Abstract
Autism is a common and heritable neuropsychiatric disorder that can be categorized into two types: syndromic and non-syndromic, the former of which are associated with other neurological disorders or syndromes. Molecular and functional links between syndromic and non-syndromic autism genes were lacking until studies aimed at understanding role of trans-synaptic adhesion molecule neuroligin, which is associated with non-syndromic autism, provided important connections. Here, we integrate data from these studies into a model of how neuroligin functions to control synaptic connectivity in the central nervous system and how neuroligin dysfunction may participate in the pathophysiology of autism. Understanding the complex functional interactions between neuroligins and other autism-associated proteins at the synapse is crucial to understand the pathology of autism. This understanding might bring us closer to development of therapeutic approaches for autism.
Autism, a common and heritable neuropsychiatric disorder, is categorized as either syndromic or non-syndromic (1, 2). Syndromic autisms, which are so defined because they occur in individuals with neurological disorders, such as Fragile X Mental Retardation (FXR), Tuberous Sclerosis, or Rett Syndrome, harbor a set of phenotypes that can be fully attributed to a mutation in a particular gene or a set of genes (2). The molecular nature of syndromic autism has been studied in detail in animal models of these diseases. There is a significant association between the function of syndromic autism genes and the pathways that regulate activity-dependent remodeling of synaptic circuits (1, 3).
Non-syndromic autism, which comprises a vast majority of autism cases, is not linked to other neurological diseases (or syndromes), but is also heritable. Genome-wide association studies and other genetic analyses revealed hundreds of previously unknown rare mutations and gene number variations linked to non-syndromic autism (4, 5). Bioinformatic analyses of functional networks that encompass rare autism genes also underscore the relevance of cellular processes that control synaptic function and plasticity in the pathology of autism (6).
Ten years ago analyses of X-linked loci that are associated with non-syndromic autism revealed mutations in the Neuroligin-3 and Neuroligin-4 genes in two Swedish autism families (7). Neuroligin family genes encode postsynaptic cell adhesion proteins (NLGs 1–4) (8). Postsynaptic NLGs interact with presynaptic neurexins (Nrxs) to form trans-synaptic adhesions (9) (Fig. 1). This interaction is thought to control bidirectional-signaling events that regulate the formation and functional maturation of synapses (8, 10, 11). In vitro studies have shown that NLGs are required for excitatory and inhibitory synapse formation (12). However, analyses of mice deficient in one or more Neuroligin genes showed that synapse formation is largely normal even in the absence of three NLGs (NLG1, 2, and 3 triple knockouts) (13). These in vivo findings initially suggested that NLGs’ role in synaptogenesis is redundant in vivo. However, the balance of NLG abundance between neighboring neurons strongly governs synapse formation in a competitive manner (14, 15). This likely explains why the total loss of a NLG (such as in knockout animals) does not result in overt synaptogenesis defects, but cell-specific knockdown of NLGs in a wild-type background leads to severe loss of synaptic connectivity in vivo (15).
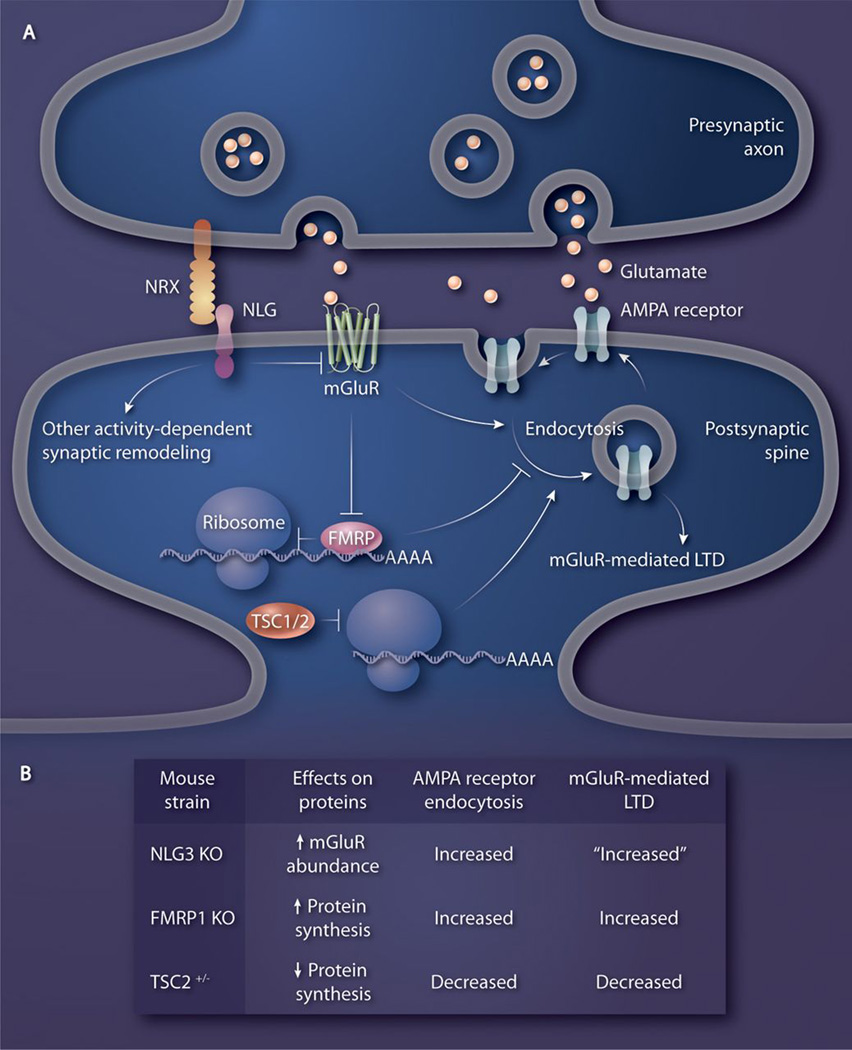
Fig. 1. Neuroligin function at the synapse links syndromic and non-syndromic autism.
NLGs interact with Nrxs trans-synaptically. NLG-dependent control of synaptic activity increases the abundance of AMPA-type ionotropic glutamate receptors on the postsynaptic membrane by reducing the abundance of mGluRs (19). Postsynaptic mGluRs, which are activated by presynaptic release of glutamate, mediate long-term depression (LTD), which involves the endocytosis of AMPA receptors. FMRP1 binds to polysome-associated mRNAs and inhibits synthesis of certain proteins that enhance endocytosis of AMPA receptors. FMRP1-driven blockage of translation is removed by mGluR activation, which triggers induction of LTD. In FMRP1-knockout mice, mGluR-mediated LTD is enhanced similarly to that in NLG3-knockout mice (24). TSC1 and 2 (TSC1/2), which are encoded by the genes mutated in Tuberous Sclerosis, inhibit mTOR-dependent synthesis of proteins at the synapse that induce AMPA receptor endocytosis (21).
The autism-associated NLG3 mutation, which was identified in one Swedish family, is a missense conversion of a highly conserved arginine residue to cysteine (NLG3 R451C) (7). At the cellular level, R451C mutation leads to diminished surface trafficking of NLG3, which reduces the effectiveness of NLG3 to induce clustering of proteins at synapses in cultured neurons (16). To determine how the R451C mutation affects synaptic connectivity in vivo, a knockin mouse model was developed (17). Analysis of protein abundance in the R451C-knockin mice showed a remarkable reduction in the amount of NLG3 in the brain. These mice displayed impaired social interactions, a core characteristic of autism, but surprisingly they had increased ability for spatial learning and memory. Cognitive changes were accompanied by a significant increase in the inhibitory synaptic transmission at the cortices of R451C-knockin mice when compared to wild-type mice. Interestingly, none of these changes were observed in the cortices of the Nlg3-null mice. From these observations, it was proposed that the R451C mutation acts through a gain-of-function mechanism (17). Although a gain-of-function mechanism is plausible, these observations can also be explained as the result of loss of NLG3 function in a cell-specific or neuronal circuit-specific manner as has been shown for NLG-1; NLG-1–dependent competition regulates cortical synaptogenesis and synapse number. Reducing the amount of NLG-1 in cortical neurons in vivo by RNA interference greatly reduced excitatory connections made onto the NLG-1-deficient cell (15). NLG3 may have a similar function in activity-dependent regulation of synaptic connectivity. Inefficient production and trafficking of NLG3 due to R451C conversion may lead to an imbalance in NLG3 abundance across the central nervous system (CNS), and this imbalance may impair the activity-dependent NLG3 signaling in a way that is worse than the absence of NLG3, such as in an NLG-3 knockout. In agreement with this possibility, studies with NLG3-knockout and R451C mice show the presence of circuit-specific and cell-specific synaptic dysfunction (18, 19). Thus, we propose that the imbalance in the amount of NLG3 across the CNS due to the R451C mutation is the main problem. The unevenness in NLG3 abundance caused by R451C mutation may be affected by genetic background, which could explain why a study that utilized another R451C-knockin line in a different mouse background could not reproduce the behavioral findings of Tabuchi et al. (17, 20).
The function of NLG3 in the mouse cerebellum was investigated using a series of genetic manipulations (19). In cerebellar Purkinje cell neurons, NLG3 is specifically localized to parallel fiber synapses, but not to climbing fiber synapses or inhibitory synapses. Electrophysiological analyses of Purkinje cells showed a small but significant reduction in miniature excitatory postsynaptic current (mEPSC) amplitudes in NLG3-knockout mice compared to wild type. Interestingly, metabotropic glutamate receptor (mGluR)-mediated long-term depression (LTD) was occluded in NLG3-knockout mice. This defect in mGluR-mediated LTD correlated with an increase in the abundance of the mGluR1a subunit in the Purkinje cells of NLG3-knockout mice (19). This connection between NLG3 function and mGluR-mediated LTD is of particular importance because it provides a molecular link between non-syndromic and syndromic autism (Fig. 1).
The importance of balanced mGluR function in normal cognition was demonstrated by comparing two syndromic autism mouse models: Tuberous Sclerosis mice heterozygous for the gene Tsc2 (Tsc2+/−), encoding tuberous sclerosis 2 (TSC2), and FXR mice lacking the gene encoding FMRP1 (FMR1−/y) (21). Both FMRP1 and TSC2 control activity-dependent local protein synthesis at synapses (Fig. 1). FMR1−/y and Tsc2+/− mice show similar behavior and impairment in learning and memory (22, 23). Surprisingly, biochemical and electrophysiological analyses of synapses in these mouse models showed opposite effects on the abundance of synaptic proteins and mGluR-mediated LTD. FMRP1-KO mice had increased abundance of synaptic proteins and enhanced mGluR-mediated LTD (24); the opposite was the case for Tsc2+/− mice. Interestingly, the aberrant behavioral phenotypes disappeared when FMR1−/y and Tsc2+/− mice were crossed with each other (21). These findings indicate that normal synaptic plasticity, which drives functional cognition and behavior, occurs in an optimal range of mGluR-mediated activity-dependent protein synthesis; deviation in either direction leads to behavioral pathologies associated with syndromic autism. Mutant phenotypes in FXR animal models have been corrected by genetic or pharmacological inhibition of mGluR5, and there are ongoing preliminary human clinical trials using drugs that inhibit mGluR5 (25). Similarly, a positive modulator of mGluR5 function can correct the synaptic and behavioral phenotypes seen in Tsc2+/− mice (21). Because aberrant mGluR-mediated LTD is also reported in NLG3-knockout mice (a model of non-syndromic autism), positive and negative modulators of mGluR function may prove beneficial for treatment of a larger number of Autism Spectrum Disorders (ASDs) than previously thought.
In NLG3-knockout mice, the increase in the abundance of mGluR1a could be rescued by Purkinje cell-specific re-expression of NLG3, suggesting that mGluR1a-mediated LTD can be normalized by this manipulation (19). In the future it would be interesting to explore whether the functional link between NLG3 and mGluRs is also present in brain regions other than the cerebellum and whether other NLGs (NLG 1, 2, and 4) are also involved in the regulation of mGluR-mediated LTD. Baudouin and colleagues also showed that in the NLG3-knockout mice the climbing fibers ectopically innervate the territory of the parallel fibers (19), which indicates that NLG3 is required to balance the climbing fiber and parallel fiber inputs onto Purkinje cells. This result supports the emerging evidence that NLGs are important for activity-dependent competitive synaptogenesis (14, 15). Re-expressing NLG3 in Purkinje cells even after development is complete (after postnatal day 30 in mice) attenuated mGluR1a to amounts similar to those in wild-type mice and eliminated the ectopic climbing fiber innervations (19), indicating that the role of NLG3 in controlling synaptic connectivity is not limited to development. This result is exciting because it suggests that malformed circuits in autistic patients may be corrected even after development is complete. Indeed, studies with syndromic autism animal models showed that disease phenotypes could be rescued by therapeutic interventions in juvenile and adult mice (22, 25).
In humans, both deletions and point mutations (such as R451C) in the Neuroligin 3 gene are linked to autism; however, no common synaptic dysfunction phenotypes had been reported in their corresponding mouse models. Földy and colleagues studied the properties of synaptic transmission in NLG3-knockout mice and NLG3 R451C mice to determine whether these non-syndromic autism models had common synaptic phenotypes (26). The analyses of the GABAergic synapses formed by hippocampal inhibitory neurons (parvalbumin and cholecystokinin-positive basket cells) onto pyramidal neurons revealed that both mutations dramatically impair tonic endocannabinoid signaling at these GABAergic synapses (26). Retrograde endocannabinoid signaling (from postsynapse to presynapse) is a key modulator of synaptic plasticity and cognitive performance. The tonic component of endocannabinoid signaling affects synaptic transmission over long time periods and controls the presynaptic release properties at these GABAergic synapses (27). Interestingly, studies with the syndromic autism model FXR mice (FMR1−/y) showed that FMRP1 deletion leads to greater endocannabinoid-mediated responses at GABAergic synapses of the dorsal striatum and the CA1 region of the hippocampus (28, 29). Moreover, loss of FMRP function leads to marked deficits in mGluR5-dependent release of endocannabinoids at excitatory synapses (30). Taken together these findings further indicate that there are common molecular mechanisms that underlie the pathophysiology of syndromic and non-syndromic autism and suggest that modulation of endocannabinoid signaling may provide valid therapeutic avenues for ASDs (27, 31).
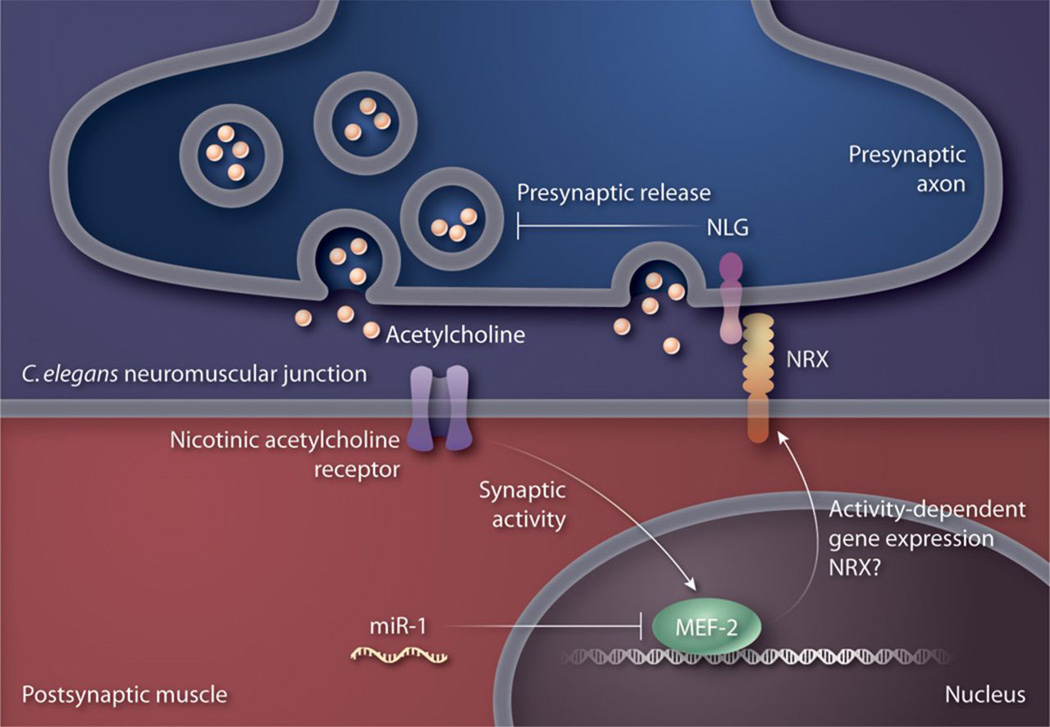
Another study, which used the Caenorhabditis elegans neuromuscular junction (NMJ) as a model, revealed a previously unknown mode of action for NLGs at the synapse and provided further links between syndromic and non-syndromic autism (32). In worms lacking the function of a specific micro RNA (miRNA), a retrograde signal coming from muscle inhibits acetylcholine release by axonal terminals of motor neurons. This retrograde signal is not present in wild-type worms, but occurs when miR-21, a muscle-specific miRNA, is inactivated. Deletion of the gene encoding the transcription factor, MEF-2 (myocyte enhancer factor-2) that is a target of miR-21, in a miR-21-mutant background abolishes this retrograde signal (33) (Fig. 2). This retrograde signal depends on the interaction between NLG-1 at the cholinergic motor neurons (presynaptic) and NRX-1 at the muscle (postsynaptic) (Fig. 2) and was eliminated in miR-21;nlg-1 or miR-21:nrx-1 double mutants. Analyses of evoked responses in nlg-1 mutants showed that NLG-1 is required for faster presynaptic release kinetics. To determine if this function of NLG-1 was restricted to worms or was conserved in mammals, Hu and colleagues re-analyzed the evoked EPSC data from NLG1, 2, and 3 triple knockout mice (13) and found that evoked EPSCs decayed significantly more slowly in the brain stems of triple knockouts when compared to double-knockout controls. Thus, NLGs shape evoked response kinetics at the worm NMJ and in mouse glutamatergic synapses and they can mediate this effect either from pre- or post-synaptic sites (Fig. 1 and Fig. 2) (32).
Fig. 2. Presynaptic NLGs control synaptic vesicle release kinetics in C. elegans neuromuscular junction.
In the C. elegans neuromuscular junction, MEF-2 activity in the muscle inhibits presynaptic release of neurotransmitter in a retrograde manner that involves an interaction between postsynaptic NRX and presynaptic NLG.
These findings have important implications for understanding the synaptic pathologies observed in ASDs. Prolonged neurotransmitter release could affect circuit development and alter temporal and spatial resolution of sensory responses that are keys to the development of proper language and social skills (34, 35). In addition, the findings of Hu et al. (32) molecularly link NLG and Nrx proteins to synaptic activity-dependent MEF-2 signaling. Mutations in MEF2C (the human homolog of worm MEF-2) cause a small percentage of Rett syndrome cases (36). Thus, even in syndromic ASDs that are not caused by mutations in NLG- or Nrx-encoding genes, manipulation of trans-synaptic Nrx-NLG signaling could be therapeutic.
In summary, NLGs and their signaling partners Nrxs regulate synaptic activity in such a way that accurate activity-dependent remodeling of circuits occurs (Fig. 1). Emerging evidence indicates that altered synaptic connectivity lies at the heart of the pathological changes that occur in the autistic brain and, due to their central role at the synapse, NLGs are key players in the pathogenesis of ASD. Manipulation of NLG function to correct altered synaptic connectivity in autism, even after development has taken place, may provide relief. Therefore, it may be feasible to develop therapeutic strategies for autism by targeting NLGs. However, we still lack a comprehensive circuit-level understanding of NLG function in the CNS and it is important to elucidate the roles NLGs play in signaling between neurons and glia. Surprisingly, NLGs and Nrxs (37) are also present on astroctyes and astroctyes secrete synapse-modulating proteins that interact with NLGs (38), suggesting a possible link between astrocytic control of synaptic connectivity and ASD.
GLOSS.
Autism is a heterogeneous neuropsychiatric disorder that is heritable. Autism is categorized into two types: syndromic and non-syndromic. Syndromic autisms are caused by mutations in single genes and are manifested within the context of neurological syndromes, such as Fragile X Syndrome. In the last decade, genetic analyses of non-syndromic autism families revealed a number of genes that are linked to this class of autism. In this Review with 2 figures, and 38 references, we focus on the Neuroligin family genes, which encode postsynaptic adhesion proteins that are critical for activity-dependent maturation of synaptic circuits. Studies investigating the role of neuroligins at synapses provide interesting new insights into the role of these proteins in the pathophysiology of autism. Moreover, these findings underscore the shared synaptic dysfunctions in syndromic and non-syndromic autism. Targeting neuroligins and the molecular pathways that they regulate may provide effective therapeutic strategies to combat autism.
ACKNOWLEDGEMENTS
We would like to thank Dr. Scott Soderling, Dr. W. Christopher Risher and Spencer McKinstry for the critical reading of the manuscript. Funding: SKS is a Ruth K. Broad International Postdoctoral Fellow. C.E. is an Esther and Joseph Klingenstein Fund Fellow and Alfred P. Sloan Fellow. CE and SKS are supported by NIH/NIDA DA031833 and Brumley Neonatal Perinatal Research Institute.
REFERENCES
- 1.Miles JH. Autism spectrum disorders--a genetics review. Genet Med. 2011;13:278–294. doi: 10.1097/GIM.0b013e3181ff67ba. published online EpubApr (10.1097/GIM.0b013e3181ff67ba). [DOI] [PubMed] [Google Scholar]
- 2.Schaaf CP, Zoghbi HY. Solving the autism puzzle a few pieces at a time. Neuron. 2011;70:806–808. doi: 10.1016/j.neuron.2011.05.025. published online EpubJun 9 (S0896-6273(11)00443-0 [pii]10.1016/j.neuron.2011.05.025). [DOI] [PubMed] [Google Scholar]
- 3.Walsh CA, Morrow EM, Rubenstein JL. Autism and brain development. Cell. 2008;135:396–400. doi: 10.1016/j.cell.2008.10.015. published online EpubOct 31 (S0092-8674(08)01306-8 [pii] 10.1016/j.cell.2008.10.015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sanders SJ, Ercan-Sencicek AG, Hus V, Luo R, Murtha MT, Moreno-De-Luca D, Chu SH, Moreau MP, Gupta AR, Thomson SA, Mason CE, Bilguvar K, Celestino-Soper PB, Choi M, Crawford EL, Davis L, Wright NR, Dhodapkar RM, DiCola M, DiLullo NM, Fernandez TV, Fielding-Singh V, Fishman DO, Frahm S, Garagaloyan R, Goh GS, Kammela S, Klei L, Lowe JK, Lund SC, McGrew AD, Meyer KA, Moffat WJ, Murdoch JD, O'Roak BJ, Ober GT, Pottenger RS, Raubeson MJ, Song Y, Wang Q, Yaspan BL, Yu TW, Yurkiewicz IR, Beaudet AL, Cantor RM, Curland M, Grice DE, Gunel M, Lifton RP, Mane SM, Martin DM, Shaw CA, Sheldon M, Tischfield JA, Walsh CA, Morrow EM, Ledbetter DH, Fombonne E, Lord C, Martin CL, Brooks AI, Sutcliffe JS, Cook EH, Jr, Geschwind D, Roeder K, Devlin B, State MW. Multiple recurrent de novo CNVs, including duplications of the 7q11.23 Williams syndrome region, are strongly associated with autism. Neuron. 2011;70:863–885. doi: 10.1016/j.neuron.2011.05.002. published online EpubJun 9 (S0896-6273(11)00374-6 [pii] 10.1016/j.neuron.2011.05.002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sanders SJ, Murtha MT, Gupta AR, Murdoch JD, Raubeson MJ, Willsey AJ, Ercan-Sencicek AG, DiLullo NM, Parikshak NN, Stein JL, Walker MF, Ober GT, Teran NA, Song Y, El-Fishawy P, Murtha RC, Choi M, Overton JD, Bjornson RD, Carriero NJ, Meyer KA, Bilguvar K, Mane SM, Sestan N, Lifton RP, Gunel M, Roeder K, Geschwind DH, Devlin B, State MW. De novo mutations revealed by whole-exome sequencing are strongly associated with autism. Nature. 2012;485:237–241. doi: 10.1038/nature10945. published online EpubMay 10 (nature10945 [pii] 10.1038/nature10945). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gilman SR, Iossifov I, Levy D, Ronemus M, Wigler M, Vitkup D. Rare de novo variants associated with autism implicate a large functional network of genes involved in formation and function of synapses. Neuron. 2011;70:898–907. doi: 10.1016/j.neuron.2011.05.021. published online EpubJun 9 (S0896-6273(11)00439-9 [pii] 10.1016/j.neuron.2011.05.021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jamain S, Quach H, Betancur C, Rastam M, Colineaux C, Gillberg IC, Soderstrom H, Giros B, Leboyer M, Gillberg C, Bourgeron T. Mutations of the Xlinked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Nat Genet. 2003;34:27–29. doi: 10.1038/ng1136. published online EpubMay (10.1038/ng1136 ng1136 [pii]). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sudhof TC. Neuroligins and neurexins link synaptic function to cognitive disease. Nature. 2008;455:903–911. doi: 10.1038/nature07456. published online EpubOct 16 (nature07456 [pii] 10.1038/nature07456). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scheiffele P, Fan J, Choih J, Fetter R, Serafini T. Neuroligin expressed in nonneuronal cells triggers presynaptic development in contacting axons. Cell. 2000;101:657–669. doi: 10.1016/s0092-8674(00)80877-6. published online EpubJun 9 (S0092-8674(00)80877-6 [pii]). [DOI] [PubMed] [Google Scholar]
- 10.Craig AM, Kang Y. Neurexin-neuroligin signaling in synapse development. Curr Opin Neurobiol. 2007;17:43–52. doi: 10.1016/j.conb.2007.01.011. published online EpubFeb (S0959-4388(07)00013-X [pii] 10.1016/j.conb.2007.01.011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baudouin S, Scheiffele P. SnapShot: Neuroligin-neurexin complexes. Cell. 2010;141:908, 908, e901. doi: 10.1016/j.cell.2010.05.024. published online EpubMay 28 (S0092-8674(10)00560-X [pii] 10.1016/j.cell.2010.05.024). [DOI] [PubMed] [Google Scholar]
- 12.Chih B, Engelman H, Scheiffele P. Control of excitatory and inhibitory synapse formation by neuroligins. Science. 2005;307:1324–1328. doi: 10.1126/science.1107470. published online EpubFeb 25 (1107470 [pii]10.1126/science.1107470). [DOI] [PubMed] [Google Scholar]
- 13.Varoqueaux F, Aramuni G, Rawson RL, Mohrmann R, Missler M, Gottmann K, Zhang W, Sudhof TC, Brose N. Neuroligins determine synapse maturation and function. Neuron. 2006;51:741–754. doi: 10.1016/j.neuron.2006.09.003. published online EpubSep 21 (S0896-6273(06)00680-5 [pii]10.1016/j.neuron.2006.09.003). [DOI] [PubMed] [Google Scholar]
- 14.Chubykin AA, Atasoy D, Etherton MR, Brose N, Kavalali ET, Gibson JR, Sudhof TC. Activity-dependent validation of excitatory versus inhibitory synapses by neuroligin-1 versus neuroligin-2. Neuron. 2007;54:919–931. doi: 10.1016/j.neuron.2007.05.029. published online EpubJun 21 (S0896-6273(07)00409-6 [pii] 10.1016/j.neuron.2007.05.029). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwon HB, Kozorovitskiy Y, Oh WJ, Peixoto RT, Akhtar N, Saulnier JL, Gu C, Sabatini BL. Neuroligin-1-dependent competition regulates cortical synaptogenesis and synapse number. Nat Neurosci. 2012;15:1667–1674. doi: 10.1038/nn.3256. published online EpubDec (nn.3256 [pii] 10.1038/nn.3256). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chih B, Afridi SK, Clark L, Scheiffele P. Disorder-associated mutations lead to functional inactivation of neuroligins. Hum Mol Genet. 2004;13:1471–1477. doi: 10.1093/hmg/ddh158. published online EpubJul 15 (10.1093/hmg/ddh158 ddh158 [pii]). [DOI] [PubMed] [Google Scholar]
- 17.Tabuchi K, Blundell J, Etherton MR, Hammer RE, Liu X, Powell CM, Sudhof TC. A neuroligin-3 mutation implicated in autism increases inhibitory synaptic transmission in mice. Science. 2007;318:71–76. doi: 10.1126/science.1146221. published online EpubOct 5 (1146221 [pii]10.1126/science.1146221). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Etherton M, Foldy C, Sharma M, Tabuchi K, Liu X, Shamloo M, Malenka RC, Sudhof TC. Autism-linked neuroligin-3 R451C mutation differentially alters hippocampal and cortical synaptic function. Proc Natl Acad Sci U S A. 2011;108:13764–13769. doi: 10.1073/pnas.1111093108. published online EpubAug 16 (1111093108 [pii] 10.1073/pnas.1111093108). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baudouin SJ, Gaudias J, Gerharz S, Hatstatt L, Zhou K, Punnakkal P, Tanaka KF, Spooren W, Hen R, De Zeeuw CI, Vogt K, Scheiffele P. Shared synaptic pathophysiology in syndromic and nonsyndromic rodent models of autism. Science. 2012;338:128–132. doi: 10.1126/science.1224159. published online EpubOct 5 (science.1224159 [pii] 10.1126/science.1224159). [DOI] [PubMed] [Google Scholar]
- 20.Chadman KK, Gong S, Scattoni ML, Boltuck SE, Gandhy SU, Heintz N, Crawley JN. Minimal aberrant behavioral phenotypes of neuroligin-3 R451C knockin mice. Autism Res. 2008;1:147–158. doi: 10.1002/aur.22. published online EpubJun (10.1002/aur.22). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Auerbach BD, Osterweil EK, Bear MF. Mutations causing syndromic autism define an axis of synaptic pathophysiology. Nature. 2011;480:63–68. doi: 10.1038/nature10658. published online EpubDec 1 (nature10658 [pii]10.1038/nature10658). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ehninger D, Han S, Shilyansky C, Zhou Y, Li W, Kwiatkowski DJ, Ramesh V, Silva AJ. Reversal of learning deficits in a Tsc2+/− mouse model of tuberous sclerosis. Nat Med. 2008;14:843–848. doi: 10.1038/nm1788. published online EpubAug (nm1788 [pii] 10.1038/nm1788). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krueger DD, Osterweil EK, Chen SP, Tye LD, Bear MF. Cognitive dysfunction and prefrontal synaptic abnormalities in a mouse model of fragile X syndrome. Proc Natl Acad Sci U S A. 2011;108:2587–2592. doi: 10.1073/pnas.1013855108. published online EpubFeb 8 (1013855108 [pii]10.1073/pnas.1013855108). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huber KM, Gallagher SM, Warren ST, Bear MF. Altered synaptic plasticity in a mouse model of fragile X mental retardation. Proc Natl Acad Sci U S A. 2002;99:7746–7750. doi: 10.1073/pnas.122205699. published online EpubMay 28 (10.1073/pnas.122205699). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krueger DD, Bear MF. Toward fulfilling the promise of molecular medicine in fragile X syndrome. Annu Rev Med. 2011;62:411–429. doi: 10.1146/annurev-med-061109-134644. 10.1146/annurev-med-061109-134644). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Foldy C, Malenka RC, Sudhof TC. Autism-associated neuroligin-3 mutations commonly disrupt tonic endocannabinoid signaling. Neuron. 2013;78:498–509. doi: 10.1016/j.neuron.2013.02.036. published online EpubMay 8 (S0896-6273(13)00225-0 [pii] 10.1016/j.neuron.2013.02.036). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krueger DD, Brose N. Evidence for a common endocannabinoid-related pathomechanism in autism spectrum disorders. Neuron. 2013;78:408–410. doi: 10.1016/j.neuron.2013.04.030. published online EpubMay 8 (S0896-6273(13)00361-9 [pii] 10.1016/j.neuron.2013.04.030). [DOI] [PubMed] [Google Scholar]
- 28.Maccarrone M, Rossi S, Bari M, De Chiara V, Rapino C, Musella A, Bernardi G, Bagni C, Centonze D. Abnormal mGlu 5 receptor/endocannabinoid coupling in mice lacking FMRP and BC1 RNA. Neuropsychopharmacology. 2010;35:1500–1509. doi: 10.1038/npp.2010.19. published online EpubJun (npp201019 [pii] 10.1038/npp.2010.19). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang L, Alger BE. Enhanced endocannabinoid signaling elevates neuronal excitability in fragile X syndrome. J Neurosci. 2010;30:5724–5729. doi: 10.1523/JNEUROSCI.0795-10.2010. published online EpubApr 21 (30/16/5724 [pii] 10.1523/JNEUROSCI.0795-10.2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jung KM, Sepers M, Henstridge CM, Lassalle O, Neuhofer D, Martin H, Ginger M, Frick A, DiPatrizio NV, Mackie K, Katona I, Piomelli D, Manzoni OJ. Uncoupling of the endocannabinoid signalling complex in a mouse model of fragile X syndrome. Nat Commun. 2012;3:1080. doi: 10.1038/ncomms2045. ncomms2045 [pii] 10.1038/ncomms2045). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Busquets-Garcia A, Gomis-Gonzalez M, Guegan T, Agustin-Pavon C, Pastor A, Mato S, Perez-Samartin A, Matute C, de la Torre R, Dierssen M, Maldonado R, Ozaita A. Targeting the endocannabinoid system in the treatment of fragile X syndrome. Nat Med. 2013;19:603–607. doi: 10.1038/nm.3127. published online EpubMay (nm.3127 [pii] 10.1038/nm.3127). [DOI] [PubMed] [Google Scholar]
- 32.Hu Z, Hom S, Kudze T, Tong XJ, Choi S, Aramuni G, Zhang W, Kaplan JM. Neurexin and neuroligin mediate retrograde synaptic inhibition in C. elegans. Science. 2012;337:980–984. doi: 10.1126/science.1224896. published online EpubAug 24 (science.1224896 [pii] 10.1126/science.1224896). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simon DJ, Madison JM, Conery AL, Thompson-Peer KL, Soskis M, Ruvkun GB, Kaplan JM, Kim JK. The microRNA miR-1 regulates a MEF-2-dependent retrograde signal at neuromuscular junctions. Cell. 2008;133:903–915. doi: 10.1016/j.cell.2008.04.035. published online EpubMay 30 (S0092-8674(08)00609-0 [pii] 10.1016/j.cell.2008.04.035). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Foss-Feig JH, Kwakye LD, Cascio CJ, Burnette CP, Kadivar H, Stone WL, Wallace MT. An extended multisensory temporal binding window in autism spectrum disorders. Exp Brain Res. 2010;203:381–389. doi: 10.1007/s00221-010-2240-4. published online EpubJun (10.1007/s00221-010-2240-4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roberts TP, Khan SY, Rey M, Monroe JF, Cannon K, Blaskey L, Woldoff S, Qasmieh S, Gandal M, Schmidt GL, Zarnow DM, Levy SE, Edgar JC. MEG detection of delayed auditory evoked responses in autism spectrum disorders: towards an imaging biomarker for autism. Autism Res. 2010;3:8–18. doi: 10.1002/aur.111. published online EpubFeb (10.1002/aur.111). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zweier M, Gregor A, Zweier C, Engels H, Sticht H, Wohlleber E, Bijlsma EK, Holder SE, Zenker M, Rossier E, Grasshoff U, Johnson DS, Robertson L, Firth HV, Ekici AB, Reis A, Rauch A. Mutations in MEF2C from the 5q14.3q15 microdeletion syndrome region are a frequent cause of severe mental retardation and diminish MECP2 and CDKL5 expression. Hum Mutat. 2010;31:722–733. doi: 10.1002/humu.21253. published online EpubJun (10.1002/humu.21253). [DOI] [PubMed] [Google Scholar]
- 37.Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, Xing Y, Lubischer JL, Krieg PA, Krupenko SA, Thompson WJ, Barres BA. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci. 2008;28:264–278. doi: 10.1523/JNEUROSCI.4178-07.2008. published online EpubJan 2 (28/1/264 [pii] 10.1523/JNEUROSCI.4178-07.2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu J, Xiao N, Xia J. Thrombospondin 1 accelerates synaptogenesis in hippocampal neurons through neuroligin 1. Nat Neurosci. 2010;13:22–24. doi: 10.1038/nn.2459. published online EpubJan (nn.2459 [pii] 10.1038/nn.2459). [DOI] [PubMed] [Google Scholar]