Abstract
Background/Aims
To develop parameters using a combination of optical coherence tomography (OCT) and videokeratography to ‘early’ detect keratoconus.
Methods
We performed videokeratography, wavefront analysis and measured OCT indices on 180 normal, 46 moderate keratoconus, 54 early keratoconus, 7 ‘forme fruste’ keratoconus and 16 keratoconus ‘suspect” eyes, to determine the most sensitive parameters for separating these groups.
Results
A combination of videokeratography and OCT indices (I-S value and Minimum pachymetry) was statistically the most significant in separating the keratoconus groups from normals (P<.001). Using a newly derived index, the Minimum pachymetry divided by the I-S value (PA/I-S index) with a cut off of 100, we could identify 100% of “early” and ‘forme fruste’ keratoconus as being abnormal with 7 normals misclassified (misclassification rate of 2.7%). By adding keratoconus ‘suspects’ to the analysis and an I-S value of 1.2 as a cut of point, we classified 5 ‘suspects’ as normal and 11 normals as abnormal (misclassification rate 7.8%). The PA/I-S index, with a cut of point of 100, reduced this misclassification rate to 4.4%.
Conclusion
These results suggest that OCT combined with videokeratography may be more useful for differentiating mild forms of keratoconus, than videokeratography alone.
INTRODUCTION
The ‘early’ detection of keratoconus is important for refractive surgery screening, the understanding of the genetics of keratoconus; and corneal collagen cross linking treatments1–5.
Typically Keratoconus can be diagnosed by well-recognized biomicroscopic and external signs6. However, some patients with ‘early’ disease do not present with clinical signs and their diagnosis requires an appreciation of subtle changes of the topography of the cornea.
Marc Amsler was the first to describe early corneal topographic changes in keratoconus patients prior to detectable clinical signs using a photographic placido disc7,8. Vdeokeratography provided an opportunity to replicate Amsler’s work and better define ‘early’ forms of keratoconus. Our group has described videokeratography patterns and indices, which can be used to ‘early’ detect keratoconus9–15. However, none of these indices are 100% accurate, in differentiating normal and ‘forme fruste’ keratoconus.
Using wavefront technology, we and others demonstrated that a combination of wavefront and videokeratograpy indices could increase the sensitivity of ‘early’ detection of keratoconus16,17. Videokeratography only examines the anterior surface of the cornea and it has been suggested that a major limitation of this technology is that it does not measure the posterior surface of the cornea, which may be important for ‘early’ keratoconus detection18,19.
Anterior segment optical coherence tomography (OCT) of the cornea that has the potential to address this concern. It maps the cornea in a 3 dimensional manner including accurate imaging of the posterior corneal surface20. It has also been shown to be very accurate and reproducible in measuring cross sectional pachymetry with broad corneal coverage and can differentiating patients with clinical keratoconus from normals21.
In this study we sought to establish a normative database of OCT indices for future research and to devise algorithms using a combination of videokeratography, and OCT indices for separating mildly abnormal corneas ‘early’ keratoconus, ‘forme fruste’ keratoconus and keratoconus ‘suspects’ from the normal population.
PATIENTS AND METHODS
Patients
This prospective comparative study included 303 eyes of patients presented to the Cornea Genetic Eye Institute, either as part of the longitudinal evaluation of keratoconus genetics study or for screening prior to refractive surgery. They included 180 normal eyes, 46 eyes with moderate keratoconus, 54 eyes with early keratoconus, 7 eyes with ‘forme fruste’ keratoconus and 16 eyes keratoconus ‘suspect’ eyes. Normal eyes were selected from patients who presented for LASIK surgery screening who were deemed suitable candidates based on videokeratography and clinical findings or their spouses. The study was conducted in concordance with the provisions of the Declaration of Helsinki.
Clinical diagnosis
For the purposes of this study a patient was labeled as moderate keratoconus, if they had stromal thinning on slit-lamp evaluation, scissoring on retinoscopy, an AB/SRAX videokeratography pattern, and an average K reading of greater than 47 Diopters but less than 55 Diopters. Patients with any form of scarring were excluded from this study. A patient was labeled as ‘early” keratoconus if they had scissoring on retinoscopy, an AB/SRAX videokeratography pattern and an average K reading of less than 47 Diopters. Patients were labeled as ‘forme fruste’ keratoconus, if they were the fellow eye of patients with keratoconus, had an AB/SRAX videokeratography pattern and no clinical signs of keratoconus. A patient was labeled as keratoconus ‘suspect’, if it was the fellow eye of a patient with keratoconus, had mild inferior steepening on topography and no clinical signs of keratoconus22 (see Table 1 for classification scheme). Any patient with an AB/SRAX videokeratography pattern; a ‘suspicious’ inferior steepening videokeratograph or a family history of keratoconus was excluded from the normal study pool11,22. All patients had a slit-lamp evaluation and a detailed ophthalmological evaluation including dilated retinoscopy. Patients also underwent the following anciliary tests: videokeratography, wavefront aberrometry and corneal OCT pachymetry.
Table 1.
Keratoconus classification scheme for the purposes of this study only
| Keratoconus(KC) subtype | Clinical findings | Average K readings | Videokeratography pattern |
|---|---|---|---|
| 1. Moderate KC | slit-lamp – stromal thinning retinoscopy – scissoring |
> 47 diopters | AB/SRAX |
| *2. ‘Early’ KC | slit-lamp – normal retinoscopy - scissoring |
< 47 diopters | AB/SRAX |
| *3. ‘Forme fruste’ KC | slit-lamp – normal retinoscopy – normal |
< 47 diopters | AB/SRAX |
| *4. KC ‘suspect’ | slit-lamp – normal | < 47 diopters | inferior steepening |
mild forms of keratoconus
Videokeratography
Videokeratography was performed using the TMS-4 (Tomey Corp, Nagoya, Japan) in the sagittal mode. All patients studied had to be out of soft contact lenses for at least 10 days and rigid contact lenses for at least 1 month prior to being mapped. All maps were printed in the absolute scale. Videokeratography indices studied were the IS value, Average K and regular astigmatism9. These three variables were selected since they had been previously described as being very sensitive for differentiating early keratoconus from normals9,10,11. The I-S value is calculated by averaging data points on rings 14, 15, and 16 of the videokeratographs generated by the TMS-4 instrument at approximately 3mm inferior to the center of the cornea at thirty degree intervals (that is, 210, 240, 270, 300, and 330 degrees) (five data points on each of the three rings). The values of these 15 data points are averaged to give a single dioptric value, I. Averaging the data points on rings 14, 15, and 16 at 3mm superior to the center of the cornea at 30 degree intervals (that is, 30, 60, 90,120, and 150 degrees) makes a similar calculation. These 15 points are averaged to give a single dioptric value, S. The superior value is subtracted from the Inferior value to give the I-S value. A positive I-S value indicates a relatively steeper inferior cornea, while a negative I- S values indicates a relatively steeper superior cornea9,10,15. For the purposes of this study we studied the absolute I-S values only.
Wavefront aberrometry
Hartmann-Shack aberrometry (LADARWave; Alcon Laboratories Inc, Ft Worth, TX) was performed on all normal and early keratoconus patients, and the relevant Zernike polynomials were recorded. For all patients, a pupil size of 6.5 mm was selected to acquire wavefront data16.
Corneal OCT pachymetry measurements
A pachymetry map of the cornea was acquired using a high-speed anterior segment Optical Coherence Tomographer Rtvue (Optovue, Fremont, CA) which operates at an 840-nanometer wavelength with a scan rate of 26,000 axial scans per second. A pachymetry scan pattern (8 radials, approximately 800 scans each: 6mm diameter) centered at the corneal vertex was divided into zones by octants and annular rings. Each eye was scanned 5 times within a single visit and the pachymetry maps were calculated. The central corneal thickness (CCT) was identified as the pachymetry reading at the center of the cornea. The maps were divided into zones by octants: superior (S), superotemporal (ST), temporal (T), inferotemporal (IT), inferior (I), inferonasal (IN), nasal (N), superonasal (SN), and annular rings (2, 5, 7, and 10 mm diameter as previously described20.
Several diagnostic parameters constructed from the OCT pachymetric map with the aim of capturing the focal and asymmetric nature of keratoconus corneal thinning were used. The parameters were calculated from the central 5 mm diameter of the pachymetry map. The octant values were averaged in the 2- to 5-mm diameter zone. OCT pachymetric data were calculated based on signals obtained from both the anterior and posterior surfaces of the cornea.
The five pachymetric diagnostic parameters were as follows: 1. Minimum-median; 2. The S-I: The average thickness of the inferior (I) octant minus that of the superior (S) octant; 3. The IT-SN: The average thickness of the IT octant minus that of the SN octant; 4. Minimum (Min); 5. Vertical location of the minimum. Focal thinning was captured by the minimum–median and minimum parameters. Asymmetric thinning was captured by the S-I and IT-SN parameters, and by the vertical location of the minimum. The method for derivation of these indices has previously been described in detail21.
Statistical methods
Statistical analyses were performed using SAS version 9.2 (SAS Institute Inc., Cary, NC). Twelve videokeratography, wavefront and OCT variables were analyzed and checked by distribution first, and then the comparisons were performed based on the distribution. The absolute value of the I-S was used in the analysis. Comparisons between two groups were tested by the Student t-test when variables had a normal distribution, or by the non-parametric Wilcoxon rank test when traits deviated from normal distribution. In order to evaluate the diagnostic performance of each variable and select sensitive indices for further analysis, the receiver operating characteristic (ROC) curve analysis was performed using data from groups of normals and keratoconus. The area under the ROC curve (AUC) was calculated for variables’ selection. Thus, we generated a combination of the most significant variables based on the highest AUC (“pachymetry/asymmetry” index or PA/I-S index which is equal to the Minimum pachymetry derived from OCT measurements divided by 4 times the I-S value (+.1)) and tested them using the same strategy for the single variable’s testing of applying either t-test or Wilcoxon test depending on the distribution. Since the I-S values of many normal eyes are close to 0, the formula with (+.1) is to make the calculation possible for those samples. The estimated normality plot of log transformed PA/I-S was also presented for each group. The significance level of 0.05 was used for the statistical tests.
Initially we tested three groups of patients and controls: the normal group, the early keratoconus group and the moderate keratoconus group, for the twelve videokeratography, wavefront and OCT variables The normal and early keratoconus groups were compared to determine which combination of variables were most sensitive in differentiating early keratoconus patients from normals. When multiple comparisons were conducted, P values were presented after adjusting for the number of multiple testing.
Once it was determined that a combination of videokeratography and OCT was more sensitive than videokeratography and wavefront, the wavefront parameters were dropped and the keratoconus ‘suspect’ and ‘forme fruste’ groups were analyzed with videokeratography and OCT only.
RESULTS
In total, twelve variables based on videokeratography, wavefront and OCT measurements were selected to perform comparisons between the study groups (Table 2). Significant differences were identified among three groups for most tested variables. I-S was the most significant index to separate normal controls and early keratoconus, while average K value seems to be the best trait to distinguish keratoconus from both normal controls, and early keratoconus. The AUC was identified for I-S value (0.99) and Minimum pachymetry (Min, 0.95), respectively. Thus, a combination of two most significant variables, I-S value and Min pachymetry, was calculated and seems to be the best for differentiating early keratoconus from normals demonstrating a modest improvement over our previously reported combination of variables of vertical Coma and I-S (16, Table 3).
Table 2.
Comparison of videokeratography and OCT measures between groups of normal controls, early keratoconus and moderate keratoconus.
| Variable | Normal (N=180) | Early (N=54) | Moderate (N=46) | AUC (Normal vs KC) | P value | ||
|---|---|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | Normal vs. Early | Normal vs. Moderate | Early vs. Moderate | ||
| Average K | 43.68(1.34) | 45.07(1.77) | 51.39(2.96) | 0.85 | <0.001 | <0.001† | <0.001* |
| Cylinder | 0.92(0.71) | 3.20(2.13) | 5.51(2.57) | 0.93 | <0.001 | <0.001 | <0.001 |
| I-S value | 0.41(0.38) | 5.95(3.03) | 6.45(2.72) | 0.99 | <0.001* | <0.001* | 0.34 |
| Coma | 0.25(0.19) | 2.38(1.57) | 2.07(0.91) | 0.96 | <0.001 | <0.001 | 0.60 |
| RMS | 6.24(3.96) | 6.83(2.83) | 8.15(3.79) | 0.59 | 0.22 | 0.18 | 0.25 |
| Sph_Abb | 0.22(0.22) | 0.28(0.30) | 0.70(0.97) | 0.57 | 0.49 | 0.11 | 0.32 |
| SN-IT | 29.42(15.58) | 67.61(22.61) | 85.13(39.66) | 0.93 | <0.001 | <0.001 | 0.03 |
| S-I | 26.88(16.39) | 65.43(28.07) | 83.96(50.35) | 0.90 | <0.001 | <0.001 | 0.06 |
| Min-Median | −24.91(11.02) | −50.31(16.97) | −74.46(35.54) | 0.94 | <0.001 | <0.001 | <0.001 |
| Min-Max | −67.44(16.92) | −105.81(31.04) | −149.70(58.41) | 0.92 | <0.001 | <0.001 | <0.001† |
| Minimum pachymetry | 537.3(35.1) | 459.7(37.6) | 413.6(52.3) | 0.95 | <0.001† | <0.001 | <0.001 |
| CCT | 548.39(33.28) | 494.09(37.39) | 459.07(42.58) | 0.89 | <0.001 | <0.001 | <0.001 |
the most significant p value;
the second most significant p value;
SD: Standard Deviation.
Table 3.
Comparison of combination of videokeratography and OCT measures between groups of normal controls, early keratoconus and moderate keratoconus.
| Variable | Normal (N=180) | Early (N=54) | Moderate (N=46) | P value | ||
|---|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | Normal vs. Early | Normal vs. Moderate | Early vs. Moderate | |
| PA/I-S† | 550(501) | 26(16) | 19(10) | <0.001* | <0.001* | 0.07 |
| K_Min† | 12.3(0.9) | 10.2(1.0) | 8.1(1.2) | <0.001 | <0.001 | <0.001* |
| Coma_IS† | 2.64(0.26) | 4.43(0.56) | 4.31(0.30) | <0.001 | <0.001 | 0.43 |
the most significant p value;
- PA/I-S=minimum pachymetry/(I-S value+0.1)/4;
- K_Min= minimum pachymetry /average K;
- Coma_IS=log((vertical coma+10)*( I-S value +1));
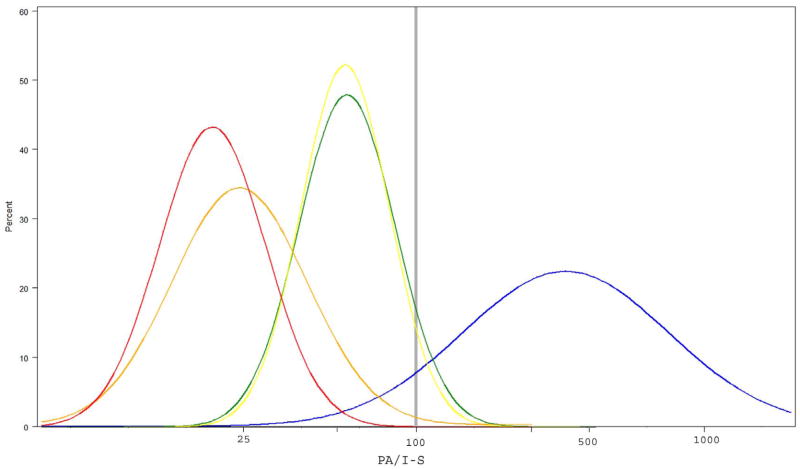
We have also formulated a novel index, we named the “pachymetry/asymmetry” index or PA/I-S index, which is equal to the Minimum pachymetry derived from OCT measurements divided by 4 times the I-S value (+.1). We estimated that 95% normals had PA/I-S value greater than 106, and 95% early keratoconus between 10 to 57 (Figure 1, Table 4). This index was developed retrospectively from the data presented here and is specific to this dataset. To test sensitivity of this new index we analyzed the ‘forme fruste’ and keratoconus ‘suspect’ groups and compared them to normals and early keratoconus groups. Using a threshold of 100 we were able to correctly classify 100% of ‘forme fruste’ keratoconus and 87.5% (14 out of 16) of keratoconus ‘suspects’ (Table 5), and successfully separate moderate keratoconus, early keratoconus, ‘forme fruste’ keratoconus, keratoconus ‘suspects’ and normal groups (Figure 1).
Figure 1.
Estimated normality plot of PA/I-S among groups. Log-scaled PA/I-S values are presented on X-axis. Blue: normal controls; Yellow: ‘Forme fruste’ keratoconus; Green: keratoconus ‘suspect’; Orange: early keratoconus; Red: moderate keratoconus. Grey line indicates 100 threshold.
Table 4.
Comparison of I-S, Minimum pachymetry and PA/I-S measures between normal controls, ‘forme fruste’, keratoconus ‘suspect’ and early keratoconus.
| Variable | Normal N=180 |
‘Forme fruste’ N=7 |
Suspect N=16 |
Early N=54 |
P value | |||
|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Normal vs. ‘Forme fruste’ | Normal vs. Suspect | ‘Forme fruste’ vs. Early | Suspect vs. Early | |
| I-S value | 0.41 (0.38) | 2.19 (0.89) | 2.25 (0.97) | 5.95 (3.03) | <0.001* | <0.001* | 0.004* | <0.001 |
| Minimum pachymetry | 537.3 (35.1) | 477.6 (35.8) | 492.4 (34.1) | 459.7 (37.6) | 0.003 | <0.001 | 0.39 | 0.03 |
| PA/I-S† | 550 (501) | 59 (21) | 61 (24) | 26 (16) | <0.001 | <0.001 | 0.004 | <0.001* |
the most significant p value
PA/I-S=minimum pachymetry/(I-S value+0.1)/4
Table 5.
Ages, I-S values, Minimum pachymetry and PA/I-S index of subjects in ‘suspect’ and ‘forme fruste’ groups.
| Group | Age | I-S value | Minimum pachymetry | PA/I-S† |
|---|---|---|---|---|
| ‘Suspect’ | 33 | 3.9 | 448 | 28 |
| 31 | 4.2 | 532 | 31 | |
| 24 | 3.6 | 471 | 32 | |
| 24 | 3 | 499 | 40 | |
| 31 | 2.7 | 470 | 42 | |
| 43 | 2.1 | 458 | 52 | |
| 27 | 2.1 | 478 | 54 | |
| 27 | 2.1 | 488 | 56 | |
| 43 | 1.8 | 439 | 58 | |
| 22 | 2.1 | 524 | 60 | |
| 33 | 1.8 | 553 | 73 | |
| 34 | 1.5 | 469 | 73 | |
| 26 | 1.5 | 498 | 78 | |
| 32 | 1.2 | 485 | 93 | |
| 24 | 1.2 | 532 | 102 | |
| 38 | 1.2 | 534 | 103 | |
| ‘Forme fruste’ | 58 | 3.9 | 472 | 30 |
| 35 | 2.7 | 467 | 42 | |
| 27 | 2.1 | 451 | 51 | |
| 21 | 2.1 | 466 | 53 | |
| 18 | 1.8 | 508 | 67 | |
| 41 | 1.2 | 437 | 84 | |
| 33 | 1.5 | 542 | 85 |
PA/I-S=minimum pachymetry/(I-S value+0.1)/4
Using this new PA/I-S index, we were also able to improve misclassification rate of keratoconus ‘suspects’, an especially hard to diagnose group of patients. Using an IS value of 1.2 we classified 5 keratoconus suspects as normal and 11 normals as keratoconus suspects for a misclassification rate of 7.8%. However, using the new PA/IS index threshold of 100 we misclassified 2 keratoconus suspects as normal and 7 normals as keratoconus suspects for a reduced misclassification rate of 4.4%. The misclassification rates within groups were 3.9% in normals and 12.5% in keratoconus suspects, respectively. Using normal subjects as negative controls and PA/I-S threshold of 100, the positive predictive value and negative predictive value of keratoconus suspects were 63.2% and 97.8%, respectively.
With the PA/I-S index, we were also able to improve diagnosis of the ‘forme fruste’ keratoconus group compared to using an I-S value alone. Using I-S value of 1.2 we would detect only 6 out of 7 (85.7%) ‘forme fruste’ keratoconus while the new “pachymetry/asymmetry” index threshold of 100 would detect all 7 out of 7 (100%) (Table 4). 100% of both early and ‘forme fruste’ keratoconus groups were classified correctly by using PA/I-S index threshold of 100 with only 7 normals misclassified as ’forme fruste’ or early keratoconus with an overall misclassification rate of 7/257 or 2.7% (Tables 4 and 5). With PA/I-S index threshold of 100, the positive predictive value and negative predictive value of ‘forme fruste’ keratoconus were 50% and 100% from negative normal subjects.
DISCUSSION
Most clinicians would agree that detecting moderate to advanced keratoconus with obvious clinical signs is relatively routine. However, detection of the ‘early’ forms of the disease in the absence of slit-lamp findings remains a challenge6,7,11,23–25.
Videokeratography has significantly enhanced our ability to detect ‘early’ disease using both pattern recognition and a series of indices to enhance pattern recognition. In order to enhance abnormal pattern recognition, our group published a normative database of videokeratography patterns using sagittal topography and a classification scheme based on videokeratography and clinical signs to bring uniformity to the study and identification of ‘early’ disease9,10,22.
In a study of families with keratoconus using videokeratography, we initially described patterns with inferior steepening in 50% of family members15. However, in our normative database of videokeratography patterns we demonstrated that 20% of normal patients also had patterns with inferior steepening22. This led to the description of the term keratoconus ‘suspect’ i.e. patterns of inferior steepening that are suspicious and would need to be followed over time to determine whether they may be at risk for developing keratoconus26.
One pattern with inferior steepening the asymmetric bowtie with skewed steep radial axis (AB/SRAX) pattern, was found in only 1/200 (0.5%) of the normal population but in almost 100% of patients with ‘early’ keratoconus22. Longitudinal analysis of videokeratography patterns of the normal fellow eye of unilateral keratoconus over an 8 year period followed by our group in a previously reported study demonstrated that 50% of eyes with an AB/SRAX pattern ultimately progress to keratoconus confirming that this videokeratography pattern is a phenotypic marker for ‘forme fruste’ keratoconus)11,27.
To determine which eyes with inferior steepening are abnormal and develop a quantitative phenotype for molecular genetic studies, we developed a series of quantitative indices: the I-S value, the SRAX index and an index using both these indices the KISA% index9,10,11. These indices are useful but have their limitations. As shown in this study the I-S value, while extremely useful and simple to use, could detect 85.7% of patients with ‘forme fruste’ keratoconus, but has a misclassification rate of 7.8% when trying to separate ‘suspects’ from normals. The KISA% index is another useful quantitative phenotype). However, it works well only in individuals who have at least 1.5D of astigmatism, which may be absent in a significant proportion of ‘suspects’. We know that as keratoconus advances the cornea becomes thinner, however, pachymetry alone is not sufficient in differentiating keratoconus from normals because of the large variation in normal pachymetry28. The pachymetry/asymmetry index is, however, derived from technologies that take into account both the anterior(videokeratography) and posterior surface of the cornea(OCT pachymetry)29,30. It is simple to calculate manually ((PA/(I-S(+.1)*4)). The Minimum pachymetry divided by 4 times the I-S value +0.1. It works very well on corneas with less than 1.5D of astigmatism, a potentially critical subgroup of ‘suspects’. It also provides a good linear index which can be used as quantitative phenotype for monitoring the progression of keratoconus and for use in genome wide association (GWA) studies of keratoconus. Since PA/I-S index is based on using the thinnest point on the cornea and the asymmetry of the cornea, it stands to reason that the more advanced the keratoconus is the lower this value will be since the pachymetry value will be lower and the I-S value will be higher. The more normal the eye the higher this value will be since the pachymetry will be larger and the I-S value will be smaller. Figures 2, 3, 4 & 5 show examples of PA/I-S index calculated for normals, ‘forme fruste’ keratoconus and early keratoconus and for a patient with suspicious topography who presented for the refractive surgery screening. The patient with abnormal topography(Figure 5) had a PA/I-S value of less than 100 (94) which would put her into the “forme fruste” category and exclude her as a candidate for LASIK surgery despite a normal clinical exam, plano − 1.50 refraction( and 20/20 correctable vision) and no scissoring on retinoscopy. In summary, in this study we demonstrate the potential for improving differentiating keratoconus ‘suspects’ and ‘forme fruste’ keratoconus from normal corneas by using a combination of videokeratography and OCT. The pachymetry/asymmetry index is derived from technologies that take into account select data points from both the anterior corneal surface (videokeratography) and posterior surface of the cornea (OCT pachymetry), which could be used to differentiate mild keratoconus subtypes from the normal population during refractive surgery screening. This study does have several limitations however, these are 1) the study group and test groups are the same; 2) we looked at individual eyes only, not individual patients where information from both eyes could be used for ‘early’ detection and 3) the forme fruste and suspect groups were small compared to the normal group. Expanded studies on larger numbers of eyes and testing our new algorithm on an independent test sample may validate our preliminary findings which suggest that adding OCT to videokeratography for refractive surgery screening will improve the detection of mildly abnormal corneas which may be at risk for refractive surgery.
Figure 2.
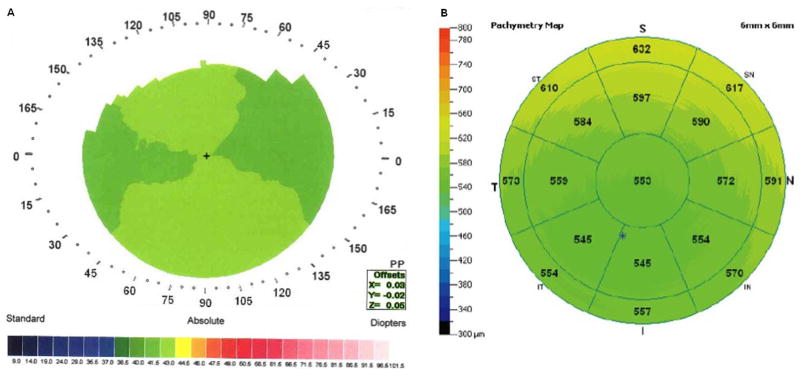
Normal cornea with high PA/I-S value (337). A: Videokeratography; B: OCT.
Figure 3.
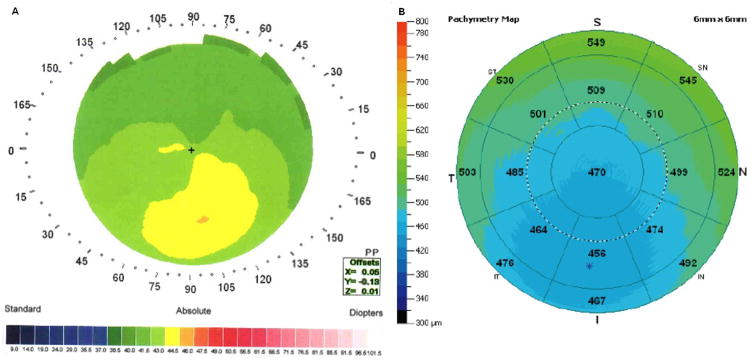
‘Forme fruste’ keratoconus with a lower PA/I-S value (53).
A: Videokeratography; B: OCT.
Figure 4.
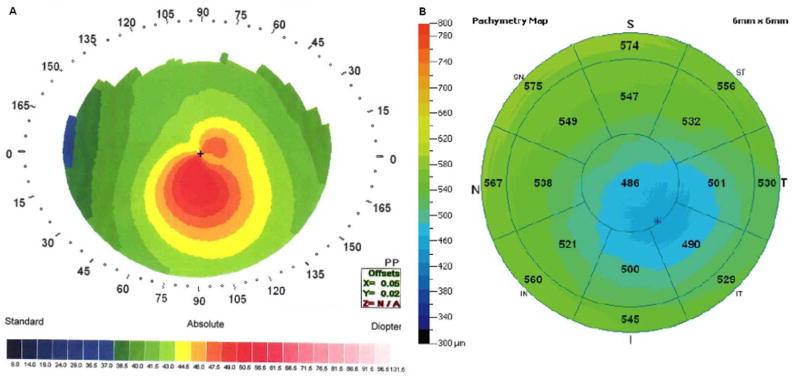
Early keratoconus with an even lower PA/I-S value (30).
A: Videokeratography; B: OCT.
Figure 5.
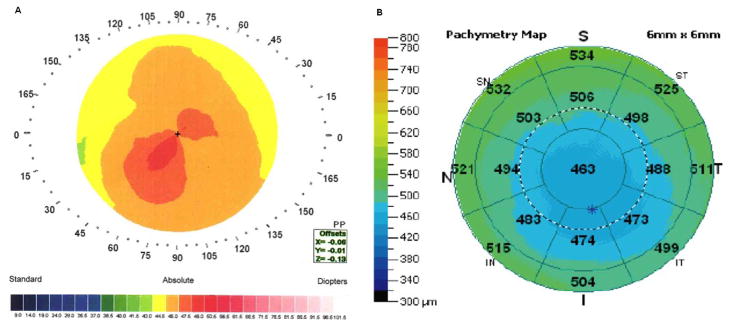
Candidate for refractive surgery with a PA/I-S value of less than 100 (94):
A: Videokeratography; B: OCT
Acknowledgments
This work was supported by a grant from the National Eye Institutes of Health NEI – RO1- 09052, the Skirball Foundation for Molecular Ophthalmology, Los Angeles, USA, and the Eye Defects Research Foundation Inc., Beverly Hills, CA, USA.
Footnotes
Contribution:
Dr. Yaron S. Rabinowitz: study design and manuscript drafting;
Dr. Xiaohui Li: statistical analysis;
Dr. Ana Laura Caiado Canedo: data interpretation;
Dr. Renato Ambrosio Jr.: data interpretation;
Dr. Yelena Bykhovskaya: manuscript revising.
Conflict of Interest: No conflicting relationship exists for any author.
References
- 1.Wilson SE, Klyce SD. Screening for corneal topographic abnormalities before refractive surgery. Ophthalmology. 1994;101(1):147–152. doi: 10.1016/s0161-6420(94)31372-8. [DOI] [PubMed] [Google Scholar]
- 2.Nielsen K, Hjortdal J, Pihlmann M, Corydon TJ. Update on the keratoconus genetics. Acta Ophthalmol. 2012 doi: 10.1111/j.1755-3768.2012.02400.x. [DOI] [PubMed] [Google Scholar]
- 3.Keating A, Pineda R, 2nd, Colby K. Corneal cross linking for keratoconus. Semin Ophthalmol. 2010 Nov 26;25(5–6):249–255. doi: 10.3109/08820538.2010.518503. [DOI] [PubMed] [Google Scholar]
- 4.Ambrosio R, Jr, Randleman JB. Screening for ectasia risk: what are we screening for and how should we screen for it? J Refract Surg. 2013 Apr 06;29(4):230–232. doi: 10.3928/1081597X-20130318-01. [DOI] [PubMed] [Google Scholar]
- 5.Randleman JB, Trattler WB, Stulting RD. Validation of the Ectasia Risk Score System for preoperative laser in situ keratomileusis screening. Am J Ophthalmol. 2008 Mar 11;145(5):813–818. doi: 10.1016/j.ajo.2007.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rabinowitz YS. Keratoconus. Surv Ophthalmol. 1998;42(4):297–319. doi: 10.1016/s0039-6257(97)00119-7. [DOI] [PubMed] [Google Scholar]
- 7.Amsler M. Le keratosonus fruste au javal. Ophthalmologica. 1938;96:77–83. [Google Scholar]
- 8.Amsler M. Keratocone classique et keratocone fruste, arguments unitaires. Ophthalmologica. 1946;111:96–101. doi: 10.1159/000300309. [DOI] [PubMed] [Google Scholar]
- 9.Rabinowitz YS. Videokeratographic indices to aid in screening for keratoconus. J Refract Surg. 1995;11(5):371–379. doi: 10.3928/1081-597X-19950901-14. [DOI] [PubMed] [Google Scholar]
- 10.Rabinowitz YS, Rasheed K. KISA% index: a quantitative videokeratography algorithm embodying minimal topographic criteria for diagnosing keratoconus. J Cataract Refract Surg. 1999;25(10):1327–1335. doi: 10.1016/s0886-3350(99)00195-9. [DOI] [PubMed] [Google Scholar]
- 11.Li X, Rabinowitz YS, Rasheed K, Yang H. Longitudinal study of the normal eyes in unilateral keratoconus patients. Ophthalmology. 2004;111(3):440–446. doi: 10.1016/j.ophtha.2003.06.020. [DOI] [PubMed] [Google Scholar]
- 12.Wygledowska-Promienska D, Zawojska I. Procedure for keratoconus detection according to the Rabinowitz-Rasheed method--personal experience. Klin Oczna. 2000 Apr 09;102(4):241–244. [PubMed] [Google Scholar]
- 13.Zghal I, Saragoussi JJ, Cotinat J, Renard G, Pouliquen Y. Quantitative topographic detection of keratoconus in the contralateral eye in clinically unilateral keratoconus. Apropos of 5 cases. J Fr Ophtalmol. 1997 Jan 01;20(4):284–291. [PubMed] [Google Scholar]
- 14.Salabert D, Cochener B, Mage F, Colin J. Keratoconus and familial topographic corneal anomalies. J Fr Ophtalmol. 1994 Jan 01;17(11):646–656. [PubMed] [Google Scholar]
- 15.Rabinowitz YS, Garbus J, McDonnell PJ. Computer-assisted corneal topography in family members of patients with keratoconus. Arch Ophthalmol. 1990;108(3):365–371. doi: 10.1001/archopht.1990.01070050063032. [DOI] [PubMed] [Google Scholar]
- 16.Jafri B, Li X, Yang H, Rabinowitz YS. Higher order wavefront aberrations and topography in early and suspected keratoconus. J Refract Surg. 2007;23(8):774–781. doi: 10.3928/1081-597X-20071001-06. [DOI] [PubMed] [Google Scholar]
- 17.Alio JL, Shabayek MH. Corneal higher order aberrations: a method to grade keratoconus. J Refract Surg. 2006 Jun 30;22(6):539–545. doi: 10.3928/1081-597X-20060601-05. [DOI] [PubMed] [Google Scholar]
- 18.Ambrosio R, Jr, Caiado AL, Guerra FP, Louzada R, Roy AS, Luz A, Dupps WJ, Belin MW. Novel pachymetric parameters based on corneal tomography for diagnosing keratoconus. J Refract Surg. 2011 Aug 02;27(10):753–758. doi: 10.3928/1081597X-20110721-01. [DOI] [PubMed] [Google Scholar]
- 19.Saad A, Gatinel D. Topographic and tomographic properties of forme fruste keratoconus corneas. Invest Ophthalmol Vis Sci. 2010 Jun 18;51(11):5546–5555. doi: 10.1167/iovs.10-5369. [DOI] [PubMed] [Google Scholar]
- 20.Qin B, Zhou XT, Huang D, Chu RY. Effects of age on ocular anterior segment dimensions measured by optical coherence tomography. Chin Med J (Engl) 2011 Jul 12;124(12):1829–1834. [PubMed] [Google Scholar]
- 21.Li Y, Meisler DM, Tang M, Lu AT, Thakrar V, Reiser BJ, Huang D. Keratoconus diagnosis with optical coherence tomography pachymetry mapping. Ophthalmology. 2008 Nov 04;115(12):2159–2166. 2652571. doi: 10.1016/j.ophtha.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rabinowitz YS, Yang H, Brickman Y, Akkina J, Riley C, Rotter JI, Elashoff J. Videokeratography database of normal human corneas. Br J Ophthalmol. 1996;80(7):610–616. doi: 10.1136/bjo.80.7.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seiler T, Quurke AW. Iatrogenic keratectasia after LASIK in a case of forme fruste keratoconus. J Cataract Refract Surg. 1998;24(7):1007–1009. doi: 10.1016/s0886-3350(98)80057-6. [DOI] [PubMed] [Google Scholar]
- 24.Hutchings H, Ginisty H, Le Gallo M, Levy D, Stoesser F, Rouland JF, Arne JL, Lalaux MH, Calvas P, Roth MP, Hovnanian A, Malecaze F. Identification of a new locus for isolated familial keratoconus at 2p24. J Med Genet. 2005;42(1):88–94. doi: 10.1136/jmg.2004.022103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Y, Huang J, Amos CI. Genetic association analysis of complex diseases incorporating intermediate phenotype information. PLoS One. 2012 Oct 25;7(10):e46612. 3477105. doi: 10.1371/journal.pone.0046612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Waring GO, Rabinowitz YS, Sugar J, Damiano R. Nomenclature for keratoconus suspects. Refract Corneal Surg. 1993;9(3):219–222. [PubMed] [Google Scholar]
- 27.Li X, Yang H, Rabinowitz YS. Keratoconus: classification scheme based on videokeratography and clinical signs. J Cataract Refract Surg. 2009;35(9):1597–1603. doi: 10.1016/j.jcrs.2009.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rabinowitz YS, Rasheed K, Yang H, Elashoff J. Accuracy of ultrasonic pachymetry and videokeratography in detecting keratoconus. J Cataract Refract Surg. 1998;24(2):196–201. doi: 10.1016/s0886-3350(98)80200-9. [DOI] [PubMed] [Google Scholar]
- 29.Li Y, Shekhar R, Huang D. Corneal pachymetry mapping with high-speed optical coherence tomography. Ophthalmology. 2006 May 03;113(5):792–799. e792, 1474520. doi: 10.1016/j.ophtha.2006.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Y, Tang M, Zhang X, Salaroli CH, Ramos JL, Huang D. Pachymetric mapping with Fourier-domain optical coherence tomography. J Cataract Refract Surg. 2010 May 12;36(5):826–831. 2872166. doi: 10.1016/j.jcrs.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]