Abstract
Hypothalamic neurons that co-express agouti-related protein (AgRP), neuropeptide Y (NPY), and γ-amino-butyric acid (GABA) are known to promote feeding and weight gain by integration of various nutritional, hormonal, and neuronal signals1,2. Ablation of these neurons leads to cessation of feeding that is accompanied by Fos activation in most regions where they project3–6. Previous experiments indicate that the ensuing starvation is due to aberrant activation of the parabrachial nucleus (PBN) and it could be prevented by facilitating GABAA receptor signaling in the PBN within a critical adaptation period5. We hypothesized that loss of GABAergic inhibition from AgRP neurons to the PBN leads to abnormal activation of the PBN, which in turn inhibits feeding. However, the source of the excitatory inputs to the PBN was unknown. Here we show that glutamatergic neurons in the nucleus tractus solitarius (NTS) and caudal serotonergic neurons control the excitability of PBN neurons and inhibit feeding. Blockade of 5-HT3 receptor signaling in the rostral NTS by either chronic administration of ondansetron or genetic inactivation of Tph2 in caudal serotonergic neurons that project to the NTS protects against starvation when AgRP neurons are ablated. Moreover, genetic inactivation of glutamatergic signaling by the NTS onto N-methyl D-aspartate (NMDA)-type glutamate receptors in the PBN prevents starvation. We also demonstrate that suppressing glutamatergic output of the PBN reinstates normal appetite after AgRP neuron ablation, whereas it promotes weight gain without AgRP neuron ablation. Hence, we identify the PBN as an important hub that integrates signals from several brain regions to bidirectionally modulate feeding and body weight.
Administration of diphtheria toxin to AgrpDTR mice that express the human diphtheria toxin (DT) receptor selectively in AgRP neurons ablates nearly all AgRP neurons in the arcuate nucleus of the hypothalamus; during that time the mice gradually cease eating, lose body weight, and die without intervention4. Importantly, chronic infusion of bretazenil, a GABAA receptor partial agonist, into the PBN for 12 days prevents starvation and allows an adaptive process to take place such that the mice eat and maintain their body weight5. Ablation of AgRP neurons not only inhibits initiation of meals, but also reduces the amount of liquid food that will be swallowed when it is delivered directly into the mouth7. Because the PBN not only responds to visceral malaise, such as food poisoning and LiCl treatment8, but also processes gustatory signals in paradigms like the conditional taste aversion or preference9,10, we predict that ablation of AgRP neurons results in unopposed activation of PBN that may mimic a nausea signal and thereby inhibit feeding. To test this hypothesis, we infused ondansetron, an anti-nausea drug that antagonizes 5-HT3 receptors11, subcutaneously or directly into the 4th ventricle starting 3 d before injecting AgrpDTR mice with DT. Despite the fact that the drug is administered orally to people, only central delivery of ondansetron prevented fatal weight loss and allowed the mice to recover (Fig. 1a and supplementary Fig. 1a). Consumption of low-fat chow pellets by ondansetron-treated mice fell and they lost ~10% of their body weight during the first week after DT treatment, but then they gradually ate more and regained body weight by 3 wk after DT treatment (Fig. 1a and supplementary Fig. 1a). The 5-HT3 receptor is an excitatory ion channel that is expressed widely in the brain, especially in the cortex and dorsal brainstem12. To examine more precisely where ondansetron acts to prevent starvation after AgRP neuron ablation, the drug was delivered bilaterally to either the PBN or the NTS (see Supplementary Fig. 2 for cannula placement). Delivery of ondansetron to the PBN did not rescue the starvation phenotype of DT-treated mice, whereas delivery to the NTS prevented starvation (Fig. 1b and supplementary Fig. 1b). The results suggest that serotonin provides some of the excitatory drive that indirectly results in hyperactivity of the PBN after loss of inhibitory input from AgRP neurons. Neurons in the NTS are known to send excitatory, glutamatergic inputs to the PBN13,14. Thus, we predicted that serotonin action on 5-HT3 receptors in the NTS promotes hyperexcitation of the PBN, which can be measured as local Fos gene activation6. Consistent with this hypothesis, Fos induction in the PBN was significantly ameliorated by administration of ondansetron in the NTS (Supplementary Fig. 3). We conclude that inhibition of 5-HT3-mediated excitatory currents in the vicinity of the NTS prevents starvation after ablation of AgRP neurons and promotes an adaptation that allows feeding to be maintained in the absence of AgRP neurons.
Figure 1. Chronic administration of ondansetron into the NTS, or genetic inactivation of serotonergic input to the NTS prevents starvation in AgRP neuron-ablated mice.
a, Body weight of AgrpDTR/+ mice or WT mice after either subcutaneous (sc, 1 mg/kg/day, n=6) or 4th ventricle (4v, 0.1 mg/kg/day, n=12) infusion of ondansetron, a 5-HT3 receptor antagonist, through osmotic minipumps. DT was injected intramuscularly twice as indicated by arrows. Minipumps were removed on Day 11 after pump content was depleted. Eight of 12 mice survived the treatment of DT and infusion of ondansetron into 4v (blue line).*p < 0.01 between AgrpDTR/+ mice treated with ondansetron sc (n = 6 non-survivors) vs 4v (n = 8 survivors) b, Body weight of DT-treated, AgrpDTR/+ mice after chronic infusion of ondansetron or vehicle into either the NTS (n=14) or PBN (n=8). DT was injected intramuscularly twice as indicated by arrows. Six of 14 mice survived the treatment of DT and infusion of ondansetron into the NTS (blue line). *p < 0.01 between AgrpDTR/+ mice infused by ondansetron into the NTS (n=6 survivors) and the PBN (n=8 non-survivors). c, Body weight of DT-treated, AgrpDTR/+;Tph2lox/lox mice after bilateral injection of CAV2-Cre virus or vehicle into the rostral part of NTS. Seven out of 12 mice survived the treatment of DT and viral infection (blue line). *p < 0.05 between mice injected with CAV2-Cre (n=7 survivors) and vehicle into the NTS (n=8 non survivors). Error bars represent the standard error of the mean (SEM) in all figures unless otherwise stated.†, mice were removed from experiment when they either lost 20% of their body weight or appeared moribund.
Tryptophan hydroxylase 2 (Tph2) catalyzes the first and rate-limiting step in serotonin biosynthesis in the central nervous system15. To examine the role of serotonin more directly, conditional Tph2lox/lox mice carrying the AgrpDTR/+ allele were generated and then injected with CAV2-Cre - a virus that is retrogradely transported from the site of injection to the cell bodies where it can inactivate the Tph2 gene only in those neurons that project to the injection site. CAV2-Cre was injected bilaterally into the NTS of Tph2lox/lox; AgrpDTR/+ mice and 8 d later they were treated with DT to ablate the AgRP neurons. This viral treatment prevented the starvation that normally occurs after ablation of AgRP neurons. Feeding and body weight fell slightly after DT treatment of the virally transduced mice, but all the mice restored normal food intake and regained body weight (Fig. 1c and Supplementary Fig. 1c). Various raphe nuclei from the virally rescued Tph2lox/lox mice were examined for serotonergic neurons that lacked Tph2, but retained L-aromatic amino acid decarboxylase (L-AADC), another marker of serotonergic cells. Many serotonergic cell bodies in the raphe obscurus (ROb) and raphe magnus (RMg) were found significantly absent of Tph2 staining (Fig. 2a–l). Quantification of the results revealed that viral treatment reduced Tph2 signaling in ROb and RMg serotonergic neurons by 60 to 80% (Fig. 2m), whereas serotonergic neurons in the dorsal raphe (DR) were unaffected (Fig. 2m and data not shown). Viral injection into NTS of Tph2lox/lox mice reduced 5-HT levels in the NTS by ~ 75% (WT = 11.65 +/− 0.92 ng/mg protein; viral injected = 2.68 +/− 0.57 ng/mg protein). Our observations are consistent with known projections of the caudal raphe neurons to brainstem structures and projections of DR neurons to forebrain regions16.
Figure 2. Serotonergic projections from the ROb and RMg to the NTS mediate starvation after ablation of AgRP neurons.
a–f, Representative immunohistochemistry pictures of Tph2 and AADC, markers of sertoninergic neurons, in AgrpDTR/+;Tph2lox/lox mice after bilateral injection of either vehicle (a–c) or a retrograding CAV2-Cre virus (d–f) into the rostral NTS. Arrowheads indicate the serotonin neurons within the ROb (B2 group) where expression of Tph2 was gone after viral infection. g-l, Representative immunostaining pictures of Tph2 and AADC at the RMg (B3 group) from the mice described above. Arrowheads indicate the serotonin neurons within the RMg where expression of Tph2 was gone after viral infection. m, Quantified immunohistochemistry results of AADC-expressing neurons that co-localized with Tph2-expressing neurons in the ROb, RMg, and DR, of the mice described in (a–f and data not shown). *p < 0.01, ANOVA. n = 6 mice per group. Scale bar (in a): a–l, 400 μm.
Because many of the neurons in the NTS are known to send glutamatergic projections to the PBN13,14, we predicted that serotonergic activation of the NTS might lead to glutamatergic activation of the PBN, which could be responsible for starvation after AgRP neuron ablation. To test this hypothesis, we inactivated the vesicular glutamate transporter 2 (Vglut2 encoded by the Slc17a6 gene) within the NTS by injecting AAV1-CreGFP virus bilaterally into the NTS of Slc17a6lox/lox;AgrpDTR/+ mice 8 d before initiation of DT treatment; see Supplementary Fig. 4 for placement of viral injections. Mice that were injected bilaterally and treated with DT maintained body weight and feeding, whereas mice that were unilaterally injected, or mice with vehicle injection starved (Fig. 3a and Supplementary Fig. 5). To further establish that glutamatergic activation of the PBN inhibits feeding in this model, we used a viral/genetic approach to reduce NMDA receptors in the PBN and thereby dampen the excitability by glutamate. Grin1lox/lox; AgrpDTR/+ mice that carry two conditional alleles of the gene encoding the essential NR1 subunit of NMDA receptors and the AgrpDTR allele were injected bilaterally with the AAV1-CreGFP in the PBN 9 d prior to ablating the AgRP neurons by DT; see Supplementary Fig. 6 for placement of viral injections. For the bilaterally injected mice, body weight slightly declined during the first 8 d after DT injection, but then recovered along with a rebound of food intake, whereas the vehicle-injected or unilaterally injected mice stopped eating and did not recover (Fig. 3b and Supplementary Fig. 7). These experiments show that either reducing glutamatergic signaling by neurons within the NTS or reducing NMDA receptors in the PBN protects against the starvation caused by ablation of AgRP neurons. Most neurons within the PBN are glutamatergic. Thus, we predicted that suppression of glutamatergic signaling by the PBN should also protect against starvation when AgRP neurons are ablated. We used the Slc17a6lox/lox;AgrpDTR/+ mice again for this experiment and injected AAV1-CreGFP into the lateral PBN 9 d prior to DT treatment (Supplementary Fig. 5). The mice with reduced Vglut2 signaling in the PBN lost body weight for the first week of DT treatment, but then gradually recovered, whereas the controls never recovered (Fig. 3c and Supplementary Fig. 8). In another cohort of mice, AAV1-CreGFP was injected into the PBN of Slc17a6lox/lox;AgrpDTR/+ mice, but they were not treated with DT. Interestingly, those mice gained ~20% in body weight along with a significant increase (~16%) of food intake over the next 3 wk (Fig. 3d and Supplementary Fig. 9). Our results indicate that enhanced glutamatergic signaling by the PBN inhibits feeding and promotes weight loss, whereas lowering the glutamatergic output of the PBN promotes weight gain through an increase in feeding, perhaps combined with reduction of energy expenditure.
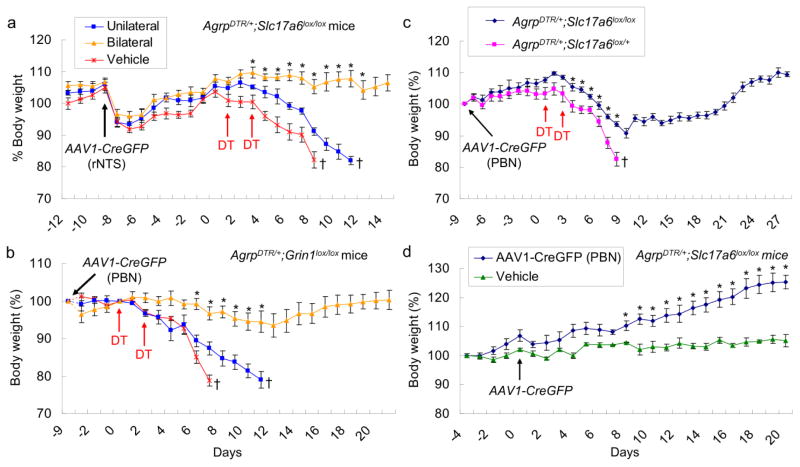
Figure 3. Viral-mediated disruption of glutamatergic circuitry between the NTS and PBN, or glutamatergic output of the PBN, rescues feeding after ablation of AgRP neurons.
a, Percentage of initial body weight of DT-treated, AgrpDTR/+;Slc17a6lox/lox mice after either bilateral (n=19) or unilateral injection (n=9) of AAV1-CreGFP virus or vehicle into the rostral NTS (n=9). AAV1-CreGFP virus reduces glutamatergic signaling from the rNTS to downstream targets, including the PBN. Fourteen of 19 mice survived of DT treatment and viral injections into the NTS (orange line). *p < 0.01, between bilateral virus injection (n=14 survivors) vs. unilateral virus injection (n= 9 non-survivors) or vehicle injection (n = 9 non-survivors). b, Percentage of initial body weight of DT-treated, AgrpDTR/+;Grin1lox/lox mice after either bilateral (n=18) or unilateral injection of AAV1-CreGFP virus (n = 8) or vehicle (n = 8) into the lateral PBN. AAV1-CreGFP virus attenuates NMDA receptor signaling in the PBN that receives dense glutamatergic projections from the NTS. Eleven of 18 mice survived of DT treatment and viral injections into the PBN (orange line). *p < 0.01, between bilateral virus injection (n=11 survivors) vs. unilateral virus injection (n = 8 non-survivors) or vehicle injection (n = 8 non-survivors). c, Percentage of initial body weight of DT-treated, AgrpDTR/+;Slc17a6lox/lox mice (n = 21) or the DT-treated, AgrpDTR/+;Slc17a6lox/+ control mice (n = 10) after bilateral injection of AAV1-CreGFP virus into the lateral PBN. AAV1-CreGFP virus abolishes glutamatergic signaling from the PBN to diverse forebrain targets. Twelve of 21 mice survived of DT treatment and viral injections into the PBN (blue lines). *p < 0.01, ANOVA; 12 survivors of Vglut2-deficient group vs 10 non-survivor control group. d, Percentage of initial body weight of AgrpDTR/+;Slc17a6lox/lox mice (n = 14) after bilateral injection of AAV1-CreGFP virus or vehicle (n = 10) into the PBN. *p < 0.01, ANOVA between the mice with precise viral injection (n = 7) and vehicle injection (n = 10).†, mice were removed from experiment when they either lost >20% body weight or appeared moribund.
Our studies reveal six manipulations that allow mice survive after AgRP neuron ablation: a) enhancement of GABAA receptor signaling in the PBN with bretazenil5, (b) suppression of 5-HT3 receptor signaling in the NTS with ondansetron, (c) disablement of serotonergic input to the NTS through viral-mediated removal of Tph2, (d) reduction of glutamatergic signaling by the NTS by removing Vglut2, (e) reduction of glutamatergic activation of the PBN by reducing NMDA receptors, or (f) reduction of efferent glutamatergic signals from the PBN. These observations support the circuit depicted in Fig. 4. We suggest that a sub-population of neurons in the PBN integrates visceral and gustatory information from the NTS with energy-balance signals emanating from AgRP neurons. The NTS responds to vagal inputs as well as gut-derived hormones, while AgRP neurons detect nutrient levels and respond to hormones such as insulin, leptin, and ghrelin17–19. Consequently, the appetitive response can be modulated by food palatability and visceral condition in a manner dictated by current energy balance.
Figure 4. Schematic illustrating circuitry that mediates loss of appetite after acute ablation of hypothalamic AgRP neurons.

AgRP neurons co-expressing AgRP, NPY, and GABA send inhibitory projections to the PBN. Serotonergic neurons residing in the RMg and ROb inhibit feeding through excitation of post-synaptic neurons in rostral NTS that express 5-HT3 receptors. This subpopulation of NTS neurons, by integration of visceral and gustatory inputs, sends excitatory glutamate signaling to the lateral PBN neurons that express NMDA receptors. Nutritional signaling from the hypothalamus and sensory signals may interact within the PBN to promote appropriate feeding responses.
Some studies suggest that serotonin exerts its anorectic effects by differential actions upon 5-HT1b and 5-HT2c receptors in the hypothalamus to stimulate melanocortin signaling20–22, while a recent study indicate that serotonergic neurons in ventral raphe nuclei respond to food restriction by elevated Fos signal23. We show here that serotonin from the ROb and RMg acts on 5-HT3 receptors in the NTS to inhibit feeding after ablation of AgRP neurons; thus, some of the anorectic effects of serotonin re-uptake inhibitors, such as fenfluramine, may also be mediated in the brainstem. Classical mapping studies reveal projections from the PBN to the amygdala, thalamus, hypothalamus, and other brain regions24. Further characterization and manipulation of PBN circuits that control feeding will be greatly facilitated by identification of genes that are expressed exclusively by the relevant sub-populations of PBN neurons. These experiments help define an important neural pathway within which some unique therapeutic targets have been characterized that could be valuable for development of novel treatments of diverse eating disorders, including nausea and anorexia nervosa25,26.
Supplementary Methods
Animal maintenance and neuron ablation
Mice were housed in a temperature- and humidity-controlled facility with a 12-h light/dark cycle. All animal care and experimental procedures were approved by the Institutional Animal Care and Use Committee at the University of Washington. In compliance with our approved protocol, all experiments were terminated if the body weight of mice fell to 80% of their original body weight or they appeared moribund. AgrpDTR/+ mice, Grin1lox/lox mice and Slc17a6lox/lox mice were generated and genetically identified by PCR of tail DNA as described previously4,27,28. A conditional Tph2 targeting construct was prepared by flanking the first exon with loxP sites along with a frt-flanked SV-Neo gene in the first intron. The construct was electroporated in G4 ES cells and correctly targeted clones were identified by Southern blotting. After removal of the Sv-Neo gene by breeding with a mouse expressing FLP recombinase, heterozygotes were bred to generate Tph2lox/lox mice that were used for viral injection. Details will be published elsewhere.
All except Tph2lox/lox mice were on the C57Bl/6 background (> 9 generations backcrossed); Tph2lox/lox mice were on a mixed 129/Sv x C57Bl/6 background. AgrpDTR/DTR male mice were bred with Slc17a6lox/lox female mice to generate AgrpDTR/+; Slc17a6lox/+ mice, which were further bred to each other to create AgrpDTR/+; Slc17a6lox/lox mice and AgrpDTR/+; Slc17a6lox/+ control mice. Similar breeding strategy was adopted when generating AgrpDTR/+; Tph2lox/lox mice and AgrpDTR/+; Grin1lox/lox mice. Mice were group housed with standard chow diet (LabDiet 5053) and water provided ad libitum until the beginning of the experiments. All experiments were performed with ~8-wk-old male mice. To ablate AgRP neurons, mice carrying the AgrpDTR allele, were injected twice with DT (50 μg/kg; intramuscular; 2 d apart; List Biological Laboratories, Campbell, CA)4. The extent of ablation (>95%) was determined by immunohistochemistry4–7.
Viral injections
Production of adeno-associated virus 1 (AAV1-CreGFP) and canine adenovirus 2 (CAV2-Cre) followed the protocols described previously29,30. For injection of CAV2-Cre or AAV1-CreGFP virus into the rostral NTS, mice were anesthetized and virus (or PBS as the vehicle) was injected bilaterally or unilaterally (1 μl of ~1010 particles/μl per each side) through a Hamilton syringe (size 5 μl, Hamilton, Reno, NV) using sterotaxic coordinates ±0.8 mm (X axis), −7.1 mm (Y axis) and −4.3 mm (Z axis). Similarly, AAV1-CreGFP virus (or PBS as the vehicle) was injected into the PBN bilaterally or unilaterally using the sterotaxic coordinates ±1.0 mm (X axis), −5.3 mm (Y axis) and −3.3 mm (Z axis). Brain samples from all mice were collected at the end of the behavioral experiment and processed for immunohistological analysis. For all viral injection experiments, only a fraction (indicated in legends) of the DT-treated mice survived AgRP-neuron ablation; subsequent evaluation of CreGFP expression revealed that failure to rescue was associated with poor placement or inadequate viral transduction.
Drug treatments
Alzet 14-d minipumps (model 1002, Durect, Cupertino, CA) loaded with 100 μL of ondansetron (6 mg/ml in saline, Sigma-Aldrich, St Louis, MO) were implanted subcutaneously on the back of anesthetized mice 4 d before DT treatment. These minipumps dispense 0.25 μL/h. Alternatively, cannulas (28 gauge, Plastics One, Roanoke, VA) were placed into 4th ventricles under anesthesia and the subcutaneous minipumps that were loaded with ondansetron (0.6 mg/ml in saline) were connected to the cannulas by tubing (PE60, Stoelting, Wood Dale, IL) that was threaded under the skin to help prevent the mice from dislodging it. For some experiments, the minipumps (ondansetron, 0.6 mg/ml) were connected to bilateral cannulas (28 gauge, Plastics One) directed to specific brain regions by using the following sterotaxic coordinates: the PBN, ±1 mm (X axis), −5.3 mm (Y axis) and −3.3 mm (Z axis); the NTS, ±0.8 mm (X axis), −7.1 mm (Y axis) and −4.3 mm (Z axis). The patency and placement of the bilateral minipump were verified at the end of each experiment and brain samples were processed for immunohistological analysis.
Food intake and body weight measurements
For feeding assays, mice were transferred to BioDAQ Food and Water Intake Monitor (Research Diets, New Brunswick, NJ) supplied with water and low-fat chow diet (D12450B, Research Diets, 3.85 Kcal/ml). The mice were allowed to acclimate for 3 d before initiating each experiment and data collection. Body weight and total food intake were recorded every 24 h. Feeding and drinking activity was recorded based on manufacturer’s suggested protocol.
Immunohistochemistry
Mice were sacrificed by CO2 asphyxiation and perfused transcardially with ice-cold PBS buffer containing 4% paraformaldehyde. Brains were dissected and post-fixed overnight at 4°C in the fixation buffer. Free-floating brain sections (25 μm) were washed in PBS and 0.1% Triton X-100 (PBST buffer) solution (3 × 15 min) and then blocked with 3% normal donkey serum in PBST for 2 to 3 h at room temperature. Rabbit anti-AgRP (1:1500 dilution; Phoenix Pharmaceuticals, Belmont, CA), rabbit anti-Fos (1: 1000 dilution; Millipore, Temecula, CA), monoclonal anti-tryptophan hydroxylase (1:1500 dilution, Sigma-Aldrich), and rabbit anti-dopa decarboxylase (1:500, Millipore; equivalent to AADC) were applied to the sections for overnight incubation at 4°C, followed by three 15-min rinses in PBST. Finally, sections were incubated in Cy2- or Cy3-labeled secondary antibody (1:300 dilution, Jackson Immunolaboratory, West Grove, PA) before visualization. Images were captured using a digital camera mounted on a Leica TCS SP1 confocal microscope (Leica Microsystems USA); all paired photos were obtained through the same system settings. For each group of mice, at least 8 sections from 4 different mice were analyzed.
Data analyses
Quantification of Tph2- and AADC-positive cells was done using the NIH Image software (National Institutes of Health). Anatomical correlations of brain sections and delineation of individual nuclei were determined by comparing landmarks of Nissl staining images with those given in the stereotaxic atlas. From the anatomically matched sections, a region of interest of the same size was further defined. Meanwhile, an optimized threshold that can discern round nuclei from partially stained ones as well as background noise was preset for all measurement. For all experiments only those mice with correct placement of cannula or viral injections were compared with the control group. Data sets collected from all experiments, unless otherwise stated, were analyzed by one-way ANOVA followed by Student-Newman-Keuls method for statistical significance and plotted as means ± standard error of mean (SEM). Post-hoc analysis was performed when group differences were significant by ANOVA at p < 0.05.
Supplementary Material
Acknowledgments
We thank Glenda Froelick, Jill Wang and Katie Battani for help with histology, Aundrea Rainwater for help with mouse breeding, Albert Quintana for propagating CAV2-Cre virus and preparing AAV1-CreGFP virus. We appreciate the helpful comments on the manuscript by Ali Guler and Matthew Carter. This work was supported in part by NIH DA024908 to R.D.P.
References
- 1.Wu Q, Palmiter RD. GABAergic signaling by AgRP neurons prevents anorexia via a melanocortin-independent mechanism. Eur J Pharmacol. 2011;660:21–27. doi: 10.1016/j.ejphar.2010.10.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. 2006;443:289–295. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- 3.Gropp E, et al. Agouti-related peptide-expressing neurons are mandatory for feeding. Nat Neurosci. 2005;8:1289–1291. doi: 10.1038/nn1548. [DOI] [PubMed] [Google Scholar]
- 4.Luquet S, Perez FA, Hnasko TS, Palmiter RD. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science. 2005;310:683–685. doi: 10.1126/science.1115524. [DOI] [PubMed] [Google Scholar]
- 5.Wu Q, Boyle MP, Palmiter RD. Loss of GABAergic signaling by AgRP neurons to the parabrachial nucleus leads to starvation. Cell. 2009;137:1225–1234. doi: 10.1016/j.cell.2009.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu Q, Howell MP, Palmiter RD. Ablation of neurons expressing agouti-related protein activates fos and gliosis in postsynaptic target regions. J Neurosci. 2008;28:9218–9226. doi: 10.1523/JNEUROSCI.2449-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu Q, Howell MP, Cowley MA, Palmiter RD. Starvation after AgRP neuron ablation is independent of melanocortin signaling. Proc Natl Acad Sci U S A. 2008;105:2687–2692. doi: 10.1073/pnas.0712062105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Swank MW, Bernstein IL. c-Fos induction in response to a conditioned stimulus after single trial taste aversion learning. Brain Res. 1994;636:202–208. doi: 10.1016/0006-8993(94)91018-9. [DOI] [PubMed] [Google Scholar]
- 9.Yamamoto T. Neural substrates for the processing of cognitive and affective aspects of taste in the brain. Arch Histol Cytol. 2006;69:243–255. doi: 10.1679/aohc.69.243. [DOI] [PubMed] [Google Scholar]
- 10.Berridge KC, Pecina S. Benzodiazepines, appetite, and taste palatability. Neurosci Biobehav Rev. 1995;19:121–131. doi: 10.1016/0149-7634(94)00026-w. [DOI] [PubMed] [Google Scholar]
- 11.Gershon MD, Tack J. The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology. 2007;132:397–414. doi: 10.1053/j.gastro.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 12.Barnes NM, Hales TG, Lummis SC, Peters JA. The 5-HT3 receptor--the relationship between structure and function. Neuropharmacology. 2009;56:273–284. doi: 10.1016/j.neuropharm.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herbert H, Moga MM, Saper CB. Connections of the parabrachial nucleus with the nucleus of the solitary tract and the medullary reticular formation in the rat. J Comp Neurol. 1990;293:540–580. doi: 10.1002/cne.902930404. [DOI] [PubMed] [Google Scholar]
- 14.Jhamandas JH, Harris KH. Excitatory amino acids may mediate nucleus tractus solitarius input to rat parabrachial neurons. Am J Physiol. 1992;263:R324–330. doi: 10.1152/ajpregu.1992.263.2.R324. [DOI] [PubMed] [Google Scholar]
- 15.Walther DJ, Bader M. A unique central tryptophan hydroxylase isoform. Biochem Pharmacol. 2003;66:1673–1680. doi: 10.1016/s0006-2952(03)00556-2. [DOI] [PubMed] [Google Scholar]
- 16.Thor KB, Helke CJ. Serotonin- and substance P-containing projections to the nucleus tractus solitarii of the rat. J Comp Neurol. 1987;265:275–293. doi: 10.1002/cne.902650210. [DOI] [PubMed] [Google Scholar]
- 17.Abizaid A, Horvath TL. Brain circuits regulating energy homeostasis. Regul Pept. 2008;149:3–10. doi: 10.1016/j.regpep.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grill HJ. Distributed neural control of energy balance: contributions from hindbrain and hypothalamus. Obesity (Silver Spring) 2006;14 (Suppl 5):216S–221S. doi: 10.1038/oby.2006.312. [DOI] [PubMed] [Google Scholar]
- 19.Berthoud HR, Morrison C. The brain, appetite, and obesity. Annu Rev Psychol. 2008;59:55–92. doi: 10.1146/annurev.psych.59.103006.093551. [DOI] [PubMed] [Google Scholar]
- 20.Heisler LK, et al. Serotonin reciprocally regulates melanocortin neurons to modulate food intake. Neuron. 2006;51:239–249. doi: 10.1016/j.neuron.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 21.Xu Y, et al. 5-HT2CRs expressed by pro-opiomelanocortin neurons regulate energy homeostasis. Neuron. 2008;60:582–589. doi: 10.1016/j.neuron.2008.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu Y, et al. A serotonin and melanocortin circuit mediates D-fenfluramine anorexia. J Neurosci. 2010;30:14630–14634. doi: 10.1523/JNEUROSCI.5412-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takase LF, Nogueira MI. Patterns of fos activation in rat raphe nuclei during feeding behavior. Brain Res. 2008;1200:10–18. doi: 10.1016/j.brainres.2008.01.036. [DOI] [PubMed] [Google Scholar]
- 24.Fulwiler CE, Saper CB. Subnuclear organization of the efferent connections of the parabrachial nucleus in the rat. Brain Res. 1984;319:229–259. doi: 10.1016/0165-0173(84)90012-2. [DOI] [PubMed] [Google Scholar]
- 25.Rask-Andersen M, Olszewski PK, Levine AS, Schioth HB. Molecular mechanisms underlying anorexia nervosa: focus on human gene association studies and systems controlling food intake. Brain Res Rev. 2010;62:147–164. doi: 10.1016/j.brainresrev.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 26.Kaye W. Neurobiology of anorexia and bulimia nervosa. Physiol Behav. 2008;94:121–135. doi: 10.1016/j.physbeh.2007.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsien JZ, Huerta PT, Tonegawa S. The essential role of hippocampal CA1 NMDA receptor-dependent synaptic plasticity in spatial memory. Cell. 1996;87:1327–1338. doi: 10.1016/s0092-8674(00)81827-9. [DOI] [PubMed] [Google Scholar]
- 28.Hnasko TS, et al. Vesicular glutamate transport promotes dopamine storage and glutamate corelease in vivo. Neuron. 2010;65:643–656. doi: 10.1016/j.neuron.2010.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kremer EJ, Boutin S, Chillon M, Danos O. Canine adenovirus vectors: an alternative for adenovirus-mediated gene transfer. J Virol. 2000;74:505–512. doi: 10.1128/jvi.74.1.505-512.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaplitt MG, et al. Long-term gene expression and phenotypic correction using adeno-associated virus vectors in the mammalian brain. Nat Genet. 1994;8:148–154. doi: 10.1038/ng1094-148. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.