Abstract
Amplification of the human epidermal growth factor receptor 2 (HER2) gene and overexpression of the HER2 protein is found in 15%-20% of patients with gastric and gastroesophageal junction cancer. The degree of HER2 overexpression and amplification varies with the location of the carcinoma, with higher expression in the gastroesophageal and proximal parts compared to the distal parts of the stomach. Further, HER2 overexpression and amplification also seems to be related to the Lauren histological classification, with higher levels found in the intestinal phenotype compared to the diffuse and mixed types. The prognostic properties of HER2 overexpression and amplification are still under debate, but a large number of studies seem to indicate that HER2 is a negative prognostic factor. The usefulness of HER2 targeted therapy in gastric cancer was demonstrated in the ToGA trial, where HER2-positive patients with advanced gastric and gastroesophageal junction adenocarcinoma were randomized to receive 5-FU/capecitabine and cisplatin, either alone or in combination with trastuzumab. A statically significant gain in overall survival was seen in patients who received the combined treatment of trastuzumab and chemotherapy. Patients with a strong overexpression of the HER2 protein (IHC3+) specifically benefited from the treatment, with a median overall survival of 17.9 mo. As a consequence of the positive results of the ToGA trial, patients with advanced gastric or gastroesophageal junction adenocarcinoma are now routinely tested for HER2. The ToGA trial must be characterized as a landmark in the treatment of gastric cancer and it has paved the way for a number of new HER2 targeted compounds such as pertuzumab, ado-trastuzumab emtansine, lapatinib, afatinib, and dacomitinib, which are currently undergoing phase II and III clinical testing. Overall, this review will discuss the current status of HER2 in gastric and gastroesophageal junction cancer and the future direction in relation to HER2 target therapy.
Keywords: Human epidermal growth factor receptor 2, Gastric cancer, Prognostic, Companion diagnostics, Trastuzumab, Pertuzumab, Ado-trastuzumab emtansine, Lapatinib
Core tip: Amplification of the human epidermal growth factor receptor 2 (HER2) gene and overexpression of the HER2 protein can be detected in 15%-20% of patients with gastric and gastroesophageal junction (GEJ) cancer. Recently, HER2 has proven to be an important target for treatment with trastuzumab in these patients, and a positive HER2 status seems to possess both prognostic and predictive properties. A number of new compounds directed towards HER2 and other members of the HER family is currently under development. This review will discuss the current status of HER2 in gastric and GEJ cancer and the future direction in relation to HER2 target therapy.
INTRODUCTION
In gastric and gastroesophageal junction (GEJ) cancer, human epidermal growth factor receptor 2 (HER2) overexpression has become an important selective biomarker for treatment with trastuzumab (Herceptin®, Roche/Genentech)[1]. The gene for the HER2 protein (also known as ErbB-2, c-erbB2, or Her2/neu) is a proto-oncogene located on the chromosome 17q. This gene encodes a 185-kDa transmembrane tyrosine kinase receptor protein, which is a member of the HER family that consists of HER1 (EFGR), HER2, HER3, and HER4. HER2 forms both homo- and heterodimers and serves as a critical dimerization partner for other members of the HER family, and leads to activation of downstream signaling pathways associated with cell proliferation, differentiation, survival, and angiogenesis[2]. Amplification of the HER2 gene and overexpression of HER2 in gastric cancer was first described in 1986[3-5], and since then a large number of studies has confirmed these findings[6].
Gastric cancer is the fourth most commonly diagnosed cancer and the second most common cause of cancer-related death worldwide[7]. Despite some advances in the prevention and treatment of the disease, the 5-year survival still remains around 20%-25% in most parts of the world. Although the incidence of gastric cancer is declining, the prognosis for the disease remains poor. The poor survival rate is mainly explained by the advanced stage of the disease at the time of diagnosis. If screening for gastric cancer was performed, as in Japan, the tumors could be detected at an earlier stage and thus surgical resection performed, which has shown to increase the 5-year survival significantly[8]. When the disease becomes metastatic the treatment is largely palliative, and different combinations of chemotherapy have resulted in a median overall survival of 8-10 mo[9]. Based on data from the ToGA trial, trastuzumab, in combination with chemotherapy, was approved in 2010 for treatment of patients with HER2 overexpressing metastatic gastric or GEJ cancer, and thus became the first targeted anti-cancer drug for treatment of this serious disease[10,11]. This short review will discuss HER2 status as a prognostic and selective biomarker in gastric and GEJ cancer, as well as current and future HER2 directed therapies.
HER2 AND GASTRIC CANCER
Different slide-based assays are available for the detection of overexpression of the HER2 protein, which is measured by immunohistochemistry (IHC), or amplification of the HER2 gene, which is measured by fluorescence in situ hybridization (FISH) or other ISH methods. Examples of a positive HER2 status by IHC and FISH are shown in Figure 1. Due to differences in tumor biology, HER2 testing in gastric cancer differs from breast cancer. The gastric cancer tissue more frequently shows HER2 heterogeneity and incomplete membrane staining, and as a consequence of this a specific gastric cancer testing protocol has been developed[12,13]. Based on the results from the ToGA trial, which will be discussed later, IHC is the primary test in gastric and GEJ cancer, with FISH being used as a reflex test in cases of an equivocal IHC2+ result. In Table 1 are shown the interpretation and scoring guideline for the HercepTest™ (Dako), which, together with the HER2 FISH pharmDx™ Kit (Dako), are the only companion diagnostic assays that are currently approved by the United States FDA in relation to testing of gastric and GEJ cancer patients for whom treatment with trastuzumab is under consideration. The reason for this is that these two assays were those used to select the patients enrolled in the ToGA trial[10]. As HER2 positivity in the ToGA trial was defined as being either IHC3+ or FISH+ and both tests were performed on almost all patients, the United States FDA requires that both assays are used in order to determine the HER2 status[14].
Figure 1.
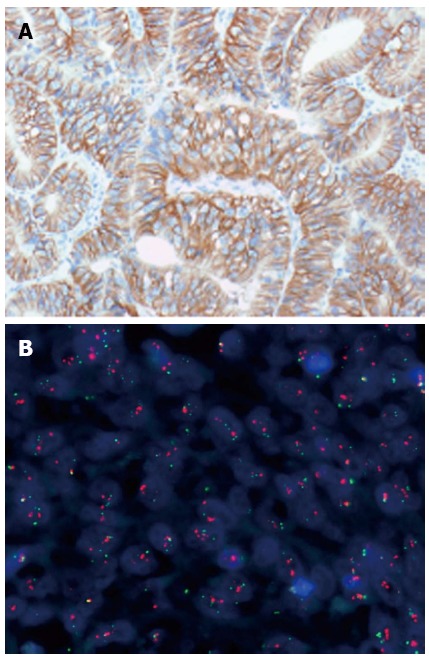
Human epidermal growth factor receptor 2 positive gastric adenocarcinoma. A: Immunohistochemistry (HercepTest™, Dako); B: Fluorescence in situ hybridization (FISH) (Human epidermal growth factor receptor 2 FISH pharmDx™ Kit, Dako).
Table 1.
Interpretation and scoring of human epidermal growth factor receptor 2 immunohistochemistry for gastric cancer, as approved by the United States Food and Drug Administration in relation to the HercepTest (Dako)
| Score | Surgical specimen staining pattern | Biopsy specimen staining pattern | HER2 overexpression assessment |
| 0 | No reactivity or membranous reactivityin < 10% of tumor cells | No reactivity or no membranous reactivity in any (or < 5 clustered) tumor cells | Negative |
| 1+ | Faint/barely perceptible membranous reactivity in ≥ 10% of tumor cells; cells are reactive only in part of their membrane | Tumor cell cluster (≥ 5 cells) with a faint/barely perceptible membranous reactivity irrespective of percentage of tumor cells stained | Negative |
| 2+ | Weak to moderate complete, basolateral or lateral membranous reactivity in ≥ 10% of tumor cells | Tumor cell cluster (≥ 5 cells) with a weak to moderate complete, basolateral, or lateral membranous reactivity irrespective of percentage of tumor cells stained | Equivocal |
| 3+ | Strong complete, basolateral or lateral membranous reactivity in ≥ 10% of tumor cells | Tumor cell cluster (≥ 5 cells) with a strong complete, basolateral, or lateral membranous reactivity irrespective of percentage of tumor cells stained | Positive |
HER2: Human epidermal growth factor receptor 2.
The prevalence of HER2 overexpression in gastric cancer varies a lot from study to study. In a larger literature survey based on 11860 patients from 38 individually published studies, the calculated weighted mean was 17.9% (95%CI: 14.8-20.9). The corresponding range for these studies was from 4.4% to 53.4%. This survey also looked at HER2 amplification; however, here the number of patients was somewhat lower. The prevalence estimate was based on 1597 patients from 8 different published studies and the calculated weighted mean was 12.2% (95%CI: 9.5-14.8). The corresponding range for these studies was from 8.7% to 18.1%[6]. The explanation for the large variation found in the HER2 positivity rate for the IHC studies is likely to be multifactorial, and here the difference in the populations studied may play a role. However, the most important factor is probably the use of non-standardized assays using different antibodies and the application of different scoring and interpretation criteria for the stained slides[6].
In the screening program related to the ToGA trial, 3807 patients were screened, which makes it the largest single study conducted on the prevalence of HER2 positivity in gastric and GEJ cancer. This program showed an overall HER2 positivity rate of 22.1%, although with a large variation from country to country. The highest prevalence rate (33.2%) was found in Australia and the lowest (5.9%) in Taiwan[11].
A number of studies have shown that HER2 overexpression and amplification are related to the Lauren histological classification, with higher levels found in the intestinal phenotype compared to the diffuse and mixed types[6,11,15-21]. This was also confirmed in the ToGA screening program, where the HER2 positivity rate was found to be statistically significantly (P < 0.001) higher in the intestinal phenotype (32.2%) compared to the diffuse (6.1%) and mixed (20.4%) types[11]. Furthermore, the degree of HER2 overexpression seems to vary with the location of the carcinoma, with higher expression in the proximal part and the GEJ compared to distal parts of the stomach[21,22]. Again, the ToGA screening program confirmed this observation with a HER2 positivity rate of 33.2% when the cancer is located in the GEJ, compared to 20.9% when located in the stomach. Again, this difference in HER2 positivity related to tumor site was statistically significant (P < 0.001)[11]. A few studies have also shown that the expression of HER2 increases with disease progression[23-26].
TOGA TRIAL
The ToGA trial must be characterized as a landmark in the treatment of gastric cancer. Following the successful completion of the study, trastuzumab, in combination with chemotherapy, became the first targeted drug to be approved for this indication. The study was designed as an open labeled, randomized multicenter phase III study in HER2-positive patients with histologically confirmed inoperable locally advanced, recurrent, or metastatic adenocarcinoma of the stomach or GEJ. HER2 positivity was defined as being either IHC positive (3+) or positive by HER2 FISH (HER2/CEN17 ratio ≥ 2). However, both an IHC and FISH test were performed on almost all patients. After inclusion in the study, patients were randomized to receive chemotherapy (5-FU or capecitabine and cisplatin) or chemotherapy plus trastuzumab. More than 3800 patients were screened for the study and 584 of these were randomized. The primary endpoint in the study was overall survival (OS), with secondary endpoints including overall response rate (ORR) and progression free survival (PFS)[10].
For the primary endpoint, the combination of chemotherapy plus trastuzumab was shown to be statistically superior to chemotherapy alone. The median OS increased from 11.1 to 13.8 mo (P = 0.0046), with a hazard ratio (HR) of 0.74 (95%CI: 0.60-0.91). The secondary endpoints of ORR and PFS showed superiority in favor of the combined treatment with chemotherapy and trastuzumab. The overall tumor response rate was 47% in combined treatment with chemotherapy and trastuzumab, compared to 35% in the group with chemotherapy alone. A pre-planned exploratory analysis looking at the effect in the different HER2 IHC categories (0, 1+, 2+, 3+) showed that the survival benefit provided by trastuzumab seemed to be dependent on the level of HER2 protein overexpression. The single subgroup of patients with the greatest survival benefit was the one with a HER2 test result of IHC3+. Here, the median OS increased to 17.9 mo for the group treated with the combination of trastuzumab and chemotherapy compared to chemotherapy alone, which achieved an OS of 12.3 mo. Overall, the survival gain for the group of patients with IHC3+ expressing tumors was nearly 6 mo. The HR for this group of patients was 0.58 (95%CI: 0.41-0.81)[10]. The results of the subgroup analysis for the different IHC scores are shown in Figure 2.
Figure 2.
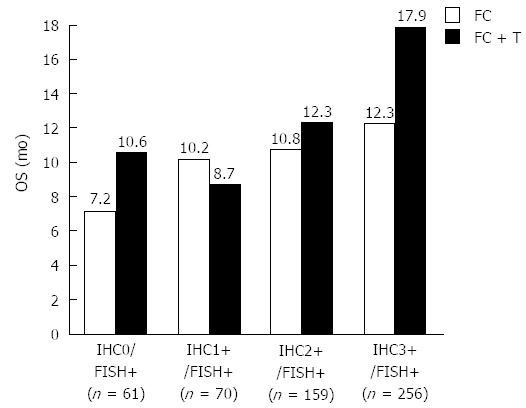
Median overall survival in months for the four individual human epidermal growth factor receptor 2 immunohistochemistry scores for the two treatment groups. OS: Overall survival; FC: Fluorouracil/capecitabine plus cisplatin; FC + T: Fluorouracil/capecitabine plus cisplatin plus trastuzumab[10]; FISH: Fluorescence in situ hybridization; IHC: Immunohistochemistry.
Based on the information from the pre-planned exploratory analysis, a post-hoc explorative analysis was performed on a subpopulation of the originally included patients. This population comprised the patients who were IHC3+ positive or IHC2+ positive, and those who were FISH positive. A total of 446 patients fulfilled these criteria, and the median OS for the group of patients who had received chemotherapy plus trastuzumab increased to 16.0 mo compared to 11.8 mo for the patients on chemotherapy alone. The HR for this analysis was 0.65 (95%CI: 0.51-0.83). The median follow-up for all the patients in the ToGA trial was reported to be 17.1 mo[10].
Concerning the selective properties of the two HER2 companion diagnostic assays, explorative analysis showed that the IHC test should be used as the primary test for selection of patients for treatment with trastuzumab. As shown in Figure 2, the effect of trastuzumab seems to be dependent on the degree of HER2 protein overexpression, with the best median OS in the group of patients with IHC3+. Furthermore, when looking at the HER2 test results for the patients enrolled in the ToGA trial, the agreement between overexpression of the HER2 protein and amplification of the gene is found to be somewhat lower in gastric cancer than that normally observed in breast cancer. A relatively high number of HER2 FISH positive cases were found among the IHC0 and IHC1+ tumors as shown in Table 2[10,11,27]. Based on the subgroup analyses in the ToGA trial, a specific HER2 testing algorithm was developed as shown in Figure 3[27]. However, since nearly 95% (533/584) of the patients enrolled in the ToGA trial had tumors that were HER2 amplified, it has been argued that the criteria for treatment with trastuzumab should be both gene amplification and protein overexpression. In relation to the approval of trastuzumab for treatment of advanced gastric cancer this was, in fact, the position taken by the United States FDA, who recommended that reflex testing with FISH should be considered for both IHC2+ and IHC3+[14]. An algorithm taking this into consideration is shown in Figure 4. A recent survey made in the United States also showed that FISH reflex testing was performed for both IHC0 and IHC1+ at some cancer centers[28]. So, despite recommendations from both scientific and regulatory sides there still seems to be no real consensus with respect to which testing algorithm to use.
Table 2.
Positive human epidermal growth factor receptor 2 status by immunohistochemistry and/or fluorescence in situ hybridization for the patients enrolled in the ToGA trial[10,11,27]
| HER2 status | n |
| IHC0/FISH+ | 61 |
| IHC1+/FISH+ | 70 |
| IHC2+ FISH+ | 159 |
| IHC3+/FISH+ | 256 |
| IHC3+/FISH- | 15 |
| IHC3+/FISH no results | 16 |
| IHC no results/FISH+ | 7 |
| Total | 584 |
N: Number of patients. HER2: Human epidermal growth factor receptor 2; IHC: Immunohistochemistry; FISH: Fluorescence in situ hybridization.
Figure 3.

Human epidermal growth factor receptor 2 testing algorithm developed based on the results of the ToGA trial. Immunohistochemistry (IHC) is the primary test with reflex testing with Fluorescence in situ hybridization (FISH) in case of an equivocal IHC result (IHC2+)[27].
Figure 4.

Human epidermal growth factor receptor 2 testing algorithm with fluorescence in situ hybridization reflex testing for both immunohistochemistry 2+ and immunohistochemistry 3+. This testing algorithm was recommended by the United States Food and Drug Administration in relation to approval of trastuzumab for advanced gastric cancer. FISH: Fluorescence in situ hybridization; IHC: Immunohistochemistry.
HER2 AS A PROGNOSTIC MARKER
As described above, the primary analysis of the ToGA trial showed a median OS of 13.8 mo for the group of patients that received the combined treatment of chemotherapy and trastuzumab compared to 11.1 mo for the group that was assigned to chemotherapy alone. In the discussion section of the paper published in the Lancet in 2010, it was mentioned that the OS of 11.1 mo in the group of patients receiving chemotherapy alone was longer than expected. As a possible explanation for this, it was stated that HER2 overexpression might already be conferring a better prognosis across the groups of patients studied. However, it was also mentioned that HER2 overexpression leading to a better prognosis, is in contrast to recent studies that have showed an association between HER2-positive tumors, poor outcome, and aggressive disease. The authors further concluded that more studies were needed to address the issue of whether HER2 has an effect on prognosis in gastric cancer, and whether it confers a good or poor prognosis[6,10].
In breast cancer, HER2 was found to be a negative prognostic factor very early on, and a number of studies have subsequently confirmed this[2,29]. However, when it comes to gastric cancer there still seems to be no definite conclusion, despite the fact that the first studies demonstrating an association between a positive HER2 status and poor prognosis appeared more than 20 years ago[26,30]. In order to address this issue, a systematic analysis of data from the literature was undertaken where a large number of studies on HER2 and gastric cancer were reviewed[6]. The studies included in this analysis should fulfill the following two criteria: 1) the number of patients in each study should be ≥ 100 and the HER2 status should have been determined either by IHC or ISH, and 2) the selected articles should include an analysis of the association between the HER2 status and survival or relevant clinicopathological characteristics. Forty-two publications with a total of 12749 patients fulfilled the two criteria and were reviewed in detail. The studies described in these 42 articles were scored according to the strength of the association between the positive HER2 status and the prognostic information reported, using a three point categorical scale.
In 17 of these 42 studies (approximately 40%) an association between positive HER2 status and poor survival (++) was found, and an additional 13 studies (approximately 31%) similarly displayed a relationship with clinicopathological characteristics (+) such as serosal invasion, lymph node metastases, disease stage, or distant metastases. In the last 12 studies (approximately 29%), no association between positive HER2 status and poor survival or clinicopathological characteristics could be detected (-). Overall, 30 (71%) out of 42 studies showed an association between a positive HER2 status and poor survival and/or relevant clinicopathological characteristics. Figure 5 illustrates the number of studies and patients in each of the three scoring categories[6].
Figure 5.
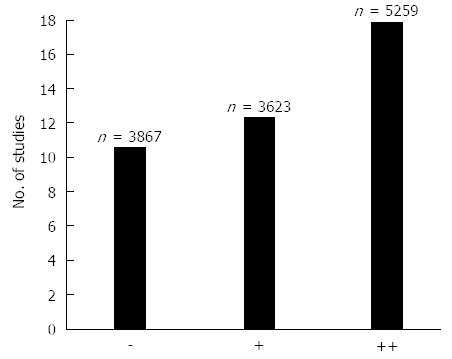
The number of studies and patients (n) in each of the three scoring categories. Symbols: Two pluses (++) indicate the strongest association with the Human epidermal growth factor receptor 2 (HER2)+ status, one plus (+) indicates a somewhat weaker association with the HER2+ status, and minus (–) indicates that no associations with the HER2+ was found[6].
Based on this analysis of the literature data, it was concluded that a clear trend towards a potential role for HER2 as a negative prognostic factor in gastric cancer was shown[6], which is also in line with another recently published systematic review based on literature data[31]. Furthermore, with reference to the publication of the ToGA trial in the Lancet, the data could not support the hypothesis that positive HER2 overexpression could act as a positive prognostic factor in gastric cancer[10]. None of the articles that fulfilled the necessary criteria in the analysis supported this hypothesis. Possible confounding factors, such as a wider use of second line treatments and a possible better prognosis related to the intestinal phenotype, should be taken into consideration when interpreting the data from the ToGA trial[32]. With regards to the latter, it is worth mentioning that approximately 75% of the patients included in the ToGA trial had tumors of the intestinal type[10], which seems to be high compared to most of the studies reported in the analysis of data from the literature. This characteristic might have influenced the OS seen in the group of patients that received chemotherapy alone. In support of the hypothesis put forward in the Lancet article, one study was identified which showed that overexpression of HER2 resulted in a better prognosis compared to those who did not overexpress the protein. However, this study was not included in the analysis due to the number of patients being < 100 (thus failing one of the criteria)[6]. Here, samples from 93 patients with advanced gastric carcinoma were investigated using IHC. Overexpression of HER2 was found in 10 patients (11%), and a multifactorial analysis showed a significantly better prognosis for those patients in relation to survival[33]. However, after the finalization of the above described analysis of data from the literature, a relatively large study has recently been published. This study comprised 381 patients, with 78 (20%) of these being found to be HER2 positive by IHC or ISH. When the HER2 status was correlated with survival data, patients with HER2 positive tumors had longer OS compared to the HER2 negative patients, however, the prognostic value disappeared in the multivariate analysis[34].
Despite the data in gastric cancer not being as consistent as shown in breast cancer, the majority of studies seem to point towards HER2 overexpression and/or amplification as being an indicator of poor prognosis[6]. In line with this conclusion, it has also been suggested recently that HER2 overexpression and/or amplification is a molecular abnormality that is linked to the development of gastric cancer[32].
POTENTIAL HER2 TARGETED DRUGS IN GASTRIC CANCER
Following the initiation and success of the ToGA trial, a number of other HER2 targeted compounds have gone into clinical development for treatment of gastric, esophageal, or gastroesophageal junction cancer. These compounds represent small molecule tyrosine kinase inhibitors (TKI) and antibodies, as well as antibody-drug conjugates (ADC). Most of these compounds, together with the stage of development, are listed in Figure 6. However, in this review, emphasis will be placed on the drugs that are further advanced in clinical development (phase II and III). An overview of these compounds and their targets are given in Table 3. Phase I compounds will only be described briefly.
Figure 6.
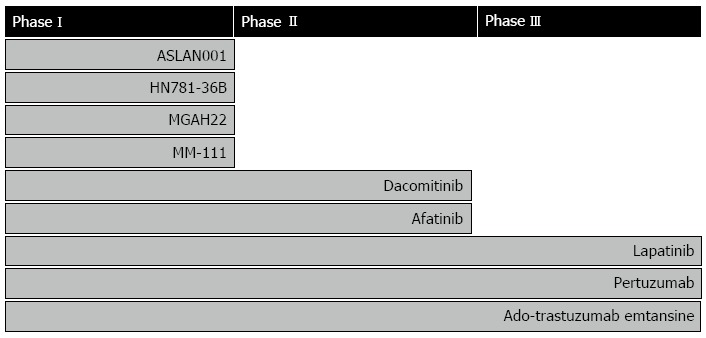
Drugs targeting human epidermal growth factor receptor 2 in clinical development for treatment of gastric, esophageal, or gastroesophageal junction cancer. The individual compounds are listed according to the stage of development[35].
Table 3.
Overview of the phase II and III compounds and their targets
| Compounds | Type of compound | Target(s) | Clinical phase |
| Dacomitinib (PF-00299804, Pfizer) | Irreversible pan-HER TKI | HER1 (EGFR) and HER2 | II |
| Afatinib (Gilotrif, Boehringer Ingelheim) | Irreversible pan-HER TKI | HER1 (EGFR), HER2, and HER4 | II |
| Pertuzumab (Perjeta, Roche/Genentech) | mAb | HER2 (subdomain II), HER2 hetero dimerization | II/III |
| Ado-trastuzumab emtansine (Kadcyla, Roche/Genentech) | ADC | HER2 (subdomain IV) | II/III |
| Lapatinib (Tyverb/Tykerb, GSK) | Reversible pan-HER TKI | HER1 (EGFR) and HER2 | III |
TKI: Tyrosine kinase inhibitors; mAb: Monoclonal antibody; ADC: Antibody-drug conjugate.
Phase I compounds
As is the case for the other phases, the compounds in phase I clinical development can be divided into small molecule inhibitors and antibodies. According to ClinicalTrials.gov there are two small molecule pan HER inhibitors, ASLAN001 (Aslan Pharmaceuticals) and HM781-36B (Hanmi Pharmaceuticals), in phase I. When it comes to the antibodies, two compounds are in phase I development, the HER2 monoclonal antibody MGAH22 (MacroGenics) and the bi-specific antibody MM-111 (Merrimack Pharmaceuticals) directed towards HER2 and HER3[35].
Dacomitinib
Dacomitinib (PF-00299804, Pfizer) is an oral pan-HER TKI. The compound irreversibly inhibits HER1 (EGFR) and HER2 tyrosine kinase, as well as blocking HER1/HER2, HER2/HER3, and HER3/HER4 heterodimerization[36,37]. In different preclinical models, dacomitinib has shown significant growth-inhibitory effects in HER2-amplified gastric cancer cells, such as SNU-216 and NCI-N87. Furthermore, the combination of dacomitinib with chemotherapeutic agents (such as 5-FU and cisplatin) or targeted agents (such as trastuzumab) showed a synergistic effect[36]. However, a clinical phase II study in HER2 positive (IHC3+ or FISH+) patients with advanced gastric cancer, where dacomitinib was given as monotherapy, showed a response rate of only 7.4% and an OS of 7.1 mo. The relatively modest clinical effect may be explained by the advanced stage of the disease and that the patients had been heavily pretreated[38].
Afatinib
Afatinib (Gilotrif, Boehringer Ingelheim) is another oral irreversible pan-HER TKI that targets HER1 (EGFR), HER2, and HER4[39]. The compound has recently obtained FDA approval for first-line treatment of patients with metastatic non-small cell lung cancer whose tumors have tested positive for EGFR mutations. The FDA has also approved therascreen EGFR RGQ Kit (Qiagen), a companion diagnostics assay, for use in the detection of EGFR exon 19 deletions or exon 21 substitution mutations[40]. In relation to gastric cancer, afatinib has demonstrated antitumor activity in a HER2 positive xenograft mouse model[41]. Additionally, results from a small clinical phase II study in HER2 positive patients with esophagogastric (EG) cancer has recently been presented. Based on data from this study, the investigators concluded that single agent afatinib showed clinical efficacy in patients with trastuzumab refractory EG cancer. However, this conclusion must be regarded as preliminary, as it was only based on data from 7 patients and more patients are expected to be enrolled in the study[42].
Lapatinib
Lapatinib (Tyverb/Tykerb, GSK) is an oral TKI, but, in contrast to both dacomitinib and afatinib, its inhibitory effect on HER1 (EGFR) and HER2 is reversible. Lapatinib is currently approved for treatment of HER2 positive metastatic breast cancer in combination with capecitabine (Xeloda, Roche) or for HER2 positive postmenopausal women with hormone receptor positive metastatic breast cancer in combination with letrozole (Femara, Novartis)[43]. The antitumor effect of lapatinib has been investigated in different gastric cancer cell lines, and it was shown to induce a selective and potent growth inhibition in the two HER2-amplified gastric cancer cell lines SNU-216 and NCI-N87. Furthermore, in the same model lapatinib combined with 5-fluorouracil, cisplatin, oxaliplatin, or paclitaxel showed an additive or synergistic effect[44]. These results provide the rationale for the clinical development of lapatinib for the treatment of HER2-positive gastric cancer. A phase II clinical trial was performed in patients with unresectable gastric adenocarcinoma, although in this protocol HER2 positivity was not an inclusion criterion. A total of 47 patients were enrolled in the study and 44 received lapatinib as monotherapy until disease progression or unacceptable toxicity. The response rate was relatively modest with 5 patients (11%) having a confirmed or unconfirmed partial response. The median OS was 4.8 mo (95%CI: 3.2-7.4)[45]. Data from the LoGIC phase III trial where lapatinib plus chemotherapy (capecitabine and oxaliplatin) was compared to chemotherapy alone in patients with HER2 positive advanced gastric, esophageal, or gastroesophageal junction adenocarcinoma has recently been presented. The median OS for the lapatinib plus chemotherapy group was 12.2 mo compared 10.5 mo for the group that received chemotherapy alone. The primary endpoint for the study with regards to HR for OS was not reached (HR: 0.91, 95%CI: 0.73-1.12, P = 0.35). The response rate was 53% for the combined group receiving lapatinib and chemotherapy, compared to 40% for the group receiving chemotherapy alone. However, a pre-specified subgroup analysis in Asian patients and patients < 60 years showed a significant improvement with a HR of 0.68 and 0.69, respectively. A total of 545 HER2 positive patients were randomized in the LoGIC study[46]. Another phase III clinical trial, TYTAN, in Asian patients with advanced gastric cancer is still ongoing. In said study, patients with HER2 amplified tumors are randomized to lapatinib plus paclitaxel or paclitaxel alone[47]. Based on the clinical data presented so far, the future role of lapatinib in gastric cancer must be regarded as unclear.
Pertuzumab
Pertuzumab (Perjeta, Roche/Genentech) is a humanized monoclonal antibody that binds to sub-domain II of the extracellular part of the HER2 protein, thereby blocking its ability to form heterodimers with other members of the HER family, including HER1 (EGFR), HER3, and HER4. Trastuzumab also binds to the extracellular part of the HER2 protein, albeit to a different sub-domain (IV), and it does not possess an inhibitory effect in relation to dimerization of HER2 with the other HER receptors[48,49]. Pertuzumab has a mechanism of action that is complementary to that of trastuzumab, and the combination of these two monoclonal antibodies has been demonstrated to be effective as a first-line treatment in metastatic breast cancer[50]. This has recently led to regulatory approval of the compound for treatment of HER2 positive metastatic breast cancer in combination with trastuzumab and docetaxel. In gastric cancer, a tumor mouse xenograft model using the HER2 positive NCI-N87 cells has been used to demonstrate the preclinical antitumor activity of pertuzumab. Based on this model, a significantly enhanced antitumor efficacy of pertuzumab in combination with trastuzumab was shown compared to monotherapy with each of the two compounds. Similar antitumor efficacy was shown using another HER2 positive cell line (4-1ST), thus paving the way for the clinical development of pertuzumab in gastric cancer[51]. Both clinical phase II and III trials in HER2 positive metastatic gastric or gastroesophageal junction adenocarcinoma have been initiated, which include the large international JACOB study. In this study, pertuzumab plus trastuzumab and chemotherapy (cisplatin, 5-FU/capecitabine) are compared to placebo plus trastuzumab and chemotherapy. It is planned that the JACOB study should enroll 780 patients at approximately 200 sites in 35 countries worldwide[52-54].
Ado-trastuzumab emtansine
Ado-trastuzumab emtansine (Kadcyla, Roche/Genentech) is a novel ADC specifically designed for the treatment of HER2-positive cancer. It is composed of the potent cytotoxic agent DM1 (a thiol-containing maytansinoid anti-microtubule agent) conjugated to trastuzumab via a specific linker molecule. Ado-trastuzumab emtansine binds to the HER2 protein (sub-domain IV) with an affinity similar to that of trastuzumab. It is hypothesized that after binding to the receptor protein, ado-trastuzumab emtansine undergoes receptor-mediated internalization, followed by intracellular release of DM1, which then exerts its cytotoxicity in the tumor cell[55]. Ado-trastuzumab emtansine has been compared with lapatinib plus capecitabine in a phase III trial in HER2-positive breast cancer patients with metastatic disease. Although these patients had previously been treated with a taxane and trastuzumab, the study showed that ado-trastuzumab emtansine significantly improved PFS and OS compared to the combination of lapatinib plus capecitabine[56]. Following the successful completion of phase III, ado-trastuzumab emtansine has recently been approved for treatment of patients with HER2-positive metastatic breast cancer who have previously received trastuzumab and/or a taxane. In gastric cancer ado-trastuzumab emtansine has been tested in a number of different preclinical in vitro and in vivo HER2 positive cell models. Using the NCI-N87 and OE-19 cells lines in vitro, ado-trastuzumab emtansine was found to be more effective than trastuzumab. In a mouse xenograft model using the same cell lines, a similar positive anti-tumor effect was found in vivo[57]. In another preclinical study, ado-trastuzumab emtansine showed pronounced antitumor activity in vivo in two other HER2 expressing cell lines (SCH and 4-1ST). Furthermore, the effect of combining ado-trastuzumab emtansine with pertuzumab has also been investigated using NCI-N87 xenografted cells, and here the combination showed a significant antitumor effect, whereas the use of the individual compounds as monotherapy did not[58]. Additionally, the positive preclinical findings led to the initiation of a clinical development program, and currently a phase II/III trial has been initiated in order to evaluate efficacy and safety of ado-trastuzumab emtansine compared to taxane treatment in patients with HER2-positive advanced gastric cancer[59].
CONCLUSION
The ToGA trial must be regarded as a landmark, not only did the study show that trastuzumab is effective in treating HER2 overexpressing gastric cancer, but it also gave us important information on the pathophysiological characteristics of the disease. As a consequence, HER2 testing of patients with advanced gastric cancer and treatment with trastuzumab has now become standard in most countries. The ToGA trial also demonstrated that overexpression of HER2 in gastric cancer possesses selective properties in relation to treatment with trastuzumab in a similar manner to what is known from breast cancer. When it comes to prognostics properties there still seems to be some discussion about the value. However, a recent large systematic analysis of data from the literature showed a clear trend towards a potential role of HER2 as a negative prognostic marker in gastric cancer. Hopefully, future additional data will clarify this issue. Despite the controversies around the prognostic properties of HER2, there seems to be much more consensus regarding the importance of the receptor as a therapeutic target in gastric cancer. A number of new compounds targeting HER2 and other members of the HER family are under development, and several of these have already reached phase III clinical studies. Pertuzumab and ado-trastuzumab emtansine, as well as some of the small molecule pan HER inhibitors, might be potentially useful for HER2 positive gastric cancer patients that have developed resistance to trastuzumab.
ACKNOWLEDGMENTS
I would like to thank Dako Denmark A/S for their permission to use the microscopic gastric adenocarcinoma images and Inge Merete Hounsgaard for her excellent linguistic support.
Footnotes
P- Reviewers: Kaumaya PTP, Merrett ND, Wilkinson N S- Editor: Cui XM L- Editor: Rutherford A E- Editor: Wang CH
References
- 1.Jørgensen JT. Targeted HER2 treatment in advanced gastric cancer. Oncology. 2010;78:26–33. doi: 10.1159/000288295. [DOI] [PubMed] [Google Scholar]
- 2.Ross JS, Slodkowska EA, Symmans WF, Pusztai L, Ravdin PM, Hortobagyi GN. The HER-2 receptor and breast cancer: ten years of targeted anti-HER-2 therapy and personalized medicine. Oncologist. 2009;14:320–368. doi: 10.1634/theoncologist.2008-0230. [DOI] [PubMed] [Google Scholar]
- 3.Yamamoto T, Ikawa S, Akiyama T, Semba K, Nomura N, Miyajima N, Saito T, Toyoshima K. Similarity of protein encoded by the human c-erb-B-2 gene to epidermal growth factor receptor. Nature. 1986;319:230–234. doi: 10.1038/319230a0. [DOI] [PubMed] [Google Scholar]
- 4.Fukushige S, Matsubara K, Yoshida M, Sasaki M, Suzuki T, Semba K, Toyoshima K, Yamamoto T. Localization of a novel v-erbB-related gene, c-erbB-2, on human chromosome 17 and its amplification in a gastric cancer cell line. Mol Cell Biol. 1986;6:955–958. doi: 10.1128/mcb.6.3.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sakai K, Mori S, Kawamoto T, Taniguchi S, Kobori O, Morioka Y, Kuroki T, Kano K. Expression of epidermal growth factor receptors on normal human gastric epithelia and gastric carcinomas. J Natl Cancer Inst. 1986;77:1047–1052. [PubMed] [Google Scholar]
- 6.Jørgensen JT, Hersom M. HER2 as a Prognostic Marker in Gastric Cancer - A Systematic Analysis of Data from the Literature. J Cancer. 2012;3:137–144. doi: 10.7150/jca.4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu B, El Hajj N, Sittler S, Lammert N, Barnes R, Meloni-Ehrig A. Gastric cancer: Classification, histology and application of molecular pathology. J Gastrointest Oncol. 2012;3:251–261. doi: 10.3978/j.issn.2078-6891.2012.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24:2137–2150. doi: 10.1200/JCO.2005.05.2308. [DOI] [PubMed] [Google Scholar]
- 9.Ohtsu A. Chemotherapy for metastatic gastric cancer: past, present, and future. J Gastroenterol. 2008;43:256–264. doi: 10.1007/s00535-008-2177-6. [DOI] [PubMed] [Google Scholar]
- 10.Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 11.Bang YJ, Chung HC, Xu JM, Lordick F, Sawaki A, Lipatov O, Al-Sakaff N, See CG, Rueschoff J, Van Cutsem E. Pathological features of advanced gastric cancer: relationship to human epidermal growth factor receptor 2 positivity in the global screening programme of the ToGA trial. J Clin Oncol. 2009;27 Suppl:Abstract 4556. [Google Scholar]
- 12.Hofmann M, Stoss O, Shi D, Büttner R, van de Vijver M, Kim W, Ochiai A, Rüschoff J, Henkel T. Assessment of a HER2 scoring system for gastric cancer: results from a validation study. Histopathology. 2008;52:797–805. doi: 10.1111/j.1365-2559.2008.03028.x. [DOI] [PubMed] [Google Scholar]
- 13.Rüschoff J, Hanna W, Bilous M, Hofmann M, Osamura RY, Penault-Llorca F, van de Vijver M, Viale G. HER2 testing in gastric cancer: a practical approach. Mod Pathol. 2012;25:637–650. doi: 10.1038/modpathol.2011.198. [DOI] [PubMed] [Google Scholar]
- 14.Food and Drug Administration. HercepTest-Summary of Safety and Effectiveness Data (SSED), October 20, 2010. Available from: http://www.accessdata.fda.gov/cdrh_docs/pdf/P980018S010b.pdf.
- 15.Ishikawa T, Kobayashi M, Mai M, Suzuki T, Ooi A. Amplification of the c-erbB-2 (HER-2/neu) gene in gastric cancer cells. Detection by fluorescence in situ hybridization. Am J Pathol. 1997;151:761–768. [PMC free article] [PubMed] [Google Scholar]
- 16.Yonemura Y, Ninomiya I, Tsugawa K, Fushida S, Fujimura T, Miyazaki I, Uchibayashi T, Endou Y, Sasaki T. Prognostic significance of c-erbB-2 gene expression in the poorly differentiated type of adenocarcinoma of the stomach. Cancer Detect Prev. 1998;22:139–146. doi: 10.1046/j.1525-1500.1998.cdoa02.x. [DOI] [PubMed] [Google Scholar]
- 17.Lee KE, Lee HJ, Kim YH, Yu HJ, Yang HK, Kim WH, Lee KU, Choe KJ, Kim JP. Prognostic significance of p53, nm23, PCNA and c-erbB-2 in gastric cancer. Jpn J Clin Oncol. 2003;33:173–179. doi: 10.1093/jjco/hyg039. [DOI] [PubMed] [Google Scholar]
- 18.Barros-Silva JD, Leitão D, Afonso L, Vieira J, Dinis-Ribeiro M, Fragoso M, Bento MJ, Santos L, Ferreira P, Rêgo S, et al. Association of ERBB2 gene status with histopathological parameters and disease-specific survival in gastric carcinoma patients. Br J Cancer. 2009;100:487–493. doi: 10.1038/sj.bjc.6604885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Song HS, Do YR, Kim IH, Sohn SS, Kwon KY. Prognostic significance of immunohistochemical expression of EGFR and C-erbB-2 oncoprotein in curatively resected gastric cancer. Cancer Res Treat. 2004;36:240–245. doi: 10.4143/crt.2004.36.4.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fan XS, Chen JY, Li CF, Zhang YF, Meng FQ, Wu HY, Feng AN, Huang Q. Differences in HER2 over-expression between proximal and distal gastric cancers in the Chinese population. World J Gastroenterol. 2013;19:3316–3323. doi: 10.3748/wjg.v19.i21.3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tanner M, Hollmén M, Junttila TT, Kapanen AI, Tommola S, Soini Y, Helin H, Salo J, Joensuu H, Sihvo E, et al. Amplification of HER-2 in gastric carcinoma: association with Topoisomerase IIalpha gene amplification, intestinal type, poor prognosis and sensitivity to trastuzumab. Ann Oncol. 2005;16:273–278. doi: 10.1093/annonc/mdi064. [DOI] [PubMed] [Google Scholar]
- 22.Polkowski W, van Sandick JW, Offerhaus GJ, ten Kate FJ, Mulder J, Obertop H, van Lanschot JJ. Prognostic value of Laurén classification and c-erbB-2 oncogene overexpression in adenocarcinoma of the esophagus and gastroesophageal junction. Ann Surg Oncol. 1999;6:290–297. doi: 10.1007/s10434-999-0290-2. [DOI] [PubMed] [Google Scholar]
- 23.Ohguri T, Sato Y, Koizumi W, Saigenji K, Kameya T. An immunohistochemical study of c-erbB-2 protein in gastric carcinomas and lymph-node metastases: is the c-erbB-2 protein really a prognostic indicator? Int J Cancer. 1993;53:75–79. doi: 10.1002/ijc.2910530115. [DOI] [PubMed] [Google Scholar]
- 24.Chariyalertsak S, Sugano K, Ohkura H, Mori Y. Comparison of c-erbB-2 oncoprotein expression in tissue and serum of patients with stomach cancer. Tumour Biol. 1994;15:294–303. doi: 10.1159/000217904. [DOI] [PubMed] [Google Scholar]
- 25.Mizutani T, Onda M, Tokunaga A, Yamanaka N, Sugisaki Y. Relationship of C-erbB-2 protein expression and gene amplification to invasion and metastasis in human gastric cancer. Cancer. 1993;72:2083–2088. doi: 10.1002/1097-0142(19931001)72:7<2083::aid-cncr2820720705>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 26.Yonemura Y, Ninomiya I, Yamaguchi A, Fushida S, Kimura H, Ohoyama S, Miyazaki I, Endou Y, Tanaka M, Sasaki T. Evaluation of immunoreactivity for erbB-2 protein as a marker of poor short term prognosis in gastric cancer. Cancer Res. 1991;51:1034–1038. [PubMed] [Google Scholar]
- 27.Chung H, Bang Y, Xu J, Lordick F, Sawaki A, Lipatov O, Lehle M, Pickl M, Rueschoff J, Van Cutsem E. Human epidermal growth factor receptor 2 (HER2) in gastric cancer (GC): results of the ToGA trial screening programme and recommendations for HER2 testing. ECCO 15-34th ESMO Multidisciplinary Congress. Berlin, Germany: ECCO; 2009. [Google Scholar]
- 28.Trosman JR, Weldon CB, Tsongalis GJ, Schink JC, Benson AB. What are NCI-designated cancer centers using for gastric and esophageal cancer HER2 testing? J Clin Oncol. 2013;31 Suppl:Abstract e15010. [Google Scholar]
- 29.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 30.Roh JK, Paik S, Chung HC, Yang W, Kim HK, Choi IJ, Kim J, Koh E, Lee KS, Min JS. Overexpression of erbB-2 protein in gastric adenocarcinoma--a potential role in therapeutic response to adjuvant 5-FU-doxorubicin regimen. Gan To Kagaku Ryoho. 1992;19:1207–1219. [PubMed] [Google Scholar]
- 31.Chua TC, Merrett ND. Clinicopathologic factors associated with HER2-positive gastric cancer and its impact on survival outcomes--a systematic review. Int J Cancer. 2012;130:2845–2856. doi: 10.1002/ijc.26292. [DOI] [PubMed] [Google Scholar]
- 32.Fornaro L, Lucchesi M, Caparello C, Vasile E, Caponi S, Ginocchi L, Masi G, Falcone A. Anti-HER agents in gastric cancer: from bench to bedside. Nat Rev Gastroenterol Hepatol. 2011;8:369–383. doi: 10.1038/nrgastro.2011.81. [DOI] [PubMed] [Google Scholar]
- 33.Jain S, Filipe MI, Gullick WJ, Linehan J, Morris RW. c-erbB-2 proto-oncogene expression and its relationship to survival in gastric carcinoma: an immunohistochemical study on archival material. Int J Cancer. 1991;48:668–671. doi: 10.1002/ijc.2910480506. [DOI] [PubMed] [Google Scholar]
- 34.Janjigian YY, Werner D, Pauligk C, Steinmetz K, Kelsen DP, Jäger E, Altmannsberger HM, Robinson E, Tafe LJ, Tang LH, et al. Prognosis of metastatic gastric and gastroesophageal junction cancer by HER2 status: a European and USA International collaborative analysis. Ann Oncol. 2012;23:2656–2662. doi: 10.1093/annonc/mds104. [DOI] [PubMed] [Google Scholar]
- 35.Janjigian YY ClinicalTrials. gov. Available from: http://www.clinicaltrials.gov/ct2/results?term=”Gastric Cancer” AND HER2&pg=3.
- 36.Gonzales AJ, Hook KE, Althaus IW, Ellis PA, Trachet E, Delaney AM, Harvey PJ, Ellis TA, Amato DM, Nelson JM, et al. Antitumor activity and pharmacokinetic properties of PF-00299804, a second-generation irreversible pan-erbB receptor tyrosine kinase inhibitor. Mol Cancer Ther. 2008;7:1880–1889. doi: 10.1158/1535-7163.MCT-07-2232. [DOI] [PubMed] [Google Scholar]
- 37.Nam HJ, Ching KA, Kan J, Kim HP, Han SW, Im SA, Kim TY, Christensen JG, Oh DY, Bang YJ. Evaluation of the antitumor effects and mechanisms of PF00299804, a pan-HER inhibitor, alone or in combination with chemotherapy or targeted agents in gastric cancer. Mol Cancer Ther. 2012;11:439–451. doi: 10.1158/1535-7163.MCT-11-0494. [DOI] [PubMed] [Google Scholar]
- 38.Oh DY, Lee KW, Cho J Y, Kang WK, Rha SY, Bang YJ. Aphase II open-label trial of dacomitinib monotherapy in patients with HER2-positive advanced gastric cancer after failure of at least one prior chemotherapy regimen. J Clin Oncol. 2012;30 suppl 4:Abstract 54. [Google Scholar]
- 39.Kwak E. The role of irreversible HER family inhibition in the treatment of patients with non-small cell lung cancer. Oncologist. 2011;16:1498–1507. doi: 10.1634/theoncologist.2011-0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Food and Drug Administration. Afatinib. July 12, 2013. Available from: http://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm360574.htm.
- 41.Janjigian YY, Viola-Villegas N, Holland JP, Divilov V, Carlin SD, Gomes-DaGama EM, Chiosis G, Carbonetti G, de Stanchina E, Lewis JS. Monitoring afatinib treatment in HER2-positive gastric cancer with 18F-FDG and 89Zr-trastuzumab PET. J Nucl Med. 2013;54:936–943. doi: 10.2967/jnumed.112.110239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Janjigian YY, Capanu M, Gromisch CM, Kelsen DP, Ku GY, Brown KT, Schattner M, Ilson DH, Solit DB, Berger MF, et al. A phase II study of afatinib in patients (pts) with metastatic human epidermal growth factor receptor (HER2)-positive trastuzumab-refractory esophagogastric (EG) cancer. J Clin Oncol. 2013;31 suppl:Abstract e15017. [Google Scholar]
- 43.Food and Drug Administration. TYKERB (lapatinib) tablets. Prescribing Information. February 2012. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/022059s013lbl.pdf.
- 44.Kim JW, Kim HP, Im SA, Kang S, Hur HS, Yoon YK, Oh DY, Kim JH, Lee DS, Kim TY, et al. The growth inhibitory effect of lapatinib, a dual inhibitor of EGFR and HER2 tyrosine kinase, in gastric cancer cell lines. Cancer Lett. 2008;272:296–306. doi: 10.1016/j.canlet.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 45.Iqbal S, Goldman B, Fenoglio-Preiser CM, Lenz HJ, Zhang W, Danenberg KD, Shibata SI, Blanke CD. Southwest Oncology Group study S0413: a phase II trial of lapatinib (GW572016) as first-line therapy in patients with advanced or metastatic gastric cancer. Ann Oncol. 2011;22:2610–2615. doi: 10.1093/annonc/mdr021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hecht JR, Bang YJ, Qin S, Chung HC, Xu JM, Park JO, Jeziorski K, Shparyk Y, Hoff PM, Sobrero AF, et al. Lapatinib in combination with capecitabine plus oxaliplatin (CapeOx) in HER2-positive advanced or metastatic gastric, esophageal, or gastroesophageal adenocarcinoma (AC): The TRIO-013/LOGiCTrial. J Clin Oncol. 2013;31 suppl:Abstract LBA4001. doi: 10.1200/JCO.2015.62.6598. [DOI] [PubMed] [Google Scholar]
- 47.Satoh T, Bang Y, Wang J, Xu J, Chung HC, Yeh K, Chen J, Mukaiyama A, Yoshida P, Ohtsu A. Interim safety analysis from TYTAN: A phase III Asian study of lapatinib in combination with paclitaxel as second-line therapy in gastric cancer. J Clin Oncol. 2010;28 Suppl:Abstract 4057. [Google Scholar]
- 48.Cho HS, Mason K, Ramyar KX, Stanley AM, Gabelli SB, Denney DW, Leahy DJ. Structure of the extracellular region of HER2 alone and in complex with the Herceptin Fab. Nature. 2003;421:756–760. doi: 10.1038/nature01392. [DOI] [PubMed] [Google Scholar]
- 49.Franklin MC, Carey KD, Vajdos FF, Leahy DJ, de Vos AM, Sliwkowski MX. Insights into ErbB signaling from the structure of the ErbB2-pertuzumab complex. Cancer Cell. 2004;5:317–328. doi: 10.1016/s1535-6108(04)00083-2. [DOI] [PubMed] [Google Scholar]
- 50.Baselga J, Cortés J, Kim SB, Im SA, Hegg R, Im YH, Roman L, Pedrini JL, Pienkowski T, Knott A, Clark E, Benyunes MC, Ross G, Swain SM; CLEOPATRA Study Group. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366:109–119. doi: 10.1056/NEJMoa1113216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yamashita-Kashima Y, Iijima S, Yorozu K, Furugaki K, Kurasawa M, Ohta M, Fujimoto-Ouchi K. Pertuzumab in combination with trastuzumab shows significantly enhanced antitumor activity in HER2-positive human gastric cancer xenograft models. Clin Cancer Res. 2011;17:5060–5070. doi: 10.1158/1078-0432.CCR-10-2927. [DOI] [PubMed] [Google Scholar]
- 52.Yamashita-Kashima Y ClinicalTrials. gov. A Study of Pertuzumab in Combination with Trastuzumab and Chemotherapy in Patients With HER2-Positive Advanced Gastric Cancer (NCT014610579) Available from: http://clinicaltrials.gov/show/NCT014610579.
- 53.Yamashita-Kashima Y ClinicalTrials. gov. A Study of Perjeta (Pertuzumab) in Combination with Herceptin (Trastuzumab) and Chemotherapy in Patients with HER2-Positive Metastatic Gastroesophageal Junction or Gastric Cancer ( NCT01774786) Available from: http://clinicaltrials.gov/show/NCT01774786.
- 54.Tabernero J, Hoff PM, Shen L, Ohtsu A, Yu R, Eng-Wong J, Kang YK. Pertuzumab (P) with trastuzumab (T) and chemotherapy (CTX) in patients (pts) with HER2-positive metastatic gastric or gastroesophageal junction (GEJ) cancer: An international phase III study (JACOB) J Clin Oncol. 2013;31:Abstract TPS4150. [Google Scholar]
- 55.Lewis Phillips GD, Li G, Dugger DL, Crocker LM, Parsons KL, Mai E, Blättler WA, Lambert JM, Chari RV, Lutz RJ, et al. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate. Cancer Res. 2008;68:9280–9290. doi: 10.1158/0008-5472.CAN-08-1776. [DOI] [PubMed] [Google Scholar]
- 56.Verma S, Miles D, Gianni L, Krop IE, Welslau M, Baselga J, Pegram M, Oh DY, Diéras V, Guardino E, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367:1783–1791. doi: 10.1056/NEJMoa1209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Barok M, Tanner M, Köninki K, Isola J. Trastuzumab-DM1 is highly effective in preclinical models of HER2-positive gastric cancer. Cancer Lett. 2011;306:171–179. doi: 10.1016/j.canlet.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 58.Yamashita-Kashima Y, Shu S, Harada N, Fujimoto-Ouchi K. Enhanced antitumor activity of trastuzumab emtansine (T-DM1) in combination with pertuzumab in a HER2-positive gastric cancer model. Oncol Rep. 2013;30:1087–1093. doi: 10.3892/or.2013.2547. [DOI] [PubMed] [Google Scholar]
- 59.Yamashita-Kashima Y ClinicalTrials. gov. A Study of Trastuzumab Emtansine versus Taxane in Patients with Advanced Gastric Cancer. Available from: http://clinicaltrials.gov/ct2/show/NCT01641939?term=Trastuzumab emtansine&rank=6.


