Abstract
AIM: To investigate the potential of promoter methylation of two tumor suppressor genes (TSGs) as biomarkers for hepatocellular carcinoma (HCC).
METHODS: A total of 189 subjects were included in this retrospective cohort, which contained 121 HCC patients without any history of curative treatment, 37 patients with chronic hepatitis B (CHB), and 31 normal controls (NCs). DNA samples were extracted from 400 μL of serum of each subject and then modified using bisulfite treatment. Methylation of the promoters of the TSGs (metallothionein 1M, MT1M; and metallothionein 1G, MT1G) was determined using methylation-specific polymerase chain reaction. The diagnostic value of combined MT1M and MT1G promoter methylation was evaluated using the area under the receiver operating characteristic curves.
RESULTS: Our results indicated that the methylation status of serum MT1M (48.8%, 59/121) and MT1G (70.2%, 85/121) promoters in the HCC group was significantly higher than that in the CHB group (MT1M 5.4%, 2/37, P < 0.001; MT1G 16.2%, 6/37, P < 0.001) and NC group (MT1M 6.5%, 2/31, P < 0.001; MT1G 12.9%, 4/27, P < 0.001). Aberrant serum MT1M promoter methylation gave higher specificity to discriminate HCC from CHB (94.6%) and NCs (93.5%), whereas combined methylation of serum MT1M and MT1G promoters showed higher diagnostic sensitivity (90.9%), suggesting that they are potential markers for noninvasive detection of HCC. Furthermore, MT1M promoter methylation was positively correlated with tumor size (rs = 0.321, P < 0.001), and HCC patients with both MT1M and MT1G promoter methylation tended to show a higher incidence of vascular invasion or metastasis (P = 0.018).
CONCLUSION: MT1M and MT1G promoter methylation may be used as serum biomarkers for noninvasive detection of HCC.
Keywords: MT1M, MT1G, Methylation, Serum biomarker, Hepatocellular carcinoma
Core tip: DNA methylation of tumor suppressor gene promoter regions appears to be a valuable biomarker in many tumors, including hepatocellular carcinoma (HCC). We found that aberrant serum metallothionein 1M (MT1M) promoter methylation gave higher specificity to discriminate HCC from chronic hepatitis B and normal controls. In contrast, combined methylation of serum MT1M and metallothionein 1G promoters showed higher diagnostic sensitivity. This indicates that they may be used as potential biomarkers for noninvasive detection of HCC.
INTRODUCTION
Hepatocellular carcinoma (HCC) is the sixth most common tumor and has the third highest mortality[1]. The areas of highest incidence are Asia and Africa, which are linked to the wide prevalence of hepatitis B virus (HBV) infection[2]. However, the incidence of HCC has been rapidly increasing in the United States and United Kingdom over the past 20 years, which is attributed to increased hepatitis C virus (HCV) infection[3,4]. In addition, aflatoxin B1 exposure and alcohol addiction are also associated with hepatocellular carcinogenesis. Despite advanced treatment, patients with HCC have a dismal 5-year survival rate of about 5%, as a result of late diagnosis[5]. Currently available screening tests to detect HCC mainly combine serum α-fetoprotein (AFP) and ultrasound (US). Regrettably, their effectiveness remains controversial, and the diagnostic rate of AFP meets with a low sensitivity and that of US depends on examiner expertise, patient data, presence of liver cirrhosis, and tumor size[6,7]. Great efforts have been made to find new biomarkers for early detection of HCC. As a result, the potential value of tumor-associated DNA methylation as a biomarker has attracted much attention[8,9].
It is well known that the silencing of tumor suppressor genes by promoter hypermethylation is responsible for carcinogenesis. Some studies have found that tumors shed methylated DNA sequences into the blood in the early stages[10-13]. Moreover, Zhang et al[14] found that methylated DNA could be detected 1-9 years before the clinical diagnosis of HCC. Thus, methylated DNA has been suggested as an ideal biomarker because of its early appearance in the disease course, as well as its easy and noninvasive detection in biological samples. In addition, the DNA methylation pattern is more stable than protein and RNA expression, which changes markedly and unpredictably[9].
The metallothioneins (MTs) are a superfamily of low-molecular-weight, cysteine-rich intracellular proteins, consisting of at least 10 functional members (MT1A, MT1B, MT1E, MT1F, MT1G, MT1H, MT1X, MT2A, MT3, and MT4)[15,16]. The role of MTs in metal homeostasis, protection against oxidative damage, cell proliferation and apoptosis, resistance to radiation and chemotherapy, as well as several aspects of the carcinogenic process, has been revealed[17-21]. Some studies have shown that MT-1 and MT-2 are frequently downregulated in HCC[22-25], and decreased MT expression might be an early event in HCC progression[22]. MT downregulation may be concerned with hypermethylation of MT promoters, as shown in rat hepatoma[26]. Moreover, others have reported that metallothionein 1M (MT1M)[27] and metallothionein 1G (MT1G)[28] are decreased in human HCC tissues by promoter hypermethylation.
Therefore, in the present study, we hypothesized that methylation of MT1M and MT1G promoters could be detected in the serum of patients with HCC, and aimed to define optimal gene sets as noninvasive markers for early detection of HCC.
MATERIALS AND METHODS
Collection of serum specimens
After obtaining informed consent, we collected 189 serum samples from 121 patients with HCC, 37 patients with chronic hepatitis B (CHB), and 31 normal controls (NCs), based on clinical and laboratory examinations. Patients with HCC and CHB were recruited from those enrolled from July 2011 to March 2013 at Qilu Hospital, Shandong University in accordance with American Association for the Study of Liver Diseases Practice Guidelines for HCC and CHB, respectively[29,30]. All cases of HCC included in our study were confirmed by pathological data. Serum samples were collected from HCC patients who did not receive curative treatments such as surgical resection, transcatheter arterial chemoembolization (TACE), or radiofrequency ablation before and during the study. Exclusion criteria included other tumors, co-infection with HCV or human immunodeficiency virus, and other causes of chronic liver diseases. The patient selection process is shown in Figure 1.
Figure 1.
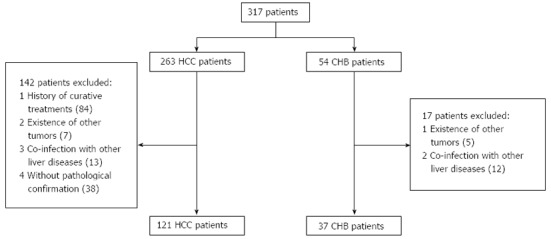
Patient selection process. HCC: Hepatocellular carcinoma; CHB: Chronic hepatitis B.
Tumor size was calibrated by computed tomography and presented as the longest diameter. AFP concentration > 20 ng/mL was regarded as abnormal[31]. The study protocol was approved by the Ethics Committee of Qilu Hospital.
Serum DNA extraction and sodium bisulfite modification
DNA was extracted from 400 μL of serum with the QIAamp DNA Blood Mini Kit (Qiagen, Hilden, Germany) following the DNA Purification from Blood or Body Fluids protocol. Bisulfite modification was performed using the EZ DNA Methylation-Gold Kit (Zymo Research, Irvine, CA, United States) according to the manufacturer’s instructions. After bisulfite treatment, all unmethylated cytosine residues were converted to uracil, whereas the methylated residues would have been resistant to this modification and remained as cytosine. The modified DNA was finally stored at -20 °C before methylation-specific polymerase chain reaction (MSP).
MSP
The primer pairs of MT1M and MT1G for MSP analysis were as described previously[27,28] (Table 1). One microliter of bisulfite-treated DNA, 0.5 μL each primer (10 μmol/L), 10.5 μL nuclease-free water, and 12.5 μL Premix Taq (Zymo Research) were mixed together to form a 25-μL MSP reaction mixture. The PCR protocol included an initial denaturation at 95 °C for 10 min, followed by 45 cycles of denaturation at 95 °C for 30 s, annealing at the respective temperature (54 °C for MT1M, 59 °C for MT1G-U, and 50 °C for MT1G-M) for 40 s, primer extension at 72 °C for 40 s, and a final extension at 72 °C for 10 min (Table 1). Water without DNA was used as a negative control. PCR products were electrophoresed on 2% agarose gels, stained with Gel Red, and visualized under UV illumination.
Table 1.
Primers for polymerase chain reaction
| Primers | Sequences | Annealing temp (°C) | Size (bp) |
| MT1M | |||
| U | 5'-TTGAAAATGGTGGGGTGA-3' | 54 | 163 |
| 5'-AAACTATACACCAAATAATACACAATATCC-3' | |||
| M | 5'-GACGTTCGCGACGTTAAG-3' | 54 | 124 |
| 5'-ACGCCGAATAATACGCAAT-3' | |||
| MT1G | |||
| U | 5'-GGGGTTGTTTTGTGGTGTGTG-3' | 59 | 135 |
| 5'-AAACACCCCACCCCACCCTT-3' | |||
| M | 5'-TTCGCGAGTCGGTGCGAAAG-3' | 50 | 96 |
| 5'-CCGCGATCCCGACCTAAACT-3' | |||
Statistical analysis
The differences in DNA methylation status of MT1M and MT1G promoters between different groups and the associations between gene methylation in HCC patients and clinical pathological variables were analyzed using the χ2 test. Correlation between MT1M and MT1G promoter methylation and tumor size was calculated by Spearman rank correlation. Diagnostic value of combined methylation of MT1M and MT1G promoters and serum AFP level was evaluated by the area under the receiver operating characteristic curves (AUC). Differences were considered significant at P < 0.05. All statistical analyses were conducted with SPSS 16.0 software.
RESULTS
Methylation status in serum
The methylation status of MT1M or MT1G promoter in 121 patients with HCC, 37 patients with CHB and 31 NCs was compared (Figure 2). The methylation percentages were higher in HCC (48.8% for MT1M and 70.2% for MT1G) than in CHB (5.4% for MT1M and 16.2% for MT1G) or NCs (6.5% for MT1M and 12.9% for MT1G) (P < 0.001). However, no differences were found for either of them between the CHB and NC groups. Representative MSP results for methylated MT1M and MT1G promoters are shown in Figure 3.
Figure 2.
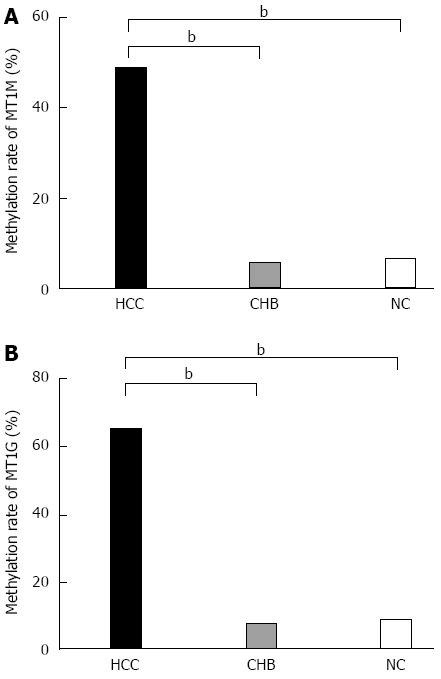
Percentage methylation of MT1M and MT1G in hepatocellular carcinoma, chronic hepatitis B and normal controls groups. A: Percentage methylation of MT1M was 48.8% (59/121) in the HCC, 5.4% (2/37) in the CHB, and 6.5% (2/31) in the NC groups; B: Percentage methylation of MT1G was 70.2% (85/121) in the HCC, 16.2% (6/37) in the CHB, and 12.9% (4/31) in the NC groups (bP < 0.001).
Figure 3.
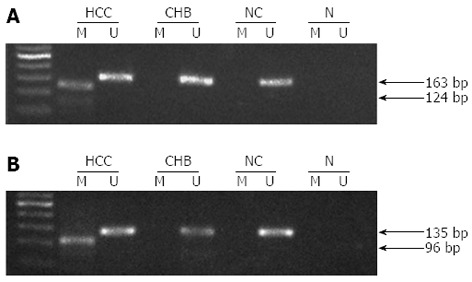
Representative methylation of metallothionein 1M and metallothionein 1G gene promoters by methylation-specific polymerase chain reaction. A: The methylated and unmethylated sequences of MT1M were 124 and 163 bp, respectively; B: The methylated and unmethylated sequences of MT1G were 96 and 135 bp, respectively. N: Negative control; M: Methylation-specific primers; U: Unmethylation-specific primers.
Correlation with clinicopathological parameters
For analysis of the correlation between methylation status of a single gene promoter in serum and clinicopathological features, there was a significant association between the methylation ratio of MT1M promoter and tumor size (P = 0.001) (Table 2). Further analysis revealed that the correlation was positive (rs = 0.321, P < 0.001) (Table 3). Moreover, advanced TNM stage (III-IV) was associated with a more elevated percentage of serum MT1M promoter methylation than early TNM stage (I-II), although the difference was not significant (P = 0.058) (Table 2). In addition, HCC patients with both MT1M and MT1G promoters methylated (18/44) tended to show a higher incidence of vascular invasion or metastasis than those with only one or neither gene methylated (16/77) (P = 0.018) (Table 4). However, no significant relationships were observed between the methylation levels of MT1M and MT1G promoters and other parameters, such as sex, age, HBV infection, serum AFP levels, tumor multiplicity or TNM stage (P > 0.05).
Table 2.
Clinicopathological data and serum metallothionein 1M and metallothionein 1G promoter methylation in hepatocellular carcinoma patients
| Characteristics | n |
MT1M |
MT1G |
||||
| 1M | 2U | P value | 1M | 2U | P value | ||
| Total number | 121 | 59 | 62 | 85 | 36 | ||
| Gender | |||||||
| Male | 100 | 50 | 50 | 0.552 | 70 | 30 | 0.896 |
| Female | 21 | 9 | 12 | 15 | 6 | ||
| Age (yr) | |||||||
| ≥ 55 | 67 | 32 | 35 | 0.807 | 46 | 21 | 0.670 |
| < 55 | 54 | 27 | 27 | 39 | 15 | ||
| HBV infection | |||||||
| Yes | 100 | 50 | 50 | 0.552 | 69 | 31 | 0.512 |
| No | 21 | 9 | 12 | 16 | 5 | ||
| Vascular invasion or metastasis | |||||||
| Yes | 44 | 26 | 18 | 0.086 | 33 | 11 | 0.387 |
| No | 77 | 33 | 44 | 52 | 25 | ||
| Histological differentiation | |||||||
| Poor | 41 | 18 | 23 | 0.610 | 26 | 15 | 0.426 |
| Moderate | 50 | 27 | 23 | 38 | 12 | ||
| Well | 30 | 14 | 16 | 21 | 9 | ||
| TNM stage | |||||||
| I-II | 64 | 26 | 38 | 0.058 | 44 | 13 | 0.115 |
| III-IV | 57 | 33 | 24 | 41 | 23 | ||
| Tumor multiplicity | |||||||
| Single | 72 | 26 | 36 | 0.741 | 52 | 20 | 0.565 |
| Multiple | 49 | 23 | 26 | 33 | 16 | ||
| Tumor size(cm) | |||||||
| ≥ 5 | 59 | 38 | 21 | 0.001 | 38 | 21 | 0.170 |
| < 5 | 62 | 21 | 41 | 47 | 15 | ||
1Methylated; 2Unmethylated.
Table 3.
Correlation of metallothionein 1M and metallothionein 1G promoter methylation with tumor size
| Gene |
Tumor size (cm) |
rs | P value | |
| MethylatedM (P25-P75) | UnmethylatedM (P25-P75) | |||
| MT1M | 6.5 (4.0-9.0) | 3.9 (2.2-6.4) | 0.321 | 0.000 |
| MT1G | 4.4 (2.9-8.0) | 5.0 (3.5-7.9) | -0.049 | 0.590 |
M: Median; P25: First quartile; P75: Third quartile; rs: Spearman correlation coefficient; MT1M: Metallothionein 1M; MT1G: Metallothionein 1G.
Table 4.
Vascular invasion or metastasis and a combination of metallothionein 1M and metallothionein 1G promoter methylation
| Characteristic | n |
MT1M and MT1G |
||
| 1M | 2U | P value | ||
| Vascular invasion or metastasis | ||||
| Yes | 44 | 18 | 26 | 0.018 |
| No | 77 | 16 | 61 | |
1Both MT1M and MT1G were methylated; 2One of MT1M and MT1G was methylated or neither of them methylated. MT1M: Metallothionein 1M; MT1G: Metallothionein 1G.
Sensitivity and specificity for single or combination methylation
There were 100 HCC patients with HBV infection (Table 2). To discriminate HBV-associated HCC from CHB, MT1M and MT1G promoter methylation showed a moderate sensitivity (MT1M, 50%, 50/100; MT1G, 69%, 69/100) but a high specificity (MT1M, 94.6%, 2/37; MT1G, 83.8%, 6/37), whereas the sensitivity and specificity of AFP were 56% (56/100) and 62.1% (23/37), respectively (Table 5). To discriminate HCC from the NC group, the specificity was still high (MT1M, 93.5%, 2/31; MT1G, 87.1%, 4/31) (Table 6). Otherwise, combined methylation of MT1M and MT1G promoters gave a sensitivity up to 90.9% (110/121) but a lower specificity to discriminate HCC from the NC (83.9%, 5/31) or CHB (81.1%, 7/37) groups (Tables 5 and 6). Moreover, the AUC of combined methylation of MT1M and MT1G promoters was 0.855 (95%CI: 0.785-0.910), which was significantly higher than that of AFP (0.754; 95%CI: 0.673-0.824) (P = 0.0446) (Figure 4).
Table 5.
Sensitivity and specificity of gene sets for hepatitis B virus (+) hepatocellular carcinoma detection in chronic hepatitis B group
| No. | Marker | TP/FN | FP/TN | Sensitivity (%)TP/(TP + FN) | Specificity (%)TN/(TN + FP) |
| 1 | AFP | 56/44 | 14/23 | 56.0 | 62.1 |
| 2 | MT1M | 50/50 | 2/35 | 50.0 | 94.6 |
| 3 | MT1G | 69/31 | 6/31 | 69.0 | 83.8 |
| 4 | MT1M/MT1G | 90/10 | 7/30 | 90.0 | 81.1 |
Sensitivity (%), TP/(TP + FN) and specificity (%), TN/(TN + FP) of each gene set were calculated and plotted. MT1M/MT1G, MT1M or MT1G promoter methylation. TP: True positive; FN: False negative; FP: False positive; TN: True negative.
Table 6.
Sensitivity and specificity of gene sets for hepatocellular carcinoma detection in normal controls group
| No. | Gene | TP/FN | FP/TN | Sensitivity (%)TP/(TP+FN) | Specificity (%)TN/(TN+FP) |
| 1 | MT1M | 59/62 | 2/29 | 48.8 | 93.5 |
| 2 | MT1G | 85/36 | 4/27 | 70.2 | 87.1 |
| 3 | MT1M/MT1G | 110/11 | 5/26 | 90.9 | 83.9 |
Sensitivity (%), TP/(TP + FN) and specificity (%), TN/(TN + FP) of each gene set were calculated and plotted. MT1M/MT1G, MT1M or MT1G promoter methylation. TP: True positive; FN: False negative; FP: False positive; TN: True negative; MT1M: Metallothionein 1M; MT1G: Metallothionein 1G.
Figure 4.
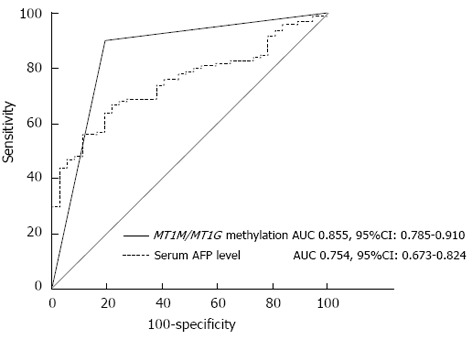
Receiver operating characteristic curves of α-fetoprotein and combined methylation of metallothionein 1M and metallothionein 1G promoters. MT1M/MT1G, MT1M or MT1G promoter methylation. AUC: Area under the ROC curve; MT1M: Metallothionein 1M; MT1G: Metallothionein 1G
DISCUSSION
DNA methylation is suggested as a promising biomarker for cancer detection. However, most studies about DNA methylation have concentrated on the analysis of tumor tissue, which is invasive and not always available, as well as one single gene, which cannot provide enough diagnostic sensitivity. In the present study, we first demonstrated that aberrant methylation status of MT1M and MT1G promoters could be detected in the serum of patients with HCC, and the frequencies were 48.8% (59/121) and 70.2% (85/121) using MSP, which were significantly higher than those in the CHB and NC groups. This was consistent with previous studies in which MT1M and MT1G promoters were methylated in HCC tissues[27,28]. From a diagnostic point of view, assaying a single gene, MT1G promoter methylation, showed a higher sensitivity of 70.2%, whereas MT1M promoter methylation gave a higher specificity to discriminate HCC from CHB (94.6%) and NCs (93.5%). However, combined methylation of MT1M and MT1G promoters significantly elevated the diagnostic sensitivity for HCC (90.9%). In addition, aberrant methylation status of MT1M and MT1G promoters was also observed in early HCC, including TNM stage I, well differentiated and small in size, as well as in patients with negative AFP. Thus, analysis of MT1M and MT1G promoter methylation showed potential value in early detection of HCC.
MT was first isolated in 1957. In addition to its function in metal homeostasis and protection against oxidative damage, several studies have focused on its role in tumors. However, large discrepancies in MT exist between different tumor types. MT expression in tumors of the lung, nasopharynx, breast, kidney, ovary, testes, thyroid, salivary gland, and urinary bladder is increased[20,21], but it is decreased in other tumors such as prostate cancer, colorectal cancer and HCC[22-25,32-34]. Compared with overall MT expression in tumors, its isoforms appear more specific and play distinct roles in different tumor types, such as breast cancer, urological malignancies, and nasopharyngeal cancer[35]. However, there are few reports on the expression of different isoforms of MT in HCC. MT1M and MT1G are two major isoforms that were recently reported to be downregulated in HCC tissues by promoter hypermethylation. Restored expression of MT1M in HCC cells impedes HCC cell growth, and low levels of MT1M are correlated with clinical TNM grade[27]. MT1G acts as a TSG in HCC and patients with MT1G promoter methylation have a poorer prognosis, although the difference is not significant[28].
In our present study, we also evaluated whether methylation status of serum MT1M and MT1G promoters in patients with HCC was associated with any clinicopathological parameter. MT1M promoter methylation was positively correlated with tumor size (rs = 0.321, P < 0.001), suggesting that methylated MT1M promoter could reflect tumor load. In addition, patients with advanced TNM stage (III-IV) showed a higher elevated percentage of serum MT1M promoter methylation than those with early TNM stage (I-II), although the difference was not significant (P = 0.058). These differences from the previous study[27] may have been due to the use of different biological samples of HCC in different regions. Surprisingly, HCC patients with combined methylation of MT1M and MT1G promoters tended to show a higher incidence of vascular invasion and lymph node or extrahepatic metastasis (P = 0.018). Tumor invasion in the portal vein is the main route for intrahepatic metastasis, which is regarded as the most frequent metastatic site of HCC[36]. Lymph node or extrahepatic metastasis is less common. Although curative resection remains a major effective method for HCC, the possibility of tumor recurrence, caused mainly by metastasis, leads to dismal prognosis. Therefore, combined methylation of serum MT1M and MT1G promoters may be a valuable prognostic marker for HCC. Also, our findings indicated that MT1M and MT1G may not only be tumor suppressors but also metastatic suppressors in HCC. However, the molecular mechanisms of this remain unclear. In previous studies, it was reported that MT1G methylation contributes to tumor invasion in prostate cancer and peripheral pulmonary adenocarcinoma[37,38]. However, to the best of our knowledge, no studies have investigated MT1M and tumor invasion. Further study is necessary to elucidate the mechanism of how MT1M and MT1G promoter methylation synergistically acts on metastasis in HCC. However, no significant differences between serum MT1M and MT1G promoter methylation and sex, age and history of HBV infection were observed, thus the analysis of serum MT1M and MT1G promoter methylation enabled the detection of HCC independent of patient settings.
Our findings demonstrated that MT isoform gene expression may be specific and reciprocal in carcinogenesis and progression of HCC. They also support the concept that the clinical significance of MT expression in HCC might be further defined if specific MT isoforms were known for individual tumors[26].
Our study had some limitations. First, the small number of HCC patients and NCs may have led to bias. Second, we do not have long-term follow-up data for HCC patients, which may reveal the predictive value of MT1M and MT1G promoter methylation in prognosis. Further study with a larger number of cases and longer follow-up is needed.
In conclusion, we demonstrated that MT1M and MT1G promoter methylation was frequently detected in serum of patients with HCC, and appeared to be a valuable diagnostic marker for noninvasive detection of HCC. Furthermore, we observed that MT1M promoter methylation was associated with tumor size and combined MT1M and MT1G promoter methylation in serum was easily detected in HCC patients with vascular invasion or metastasis, suggesting that it may be a useful prognostic marker as well.
COMMENTS
Background
Hepatocellular carcinoma (HCC) is one of the most common fatal tumors worldwide. Currently available screening tools for the diagnosis of HCC mainly depend on serum ɑ-fetoprotein and ultrasound. However, the sensitivities and specificities of the two tools remain controversial.
Research frontiers
Great efforts have been devoted to searching for new biomarkers for early diagnosis of HCC. In the present study, we demonstrated that MT1M and MT1G promoter methylation might be noninvasive biomarkers for diagnosis of HCC.
Innovations and breakthroughs
The authors demonstrated aberrant methylation of serum MT1M and MT1G promoters in HCC and reported the potential value of the two gene promoters as biomarkers for noninvasive and early diagnosis of HCC.
Applications
Serum MT1M and MT1G gene promoter methylation might be applied in the early diagnosis of HCC as novel and noninvasive biomarkers.
Terminology
MT1M and MT1G are two major isoforms in the metallothionein superfamily, and are low-molecular-weight, cysteine-rich intracellular proteins. DNA methylation is an epigenetic event to alter gene expression and function, which refers to the covalent addition of a methyl group without changing the order of bases. A biomarker is a substance used as an indicator of a biological state.
Peer review
This was a diagnostic trial. MT1M and MT1G promoter methylation is reported as serum biomarkers for HCC, which might be interesting for clinical practice.
Footnotes
Supported by National Natural Science Foundation of China, No. 81171579, No. 81201287 and No. 81371832
P- Reviewers: Luo GH, Peng T, Yu TH S- Editor: Qi Y L- Editor: Wang TQ E- Editor: Wang CH
References
- 1.Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245–1255. doi: 10.1016/S0140-6736(11)61347-0. [DOI] [PubMed] [Google Scholar]
- 2.Srivatanakul P, Sriplung H, Deerasamee S. Epidemiology of liver cancer: an overview. Asian Pac J Cancer Prev. 2004;5:118–125. [PubMed] [Google Scholar]
- 3.El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;340:745–750. doi: 10.1056/NEJM199903113401001. [DOI] [PubMed] [Google Scholar]
- 4.Taylor-Robinson SD, Foster GR, Arora S, Hargreaves S, Thomas HC. Increase in primary liver cancer in the UK, 1979-94. Lancet. 1997;350:1142–1143. doi: 10.1016/S0140-6736(05)63789-0. [DOI] [PubMed] [Google Scholar]
- 5.Mohamed NA, Swify EM, Amin NF, Soliman MM, Tag-Eldin LM, Elsherbiny NM. Is serum level of methylated RASSF1A valuable in diagnosing hepatocellular carcinoma in patients with chronic viral hepatitis C? Arab J Gastroenterol. 2012;13:111–115. doi: 10.1016/j.ajg.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 6.De Masi S, Tosti ME, Mele A. Screening for hepatocellular carcinoma. Dig Liver Dis. 2005;37:260–268. doi: 10.1016/j.dld.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 7.Farinati F, Marino D, De Giorgio M, Baldan A, Cantarini M, Cursaro C, Rapaccini G, Del Poggio P, Di Nolfo MA, Benvegnù L, et al. Diagnostic and prognostic role of alpha-fetoprotein in hepatocellular carcinoma: both or neither? Am J Gastroenterol. 2006;101:524–532. doi: 10.1111/j.1572-0241.2006.00443.x. [DOI] [PubMed] [Google Scholar]
- 8.Rivenbark AG, Coleman WB. The use of epigenetic biomarkers for preclinical detection of hepatocellular carcinoma: potential for noninvasive screening of high-risk populations. Clin Cancer Res. 2007;13:2309–2312. doi: 10.1158/1078-0432.CCR-07-0086. [DOI] [PubMed] [Google Scholar]
- 9.Zhu J. DNA methylation and hepatocellular carcinoma. J Hepatobiliary Pancreat Surg. 2006;13:265–273. doi: 10.1007/s00534-005-1054-4. [DOI] [PubMed] [Google Scholar]
- 10.Wong IH, Lo YM, Yeo W, Lau WY, Johnson PJ. Frequent p15 promoter methylation in tumor and peripheral blood from hepatocellular carcinoma patients. Clin Cancer Res. 2000;6:3516–3521. [PubMed] [Google Scholar]
- 11.Wong IH, Lo YM, Zhang J, Liew CT, Ng MH, Wong N, Lai PB, Lau WY, Hjelm NM, Johnson PJ. Detection of aberrant p16 methylation in the plasma and serum of liver cancer patients. Cancer Res. 1999;59:71–73. [PubMed] [Google Scholar]
- 12.Yeo W, Wong N, Wong WL, Lai PB, Zhong S, Johnson PJ. High frequency of promoter hypermethylation of RASSF1A in tumor and plasma of patients with hepatocellular carcinoma. Liver Int. 2005;25:266–272. doi: 10.1111/j.1478-3231.2005.01084.x. [DOI] [PubMed] [Google Scholar]
- 13.Wang J, Qin Y, Li B, Sun Z, Yang B. Detection of aberrant promoter methylation of GSTP1 in the tumor and serum of Chinese human primary hepatocellular carcinoma patients. Clin Biochem. 2006;39:344–348. doi: 10.1016/j.clinbiochem.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 14.Zhang YJ, Wu HC, Shen J, Ahsan H, Tsai WY, Yang HI, Wang LY, Chen SY, Chen CJ, Santella RM. Predicting hepatocellular carcinoma by detection of aberrant promoter methylation in serum DNA. Clin Cancer Res. 2007;13:2378–2384. doi: 10.1158/1078-0432.CCR-06-1900. [DOI] [PubMed] [Google Scholar]
- 15.West AK, Stallings R, Hildebrand CE, Chiu R, Karin M, Richards RI. Human metallothionein genes: structure of the functional locus at 16q13. Genomics. 1990;8:513–518. doi: 10.1016/0888-7543(90)90038-v. [DOI] [PubMed] [Google Scholar]
- 16.Stennard FA, Holloway AF, Hamilton J, West AK. Characterisation of six additional human metallothionein genes. Biochim Biophys Acta. 1994;1218:357–365. doi: 10.1016/0167-4781(94)90189-9. [DOI] [PubMed] [Google Scholar]
- 17.Miles AT, Hawksworth GM, Beattie JH, Rodilla V. Induction, regulation, degradation, and biological significance of mammalian metallothioneins. Crit Rev Biochem Mol Biol. 2000;35:35–70. doi: 10.1080/10409230091169168. [DOI] [PubMed] [Google Scholar]
- 18.Cai L, Satoh M, Tohyama C, Cherian MG. Metallothionein in radiation exposure: its induction and protective role. Toxicology. 1999;132:85–98. doi: 10.1016/s0300-483x(98)00150-4. [DOI] [PubMed] [Google Scholar]
- 19.Babula P, Masarik M, Adam V, Eckschlager T, Stiborova M, Trnkova L, Skutkova H, Provaznik I, Hubalek J, Kizek R. Mammalian metallothioneins: properties and functions. Metallomics. 2012;4:739–750. doi: 10.1039/c2mt20081c. [DOI] [PubMed] [Google Scholar]
- 20.Pedersen MØ, Larsen A, Stoltenberg M, Penkowa M. The role of metallothionein in oncogenesis and cancer prognosis. Prog Histochem Cytochem. 2009;44:29–64. doi: 10.1016/j.proghi.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 21.Theocharis SE, Margeli AP, Klijanienko JT, Kouraklis GP. Metallothionein expression in human neoplasia. Histopathology. 2004;45:103–118. doi: 10.1111/j.1365-2559.2004.01922.x. [DOI] [PubMed] [Google Scholar]
- 22.Jacob ST, Majumder S, Ghoshal K. Suppression of metallothionein-I/II expression and its probable molecular mechanisms. Environ Health Perspect. 2002;110 Suppl 5:827–830. doi: 10.1289/ehp.02110s5827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cai L, Wang GJ, Xu ZL, Deng DX, Chakrabarti S, Cherian MG. Metallothionein and apoptosis in primary human hepatocellular carcinoma (HCC) from northern China. Anticancer Res. 1998;18:4667–4672. [PubMed] [Google Scholar]
- 24.Datta J, Majumder S, Kutay H, Motiwala T, Frankel W, Costa R, Cha HC, MacDougald OA, Jacob ST, Ghoshal K. Metallothionein expression is suppressed in primary human hepatocellular carcinomas and is mediated through inactivation of CCAAT/enhancer binding protein alpha by phosphatidylinositol 3-kinase signaling cascade. Cancer Res. 2007;67:2736–2746. doi: 10.1158/0008-5472.CAN-06-4433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang GW, Yang LY. Metallothionein expression in hepatocellular carcinoma. World J Gastroenterol. 2002;8:650–653. doi: 10.3748/wjg.v8.i4.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tao X, Zheng JM, Xu AM, Chen XF, Zhang SH. Downregulated expression of metallothionein and its clinicopathological significance in hepatocellular carcinoma. Hepatol Res. 2007;37:820–827. doi: 10.1111/j.1872-034X.2007.00113.x. [DOI] [PubMed] [Google Scholar]
- 27.Mao J, Yu H, Wang C, Sun L, Jiang W, Zhang P, Xiao Q, Han D, Saiyin H, Zhu J, et al. Metallothionein MT1M is a tumor suppressor of human hepatocellular carcinomas. Carcinogenesis. 2012;33:2568–2577. doi: 10.1093/carcin/bgs287. [DOI] [PubMed] [Google Scholar]
- 28.Kanda M, Nomoto S, Okamura Y, Nishikawa Y, Sugimoto H, Kanazumi N, Takeda S, Nakao A. Detection of metallothionein 1G as a methylated tumor suppressor gene in human hepatocellular carcinoma using a novel method of double combination array analysis. Int J Oncol. 2009;35:477–483. doi: 10.3892/ijo_00000359. [DOI] [PubMed] [Google Scholar]
- 29.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50:661–662. doi: 10.1002/hep.23190. [DOI] [PubMed] [Google Scholar]
- 31.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 32.Garrett SH, Sens MA, Shukla D, Flores L, Somji S, Todd JH, Sens DA. Metallothionein isoform 1 and 2 gene expression in the human prostate: downregulation of MT-1X in advanced prostate cancer. Prostate. 2000;43:125–135. doi: 10.1002/(sici)1097-0045(20000501)43:2<125::aid-pros7>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 33.Zhang L, Zhou W, Velculescu VE, Kern SE, Hruban RH, Hamilton SR, Vogelstein B, Kinzler KW. Gene expression profiles in normal and cancer cells. Science. 1997;276:1268–1272. doi: 10.1126/science.276.5316.1268. [DOI] [PubMed] [Google Scholar]
- 34.Baba HA, Grabellus F, August C, Plenz G, Takeda A, Tjan TD, Schmid C, Deng MC. Reversal of metallothionein expression is different throughout the human myocardium after prolonged left-ventricular mechanical support. J Heart Lung Transplant. 2000;19:668–674. doi: 10.1016/s1053-2498(00)00074-7. [DOI] [PubMed] [Google Scholar]
- 35.Cherian MG, Jayasurya A, Bay BH. Metallothioneins in human tumors and potential roles in carcinogenesis. Mutat Res. 2003;533:201–209. doi: 10.1016/j.mrfmmm.2003.07.013. [DOI] [PubMed] [Google Scholar]
- 36.Ye QH, Qin LX, Forgues M, He P, Kim JW, Peng AC, Simon R, Li Y, Robles AI, Chen Y, et al. Predicting hepatitis B virus-positive metastatic hepatocellular carcinomas using gene expression profiling and supervised machine learning. Nat Med. 2003;9:416–423. doi: 10.1038/nm843. [DOI] [PubMed] [Google Scholar]
- 37.Chung JH, Lee HJ, Kim BH, Cho NY, Kang GH. DNA methylation profile during multistage progression of pulmonary adenocarcinomas. Virchows Arch. 2011;459:201–211. doi: 10.1007/s00428-011-1079-9. [DOI] [PubMed] [Google Scholar]
- 38.Henrique R, Jerónimo C, Hoque MO, Nomoto S, Carvalho AL, Costa VL, Oliveira J, Teixeira MR, Lopes C, Sidransky D. MT1G hypermethylation is associated with higher tumor stage in prostate cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:1274–1278. doi: 10.1158/1055-9965.EPI-04-0659. [DOI] [PubMed] [Google Scholar]


