Abstract
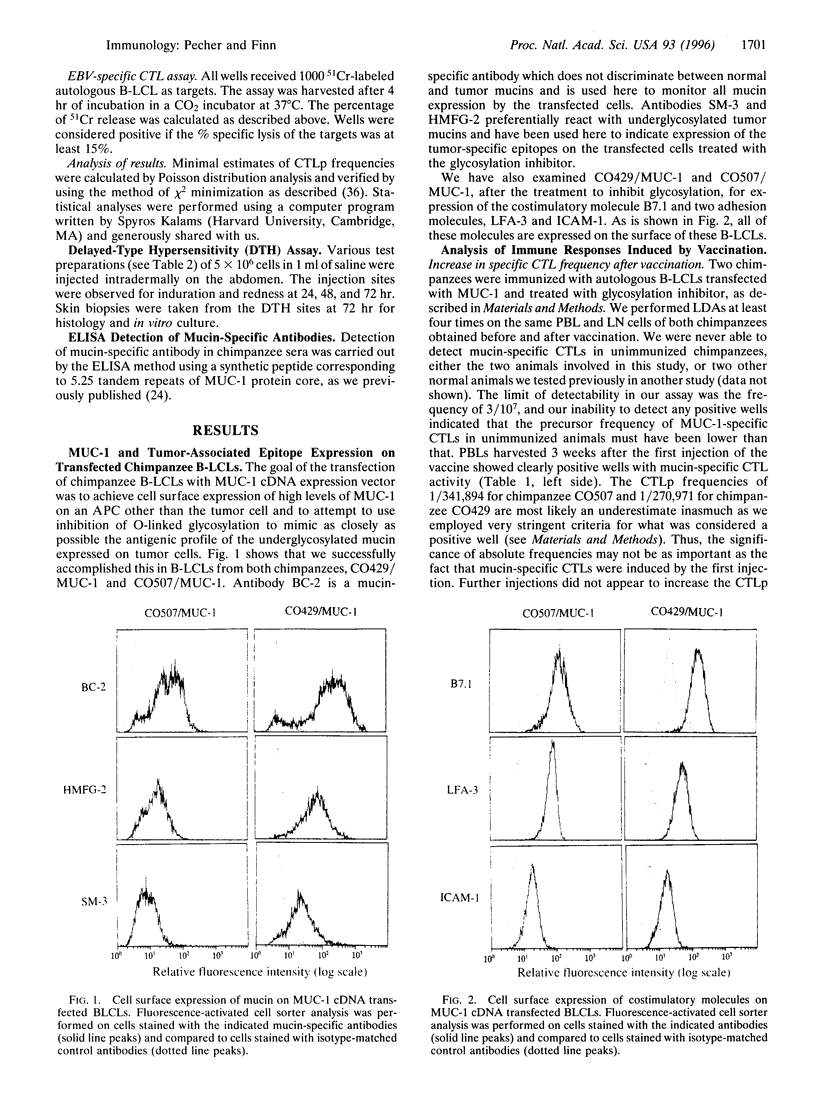
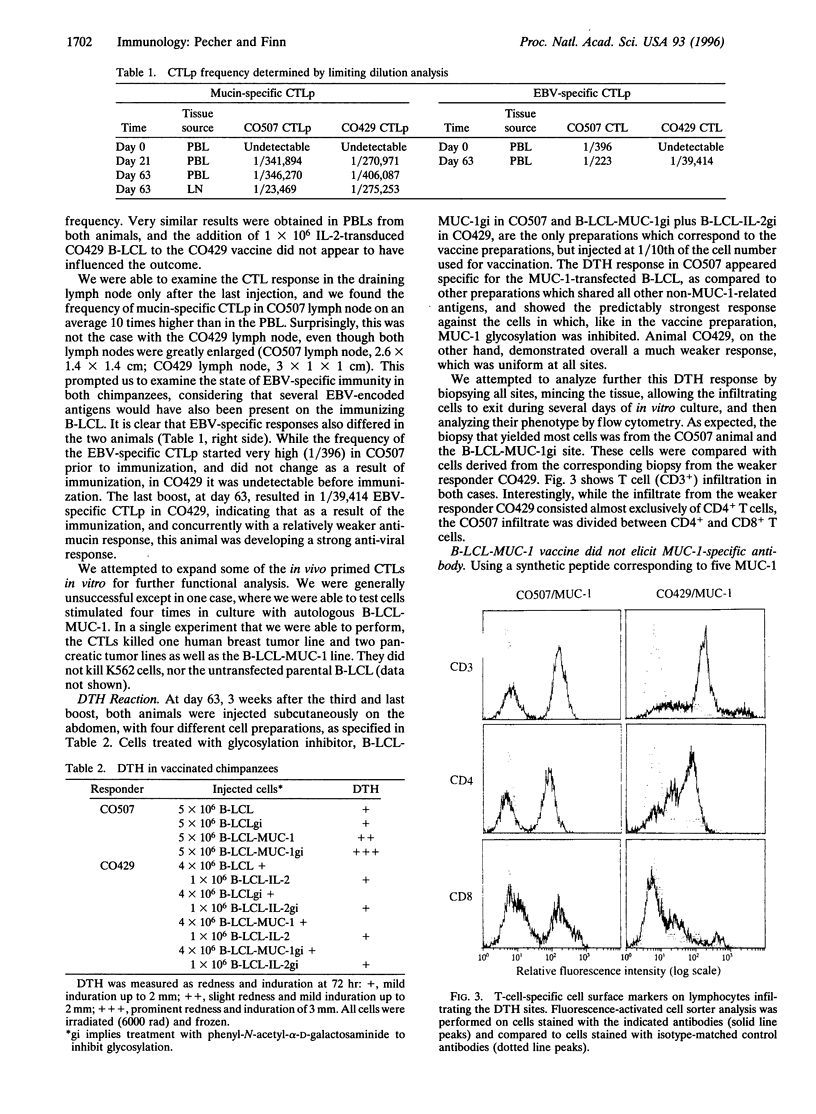
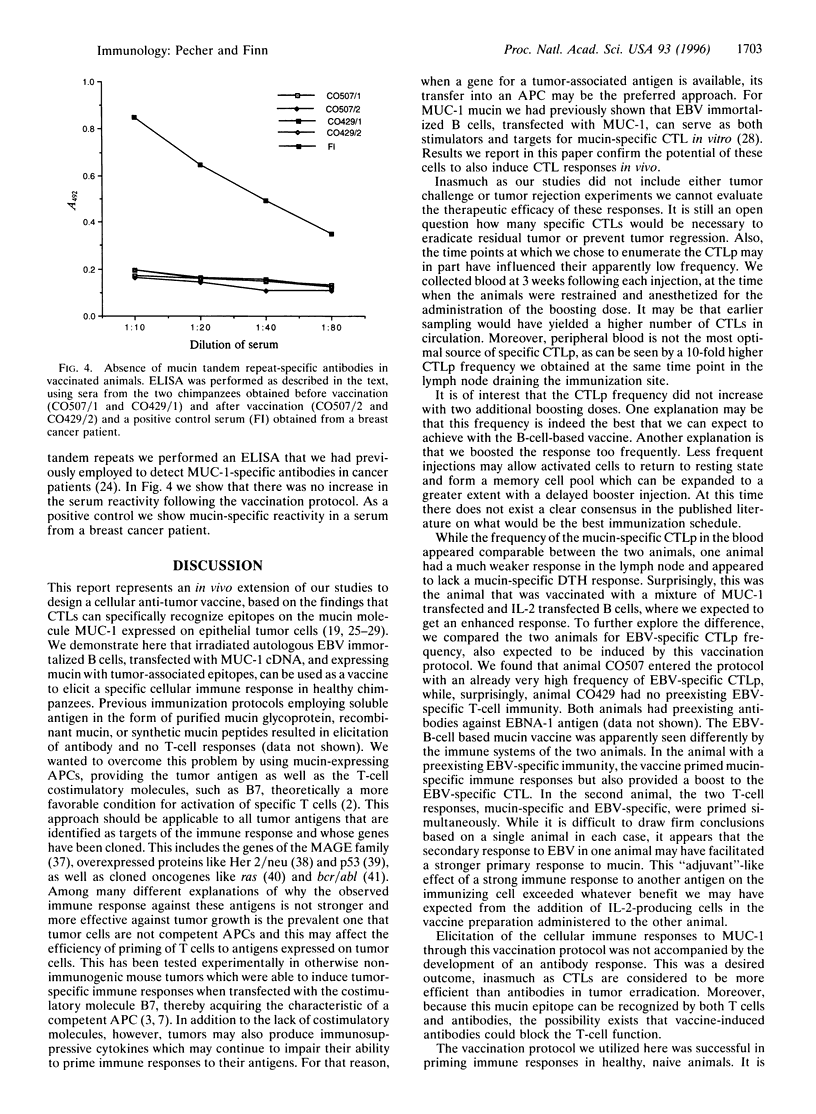
Aberrant glycosylation of the mucin molecule (encoded by the gene MUC-1) on human epithelial cell tumors leads to the exposure of tumor-associated epitopes recognized by patients' antibodies and cytotoxic T cells. Consequently, these epitopes could be considered targets for immunotherapy. We designed a cellular vaccine, employing, instead of tumor cells, autologous Epstein-Barr virus (EBV)-immortalized B cells as carriers of tumor-associated mucin, to take advantage of their costimulatory molecules for T-cell activation. The vaccine was tested in chimpanzees because of the identity of the human and chimpanzee MUC-1 tandem repeat sequence. EBV-immortalized B cells derived from two chimpanzees were transfected with MUC-1 cDNA, treated with glycosylation inhibitor phenyl-N-acetyl-alpha-D-galactosaminide to expose tumor-associated epitopes, irradiated, and injected subcutaneously four times at 3-week intervals. One vaccine preparation also contained cells transduced with the interleukin 2 (IL-2) cDNA and producing low levels of IL-2. Already after the first injection we found in the peripheral blood measurable frequency of cytotoxic T-cell precursors specific for underglycosylated mucin. The highest frequency observed was after the last boost, in the lymph node draining the vaccination site. Delayed-type hypersensitivity reaction to the injected immunogens was also induced, whereas no appearance of mucin-specific antibodies was seen. Long-term observation of the animals yielded no signs of adverse effects of this immunization. Autologous antigen-presenting cells, like EBV-immortalized B cells, expressing tumor-associated antigens are potentially useful immunogens for induction of cellular anti-tumor responses in vivo.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Austyn J. M. The dendritic cell system and anti-tumour immunity. In Vivo. 1993 May-Jun;7(3):193–201. [PubMed] [Google Scholar]
- Barnd D. L., Lan M. S., Metzgar R. S., Finn O. J. Specific, major histocompatibility complex-unrestricted recognition of tumor-associated mucins by human cytotoxic T cells. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7159–7163. doi: 10.1073/pnas.86.18.7159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boon T., Cerottini J. C., Van den Eynde B., van der Bruggen P., Van Pel A. Tumor antigens recognized by T lymphocytes. Annu Rev Immunol. 1994;12:337–365. doi: 10.1146/annurev.iy.12.040194.002005. [DOI] [PubMed] [Google Scholar]
- Boussiotis V. A., Freeman G. J., Gribben J. G., Daley J., Gray G., Nadler L. M. Activated human B lymphocytes express three CTLA-4 counterreceptors that costimulate T-cell activation. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):11059–11063. doi: 10.1073/pnas.90.23.11059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bu D., Domenech N., Lewis J., Taylor-Papadimitriou J., Finn O. J. Recombinant vaccinia mucin vector: in vitro analysis of expression of tumor-associated epitopes for antibody and human cytotoxic T-cell recognition. J Immunother Emphasis Tumor Immunol. 1993 Aug;14(2):127–135. [PubMed] [Google Scholar]
- Chen L., Ashe S., Brady W. A., Hellström I., Hellström K. E., Ledbetter J. A., McGowan P., Linsley P. S. Costimulation of antitumor immunity by the B7 counterreceptor for the T lymphocyte molecules CD28 and CTLA-4. Cell. 1992 Dec 24;71(7):1093–1102. doi: 10.1016/s0092-8674(05)80059-5. [DOI] [PubMed] [Google Scholar]
- Chen L., McGowan P., Ashe S., Johnston J., Li Y., Hellström I., Hellström K. E. Tumor immunogenicity determines the effect of B7 costimulation on T cell-mediated tumor immunity. J Exp Med. 1994 Feb 1;179(2):523–532. doi: 10.1084/jem.179.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W., Peace D. J., Rovira D. K., You S. G., Cheever M. A. T-cell immunity to the joining region of p210BCR-ABL protein. Proc Natl Acad Sci U S A. 1992 Feb 15;89(4):1468–1472. doi: 10.1073/pnas.89.4.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disis M. L., Calenoff E., McLaughlin G., Murphy A. E., Chen W., Groner B., Jeschke M., Lydon N., McGlynn E., Livingston R. B. Existent T-cell and antibody immunity to HER-2/neu protein in patients with breast cancer. Cancer Res. 1994 Jan 1;54(1):16–20. [PubMed] [Google Scholar]
- Doménech N., Henderson R. A., Finn O. J. Identification of an HLA-A11-restricted epitope from the tandem repeat domain of the epithelial tumor antigen mucin. J Immunol. 1995 Nov 15;155(10):4766–4774. [PubMed] [Google Scholar]
- Finkelman F. D., Lees A., Morris S. C. Antigen presentation by B lymphocytes to CD4+ T lymphocytes in vivo: importance for B lymphocyte and T lymphocyte activation. Semin Immunol. 1992 Aug;4(4):247–255. [PubMed] [Google Scholar]
- Finn O. J. Antigen-specific, MHC-unrestricted T cells. Biotherapy. 1992;4(4):239–249. doi: 10.1007/BF02172653. [DOI] [PubMed] [Google Scholar]
- Fontenot J. D., Mariappan S. V., Catasti P., Domenech N., Finn O. J., Gupta G. Structure of a tumor associated antigen containing a tandemly repeated immunodominant epitope. J Biomol Struct Dyn. 1995 Oct;13(2):245–260. doi: 10.1080/07391102.1995.10508837. [DOI] [PubMed] [Google Scholar]
- Fontenot J. D., Tjandra N., Bu D., Ho C., Montelaro R. C., Finn O. J. Biophysical characterization of one-, two-, and three-tandem repeats of human mucin (muc-1) protein core. Cancer Res. 1993 Nov 15;53(22):5386–5394. [PubMed] [Google Scholar]
- Gansbacher B., Zier K., Daniels B., Cronin K., Bannerji R., Gilboa E. Interleukin 2 gene transfer into tumor cells abrogates tumorigenicity and induces protective immunity. J Exp Med. 1990 Oct 1;172(4):1217–1224. doi: 10.1084/jem.172.4.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendler S. J., Lancaster C. A., Taylor-Papadimitriou J., Duhig T., Peat N., Burchell J., Pemberton L., Lalani E. N., Wilson D. Molecular cloning and expression of human tumor-associated polymorphic epithelial mucin. J Biol Chem. 1990 Sep 5;265(25):15286–15293. [PubMed] [Google Scholar]
- Gendler S., Taylor-Papadimitriou J., Duhig T., Rothbard J., Burchell J. A highly immunogenic region of a human polymorphic epithelial mucin expressed by carcinomas is made up of tandem repeats. J Biol Chem. 1988 Sep 15;263(26):12820–12823. [PubMed] [Google Scholar]
- Girling A., Bartkova J., Burchell J., Gendler S., Gillett C., Taylor-Papadimitriou J. A core protein epitope of the polymorphic epithelial mucin detected by the monoclonal antibody SM-3 is selectively exposed in a range of primary carcinomas. Int J Cancer. 1989 Jun 15;43(6):1072–1076. doi: 10.1002/ijc.2910430620. [DOI] [PubMed] [Google Scholar]
- Gum J. R., Byrd J. C., Hicks J. W., Toribara N. W., Lamport D. T., Kim Y. S. Molecular cloning of human intestinal mucin cDNAs. Sequence analysis and evidence for genetic polymorphism. J Biol Chem. 1989 Apr 15;264(11):6480–6487. [PubMed] [Google Scholar]
- Hock H., Dorsch M., Kunzendorf U., Qin Z., Diamantstein T., Blankenstein T. Mechanisms of rejection induced by tumor cell-targeted gene transfer of interleukin 2, interleukin 4, interleukin 7, tumor necrosis factor, or interferon gamma. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):2774–2778. doi: 10.1073/pnas.90.7.2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannides C. G., Fisk B., Jerome K. R., Irimura T., Wharton J. T., Finn O. J. Cytotoxic T cells from ovarian malignant tumors can recognize polymorphic epithelial mucin core peptides. J Immunol. 1993 Oct 1;151(7):3693–3703. [PubMed] [Google Scholar]
- Janeway C. A., Jr, Ron J., Katz M. E. The B cell is the initiating antigen-presenting cell in peripheral lymph nodes. J Immunol. 1987 Feb 15;138(4):1051–1055. [PubMed] [Google Scholar]
- Jerome K. R., Barnd D. L., Bendt K. M., Boyer C. M., Taylor-Papadimitriou J., McKenzie I. F., Bast R. C., Jr, Finn O. J. Cytotoxic T-lymphocytes derived from patients with breast adenocarcinoma recognize an epitope present on the protein core of a mucin molecule preferentially expressed by malignant cells. Cancer Res. 1991 Jun 1;51(11):2908–2916. [PubMed] [Google Scholar]
- Jerome K. R., Bu D., Finn O. J. Expression of tumor-associated epitopes on Epstein-Barr virus-immortalized B-cells and Burkitt's lymphomas transfected with epithelial mucin complementary DNA. Cancer Res. 1992 Nov 1;52(21):5985–5990. [PubMed] [Google Scholar]
- Jerome K. R., Domenech N., Finn O. J. Tumor-specific cytotoxic T cell clones from patients with breast and pancreatic adenocarcinoma recognize EBV-immortalized B cells transfected with polymorphic epithelial mucin complementary DNA. J Immunol. 1993 Aug 1;151(3):1654–1662. [PubMed] [Google Scholar]
- Kotera Y., Fontenot J. D., Pecher G., Metzgar R. S., Finn O. J. Humoral immunity against a tandem repeat epitope of human mucin MUC-1 in sera from breast, pancreatic, and colon cancer patients. Cancer Res. 1994 Jun 1;54(11):2856–2860. [PubMed] [Google Scholar]
- Kuan S. F., Byrd J. C., Basbaum C., Kim Y. S. Inhibition of mucin glycosylation by aryl-N-acetyl-alpha-galactosaminides in human colon cancer cells. J Biol Chem. 1989 Nov 15;264(32):19271–19277. [PubMed] [Google Scholar]
- Lan M. S., Hollingsworth M. A., Metzgar R. S. Polypeptide core of a human pancreatic tumor mucin antigen. Cancer Res. 1990 May 15;50(10):2997–3001. [PubMed] [Google Scholar]
- Li Y., McGowan P., Hellström I., Hellström K. E., Chen L. Costimulation of tumor-reactive CD4+ and CD8+ T lymphocytes by B7, a natural ligand for CD28, can be used to treat established mouse melanoma. J Immunol. 1994 Jul 1;153(1):421–428. [PubMed] [Google Scholar]
- Peace D. J., Chen W., Nelson H., Cheever M. A. T cell recognition of transforming proteins encoded by mutated ras proto-oncogenes. J Immunol. 1991 Mar 15;146(6):2059–2065. [PubMed] [Google Scholar]
- Ron Y., Sprent J. T cell priming in vivo: a major role for B cells in presenting antigen to T cells in lymph nodes. J Immunol. 1987 May 1;138(9):2848–2856. [PubMed] [Google Scholar]
- Russell S. J. Lymphokine gene therapy for cancer. Immunol Today. 1990 Jun;11(6):196–200. doi: 10.1016/0167-5699(90)90081-j. [DOI] [PubMed] [Google Scholar]
- Steinman R. M. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Makiguchi Y., Hinoda Y., Kakiuchi H., Nakagawa N., Imai K., Yachi A. Expression of MUC1 on myeloma cells and induction of HLA-unrestricted CTL against MUC1 from a multiple myeloma patient. J Immunol. 1994 Sep 1;153(5):2102–2109. [PubMed] [Google Scholar]
- Taswell C. Limiting dilution assays for the determination of immunocompetent cell frequencies. I. Data analysis. J Immunol. 1981 Apr;126(4):1614–1619. [PubMed] [Google Scholar]
- Townsend S. E., Allison J. P. Tumor rejection after direct costimulation of CD8+ T cells by B7-transfected melanoma cells. Science. 1993 Jan 15;259(5093):368–370. doi: 10.1126/science.7678351. [DOI] [PubMed] [Google Scholar]
- Weaver C. T., Unanue E. R. The costimulatory function of antigen-presenting cells. Immunol Today. 1990 Feb;11(2):49–55. doi: 10.1016/0167-5699(90)90018-5. [DOI] [PubMed] [Google Scholar]
- Xing P. X., Reynolds K., Tjandra J. J., Tang X. L., McKenzie I. F. Synthetic peptides reactive with anti-human milk fat globule membrane monoclonal antibodies. Cancer Res. 1990 Jan 1;50(1):89–96. [PubMed] [Google Scholar]
- Xing P. X., Tjandra J. J., Reynolds K., McLaughlin P. J., Purcell D. F., McKenzie I. F. Reactivity of anti-human milk fat globule antibodies with synthetic peptides. J Immunol. 1989 May 15;142(10):3503–3509. [PubMed] [Google Scholar]
- Yanuck M., Carbone D. P., Pendleton C. D., Tsukui T., Winter S. F., Minna J. D., Berzofsky J. A. A mutant p53 tumor suppressor protein is a target for peptide-induced CD8+ cytotoxic T-cells. Cancer Res. 1993 Jul 15;53(14):3257–3261. [PubMed] [Google Scholar]
- van der Bruggen P., Traversari C., Chomez P., Lurquin C., De Plaen E., Van den Eynde B., Knuth A., Boon T. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science. 1991 Dec 13;254(5038):1643–1647. doi: 10.1126/science.1840703. [DOI] [PubMed] [Google Scholar]


