents project to neurons in the vestibular nuclei, which in turn project to spinal motoneurons (Figure 2A). However, there is compelling evidence that more complicated circuitry makes a dominant contribution to these reflexes (reviewed in [27, 54]).
Studies initially used in vitro, reduced, and anesthetized preparations to characterize the intrinsic electrophysiology of the VSR pathways. However, more recent experiments in alert animals have emphasized the importance of extra-vestibular signals in shaping the sensorimotor transformations that mediate the VSR reflexes. In particular, while vestibular afferents showed no differences in sensitivity [12, 70–71] or firing rate variability [81] during active movements, vestibular-only (VO) neurons show striking differences in the two conditions [55, 82–83]. Specifically, VO neurons robustly respond to passive head movements but during active head movements their responses are markedly (70%) attenuated. Since these neurons project into VSR pathways (Figure 2A), this finding has led to the proposal that the VCR is turned off during voluntary head movements [25, 83].
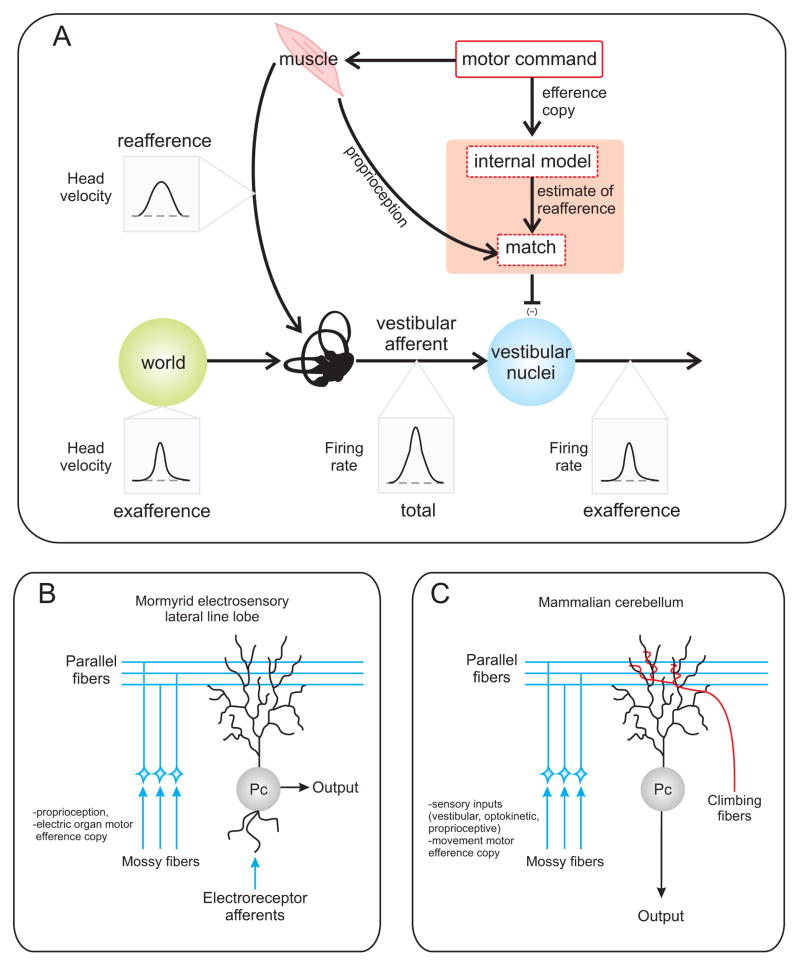
Progress has been made towards understanding the mechanism responsible for the selective cancellation of neuronal responses to active head motion. Notably, by experimentally controlling the correspondence between intended and actual head movement [83–84], it has been shown that a cancellation signal is exclusively generated in conditions where the activation of neck proprioceptors matches the motor-generated expectation (Figure 5A). This result provides support for the idea that an internal model of the sensory consequences of active head motion is used to selectively suppress reafference (i.e. the vestibular stimulation that results from our own actions) at the level of the vestibular nuclei. This general mechanism has notable similarity to that used by mormyrid fish to cancel electrosensory reafference. The cerebellum-like structures of these fish act as adaptive filters, removing predictable features of the sensory input (for review, see [85]). Recently, a combination of in vitro, in vivo, and computational studies have provided direct insight into how anti-Hebbian synaptic plasticity underlies cancellation of the electrosensory consequences of the fish’s own behavior [86–87]. It remains to be determined whether a similar strategy is used in the mammalian cerebellum to selectively suppress vestibular reafference (Figure 5B versus C).
Figure 5. Neural mechanism for the attenuation of vestibular reafference.
To produce an active head movement, the brain sends a motor command to the neck muscle. The activation of the neck muscle moves the head, which in turn results in vestibular stimulation (i.e., vestibular reafference). In addition, the brain has access to an efference copy of the motor command and/or feedback from neck proprioceptors. a) Vestibular reafference is cancelled when neck proprioceptive feedback matches the expected sensory consequence of neck motor command (red shaded box: internal model followed by a putative matching operation). In this condition, a cancellation signal is sent to VO neurons in the vestibular nuclei. b) In the cerebellum-like structures of the mormyrid fish, the principals cells (Pc) receive an efference copy of the motor commands to the electric organ via parallel fibers, as well as afferent input from the electrosensory receptors. To remove predictable features of the sensory input (i.e., electrosensory reafference), anti-Hebbian plasticity at parallel fiber synapses generates “negative images” that act to cancel predictable patterns of electrosensory input [86–87]. c) It remains to be determined whether a similar strategy is used by the mammalian cerebellum to selectively suppress vestibular reafference. Parallel fibers carry sensory as well as motor information to Purkinje cells (Pc), and climbing fibers are thought to encode a motor performance error signal (see review [26]). While climbing fiber activity paired in time with mossy fiber-parallel fiber activity is thought to weaken the associated parallel fiber synapse, a recent report suggest that instructive signals carried by parallel fiber activity alone may be sufficient to induce synaptic changes [94].
The differential processing of active versus passive head movements has important implications for voluntary motor control versus balance. While it is helpful to stabilize the head/body to compensate for unexpected movements (such as those experienced while riding on the subway), the stabilizing commands produced by an intact VSR would be counterproductive during active movements. Accordingly, turning off vestibulospinal reflexes is functionally advantageous. Moreover, because VO neurons can continue to reliably encode information about passive self-motion during the execution of voluntary head turns [55, 82], vestibulo-spinal pathways continue to selectively adjust postural tone in response to head movement that the brain does not expect. Such selectivity is fundamental to ensuring accurate motor control. For example, the ability to recover from tripping over an obstacle while walking or running requires a selective but robust postural response to the unexpected component of vestibular stimulation. Finally, the differential processing of active versus passive head movements is also likely to have important implications for the computation of self-motion since VO neurons have reciprocal interconnections with regions of the vestibular cerebellum (see above) and vestibular thalamus [88].
Voluntary Behavior: Steering, Reaching and navigation
As reviewed above, the processing of self-motion information is inherently multimodal; the integration of vestibular and extra-vestibular inputs has important implications for the control of the vestibulo-ocular and vestibulospinal reflexes which function to ensure stable gaze and posture, as well as for the processing of self-motion information for higher-order functions. Recent studies of more complex behaviors including voluntary orienting movements, steering navigation, and even reaching have furthered our understanding of the vestibular system’s pervasive role in voluntary motor control.
During self-motion, the ability to distinguish between actively-generated and passively-applied head movements is not only important for shaping motor commands, but is also critical for ensuring perceptual stability (see [89] for review). The active movements produced by orienting head and body movements are differentially encoded at the first central stage of vestibular processing. How is self-motion encoded when it is voluntarily controlled in less direct ways – for example by driving a car? Single unit experiments in monkeys reveal that all vestibular nuclei neurons respond to vestibular input during ‘self-generated’ driving as if it had been externally applied [34, 55]. However, cortical neurons in MSTd show enhanced responses to virtual (i.e. visual) self-movement when monkeys steer a straight-ahead course, using optic flow cues [90]. Thus, at this higher level stage of processing, the brain appears to combine steering-related (i.e., motor/motor preparation) signals with self-motion (i.e., vestibular, proprioceptive and visual) information. It remains to be determined whether further training in a task such as steering would lead to the construction of an accurate internal model of the vehicle being driven (in this case the monkey’s motion platform) and, in turn, suppression of sensory responses earlier in vestibular processing.
Finally, a current emerging area of interest is the role of self-motion (i.e., vestibular) information in ensuring behavioral accuracy during complex voluntary behaviors, for example, navigation and reaching. The discovery that vestibular reafference is suppressed early in processing has important implications for understanding how self-motion information is encoded during these every day activities. For example, head direction cells in the hippocampal formation combine extra-vestibular information with vestibular input to compute distinct estimates of heading direction during active versus passive navigation [91–92]. In addition, there is accumulating evidence that the brain uses vestibular signals to generate the appropriate reaching motor command required to maintain accuracy during self-motion (for a review see [93]). More work is needed to understand how the brain integrates vestibular versus extra-vestibular cues during the voluntary self-motion produced during these every day activities.
Summary
During everyday life, the brain combines vestibular and extra-vestibular cues – for example visual and/or proprioceptive information – to construct an estimate of self-motion. Significant progress has recently been made towards answering three interrelated questions: What neural code is used to represent vestibular sensory information? How does the interaction of the organism with the environment shape and alter encoding? How is the processing of self-motion information adjusted to meet the needs of specific tasks?
First, the central vestibular system receives input from two parallel information channels: regular afferents transmit detailed information about head rotations through precise spike timing, whereas irregular afferents respond to high-frequency features exclusively through changes in firing rate. Second, the brain combines information from the vestibular sensors with extra-vestibular cues, such as proprioception and motor efference signals, at the earliest stages of central vestibular processing to compute estimates of self-motion. As an organism interacts with its environment, the resulting multimodal inflow is used to provide i) robust estimates of self-motion (for example, when visual as well as vestibular cues are available), and ii) estimates of the motion of neighboring parts of the body (e.g. body versus head motion) to ensure stable posture and perception. Third and finally, vestibular processing is shaped as a function of context during reflex behavior, as well as during more complex voluntary behaviors such as orienting, steering, navigation and reaching. Taken together, recent results provide new evidence that action alters the brain’s sensory encoding of self-motion at the earliest stages to ensure the accurate control of behavior in everyday life. Future studies need to consider not only how the neural code is used to represent self-motion by central pathways when multiple inputs are combined, but also how differences in the behavioral context govern the nature of what defines the optimal computation (Box 1). A better understanding of how the brain encodes and processes self-motion will provide vital insight into the fundamental question of how we anticipate the consequences of current or potential actions, and in turn stimulate a reevaluation of the traditional separation between action and perception
Box 1. Outstanding questions.
What neural code is used to represent vestibular sensory information?
One assumption of prior analyses is that neurons encode information in a linear manner. However, recent analyses reveal that irregular afferents and PVP neurons are characterized by marked phase locking in response to motion ≥20 Hz [9, 32], suggesting a role for non-linear coding in the sensorimotor transformations that mediate the VOR at higher frequencies. Similarly, a preliminary report suggests that phase locking in VO neurons is regulated by variability (e.g., synaptic noise) [39]. More work is required to understand the strategy used to encode behaviorally relevant vestibular stimuli.
What is the functional role of the information encoded by vestibular cerebellum during self-motion?
The head and body motion signals encoded by vestibular cerebellum are known to play an important role in the production of accurate postural control. However, the vestibular cerebellum also sends ascending projections to the posterolateral ventral nucleus of the thalamus. Patients with midline cerebellar lesions exhibit reduced vestibular perception [95], and a prediction would be that body motion perception would be also impaired in these patients.
What information is encoded by cortical areas that contribute to the perception of self-motion? Do these areas distinguish actively generated from passive self-motion?
Neurons at the first central stage of vestibular processing (VN) can distinguish between self-generated and passive movements. Further studies of the cellular mechanisms which underlie this computation, as well as the functional significance of the information that is ultimately sent upstream for subsequent computation, will be key to understanding how the brain perceives self-motion.
How is self-motion information encoded by the hippocampal formation during navigation?
Vestibular input is required for the generation of the directional signal encoded by head direction cells during navigation [91–92], and directional tuning is thought to be created by means of on-line integration of the animal’s angular head velocity (reviewed [96]). To date, however, most studies report a relative response increase during active motion [97–99]; (for an exception, see [100]), which is unexpected given that passive, not active, motion is more robustly encoded in early vestibular processing (Fig. 5A). Interestingly, hippocampal place cells are characterized by the similar discrepancy (i.e., a relative response increase during active motion; compare [101–102]). Thus, how the hippocampal formation combines extra-vestibular information with vestibular input to encode self-motion during navigation remains an open question.
How is vestibular information processed to predict the consequence of the rotation dynamics during reaching?
Changes in vestibular input can affect on-going reaching movements [103–105]. In addition, vestibular signals that could potentially influence reach planning and executions have been described in somatosensory cortex, as well as parietal cortex [106–108]. While the relative influences of vestibular versus extra-vestibular (i.e. motor efference copy and proprioceptive information) remain to be precisely determined, current evidence suggest that during reaching, arm movements are altered in a manner consistent with the hypothesis that vestibular signals are used to predict Coriolis forces [109–110].
Acknowledgments
The author acknowledges support from the Canadian Institutes of Health Research (CIHR), National Institutes of Health (DC002390), and Le Fonds québécois de la recherche sur la nature et les technologies (FQNRT), and thanks Jerome Carriot, Diana Mitchell, Jessica Brooks, Mohsen Jamali, Alexis Dale, Carla Kalkhoven, Adam Schneider for help with figures and comments on the manuscript.
References
- 1.Angelaki DE, Cullen KE. Vestibular system: the many facets of a multimodal sense. Annual review of neuroscience. 2008;31:125–150. doi: 10.1146/annurev.neuro.31.060407.125555. [DOI] [PubMed] [Google Scholar]
- 2.Goldberg JM. Afferent diversity and the organization of central vestibular pathways. Experimental brain research. Experimentelle Hirnforschung. Experimentation cerebrale. 2000;130:277–297. doi: 10.1007/s002210050033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eatock RA, Songer JE. Vestibular Hair Cells and Afferents: Two Channels for Head Motion Signals. Annual review of neuroscience. 2011;34:501–534. doi: 10.1146/annurev-neuro-061010-113710. [DOI] [PubMed] [Google Scholar]
- 4.Kalluri R, et al. Ion channels set spike timing regularity of Mammalian vestibular afferent neurons. Journal of neurophysiology. 2010;104:2034–2051. doi: 10.1152/jn.00396.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Armand M, Minor LB. Relationship between time- and frequency-domain analyses of angular head movements in the squirrel monkey. Journal of computational neuroscience. 2001;11:217–239. doi: 10.1023/a:1013771014232. [DOI] [PubMed] [Google Scholar]
- 6.Huterer M, Cullen KE. Vestibuloocular reflex dynamics during high-frequency and high-acceleration rotations of the head on body in rhesus monkey. Journal of neurophysiology. 2002;88:13–28. doi: 10.1152/jn.2002.88.1.13. [DOI] [PubMed] [Google Scholar]
- 7.Haque A, et al. Spatial tuning and dynamics of vestibular semicircular canal afferents in rhesus monkeys. Experimental brain research. Experimentelle Hirnforschung. Experimentation cerebrale. 2004;155:81–90. doi: 10.1007/s00221-003-1693-0. [DOI] [PubMed] [Google Scholar]
- 8.Hullar TE, et al. Responses of irregularly discharging chinchilla semicircular canal vestibular-nerve afferents during high-frequency head rotations. Journal of neurophysiology. 2005;93:2777–2786. doi: 10.1152/jn.01002.2004. [DOI] [PubMed] [Google Scholar]
- 9.Ramachandran R, Lisberger SG. Transformation of vestibular signals into motor commands in the vestibuloocular reflex pathways of monkeys. Journal of neurophysiology. 2006;96:1061–1074. doi: 10.1152/jn.00281.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sadeghi SG, et al. Dynamics of the horizontal vestibuloocular reflex after unilateral labyrinthectomy: response to high frequency, high acceleration, and high velocity rotations. Experimental brain research. Experimentelle Hirnforschung. Experimentation cerebrale. 2006;175:471–484. doi: 10.1007/s00221-006-0567-7. [DOI] [PubMed] [Google Scholar]
- 11.Sadeghi SG, et al. Neural variability, detection thresholds, and information transmission in the vestibular system. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:771–781. doi: 10.1523/JNEUROSCI.4690-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sadeghi SG, et al. Response of vestibular-nerve afferents to active and passive rotations under normal conditions and after unilateral labyrinthectomy. Journal of neurophysiology. 2007;97:1503–1514. doi: 10.1152/jn.00829.2006. [DOI] [PubMed] [Google Scholar]
- 13.Massot C, et al. Information transmission and detection thresholds in the vestibular nuclei: single neurons vs. population encoding. Journal of neurophysiology. 2011;105:1798–1814. doi: 10.1152/jn.00910.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reinagel P, Reid RC. Temporal coding of visual information in the thalamus. J Neurosci. 2000;20:5392–5400. doi: 10.1523/JNEUROSCI.20-14-05392.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Desbordes G, et al. Timing precision in population coding of natural scenes in the early visual system. PLoS Biol. 2008;6:e324. doi: 10.1371/journal.pbio.0060324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haider B, et al. Synaptic and network mechanisms of sparse and reliable visual cortical activity during nonclassical receptive field stimulation. Neuron. 2010;65:107–121. doi: 10.1016/j.neuron.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kayser C, et al. Millisecond encoding precision of auditory cortex neurons. Proc Natl Acad Sci U S A. 2010;107:16976–16981. doi: 10.1073/pnas.1012656107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bizley JK, et al. Neural ensemble codes for stimulus periodicity in auditory cortex. J Neurosci. 2010;30:5078–5091. doi: 10.1523/JNEUROSCI.5475-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharpee TO, et al. Hierarchical representations in the auditory cortex. Curr Opin Neurobiol. 2011;21:761–767A. doi: 10.1016/j.conb.2011.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johansson RS, Birznieks I. First spikes in ensembles of human tactile afferents code complex spatial fingertip events. Nat Neurosci. 2004;7:170–177. doi: 10.1038/nn1177. [DOI] [PubMed] [Google Scholar]
- 21.Jones LM, et al. Robust temporal coding in the trigeminal system. Science. 2004;304:1986–1989. doi: 10.1126/science.1097779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scaglione A, et al. Trial-to-trial variability in the responses of neurons carries information about stimulus location in the rat whisker thalamus. Proc Natl Acad Sci U S A. 2011;108:14956–14961. doi: 10.1073/pnas.1103168108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ito I, et al. Sparse odor representation and olfactory learning. Nat Neurosci. 2008;11:1177–1184. doi: 10.1038/nn.2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giridhar S, et al. Timescale-dependent shaping of correlation by olfactory bulb lateral inhibition. Proc Natl Acad Sci U S A. 2011;108:5843–5848. doi: 10.1073/pnas.1015165108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cullen KE, Roy JE. Signal processing in the vestibular system during active versus passive head movements. Journal of neurophysiology. 2004;91:1919–1933. doi: 10.1152/jn.00988.2003. [DOI] [PubMed] [Google Scholar]
- 26.Cullen KE. In: Procedural learning: VOR. Byrne John H., editor. Academic Press/Elsevier; 2008. Learning and Memory: A Comprehensive Reference. [Google Scholar]
- 27.Goldberg JM, Cullen KE. Vestibular control of the head: possible functions of the vestibulocollic reflex. Experimental brain research. Experimentelle Hirnforschung. Experimentation cerebrale. 2011;210:331–345. doi: 10.1007/s00221-011-2611-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reisine H, Raphan T. Unit activity in the vestibular nuclei of monkeys during offvertical axis rotation. Annals of the New York Academy of Sciences. 1992;656:954–956. doi: 10.1111/j.1749-6632.1992.tb25305.x. [DOI] [PubMed] [Google Scholar]
- 29.Grusser OJ, et al. Vestibular neurones in the parieto-insular cortex of monkeys (Macaca fascicularis): visual and neck receptor responses. J Physiol. 1990;430:559–583. doi: 10.1113/jphysiol.1990.sp018307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lang W, et al. Vestibular projections to the monkey thalamus: an autoradiographic study. Brain research. 1979;177:3–17. doi: 10.1016/0006-8993(79)90914-4. [DOI] [PubMed] [Google Scholar]
- 31.Dickman JD, Angelaki DE. Dynamics of vestibular neurons during rotational motion in alert rhesus monkeys. Experimental brain research. Experimentelle Hirnforschung. Experimentation cerebrale. 2004;155:91–101. doi: 10.1007/s00221-003-1692-1. [DOI] [PubMed] [Google Scholar]
- 32.Ramachandran R, Lisberger SG. Neural substrate of modified and unmodified pathways for learning in monkey vestibuloocular reflex. Journal of neurophysiology. 2008;100:1868–1878. doi: 10.1152/jn.90498.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sadeghi SG, et al. Neural correlates of motor learning in the vestibulo-ocular reflex: dynamic regulation of multimodal integration in the macaque vestibular system. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30(30):10158–68. doi: 10.1523/JNEUROSCI.1368-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roy JE, Cullen KE. Vestibuloocular reflex signal modulation during voluntary and passive head movements. Journal of neurophysiology. 2002;87:2337–2357. doi: 10.1152/jn.2002.87.5.2337. [DOI] [PubMed] [Google Scholar]
- 35.Boyle R, et al. Inputs from regularly and irregularly discharging vestibular nerve afferents to secondary neurons in squirrel monkey vestibular nuclei. III. Correlation with vestibulospinal and vestibuloocular output pathways. Journal of neurophysiology. 1992;68:471–484. doi: 10.1152/jn.1992.68.2.471. [DOI] [PubMed] [Google Scholar]
- 36.Highstein SM, et al. Inputs from regularly and irregularly discharging vestibular nerve afferents to secondary neurons in the vestibular nuclei of the squirrel monkey. II. Correlation with output pathways of secondary neurons. Journal of neurophysiology. 1987;58:719–738. doi: 10.1152/jn.1987.58.4.719. [DOI] [PubMed] [Google Scholar]
- 37.Grabherr L, et al. Vestibular thresholds for yaw rotation about an earth-vertical axis as a function of frequency. Experimental brain research. Experimentelle Hirnforschung. Experimentation cerebrale. 2008;186:677–681. doi: 10.1007/s00221-008-1350-8. [DOI] [PubMed] [Google Scholar]
- 38.Stein RB, et al. Neuronal variability: noise or part of the signal? Nat Rev Neurosci. 2005;6:389–397. doi: 10.1038/nrn1668. [DOI] [PubMed] [Google Scholar]
- 39.Schneider AD, et al. In vivo conditions induce faithful encoding of stimuli by recording nonlinear synchronization in vestibular sensory neurons. PLoS Computational Biology. 2011;7:e1002120. doi: 10.1371/journal.pcbi.1002120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.London M, et al. Sensitivity to perturbations in vivo implies high noise and suggests rate coding in cortex. Nature. 2010;466:123–127. doi: 10.1038/nature09086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gabbiani F, et al. From stimulus encoding to feature extraction in weakly electric fish. Nature. 1996;384:564–567. doi: 10.1038/384564a0. [DOI] [PubMed] [Google Scholar]
- 42.Rieke F, et al. Spikes: exploring the neural code. MIT Press; 1996. [Google Scholar]
- 43.Waespe W, Henn V. Neuronal activity in the vestibular nuclei of the alert monkey during vestibular and optokinetic stimulation. Experimental brain research. Experimentelle Hirnforschung. Experimentation cerebrale. 1977;27:523–538. doi: 10.1007/BF00239041. [DOI] [PubMed] [Google Scholar]
- 44.Beraneck M, Cullen KE. Activity of vestibular nuclei neurons during vestibular and optokinetic stimulation in the alert mouse. Journal of neurophysiology. 2007;98:1549–1565. doi: 10.1152/jn.00590.2007. [DOI] [PubMed] [Google Scholar]
- 45.Bryan AS, Angelaki DE. Optokinetic and vestibular responsiveness in the macaque rostral vestibular and fastigial nuclei. Journal of neurophysiology. 2009;101:714–720. doi: 10.1152/jn.90612.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cullen KE, et al. Firing behavior of brain stem neurons during voluntary cancellation of the horizontal vestibuloocular reflex. II. Eye movement related neurons. Journal of neurophysiology. 1993;70:844–856. doi: 10.1152/jn.1993.70.2.844. [DOI] [PubMed] [Google Scholar]
- 47.Scudder CA, Fuchs AF. Physiological and behavioral identification of vestibular nucleus neurons mediating the horizontal vestibuloocular reflex in trained rhesus monkeys. Journal of neurophysiology. 1992;68:244–264. doi: 10.1152/jn.1992.68.1.244. [DOI] [PubMed] [Google Scholar]
- 48.Angelaki DE, et al. Visual and vestibular cue integration for heading perception in extrastriate visual cortex. The Journal of physiology. 2011;589:825–833. doi: 10.1113/jphysiol.2010.194720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gu Y, et al. A functional link between area MSTd and heading perception based on vestibular signals. Nature neuroscience. 2007;10:1038–1047. doi: 10.1038/nn1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Takahashi K, et al. Multimodal coding of three-dimensional rotation and translation in area MSTd: comparison of visual and vestibular selectivity. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:9742–9756. doi: 10.1523/JNEUROSCI.0817-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Manzoni D. The cerebellum and sensorimotor coupling: looking at the problem from the perspective of vestibular reflexes. Cerebellum. 2007;6:24–37. doi: 10.1080/14734220601132135. [DOI] [PubMed] [Google Scholar]
- 52.Guldin WO, Grusser OJ. Is there a vestibular cortex? Trends Neurosci. 1998;21:254–259. doi: 10.1016/s0166-2236(97)01211-3. [DOI] [PubMed] [Google Scholar]
- 53.Wilson VJ, et al. Cortical influences on the vestibular nuclei of the cat. Exp Brain Res. 1999;125:1–13. doi: 10.1007/s002210050651. [DOI] [PubMed] [Google Scholar]
- 54.Wilson VJ, Schor RH. The neural substrate of the vestibulocollic reflex. What needs to be learned. Experimental brain research. Experimentelle Hirnforschung. Experimentation cerebrale. 1999;129:483–493. doi: 10.1007/s002210050918. [DOI] [PubMed] [Google Scholar]
- 55.Roy JE, Cullen KE. Selective processing of vestibular reafference during self-generated head motion. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001;21:2131–2142. doi: 10.1523/JNEUROSCI.21-06-02131.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gdowski GT, McCrea RA. Neck proprioceptive inputs to primate vestibular nucleus neurons. Experimental brain research. Experimentelle Hirnforschung. Experimentation cerebrale. 2000;135:511–526. doi: 10.1007/s002210000542. [DOI] [PubMed] [Google Scholar]
- 57.Sadeghi SG, et al. Different neural strategies for multimodal integration: comparison of two macaque monkey species. Experimental brain research. Experimentelle Hirnforschung. Experimentation cerebrale. 2009;195:45–57. doi: 10.1007/s00221-009-1751-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brooks JX, Cullen KE. Multimodal integration in rostral fastigial nucleus provides an estimate of body movement. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:10499–10511. doi: 10.1523/JNEUROSCI.1937-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Furuya N, et al. Functional organization of vestibulofastigial projection in the horizontal semicircular canal system in the cat. Experimental brain research. Experimentelle Hirnforschung. Experimentation cerebrale. 1975;24:75–87. doi: 10.1007/BF00236018. [DOI] [PubMed] [Google Scholar]
- 60.Shimazu H, Smith CM. Cerebellar and labyrinthine influences on single vestibular neurons identified by natural stimuli. Journal of neurophysiology. 1971;34:493–508. doi: 10.1152/jn.1971.34.4.493. [DOI] [PubMed] [Google Scholar]
- 61.Kennedy PM, Inglis JT. Interaction effects of galvanic vestibular stimulation and head position on the soleus H reflex in humans. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2002;113:1709–1714. doi: 10.1016/s1388-2457(02)00238-9. [DOI] [PubMed] [Google Scholar]
- 62.Tokita T, et al. Modulation by head and trunk positions of the vestibulo-spinal reflexes evoked by galvanic stimulation of the labyrinth. Observations by labyrinthine evoked EMG. Acta otolaryngologica. 1989;107:327–332. doi: 10.3109/00016488909127516. [DOI] [PubMed] [Google Scholar]
- 63.Tokita T, et al. Studies on vestibulo-spinal reflexes by examination of labyrinthine-evoked EMGs of lower limbs. Acta Otolaryngol Suppl. 1991;481:328–332. doi: 10.3109/00016489109131414. [DOI] [PubMed] [Google Scholar]
- 64.Kammermeier S, et al. Vestibular-neck interaction in cerebellar patients. Ann N Y Acad Sci. 2009;1164:394–399. doi: 10.1111/j.1749-6632.2009.03861.x. [DOI] [PubMed] [Google Scholar]
- 65.Mergner T, et al. Human perception of horizontal trunk and head rotation in space during vestibular and neck stimulation. Experimental brain research. Experimentelle Hirnforschung. Experimentation cerebrale. 1991;85:389–404. doi: 10.1007/BF00229416. [DOI] [PubMed] [Google Scholar]
- 66.Buttner U, Kremmyda O. Smooth pursuit eye movements and optokinetic nystagmus. Developments in ophthalmology. 2007;40:76–89. doi: 10.1159/000100350. [DOI] [PubMed] [Google Scholar]
- 67.Grossman GE, et al. Frequency and velocity of rotational head perturbations during locomotion. Experimental brain research. Experimentelle Hirnforschung. Experimentation cerebrale. 1988;70:470–476. doi: 10.1007/BF00247595. [DOI] [PubMed] [Google Scholar]
- 68.Sylvestre PA, Cullen KE. Quantitative analysis of abducens neuron discharge dynamics during saccadic and slow eye movements. Journal of neurophysiology. 1999;82:2612–2632. doi: 10.1152/jn.1999.82.5.2612. [DOI] [PubMed] [Google Scholar]
- 69.Cullen KE, et al. Time course of vestibuloocular reflex suppression during gaze shifts. Journal of neurophysiology. 2004;92:3408–3422. doi: 10.1152/jn.01156.2003. [DOI] [PubMed] [Google Scholar]
- 70.Cullen KE, Minor LB. Semicircular canal afferents similarly encode active and passive head-on-body rotations: implications for the role of vestibular efference. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:RC226. doi: 10.1523/JNEUROSCI.22-11-j0002.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jamali M, et al. Response of vestibular nerve afferents innervating utricle and saccule during passive and active translations. Journal of neurophysiology. 2009;101:141–149. doi: 10.1152/jn.91066.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McCrea RA, Gdowski GT. Firing behaviour of squirrel monkey eye movement-related vestibular nucleus neurons during gaze saccades. The Journal of physiology. 2003;546:207–224. doi: 10.1113/jphysiol.2002.027797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Roy JE, Cullen KE. A neural correlate for vestibulo-ocular reflex suppression during voluntary eye-head gaze shifts. Nature neuroscience. 1998;1:404–410. doi: 10.1038/1619. [DOI] [PubMed] [Google Scholar]
- 74.Fuchs AF, et al. Behavior of the position vestibular pause (PVP) interneurons of the vestibuloocular reflex during head-free gaze shifts in the monkey. J Neurophysiol. 2005;94:4481–4490. doi: 10.1152/jn.00101.2005. [DOI] [PubMed] [Google Scholar]
- 75.Gandhi NJ, et al. Coordination of eye and head components of movements evoked by stimulation of the paramedian pontine reticular formation. Exp Brain Res. 2008;189:35–47. doi: 10.1007/s00221-008-1401-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ackerley R, Barnes GR. The interaction of visual, vestibular and extra-retinal mechanisms in the control of head and gaze during head-free pursuit. The Journal of physiology. 2011;589:1627–1642. doi: 10.1113/jphysiol.2010.199471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen-Huang C, McCrea RA. Effects of viewing distance on the responses of vestibular neurons to combined angular and linear vestibular stimulation. Journal of neurophysiology. 1999;81:2538–2557. doi: 10.1152/jn.1999.81.5.2538. [DOI] [PubMed] [Google Scholar]
- 78.Meng H, et al. Pursuit--vestibular interactions in brain stem neurons during rotation and translation. Journal of neurophysiology. 2005;93:3418–3433. doi: 10.1152/jn.01259.2004. [DOI] [PubMed] [Google Scholar]
- 79.Meng H, Angelaki DE. Neural correlates of the dependence of compensatory eye movements during translation on target distance and eccentricity. Journal of neurophysiology. 2006;95:2530–2540. doi: 10.1152/jn.01087.2005. [DOI] [PubMed] [Google Scholar]
- 80.Zhou W, et al. Multiplicative computation in the vestibulo-ocular reflex (VOR) Journal of neurophysiology. 2007;97:2780–2789. doi: 10.1152/jn.00812.2006. [DOI] [PubMed] [Google Scholar]
- 81.Cullen KE, et al. Internal models of self-motion: computations that suppress vestibular reafference in early vestibular processing. Exp Brain Res. 2011;210:377–388. doi: 10.1007/s00221-011-2555-9. [DOI] [PubMed] [Google Scholar]
- 82.McCrea RA, et al. Firing behavior of vestibular neurons during active and passive head movements: vestibulo-spinal and other non-eye-movement related neurons. Journal of neurophysiology. 1999;82:416–428. doi: 10.1152/jn.1999.82.1.416. [DOI] [PubMed] [Google Scholar]
- 83.Roy JE, Cullen KE. Dissociating self-generated from passively applied head motion: neural mechanisms in the vestibular nuclei. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:2102–2111. doi: 10.1523/JNEUROSCI.3988-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cullen KE, et al. How actions alter sensory processing: reafference in the vestibular system. Ann N Y Acad Sci. 2009;1164:29–36. doi: 10.1111/j.1749-6632.2009.03866.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bastian J, Zakon HH. Plasticity of sense organs and brain. In: Bullock TH, HC, Popper AN, Fay RR, editors. Electroreception. Springer; 2005. [Google Scholar]
- 86.Bell CC, et al. Cerebellum-like structures and their implications for cerebellar function. Annu Rev Neurosci. 2008;31:1–24. doi: 10.1146/annurev.neuro.30.051606.094225. [DOI] [PubMed] [Google Scholar]
- 87.Requarth T, Sawtell NB. Neural mechanisms for filtering self-generated sensory signals in cerebellum-like circuits. Curr Opin Neurobiol. 2011;21:602–608. doi: 10.1016/j.conb.2011.05.031. [DOI] [PubMed] [Google Scholar]
- 88.Marlinski V, McCrea RA. Self-motion signals in vestibular nuclei neurons projecting to the thalamus in the alert squirrel monkey. Journal of neurophysiology. 2009;101:1730–1741. doi: 10.1152/jn.90904.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cullen KE. Sensory signals during active versus passive movement. Current opinion in neurobiology. 2004;14:698–706. doi: 10.1016/j.conb.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 90.Page WK, Duffy CJ. Cortical neuronal responses to optic flow are shaped by visual strategies for steering. Cerebral cortex. 2008;18:727–739. doi: 10.1093/cercor/bhm109. [DOI] [PubMed] [Google Scholar]
- 91.Muir GM, et al. Disruption of the head direction cell signal after occlusion of the semicircular canals in the freely moving chinchilla. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:14521–14533. doi: 10.1523/JNEUROSCI.3450-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Stackman RW, Taube JS. Firing properties of head direction cells in the rat anterior thalamic nucleus: dependence on vestibular input. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1997;17:4349–4358. doi: 10.1523/JNEUROSCI.17-11-04349.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lackner JR, DiZio P. Motor control and learning in altered dynamic environments. Current opinion in neurobiology. 2005;15:653–659. doi: 10.1016/j.conb.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 94.Ke MC, et al. Elimination of climbing fiber instructive signals during motor learning. Nat Neurosci. 2009;12:1171–1179. doi: 10.1038/nn.2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bronstein AM, et al. Reduced self-motion perception in patients with midline cerebellar lesions. Neuroreport. 2008;19:691–693. doi: 10.1097/WNR.0b013e3282fbf9f6. [DOI] [PubMed] [Google Scholar]
- 96.Taube JS. The head direction signal: origins and sensory-motor integration. Annual review of neuroscience. 2007;30:181–207. doi: 10.1146/annurev.neuro.29.051605.112854. [DOI] [PubMed] [Google Scholar]
- 97.Zugaro MB, et al. Peak firing rates of rat anterodorsal thalamic head direction cells are higher during faster passive rotations. Hippocampus. 2002;12:481–486. doi: 10.1002/hipo.10022. [DOI] [PubMed] [Google Scholar]
- 98.Stackman RW, et al. Passive transport disrupts directional path integration by rat head direction cells. Journal of neurophysiology. 2003;90:2862–2874. doi: 10.1152/jn.00346.2003. [DOI] [PubMed] [Google Scholar]
- 99.Bassett JP, et al. Passive movements of the head do not abolish anticipatory firing properties of head direction cells. Journal of neurophysiology. 2005;93:1304–1316. doi: 10.1152/jn.00490.2004. [DOI] [PubMed] [Google Scholar]
- 100.Shinder ME, Taube JS. Active and passive movement are encoded equally by head direction cells in the anterodorsal thalamus. J Neurophysiol. 2011;106:788–800. doi: 10.1152/jn.01098.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Song EY, et al. Role of active movement in place-specific firing of hippocampal neurons. Hippocampus. 2005;15:8–17. doi: 10.1002/hipo.20023. [DOI] [PubMed] [Google Scholar]
- 102.Terrazas A, et al. Self-motion and the hippocampal spatial metric. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:8085–8096. doi: 10.1523/JNEUROSCI.0693-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Karnath HO. Subjective body orientation in neglect and the interactive contribution of neck muscle proprioception and vestibular stimulation. Brain. 1994;117:1001–1012. doi: 10.1093/brain/117.5.1001. [DOI] [PubMed] [Google Scholar]
- 104.Mars F, et al. Vestibular contribution to combined arm and trunk motion. Experimental brain research. Experimentelle Hirnforschung. Experimentation cerebrale. 2003;150:515–519. doi: 10.1007/s00221-003-1485-6. [DOI] [PubMed] [Google Scholar]
- 105.Bresciani JP, et al. Galvanic vestibular stimulation in humans produces online arm movement deviations when reaching towards memorized visual targets. Neuroscience letters. 2002;318:34–38. doi: 10.1016/s0304-3940(01)02462-4. [DOI] [PubMed] [Google Scholar]
- 106.Kawano K, Sasaki M. Response properties of neurons in posterior parietal cortex of monkey during visual-vestibular stimulation. II. Optokinetic neurons. Journal of neurophysiology. 1984;51:352–360. doi: 10.1152/jn.1984.51.2.352. [DOI] [PubMed] [Google Scholar]
- 107.Bottini G, et al. Identification of the central vestibular projections in man: a positron emission tomography activation study. Experimental brain research. Experimentelle Hirnforschung. Experimentation cerebrale. 1994;99:164–169. doi: 10.1007/BF00241421. [DOI] [PubMed] [Google Scholar]
- 108.Andersen RA, et al. The contributions of vestibular signals to the representations of space in the posterior parietal cortex. Annals of the New York Academy of Sciences. 1999;871:282–292. doi: 10.1111/j.1749-6632.1999.tb09192.x. [DOI] [PubMed] [Google Scholar]
- 109.Bockisch CJ, Haslwanter T. Vestibular contribution to the planning of reach trajectories. Experimental brain research. Experimentelle Hirnforschung. Experimentation cerebrale. 2007;182:387–397. doi: 10.1007/s00221-007-0997-x. [DOI] [PubMed] [Google Scholar]
- 110.Guillaud E, et al. Prediction of the body rotation-induced torques on the arm during reaching movements: evidence from a proprioceptively deafferented subject. Neuropsychologia. 2011;49:2055–2059. doi: 10.1016/j.neuropsychologia.2011.03.035. [DOI] [PubMed] [Google Scholar]