Abstract
This report presents the case of an 8.5-year-old boy with Down syndrome after experiencing extensive caustic injury to the oesophagus and stomach resulting from the accidental ingestion of concentrated sulphuric acid. The patient had undergone 32 unsuccessful endoscopic oesophageal stricture dilatations and stenting procedures performed over a period of 15 mo following the accident. Surgical reconstruction of the oesophagus was not possible due to previous gastric and cardiac surgeries for congenital conditions. Before referring the patient for salivary fistula surgery, the patient received a nasogastric tube with perforations located above the upper margin of the oesophageal stenosis for the passage of saliva and fluid. The tube was well tolerated and improved swallowing; however the backflow of gastric contents caused recurrent infections of the respiratory tract. To overcome these problems, we developed a double lumen, varying diameter, perforated tube for protection of the oesophageal closure. This nasogastric tube was found to be safe and decreased the need for hospitalization and further endoscopic procedures. This newly developed tube can thus be considered as a treatment option for patients with recurrent oesophageal stenosis and contraindications for surgical oesophageal reconstruction.
Keywords: Corrosive oesophageal stenosis, Oesophageal dilatation, Oesophageal stenting, Nasogastric tube
Core tip: This report presents the design and use of a perforated nasogastric tube for passage of saliva and fluids in a paediatric patient who was unsuitable for oesophageal reconstructive surgery. The perforated tube was safe, well tolerated and reduced the need for hospitalization and endoscopic oesophageal dilatation. Therefore, this newly developed perforated nasogastric tube can be used as an alternative method for corrosive oesophageal stenosis therapy in patients who cannot undergo reconstructive surgery.
INTRODUCTION
Corrosive agents can result in stricture of the oesophagus and/or stomach, requiring endoscopic treatment to restore the easy passage of at least semi-solid food through the oesophagus[1,2]. Alternative therapies for treatment of these strictures include the use of stents or reconstructive surgery with an intestinal loop. This report presents the case of a paediatric patient with massive chemical injury of the upper gastrointestinal tract who underwent several methods of endoscopic and stenting therapy and could not be treated surgically. The patient was finally stabilized by the implementation of a newly developed perforated nasogastric tube for the protection of a complete oesophageal closure.
CASE REPORT
An 8.5-year-old boy with trisomy 21 was transferred to our hospital 1 mo after accidentally ingesting concentrated sulphuric acid (battery electrolyte). The accident resulted in massive chemical injury of the oesophagus and stomach, with extensive multi-level oesophageal stricture and critical pyloric stenosis. The patient’s medical history included a total colon resection for aganglionosis and cardiac surgery for a congenital heart defect. The patient required surgical pyloric bypass and gastrostomy with endless thread placement for further oesophageal stricture dilatations. The patient had undergone 18 procedures of thread-guided oesophageal dilatation and several endoscopies with Savary-Gillard dilatation over the next 10 mo. The effect of dilatation was short, with oesophageal re-stenosis present within 7-10 d of the procedure.
Eleven months after the initial chemical injury, the patient underwent implantation of a coated, metal oesophageal stent (HANAROSTENT; M.I. Tech, Gyeonggi-do, South Korea). This stent migrated to the stomach 3 d after placement and was replaced by another coated, metal stent (WallFlex; Boston Scientific, MA, United States). The patient complained of pain and a foreign body sensation in the chest. The patient tolerated solid food until week 6 and subsequently developed dysphagia related to mucosal hypertrophy and stent orifice occlusion. Removal of the stent resulted in a complete restoration of the oesophageal lumen. However, oesophageal re-stenosis occurred within 2 wk and the patient was returned to endoscopic dilatation treatment. The effects of these procedures were very short and symptoms of re-stenosis were present within a few days of the dilatation.
The lack of effective treatment and severe symptoms of oesophageal stenosis (i.e., the patient could not swallow saliva) prompted the insertion of a 16 Fr nasogastric tube with several perforations above the stricture level (Figure 1). The nasogastric tube resulted in an improvement in swallowing, and the patient’s mother reported that his clothing was no longer moistened during the day, and his pillow remained dry during sleeping. Although the patient could easily swallow saliva and liquids, a regurgitation of gastric contents through the perforations located above the stricture was frequently observed. The tube was removed after 6 wk. The oesophageal mucosa was white and fragile and bled easily, but did not have deep ulcerations. An endoscope could be introduced into the stomach without forced traction; although, the effect was not long-lasting and oesophageal re-stenosis reappeared after 2 wk.
Figure 1.
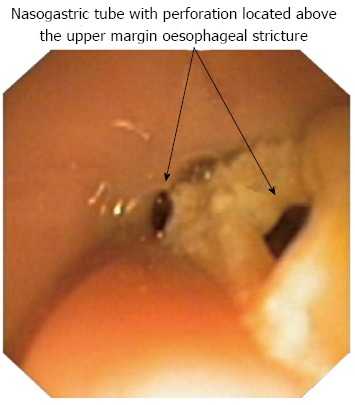
Insertion of nasogastric tube with perforations located above the stricture margin.
A new 16 Fr nasogastric tube was inserted, which was replaced 3 times and remained in place for 16 mo. Within this time period, the patient’s weight and height increased. The patient’s condition was satisfactory, and he was able to swallow saliva and liquid food. The method was generally well accepted by the patient, except for complaints regarding the thick tube extending from the nose and the frequent backflow of gastric contents to the mouth, which caused coughing and recurrent mild infections of the respiratory tract. This therapy decreased the number and duration of hospitalizations, as well as the number of endoscopic procedures (Table 1).
Table 1.
Comparison of treatments
| Oesophageal dilatation and stenting (Sep 2010-Nov 2011) | Perforated tube protecting oesophageal closure (Nov 2011-Apr 2013) | Tube developed at our institution (Apr 2013-Jan 2014) | |
| Duration of therapy, mo | 15 | 18 | 9 |
| Hospitalizations, n | 27 | 8 | 1 |
| Duration of hospitalization (d) | 112 | 32 | 3 |
| Procedures, n | 32 | 8 | 1 |
The improvement in the patient’s condition prompted the development at our institution of a specific double lumen, varying diameter, perforated, oesophageal closure protection tube (Figure 2). The closure protection part of the tube was built coaxially over a thin (8 Fr) nasogastric tube. The proximal end of the tube was fixed to the nose and the distal end was located in the stomach. The diameter and length of the portion located within the oesophageal stricture can be adjusted to the stenosis. The conical proximal end is perforated, allowing for the passage of fluids. The newly developed tube was inserted into the patient in April 2013, allowing him to easily swallow saliva and tolerate liquid diet. The coughing and respiratory tract infections related to the backflow of gastric contents were eliminated. The tube was replaced with a new, identical one after 5 mo due to partial occlusion of the tube perforations by food. The patient has continued to do well and has been gaining weight (Figure 3). During the 18 mo of therapy with a perforated nasogastric tube and 9 mo of therapy with the tube developed at our institution, there have been no complications that required hospitalization and the patient did not develop pneumonia or local complications related to the presence of the tube in the oesophagus. The implementation and removal of the tube developed at our institution was easy and not traumatic. Moreover, there was no hypertrophy of the oesophageal mucosa or tube migration.
Figure 2.
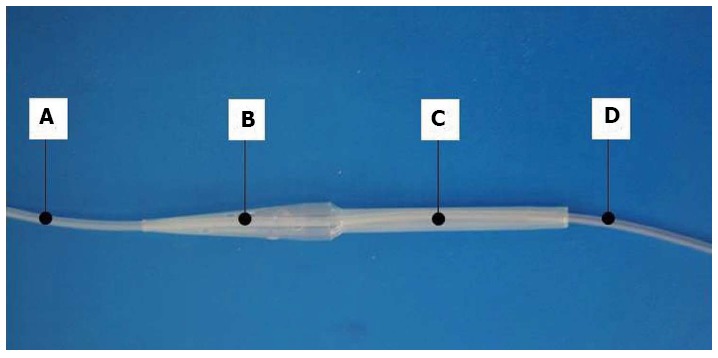
Newly developed nasogastric tube. A perforated nasogastric oesophageal closure protecting tube made of a polyamide polymer, with a double lumen and varying diameter, was developed at the Children’s Memorial Health Institute. A: Proximal part of the 8 Fr nasogastric tube; B: Conical, perforated segment setting the tube above the stenosis; C: Portion of the tube located within the stenosis and preventing it from narrowing; D: Distal end of the 8 Fr nasogastric tube to be introduced into the stomach. Liquid diet can be administered into the stomach directly by the nasogastric tube, while oral liquids and saliva can drain through the perforation in the conical portion (B) into the portion located within the stenosis (C), and further into healthy oesophagus below the stenosis. The technology of tube construction, manufacturing and prototype tube production were performed by Balton Ltd. The tube has been registered at the Polish Patent Office (No. P.399031)[9].
Figure 3.
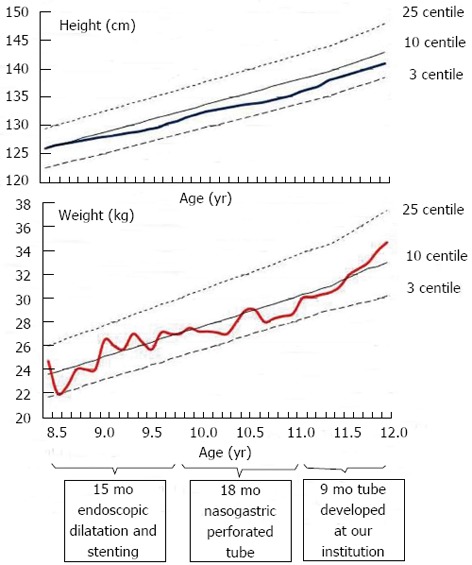
Patient’s grow charts. Height and weight of the patient during three phases of therapy: oesophageal dilatation and stenting (15 mo); nasogastric tube with perforation for saliva and fluid passage (18 mo); double lumen, varying diameter nasogastric tube developed at our institution (9 mo).
DISCUSSION
The patient described in this report had massive post-corrosive injury of the oesophagus and stomach and did not respond to oesophageal dilatation therapy. Such patients are usually referred for surgical replacement of the disintegrated oesophagus with an intestinal loop[3,4]. However, surgical replacement was not possible in this case, as the patient had previously undergone a total colectomy for treatment of Hirschprung’s disease. For these reasons, alternative methods of therapy were attempted.
Oesophageal stenting was performed using commercially available coated metal stents designed for use in adults. The stent implementation was uncomplicated, but importantly caused discomfort to the patient, and migrated or caused mucosal hypertrophy and stent orifice occlusion. The oesophageal stenting did not bring long-term benefits to our patient, who frequently returned for repeated oesophageal dilatations. Thus, the patient was considered for a salivary fistula. A nasogastric tube with perforations for saliva and liquid passage was inserted to postpone referring the patient for salivary fistula surgery, with good results. The tube remained in the oesophagus for several months, decreasing the need for repeated dilatation procedures. Although the patient could easily swallow, the large diameter of the tube extending from the nose and reflux of gastric contents to the mouth caused discomfort.
These experiences prompted us to develop a tube with varying diameters, allowing the dilatation of selected parts of the oesophagus. The purpose of the tube is to provide long-term, artificial conditions allowing oesophageal wall remodelling and final scar formation. The advantages of such a tube are the lack of margins that cause mucosal irritation and ease of removal. A similar approach has already been described in the literature[5-8]. Atabek et al[5] constructed a semi-tube oesophageal stent secured with a 4 Fr urethral catheter with the proximal end fixed to the nose and the distal end fixed to a gastrostomy tube, which was successfully used in 8 of 11 children. The margins of the semi-tube stent extended approximately 1 cm out of stricture border. Mutaf described a series of 69 children who received stenting after two unsuccessful post-injury dilatations[7]. Theses stents had an increasing diameter of 5 to 10 mm and grooves allowing oral liquid diet feeding. The stents were left in place for 1 year with no serious complications, and 69% of the treated children regained the ability to tolerate oral feeding without the need for further dilatation. Foschia et al[8] built a coaxial silicon stent over a 12-14 Fr nasogastric tube, the ends of which were tailored to allow food bolus passage. The stents were used after at least five unsuccessful post-injury dilatations and were effective in 70/79 patients.
The stent described in this case report is built over the nasogastric feeding tube fixed to the nose. Unlike the other stents described above, it has an additional soft, conical portion fixing the stent above the upper stricture margin. This conical portion has perforations that allow food passage. The length of the stent can be adjusted to the length of the oesophageal stricture, and thus there is no irritation or hypertrophy of the oesophageal wall mucosa. The stents used by other authors have been implemented relatively shortly after the corrosive injury and were successful in the majority of subjects. Our patient had stent implementation after a long period of unsuccessful therapy. We plan to gradually increase the diameter of the stent and to leave it in place for many months prior to attempting permanent removal.
The method described in this report decreased the need for repeated oesophageal dilatation, allowed for oral liquid diet feeding, and eliminated the need for saliva fistula surgery. Implementation and removal of this newly developed tube were easy, and no tube migration or complications related to the prolonged stay of the tube in the oesophagus were observed. This therapy can be considered an alternative treatment for patients with difficult corrosive oesophageal stricture, for whom oesophagus reconstruction cannot be performed.
COMMENTS
Case characteristics
An 8.5-year-old patient with extensive upper gastrointestinal tract chemical injury and contraindications for surgical oesophageal reconstruction.
Clinical diagnosis
Recurrent dysphagia not responding to endoscopic therapy.
Differential diagnosis
Congenital oesophageal ring, gastro-oesophageal reflux disease, achalasia, oesophageal tumour.
Laboratory diagnosis
All laboratory tests were within normal ranges.
Imaging diagnosis
Radiography and endoscopy showed extensive oesophageal stricture.
Pathological diagnosis
Post-chemical injury scarification of oesophageal mucosa.
Treatment
Patient underwent several unsuccessful sessions of endoscopic oesophageal stricture dilatation and stenting.
Related reports
The patient markedly improved after implementation of a nasogastric tube with perforations located above the stricture margin, which allowed for saliva and fluid passage. However, the large diameter of the tube extending from the nose was inconvenient for the patient and backflow of gastric contents to the mouth was observed.
Term explanation
The newly developed coaxial, double lumen, varying diameter tube protects the oesophageal closure and provides easy passage of saliva and fluid through the stricture; the design of the tube protects against the backflow of gastric contents to the mouth.
Experiences and lessons
The report presents a difficult to treat post-corrosive injury of the oesophagus.
Peer review
This report demonstrates the successful use of a newly developed nasogastric tube that may be an alternative therapy option in selected cases.
Footnotes
P- Reviewers: Psarras K, Rupp C., Stanciu C S- Editor: Qi Y L- Editor: A E- Editor: Wu HL
References
- 1.Kmiotek J, Woynarowski M, Celińska-Cedro D. Guidelines for the therapy of chemical esophageal burns. Standardy Medyczne. 2009;11:100–108. [Google Scholar]
- 2.Egan JV, Baron TH, Adler DG, Davila R, Faigel DO, Gan SL, Hirota WK, Leighton JA, Lichtenstein D, Qureshi WA, et al. Esophageal dilation. Gastrointest Endosc. 2006;63:755–760. doi: 10.1016/j.gie.2006.02.031. [DOI] [PubMed] [Google Scholar]
- 3.Szymczak M, Łyszkowska M, Broniszczak D, Kamiński A. Assessment of the physical development of children after esophageal reconstruction 10 years follow-up. Pediatr Wsp. 2010;12:109–112. [Google Scholar]
- 4.Szymczak M, Kamiński A, Kaliciński P, Broniszczak D. Porównanie wyników różnych sposobów rekonstrukcji przełyku z jelita u dzieci operowanych w Instytucie “Pomnik-Centrum Zdrowia Dziecka”. Przegl Chir Dziec. 2008;3:1–8. [Google Scholar]
- 5.Atabek C, Surer I, Demirbag S, Caliskan B, Ozturk H, Cetinkursun S. Increasing tendency in caustic esophageal burns and long-term polytetrafluorethylene stenting in severe cases: 10 years experience. J Pediatr Surg. 2007;42:636–640. doi: 10.1016/j.jpedsurg.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 6.Bicakci U, Tander B, Deveci G, Rizalar R, Ariturk E, Bernay F. Minimally invasive management of children with caustic ingestion: less pain for patients. Pediatr Surg Int. 2010;26:251–255. doi: 10.1007/s00383-009-2525-5. [DOI] [PubMed] [Google Scholar]
- 7.Mutaf O. Treatment of corrosive esophageal strictures by long-term stenting. J Pediatr Surg. 1996;31:681–685. doi: 10.1016/s0022-3468(96)90674-0. [DOI] [PubMed] [Google Scholar]
- 8.Foschia F, De Angelis P, Torroni F, Romeo E, Caldaro T, di Abriola GF, Pane A, Fiorenza MS, De Peppo F, Dall’Oglio L. Custom dynamic stent for esophageal strictures in children. J Pediatr Surg. 2011;46:848–853. doi: 10.1016/j.jpedsurg.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 9.Patent Office of the Republic of Poland. 013-11-12, 23/2013, P003-Zgłoszenia wynalazków lub wzorów użytkowych A1. Available from: http://regserv.uprp.pl/register/application?number=P.399031.


