Abstract
Invasive micropapillary carcinoma (IMPC) is a rare histological type of tumor, first described in invasive ductal breast cancer, than in malignancies in other organs such as lungs, urinary bladder, ovaries or salivary glands. Recent literature data shows that this histological lesion has also been found in cancers of the gastrointestinal system. The micropapillary components are clusters of neoplastic cells that closely adhere to each other and are located in distinct empty spaces. Moreover, clusters of neoplastic cells do not have a fibrous-vascular core. The IMPC cells show reverse polarity resulting in typical ‘’inside-out’’ structures that determines secretary properties, disturbs adhesion and conditions grade of malignancy in gastrointestinal (GI) tract. Invasive micropapillary carcinoma in this location is associated with metastases to local lymph nodes and lymphovascular invasion. IMPC can be a prognostic factor for patients with cancers of the stomach, pancreas and with colorectal cancer since it is related with disease-free and overall survival. The purpose of this review is to present the characterization of invasive micropapillary carcinoma in colon, rectum, stomach and others site of GI tract, and to determine the immunohistological indentification of IMPC in those localization.
Keywords: Invasive micropapillary carcinoma, MUC-1, Lymph node metastases
Core tip: We summarize the recent literature reports about individual cases and study groups of invasive micropapillary carcinoma. We postulated that invasive micropapillary carcinoma is still a great diagnostic challenge in pathomorphology and due to its high aggressiveness should be treated as a distinct histological subtype of carcinomas in gastrointestinal tract.
INTRODUCTION
Invasive micropapillary carcinoma (IMPC) is a rare histological type of tumor, first described in invasive ductal breast cancer[1]. Recent reports have confirmed that the micropapillary component can also occur in malignancies in other organs such as lungs, urinary bladder, ovaries or salivary glands[2-5]. According to the World Health Organization (WHO) classification, invasive micropapillary carcinoma was identified as a distinct histopathological subtype of the breast and urinary tract[6,7]. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society has considered the micropapillary component to be a new subtype of invasive glandular lung cancer with poor prognosis[8]. Moreover, this histological lesion has also been documented in cancers of the gastrointestinal system[9-27]. In the current WHO Classification of the Digestive System Tumors, IMPC is described as a rare histological structure found in papillary-type gastric adenocarcinoma[28]. Irrespective of location, the micropapillary component is present in high-grade tumors with local lymph node involvement and neoplastic emboli in blood and lymphatic vessels[2-5,18,21,29,30]. The analysis of literature reports concerning gastrointestinal malignancies, including IMPC in our review, may widen the knowledge of this histological structure in malignant tumors affecting these organs.
MORPHOLOGY
The micropapillary components are clusters of neoplastic cells that closely adhere to each other and are located in distinct empty spaces that resemble small dilated lymphatic vessels[12,15,19,21]. In order to differentiate cancer cells invading the lymphatic vessels from IMPC, immunohistochemical staining is performed with the use of endothelial cell-specific antibodies. Lack of color reaction of these proteins in the lymphovascular-like pattern helps exclude the presence of the vessels (Table 1). Moreover, IMPC cells are usually small, round or oval, but they are also columnar to polygonal[24,25,31]. Single cells are characterized by distinct massive acidophilic cytoplasm with numerous fine granules[21,32]. The nucleus/cytoplasm ratio is found to increase. The nuclei are acinar, with well visible nucleoli and unevenly dispersed chromatin clumps[16]. They show moderate to high pleomorphism and moderate degree of atypia, as well as diverse mitotic activity[17,21,24,25]. Clusters of neoplastic cells do not have a fibrous-vascular core[16,19]. They are separated by bands of fibrous tissue, resembling sponge in structure. Nests can also occur as single focal spaces filled up with flattened fusiform tumor cells[10,32].
Table 1.
Profile of invasive micropapillary carcinomas in gastrointestinal tract
| Immunohistochemical marker | Invasive micropapillary carcinoma | Conventional carcinoma | Ref. |
| EMA | Outer membranous | Luminal | [10,13,15,19,26,29] |
| MUC-1 | Outer membranous | Luminal | [10,12,19,25,29,30] |
| CD10 | Stroma-facing surface | Membranous | [16,31] |
| Vilin | Stroma-facing surface | Membranous | [16] |
| E-catherin | Cytoplasmic | Membranous | [13,25,33,41] |
| β-catenin | Nulcear/cytoplasmic less frequent | Nulcear/cytoplasmic more frequent | [41] |
| Lymphvascular epithelial cells | |||
| D2-40 | Negative | Positive | [12,13,15,19,21,22] |
| CD34 | Negative | Positive | [16,21] |
| CD11 | Negative | Positive | [16] |
Micropapilla are present on the invasive edges of the tumor, more seldom in its center[12,29,32,33]. The micropapillary structure may constitute one of the morphological tumor components and occur with other histological types, or it can be the only morphological exponent[16,19,29]. However, cancers composed only of the micropapillary component are very rare[29]. Moreover, all the above mentioned morphological features of the micropapillary component are visible in metastases to the lymphatic vessels, lymph nodes and distant organs[32] (Figure 1).
Figure 1.
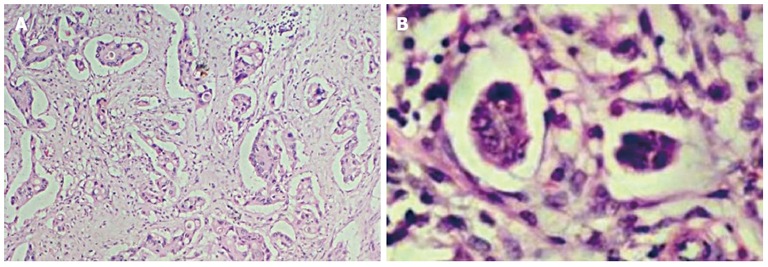
Characterization of typical invasive micropapillary carcinoma structures. A: Invasive micropapillary carcinoma in the invasive edges of the tumor; B: Morphologically, clusters of small rounded neoplastic cells without fibrous-vascular core. Hematoxylin and eosin stain, × 40, × 400, respectively.
The IMPC cells show reverse polarity resulting in typical “inside-out’’ structures, i.e. their basal surface has the properties of the upper part. The electron microscopic examination of this structure has confirmed that the outer surface of the cells is covered with numerous microvilli and shows secretory activity towards the surrounding stroma. Moreover, a slight amount of mucous secretion has been found in the spaces that enclose tumor cell nests[34,35]. These observations are also supported by immunohistochemical investigations with the use of anti- MUC1, EMA, CD10, villin antibodies (Table 1). Glycoprotein 1 (MUC-1) is mainly present on the outer surface of epithelial cells in patients with IMPC as compared to the color reaction located in the apical part in normal glandular cells. MUC-1 is responsible for the maintenance of cell integrity in normal glands[27]. The specific reaction of MUC-1 in the micropapillary component located at the invasion front allows differentiation of these structures from tumor budding[10]. Hudson et al[36] observed type I collagen fiber contraction and MUC-1 induced disorders of cytokeratin expression. At the same time, MUC-1 neutralizes the effects of fine intercellular adhesion molecules, e.g., E-cadherin and β-catenin[37]. Reports on the likely contribution of MUC-1 to IMPC adhesion seem to confirm the investigations in which an increase in the expression of this glycoprotein was related to cell-cell adhesion disorders and cell interactions with the extracellular matrix[38,39]. E-cadherin is responsible for epithelial cell integrity. A decrease in its expression is associated with a greater potential of tumor cells to metastasize. Deficiency of this protein was noted in a higher percentage of patients with gastric IMPC than in the control[13,40]. However, positive expression of E-cadherin was found in the cytoplasm of cells of the micropapillary structures as compared to the membranous reaction of this protein in normal ducts and neoplastic glandular ducts[25]. Moreover, the assessment of β-catenin expression revealed its deficiency in the cytoplasm and/or nucleus in a high percentage of patients with gastric and colon IMPC as compared to the adenocarcinoma groups[40]. It is suggested that disturbances in β-catenin distribution, its deficiency, may condition IMPC aggressiveness[40]. It can be assumed that also MUC-2 plays a crucial role in the maintenance of IMPC integrity as it joins tumor cells and acts as a physical protective barrier against their spread[41]. Lack or low percentage of positive expression of MUC-2 was observed in patients with IMPC in gastric cancer[17,21,31]. Its deficiency facilitates secretion of metaloproteinases by IMPC that determines cancer spread in the stroma and via vessels to local lymph nodes[27] (Table 1, Figure 2).
Figure 2.
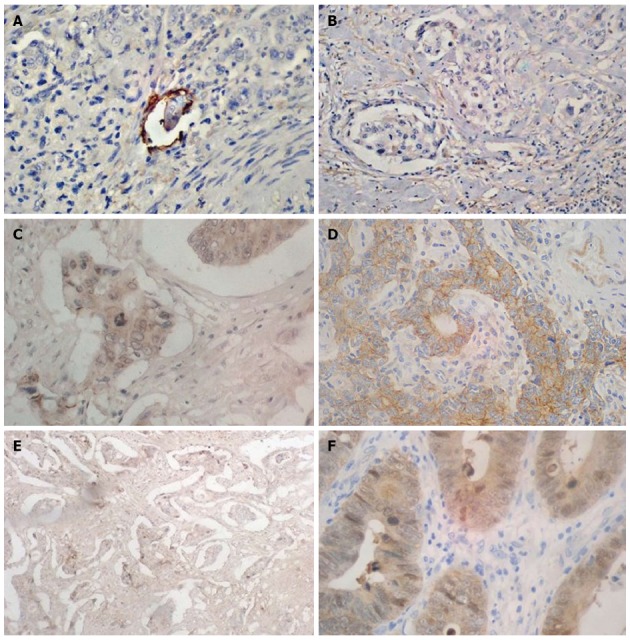
Immunohistochemical characteristics of invasive micropapillary carcinoma structures. Positive immunoreaction of lymphatic endothelial cell performed by D2-40 (A) and lack of reaction in invasive micropapillary carcinoma (IMPC) structures (B). Immunohistochemical expression of E-catherin in membrane of conventional adenocarcinoma (C) and in cytoplasm of IMPC (D). The cytoplasmic expression of Beta-catenin was observed more frequent in typical adenocarcinoma (F) compare to IMPC (E). Magnification × 400.
Many data suggest that IMPC polarity reversal determines secretory properties, disturbs adhesion and conditions grade of malignancy. All the above mentioned exponents are responsible for stromal and vascular invasion of tumor cells, resulting in easier spread of cancer cells and lymph node involvement.
INVASIVE MICROPAPILLARY CARCINOMA OF COLON AND RECTUM
Since the description of the micropapillary structure as a separate subtype of breast cancers[1], this component have been searched for in other organs, including the colon. Up to now, approximately 265 cases of colon cancer with IMPC have been reported, accounting for 9%-19% of all colon cancers. They are most common in males aged 53-72 years[20,32,10,42,43]. Otsubo et al[30] observed a single case of IMPC in a 26-year-old woman. Clinical symptoms in patients with IMPC have not been disease-specific and mainly include abdominal pain, anemia, vomiting, diarrhea, constipation and bleeding from the rectum[10,12-16,19,20,29,43].
These tumors are known to grow as polyp-like forms that narrow the lumen of the respective organ with a tendency to exophytic growth or a lesion with a centrally ulcerating crater[16,18,19,29]. Unfortunately, macroscopically the lesions do not allow IMPC differentiation from other subtypes. Regardless of the macroscopic picture, all tumors have a characteristic image of the micropapillary structure in light microscopy. IMPC can develop throughout the large intestine, although most lesions are located in the colon and 50 percent of them in the ascending colon[20,19,32,42]. In the remaining substantial proportion of cases, the rectum is affected[20,29]. The micropapillary component involves a wide range of 5%-95% of tumor volume and is usually situated in its invasion front[10,19,32]. Moreover, Verdú et al[10] described the coexistence of early sigmoid cancer with IMPC in a pedunculated polyp obtained during colonoscopy, in which the component constituted the major tumor morphological exponent. Kondo et al[15] noted a slight focus of IMPC growing in a tubulovillous adenoma. Lino-Silva et al[29] observed a single case of pure rectal IMPC, with the micropapillary structure involving > 95% of the whole lesion[29]. IMPC accompanies neoplastic lesions with clearly defined edges and various differentiation grades. In most cases, IMPC coexisted with moderately differentiated tumors (G2), with cells lacking mucous secretion[12,19,20,32]. Many of these cancers infiltrated through the muscle to the subserous layer[20,32,42].
The presence of IMPC in colorectal tumors is associated with aggressive behavior of the neoplasm. In all the reported cases, tumors invaded blood and lymphatic vessels, whereas the remaining groups showed moderate grade of invasion of these structures[12,13,18-20,29,30,32]. The involvement of lymph nodes has been estimated at 63%-100% of all cases[6,5,10,17,43]. Kim et al[20] showed metastases to local lymph nodes in 2 out of 3 patients with IMPC tumor infiltrating the submucous membrane (pT1). Their findings indicate the importance of adequately early diagnosis of IMPC lesion in biopsy and operative material, which may condition high risk of metastases. In the majority of patients, IMPC invasion involved a considerable proportion of lymph nodes and was the only histological exponent of the metastases formed[16,19,20]. Moreover, several patients showed metastases of micropapillary structures to the peritoneum and other organs, such as lungs and liver[10,13,20,29,32]. The multivariate analysis of variance revealed that the presence of the micropapillary component, apart from invasion of the lymphatic/blood vessels and infiltration depth, is an independent prognostic factor determining cancer metastases[17].
The diagnosis of the micropapillary component is closely associated with worse prognosis[42,43]. Stage I and II IMPC patients experience shorter survival as compared to the non-IMPC groups and have equally poor prognosis as those in Stage III and IV. The survival rates among these patients after 1, 3, 5 and 10 years were 67%, 53%, 50% and 50%, respectively, as compared to the groups without IMPC (81%, 75%, 73%, 70%, respectively)[44]. Five-year survival rate was also much lower in patients with IMPC than in those with microsatellite instability-high carcinoma and microsatellite stable one[43]. The molecular profile of IMPC indicates that these patients have higher proportion of TP53 alternations, and that microsatellite instability is much rarer[10]. Therefore, it is assumed that like 85% of colorectal cancers, IMPC develops via classical chromosomal instability (CIN)[10]. This confirms that IMPC shows considerable aggressiveness and is associated with shorter survival. The colorectal IMPC group profile is presented in Tables 2 and 3.
Table 2.
Characteristics of individual cases of colorectal invasive micropapillary carcinoma
| Parameter | Sakamoto et al[19], 2005 | Kuroda et al[18], 2007 | Kondo et al[15], 2008 | Wen et al[16], 2008 | Hisanori et al[13], 2009 | Sonoo et al[12], 2009 | Otsubo et al[30], 2011 |
| Age/ Sex | 67/M | 70/F | 70/M | 72/F | 71/F | 64/M | 26/F |
| 68/F | |||||||
| 53/F | |||||||
| Location | |||||||
| Colon | 3 | 1 | 1 | 1 | 1 | 1 | 1 |
| Rectum | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Unspecified | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Tumor size | |||||||
| < 5 cm | 1 | 1 | 1 | 1 | 1 | 1 | |
| > 5 cm | 0 | 0 | 0 | 0 | 0 | 0 | |
| Percentage of IMPC | PD | 40% | 5% | PD | PD | 80% | ND |
| Adenocarcinoma type | |||||||
| Nonmucinous | 2 | 1 | 1 | 1 | 1 | 1 | 1 |
| Mucinous | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Grade of malignancy (G) | G3-2 | ND | G1 | ND | ND | G2 | G4 |
| G4-1 | |||||||
| pT stage (T) | T3-3 | T3 | T1 | T3 | T1 | T1 | T3 |
| Lymphovascular invasion (Yes/No) | Yes-2 | Yes | Yes | ND | Yes | Yes | Yes |
| No-1 | |||||||
| Lymph node metastasis (N) (Yes/No) | Yes-3 | Yes | No | Yes | Yes | Yes | Yes |
| Distant metastasis (M) (Yes/No) | No | No | No | No | Yes | No | Yes |
PD: Predominant; ND: No data; M: Male; F: Female.
Table 3.
Characteristics of study group diagnosed with colorectal invasive micropapillary carcinoma
| Parameter | Kim et al[20], 2006 | Haupt et al[32], 2007 | Xu et al[44], 2009 | Verdú et al[10], 2011 | Lino-Silva et al[42], 2012 |
| No. of patients | 55 | 34 | 30 | 60 | 15 |
| Mean age, yr | 64 | 66 | 57 | 65.8 | 56 |
| Sex | F-15 | F-15 | F-13 | F- 23 | F-8 |
| M-40 | M-19 | M-17 | M-37 | M-7 | |
| Location | |||||
| Colon | 33 | 30 | 12 | 24 | 15 |
| Rectum | 22 | 2 | 18 | 36 | 0 |
| Unspecified | 0 | 2 | 0 | 0 | 0 |
| Percentage of IMPC | 5%-80% | 5%-60% | 5%-75% | 5%-30% | 10%-80% |
| Adenocarcinoma type | |||||
| Nonmucinous | 55 | 29 | 29 | 57 | 15 |
| Mucinous | 0 | 5 | 1 | 3 | 0 |
| Grade of malignancy (G) | |||||
| G1 | 5 | 1 | 13 | 23 | 0 |
| G2 | 43 | 26 | 0 | 30 | 0 |
| G3 | 7 | 7 | 0 | 7 | 6 |
| G4 | 0 | 0 | 17 | 0 | 9 |
| pT stage (T) | |||||
| T1 | 2 | 3 | 0 | 3 | 0 |
| T2 | 4 | 5 | 5 | 9 | 0 |
| T3 | 45 | 24 | 25 | 38 | 9 |
| T4 | 4 | 2 | 0 | 10 | 6 |
| Lymphovascular invasion | Yes-25 | Yes-14 | Yes-10 | Yes-28 | Yes-5 |
| (LV) (Yes/No) | No-30 | No-20 | No-20 | No- 32 | No-10 |
| Lymph node metastasis (N) | Yes-41 | Yes-25 | Yes-19 | Yes- 38 | Yes-15 |
| (Yes/No) | No-14 | No-9 | No-11 | No-12 | No-0 |
| Distant metastasis (M) | Yes-13 | Yes-4 | Yes- 1 | Yes-10 | Yes-3 |
| (Yes/No) | No-42 | No-30 | No-29 | No-50 | No-12 |
| Correlations | N, M, TNM stage | N | G, N, TB | T, TNM stage, N,M, VI, PI | G, N, TNM stage |
PD: Predominant; ND: No data; M: Male; F: Female; VI: Venous vessel invasion: PI: Perineural invasion.
INVASIVE MICROPAPILLARY CARCINOMA OF THE STOMACH
Considering a very high incidence of gastric cancer, tumors with IMPC account only for 0.07%-13.40% of gastric cancer cases[31,45]. The IMPC morbidity peak is observed at the age of 60-70, with the predominance of males being affected. IMPC is most frequently located in the lower-third, then in the middle and upper-third of the stomach. The micropapillary structure coexists with other histological types, including a considerable percentage of papillary and tubular carcinomas[21,40]. A higher percentage of IMPC are noted in tumors of the intestinal type than diffuse type according to Lauren classification[40]. The analysis of mucin profile has shown that the highest percentage of IMPC patients show gastric type (9/17) and null type (6/17) as compared to the other subtypes[21]. The IMPC structure most frequently accompanies moderately (G3) and low-differentiated cancers (G2)[22,40]. In a study by Fujita et al[22], 1/3 of the cases had papillary adenocarcinoma well-differentiated with IMPC.
The content of micropapillae in the stomach ranges between 5%-90% of tumor tissue, although pure IMPC has never been found in this organ[21,31,40]. It has been proven that the determination of the ratio of IMPC to the remaining part of the tumor has no effect on the clinicopathological parameters. However, even the smallest IMPC lesion found indicates tumor aggressiveness[40]. The occurrence of IMPC correlates with the degree of invasion of lymphatic and blood vessels, and with the number of metastases to lymph nodes. The invasion of blood and lymphatic vessels has been found in 78%-91% of cases[17,21,22,31,40]. Tumors in stage I and II IMPC patients showed higher percentage of vessel invasion than those without the component[17]. In the majority of cases, IMPC reaches the subserous layer (pT3) or infiltrates by continuity other tissues and organs (pT4)[22,40]. The proportion of lymph node involvement is very high[21,22]. Stage I and II patients with the IMPC component showed a considerably higher percentage of metastases to local lymph nodes as compared to the IMPC-free groups[40]. Statistical significance of infiltration depth and tumor size has been found in the prognostication of lymph node involvement[40] (Table 4).
Table 4.
Invasive micropapillary carcinoma of the stomach
| Parameter | Shimoda et al[17], 2008 | Roh et al[31], 2010 | Eom et al[40], 2011 | Ushiku et al[21], 2011 | Fujita et al[22], 2012 | Ninomiya et al[46], 2013 | Ohtsuki et al[33], 2013 |
| No. of patients | 1 | 11 | 72 | 17 | 14 | 1 | 4 |
| Mean age, yr | 74 | 66 | 70 | 67 | 62 | 69 | 69-79 |
| Sex | M | F-3 | F-18 | F-3 | F-4 | M | F-2 |
| M-8 | M-54 | M-14 | M-10 | M-2 | |||
| Location | |||||||
| Upper-third | 0 | 1 | 8 | 6 | 3 | 1 | 2 |
| Middle | 1 | 2 | 20 | 6 | 7 | 0 | 1 |
| Lower-third | 0 | 8 | 44 | 5 | 4 | 0 | 0 |
| Tumor size | |||||||
| < 5 cm | 1 | 5 | 40 | 17 | 14 | 1 | 2 |
| > 5 cm | 0 | 6 | 32 | 0 | 0 | 0 | 3 |
| Percentage of IMPC | PD | 5%-70% | 5%-80% | 10%-90% | > 10% | PD | ND |
| Adenocarcinoma type | |||||||
| Tubular | Absent | Both | 21 | 16 | 9 | ND | 2 |
| Papillary | 9/11 | 43 | 12 | 4 | 2-mixed | ||
| Grade of malignacies (G) | |||||||
| G1 | 0 | ND | 11 | ND | 6 | ND | ND |
| G2 | 0 | 2/11 | 38 | 7 | |||
| G3 | 1 | ND | 23 | 1 | |||
| G4 | 0 | ND | 0 | 0 | |||
| pT stage (T) | |||||||
| T1 | 0 | 3 | 20 | 2 | 0 | 0 | 0 |
| T2 | 0 | 4 | 52 | 8 | 6 | 0 | 1 |
| T3 | 1 | 2 | 5 | 8 | 1 | 2 | |
| T4 | 0 | 2 | 2 | 0 | 0 | 4 | |
| Lympho-vascular invasion (LV) (Yes/No) | Yes | Yes-10 | Yes-56 | Yes-17 | Yes11 | Yes | Yes-1 |
| No-1 | No-16 | No-0 | No-3 | No-3 | |||
| Lymph node metastasis (N) (Yes/No) | Yes | Yes- 4 | Yes-62 | Yes-14 | Yes | Yes | Yes-3 |
| No-7 | No-10 | No-3 | No-1 | ||||
| Distant metastasis (M) (Yes/No) | No | ND | ND | ND | No | No | ND |
PD: Predominant; ND: No data: M: Male; F: Female.
Not only is IMPC related to metastasizing but also to poor outcome of patients. IMPC patients have shorter survival as compared to those without the micropapillary component (59.3% vs 80.6%). Significantly shorter 1-, 3-, 5-year overall survival (83%, 55%, 30%) was also noted in comparison with non-IMPC (87%, 70%, 67%). Among stage I and II patients, the likelihood of overall and disease-free 5-year survival was much lower than in the non-IMPC cases[40]. The IMPC parameter as a potential factor in the prognosis of survival of these patients has also been evaluated[40]. As revealed by univariate analyses performed by Fujita et al[22], the IMPC component, invasion grade, infiltration of lymphatic vessels and lymph node involvement are the major factors determining survival. These parameters play an especially important role in stage I and II patients, since they are associated with much worse prognosis. On the other hand, according to the multivariate analysis, IMPC is an independent prognostic factor of survival[22]. These observations, however, have not been confirmed by other researchers[31,46].
INVASIVE MICROPAPILLARY CARCINOMA OF OTHER LOCATIONS IN GI TRACT
The micropapillary structure in the ampullopancreatobiliary region of the pancreas was first described by Khayyata et al[25], who found this lesion in 4.1% of all cancers in this location, with the majority of lesions observed in the ampullary region (11%), and the remaining ones in the pancreas (3%). The author classified primary IMPC lesions as focal (20%-50% of the tumor), predominant (51%-80%) or diffuse (> 80%). IMPC above 80% is rare, being present in 3% of periampullary cases and in 1% of pancreatic cases[25]. Kitagawa et al[26] observed a single case of pure IMPC in the pancreas without typical adenocarcinoma tissue. IMPC was also found in the ampulla of Vater (1.3% of these cancers) and in bile duct[23,24]. Kondo et al[23] suggested that the adhesion of the mucous membrane invaded by adenocarcinoma with IMPC to bile ducts may condition the occurrence of this lesion type mainly in the region of the pancreatic head. It cannot also be excluded that the location may be determined by the etiologic factors themselves, including the properties of bile content, e.g., reflux[23]. Therefore, these neoplastic lesions frequently lead to bile duct obstruction, cholestasis and jaundice, and patients complain of general malaise[23-26].
Macroscopically, the lesions have diverse descriptions, from whitish and greyish nodular tumor exhibiting soft consistency in the pancreas to irregular lesions narrowing the lumen of the bile duct[24-26]. In some cases, the micropapillary component was found to coexist with classical types of adenocarcinoma[23,25]. The tumor size found was 0.02-3.2 cm[23-26]. In two documented cases, tumor infiltrated the submucous membrane (pT1). Kondo et al[23] found a tumor that was limited to the muscular membrane (pT2) of the Oddi’s sphincter[24,26]. However, in 16 patients with IMPC in the pancreas, the cancer was classified as G3 according to the grading scheme of pancreatic adenocarcinoma. In some patients, lymphatic and blood vessels were invaded by clusters of cancer cells, including vascular microinvasion in the submucous membrane of the duodenum or papilla in 3 patients (< 5% IMPC in tumor)[25]. In the microscopic picture, the micropapillary structures showed high similarity to those observed in other organs. Contrary to other locations, in the presence of pancreatic micropapillae there was a massive inflammatory infiltrate composed of neutrophilic granulocytes that formed focal intraepithelial microabscesses, and clusters of these cells in the stroma[23,25]. Moreover, in one case, moderate infiltrate composed of eosinophils was observed to surround the micropapillary structures[24].
In most cases, local lymph node involvement was found[23,24,26]. The presence of the predominant part or pure form of IMPC was noted in an early stage, which may indicate metastasizing to numerous lymph nodes and distant organs (liver, intestine, gallbladder) in these cases[24,26]. Unfortunately, as this conclusion was based on very few observations they have to be verified on a larger group of patients. In the best-described group of patients with pancreatic IMPC there were 11/15 (73%) metastases to local lymph nodes as compared to the conventional carcinoma group (55%). In 4 patients from this group, distant metastases to the liver and lungs were found. Most metastases, both local and distant had micropapillary structures[25] (Table 5).
Table 5.
Histopathological analysis of invasive micropapillary carcinoma in other sites of the gastrointestinal tract
| Parameter | Khayyata et al[25], 2005 | Kitagawa et al[26], 2007 | Kondo et al[23], 2009 | Fujita et al[24], 2010 |
| No. of patients | 16 | 1 | 1 | 1 |
| Mean age, yr | 69 | 67 | 75 | 53 |
| Sex | F-6 | M | F | M |
| M-10 | ||||
| Location | Ampullo-pancreatobiliary region | Pancreatic head | Bile duct | Ampulla of Vater |
| Tumor size | ||||
| < 5 cm | 14 | 1 | 1 | 1 |
| > 5 cm | ND | 0 | 0 | 0 |
| Percentage of IMPC | > 20% | PD | ND | PD |
| pT stage (T) | ||||
| T1 | 0 | 1 | 0 | 1 |
| T2 | 0 | 0 | 1 | 0 |
| T3 | 0 | 0 | 0 | 0 |
| T4 | 13 | 0 | 0 | 0 |
| Lymphovascular invasion | Yes-3 | Yes | Yes | Yes |
| (Yes/No) | No-13 | |||
| Lymph node metastasis (N) | Yes-11 | Yes | Yes | Yes |
| (Yes/No) | No-4 | |||
| Distant metastasis (M) | Yes-4 | Yes | ND | Yes |
| (Yes/No) | No-12 |
PD: Predominant; ND: No data; M: Male; F: Female.
All patients underwent surgical treatment. Only in the case of pure pancreatic IMPC, pancreaticoduodenectomy was accompanied by chemotherapy with gemcitabine[26]. Pharmacotherapy in that case proved to have similar effects to those observed in patients treated for typical pancreatic cancer[26]. The analysis of survival of patients with pancreatic IMPC revealed a slightly shorter survival than in ordinary ductal carcinoma of the pancreas. The mean survival of patients with pancreatic IMPC was 8 mo and seems to be comparable to that observed in patients with poorly differentiated carcinoma at the same site[25]. The follow-up of patients lasted 12, 20, 17 and 42 mo for bile duct, ampulla of Vater, pancreas and pure pancreatic IMPC, respectively[23-25]. Only 21% of patients treated surgically live without relapse, whereas the remaining percentage of patients died due to advanced cancer or multiorgan failure[26].
PRIMARY SITE OF IMPC
The micropapillary structure does not belong to the location of any definite organ. Even though in most cases IMPC can be observed in traditional histological types of cancers, being characteristic of a respective location, and may suggest its origin, the presence of pure IMPC in the form of primary site or metastasis does not allow definite lesion localization. Therefore, the primary distribution of this type of cancer has to be confirmed by the whole panel of immunohistochemical investigations.
In immunohistochemical analyses of micropapillae in the digestive system, protein expression was similar to that of conventional adenocarcinoma. Cytokeratin 20 (CK20) (+) CK7 (-) was suggested to be an adequate marker profile for IMPC of the colon[11,16,18-20,29] (Figure 3). Research also proved a considerable proportion of positive expression of intestinal differentiation marker (CDX2) and carcinoembryonic antigen (CEA) in IMPC as compared to conventional carcinoma[16,18,20,42]. Moreover, in one case colon IMPC showed positive expression of Cancer Antigen 125 (CA 125), which did not exclude the presence of this structure in the ovary and urinary bladder[18]. On the other hand, the CK20 (-) CK7(+) variant was found to condition the primary site in the stomach[17,22,31]. Additionally, positive reactions of proteins with mucins (MUC-5, MUC-6) have been observed in the stomach and colon[17,21,22,31]. However, MUC-2 has been found to show positive reaction in the colon IMPC, but not in gastric IMPC[21,22,31,34,42]. IMPC both in the large intestine and in the stomach is characterized by a high proliferative index. IMPC cells exhibit positive expression of Ki-67 and p53 in most cases[20-22,31,44]. Also DNA mismatch repair protein such as MutL homolog 1, MutS protein homolog 2 and MutS homolog 6 have shown positive expression[16,20]. In pancreatic IMPC the profile contains CK20(-) CK7(+)[26]. Moreover, positive expression of carbohydrate antigen 19-9 (CA 19-9) and negative expression for CEA has been found in this organ[26]. The proposed IMPC profile is based on few reports and requires more detailed analysis on a larger study group.
Figure 3.
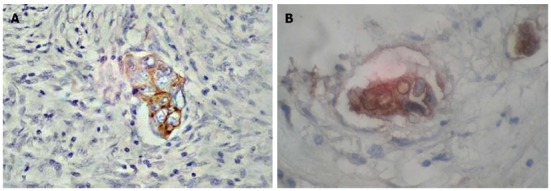
Immunohistochemical characteristics of invasive micropapillary carcinoma of colon. Positive reaction of CK20 (A) and CEA (B). Magnification × 400.
IMPC differentiation in the digestive system requires the knowledge of immunohistochemical profiles specific to other locations. The CK20(-) CK7(+) thyroid transcription factor-1 (TTF-1) (+) surfactant apoprotein A (SP-A)(+) profile indicates the location of IMPC in the urinary bladder, and CK20(-) CK7(+) estrogen receptor (ER) (+/-) progesteron receptor (PgR) (+/-) is present in the breast and ovaries. IMPC located in the salivary glands expresses CK20(-) CK7(+)[16,47]. The immunohistochemical characteristics of IMPC structures with respect to location has been presented in Table 6.
Table 6.
Immunohistochemical identification of the primary site of invasive micropapillary carcinoma
| Protein/marker | Colon and rectum | Stomach | Pancreas1 | Breast | Bladder | Ovary | Lung | Salivary glands |
| CK20 | + | - | - | + | + | + | + | + |
| CK7 | - | + | + | - | + | - | - | - |
| TTF-1 | - | - | ND | - | - | - | + | ND |
| SP-A | - | - | ND | - | - | - | + | ND |
| ER | - | +/- | ND | +/- | - | +/- | - | - |
| PgR | - | - | ND | +/- | - | +/- | - | - |
| CA 125 | +/- | ND | ND | - | + | + | ND | ND |
| CA 19-9 | ND | ND | + | ND | ND | ND | ND | ND |
| CEA | + | ND | - | ND | ND | ND | ND | ND |
And other sites in gastrointestinal tract. ND: No data.
The determination of the primary site of IMPC, due to high metastasizing capacity and advanced clinical status of most cases facilitates proper therapy.
CONCLUSION
In summary, an increasing number of reports confirming the morphological distinction of the micropapillary structure as compared to other histological types indicate an essential impact of this structure on the pathomorphological diagnosis. The morphological properties of IMPC condition the lymphovascular invasion and metastases to regional lymph nodes. This has been confirmed above all by the studies in which numerous metastases were observed in early neoplastic lesions. IMPC can be a prognostic factor for patients with cancers of the stomach, pancreas and with colorectal cancer since it is associated with disease-free and overall survival. Nowadays, IMPC is a great diagnostic challenge, and due to its high aggressiveness, the histology of cancer lesions in the digestive tract requires careful analysis.
Footnotes
P- Reviewers: Jafari A, MatsushitaK, Zhao J S- Editor: Wen LL L- Editor: A E- Editor: Zhang DN
References
- 1.Siriaunkgul S, Tavassoli FA. Invasive micropapillary carcinoma of the breast. Mod Pathol. 1993;6:660–662. [PubMed] [Google Scholar]
- 2.Amin MB, Ro JY, el-Sharkawy T, Lee KM, Troncoso P, Silva EG, Ordóñez NG, Ayala AG. Micropapillary variant of transitional cell carcinoma of the urinary bladder. Histologic pattern resembling ovarian papillary serous carcinoma. Am J Surg Pathol. 1994;18:1224–1232. doi: 10.1097/00000478-199412000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Amin MB, Tamboli P, Merchant SH, Ordóñez NG, Ro J, Ayala AG, Ro JY. Micropapillary component in lung adenocarcinoma: a distinctive histologic feature with possible prognostic significance. Am J Surg Pathol. 2002;26:358–364. doi: 10.1097/00000478-200203000-00010. [DOI] [PubMed] [Google Scholar]
- 4.Nagao T, Gaffey TA, Visscher DW, Kay PA, Minato H, Serizawa H, Lewis JE. Invasive micropapillary salivary duct carcinoma: a distinct histologic variant with biologic significance. Am J Surg Pathol. 2004;28:319–326. doi: 10.1097/00000478-200403000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Laurent I, Uzan C, Gouy S, Pautier P, Duvillard P, Morice P. Results after conservative treatment of serous borderline tumors of the ovary with a micropapillary pattern. Ann Surg Oncol. 2008;15:3561–3566. doi: 10.1245/s10434-008-0159-9. [DOI] [PubMed] [Google Scholar]
- 6.Lakhani SR, Ellis IO, Schnitt SJ, Tan PH, van de Vijver MJ. WHO Classification of Tumours of the Breast. 4th ed. Lyon: IARC Press; 2012. [Google Scholar]
- 7.Pan CC, Chang YH, Chen KK, Yu HJ, Sun CH, Ho DM. Prognostic significance of the 2004 WHO/ISUP classification for prediction of recurrence, progression, and cancer-specific mortality of non-muscle-invasive urothelial tumors of the urinary bladder: a clinicopathologic study of 1,515 cases. Am J Clin Pathol. 2010;133:788–795. doi: 10.1309/AJCP12MRVVHTCKEJ. [DOI] [PubMed] [Google Scholar]
- 8.Travis WD, Brambilla E, Riely GJ. New pathologic classification of lung cancer: relevance for clinical practice and clinical trials. J Clin Oncol. 2013;31:992–1001. doi: 10.1200/JCO.2012.46.9270. [DOI] [PubMed] [Google Scholar]
- 9.Shibuya H, Matsuda K, Shimada R, Horiuchi A, Iinuma H, Hayama T, Yamada H, Nozawa K, Ishihara S, Watanabe T. Invasive micropapillary carcinoma of the ascending colon--a report of a case. Int Surg. 2011;96:82–86. doi: 10.9738/1355.1. [DOI] [PubMed] [Google Scholar]
- 10.Verdú M, Román R, Calvo M, Rodón N, García B, González M, Vidal A, Puig X. Clinicopathological and molecular characterization of colorectal micropapillary carcinoma. Mod Pathol. 2011;24:729–738. doi: 10.1038/modpathol.2011.1. [DOI] [PubMed] [Google Scholar]
- 11.Kasashima S, Kawashima A, Zen Y. Invasive micropapillary carcinoma of the colon in ascitic fluid: a case report. Acta Cytol. 2010;54:803–806. [PubMed] [Google Scholar]
- 12.Sonoo H, Kameyama M, Inatugi N, Nonomura A, Enomoto Y. Pedunculated polyp of early sigmoid colon cancer with invasive micropapillary carcinoma. Jpn J Clin Oncol. 2009;39:523–527. doi: 10.1093/jjco/hyp051. [DOI] [PubMed] [Google Scholar]
- 13.Hisamori S, Nagayama S, Kita S, Kawamura J, Yoshizawa A, Sakai Y. Rapid progression of submucosal invasive micropapillary carcinoma of the colon in progressive systemic sclerosis: report of a case. Jpn J Clin Oncol. 2009;39:399–405. doi: 10.1093/jjco/hyp015. [DOI] [PubMed] [Google Scholar]
- 14.Trabelsi A, Ali AB, Yacoub-Abid LB, Stita W, Mokni M, Korbi S. Primary invasive micropapillary carcinoma of the colon: case report and review of the literature. Pathologica. 2008;100:428–430. [PubMed] [Google Scholar]
- 15.Kondo T. Colon invasive micropapillary carcinoma arising in tubulovillous adenoma. Pol J Pathol. 2008;59:183–185. [PubMed] [Google Scholar]
- 16.Wen P, Xu Y, Frankel WL, Shen R. Invasive micropapillary carcinoma of the sigmoid colon: distinct morphology and aggressive behavior. Int J Clin Exp Pathol. 2008;1:457–460. [PMC free article] [PubMed] [Google Scholar]
- 17.Shimoda M, Okada Y, Hayashi Y, Hatano S, Kawakubo H, Omori T, Ishii S, Sugiura H. Primary invasive micropapillary carcinoma of the stomach. Pathol Int. 2008;58:513–517. doi: 10.1111/j.1440-1827.2008.02265.x. [DOI] [PubMed] [Google Scholar]
- 18.Kuroda N, Oonishi K, Ohara M, Hirouchi T, Mizuno K, Hayashi Y, Lee GH. Invasive micropapillary carcinoma of the colon: an immunohistochemical study. Med Mol Morphol. 2007;40:226–230. doi: 10.1007/s00795-007-0353-z. [DOI] [PubMed] [Google Scholar]
- 19.Sakamoto K, Watanabe M, De La Cruz C, Honda H, Ise H, Mitsui K, Namiki K, Mikami Y, Moriya T, Sasano H. Primary invasive micropapillary carcinoma of the colon. Histopathology. 2005;47:479–484. doi: 10.1111/j.1365-2559.2005.02241.x. [DOI] [PubMed] [Google Scholar]
- 20.Kim MJ, Hong SM, Jang SJ, Yu E, Kim JS, Kim KR, Gong G, Ro JY. Invasive colorectal micropapillary carcinoma: an aggressive variant of adenocarcinoma. Hum Pathol. 2006;37:809–815. doi: 10.1016/j.humpath.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 21.Ushiku T, Matsusaka K, Iwasaki Y, Tateishi Y, Funata N, Seto Y, Fukayama M. Gastric carcinoma with invasive micropapillary pattern and its association with lymph node metastasis. Histopathology. 2011;59:1081–1089. doi: 10.1111/j.1365-2559.2011.04055.x. [DOI] [PubMed] [Google Scholar]
- 22.Fujita T, Gotohda N, Kato Y, Kinoshita T, Takahashi S, Konishi M, Daiko H, Nishimura M, Kuwata T, Ochiai A, et al. Clinicopathological features of stomach cancer with invasive micropapillary component. Gastric Cancer. 2012;15:179–187. doi: 10.1007/s10120-011-0094-5. [DOI] [PubMed] [Google Scholar]
- 23.Kondo T. Bile duct adenocarcinoma with minor micropapillary component: a case report. Cases J. 2009;2:51. doi: 10.1186/1757-1626-2-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fujita T, Konishi M, Gotohda N, Takahashi S, Nakagohri T, Kojima M, Kinoshita T. Invasive micropapillary carcinoma of the ampulla of Vater with extensive lymph node metastasis: Report of a case. Surg Today. 2010;40:1197–1200. doi: 10.1007/s00595-010-4330-0. [DOI] [PubMed] [Google Scholar]
- 25.Khayyata S, Basturk O, Adsay NV. Invasive micropapillary carcinomas of the ampullo-pancreatobiliary region and their association with tumor-infiltrating neutrophils. Mod Pathol. 2005;18:1504–1511. doi: 10.1038/modpathol.3800460. [DOI] [PubMed] [Google Scholar]
- 26.Kitagawa H, Nakamura M, Tani T, Tajima H, Nakagawara H, Ohnishi I, Takamura H, Kayahara M, Ohta T, Zen Y, et al. A pure invasive micropapillary carcinoma of the pancreatic head: long disease-free survival after pancreatoduodenectomy and adjuvant chemotherapy with gemcitabine. Pancreas. 2007;35:190–192. doi: 10.1097/01.mpa.0000250142.02768.c7. [DOI] [PubMed] [Google Scholar]
- 27.Nassar H, Pansare V, Zhang H, Che M, Sakr W, Ali-Fehmi R, Grignon D, Sarkar F, Cheng J, Adsay V. Pathogenesis of invasive micropapillary carcinoma: role of MUC1 glycoprotein. Mod Pathol. 2004;17:1045–1050. doi: 10.1038/modpathol.3800166. [DOI] [PubMed] [Google Scholar]
- 28.Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO Classification of Tumours of the Digestive System. 4th ed. Lyon: IARC Press; 2010. pp. 43–44. [Google Scholar]
- 29.Lino-Silva LS. Pure micropapillary rectal carcinoma with CK7 and CK20 coexpression and loss of CDX2 reactivity. Int J Morphol. 2012;30:25–29. [Google Scholar]
- 30.Otsubo K, Kubo N, Nakashima N, Izumi M, Nakamori M, Koto H. A juvenile case of pulmonary lymphangitic carcinomatosis caused by sigmoid colon cancer with a component of micropapillary carcinoma. Intern Med. 2011;50:2361–2365. doi: 10.2169/internalmedicine.50.5170. [DOI] [PubMed] [Google Scholar]
- 31.Roh JH, Srivastava A, Lauwers GY, An J, Jang KT, Park CK, Sohn TS, Kim S, Kim KM. Micropapillary carcinoma of stomach: a clinicopathologic and immunohistochemical study of 11 cases. Am J Surg Pathol. 2010;34:1139–1146. doi: 10.1097/PAS.0b013e3181e7043b. [DOI] [PubMed] [Google Scholar]
- 32.Haupt B, Ro JY, Schwartz MR, Shen SS. Colorectal adenocarcinoma with micropapillary pattern and its association with lymph node metastasis. Mod Pathol. 2007;20:729–733. doi: 10.1038/modpathol.3800790. [DOI] [PubMed] [Google Scholar]
- 33.Ohtsuki Y, Kuroda N, Yunoki S, Murakami S, Mizukami Y, Okada Y, Iguchi M, Lee GH, Furihata M. Immunohistochemical analysis of invasive micropapillary carcinoma pattern in four cases of gastric cancer. Med Mol Morphol. 2013;46:114–121. doi: 10.1007/s00795-013-0037-9. [DOI] [PubMed] [Google Scholar]
- 34.Seidman JD, Kurman RJ. Subclassification of serous borderline tumors of the ovary into benign and malignant types. A clinicopathologic study of 65 advanced stage cases. Am J Surg Pathol. 1996;20:1331–1345. doi: 10.1097/00000478-199611000-00004. [DOI] [PubMed] [Google Scholar]
- 35.Luna-Moré S, Gonzalez B, Acedo C, Rodrigo I, Luna C. Invasive micropapillary carcinoma of the breast. A new special type of invasive mammary carcinoma. Pathol Res Pract. 1994;190:668–674. doi: 10.1016/S0344-0338(11)80745-4. [DOI] [PubMed] [Google Scholar]
- 36.Hudson MJ, Stamp GW, Chaudhary KS, Hewitt R, Stubbs AP, Abel PD, Lalani EN. Human MUC1 mucin: a potent glandular morphogen. J Pathol. 2001;194:373–383. doi: 10.1002/1096-9896(200107)194:3<373::AID-PATH898>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 37.Hilkens J, Vos HL, Wesseling J, Boer M, Storm J, van der Valk S, Calafat J, Patriarca C. Is episialin/MUC1 involved in breast cancer progression? Cancer Lett. 1995;90:27–33. doi: 10.1016/0304-3835(94)03674-8. [DOI] [PubMed] [Google Scholar]
- 38.Wesseling J, van der Valk SW, Vos HL, Sonnenberg A, Hilkens J. Episialin (MUC1) overexpression inhibits integrin-mediated cell adhesion to extracellular matrix components. J Cell Biol. 1995;129:255–265. doi: 10.1083/jcb.129.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wesseling J, van der Valk SW, Hilkens J. A mechanism for inhibition of E-cadherin-mediated cell-cell adhesion by the membrane-associated mucin episialin/MUC1. Mol Biol Cell. 1996;7:565–577. doi: 10.1091/mbc.7.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eom DW, Kang GH, Han SH, Cheon GJ, Han KH, Oh HS, Kim JH, Jang HJ, Hong SM. Gastric micropapillary carcinoma: A distinct subtype with a significantly worse prognosis in TNM stages I and II. Am J Surg Pathol. 2011;35:84–91. doi: 10.1097/PAS.0b013e3181ff61e2. [DOI] [PubMed] [Google Scholar]
- 41.Adsay NV, Merati K, Nassar H, Shia J, Sarkar F, Pierson CR, Cheng JD, Visscher DW, Hruban RH, Klimstra DS. Pathogenesis of colloid (pure mucinous) carcinoma of exocrine organs: Coupling of gel-forming mucin (MUC2) production with altered cell polarity and abnormal cell-stroma interaction may be the key factor in the morphogenesis and indolent behavior of colloid carcinoma in the breast and pancreas. Am J Surg Pathol. 2003;27:571–578. doi: 10.1097/00000478-200305000-00002. [DOI] [PubMed] [Google Scholar]
- 42.Lino-Silva LS, Salcedo-Hernández RA, Caro-Sánchez CH. Colonic micropapillary carcinoma, a recently recognized subtype associated with histological adverse factors: clinicopathological analysis of 15 cases. Colorectal Dis. 2012;14:e567–e572. doi: 10.1111/j.1463-1318.2012.03013.x. [DOI] [PubMed] [Google Scholar]
- 43.Lee HJ, Eom DW, Kang GH, Han SH, Cheon GJ, Oh HS, Han KH, Ahn HJ, Jang HJ, Han MS. Colorectal micropapillary carcinomas are associated with poor prognosis and enriched in markers of stem cells. Mod Pathol. 2013;26:1123–1131. doi: 10.1038/modpathol.2012.163. [DOI] [PubMed] [Google Scholar]
- 44.Xu F, Xu J, Lou Z, Di M, Wang F, Hu H, Lai M. Micropapillary component in colorectal carcinoma is associated with lymph node metastasis in T1 and T2 Stages and decreased survival time in TNM stages I and II. Am J Surg Pathol. 2009;33:1287–1292. doi: 10.1097/PAS.0b013e3181a5387b. [DOI] [PubMed] [Google Scholar]
- 45.Lee JH, Kim JH, Choi JW, Kim YS. The presence of a micropapillary component predicts aggressive behaviour in early and advanced gastric adenocarcinomas. Pathology. 2010;42:560–563. doi: 10.3109/00313025.2010.508790. [DOI] [PubMed] [Google Scholar]
- 46.Ninomiya S, Sonoda K, Shiroshita H, Bandoh T, Arita T. Five-year survival after surgery for invasive micropapillary carcinoma of the stomach. Case Rep Surg. 2013;2013:560712. doi: 10.1155/2013/560712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lotan TL, Ye H, Melamed J, Wu XR, Shih IeM, Epstein JI. Immunohistochemical panel to identify the primary site of invasive micropapillary carcinoma. Am J Surg Pathol. 2009;33:1037–1041. doi: 10.1097/PAS.0b013e3181962dcd. [DOI] [PMC free article] [PubMed] [Google Scholar]


