Abstract
Sexual dimorphism in the brain and cognition is a topic of widespread interest. Many studies of sex differences have focused on visuospatial and verbal abilities but few studies have investigated sex differences in executive functions. We examined two key components of executive function—response inhibition and response monitoring—in healthy men (n=285) and women (n=346) performing the Stop-signal task. In this task, participants are required to make a key press to a stimulus, unless a tone is presented at some delay following the initial stimulus presentation; on these infrequent trials, participants are instructed to inhibit their planned response. Response inhibition was assessed with an estimate of the latency needed to inhibit a response (stop-signal reaction time), and response monitoring was measured by calculating the degree to which participants adjusted their reaction times based on the immediately preceding trial (e.g. speeding following correct trials and slowing following errors). There were no sex differences in overall accuracy or response inhibition but women showed greater sensitivity to trial history. Women sped up more than men following correct “Go” trials, and slowed down more than men following errors. These small but statistically significant effects (Cohen’s d=0.25–0.3) suggest more flexible adjustments in speed-accuracy trade-offs in women and greater cognitive flexibility associated with the responsive control of action.
1. Introduction
Sexual dimorphism in the brain and cognition is a topic of great public and scientific interest. Findings from human and animal studies have indicated sex differences ranging from molecular to behavioral levels, although these differences are often subtle and inconsistent (Eliot, 2011). A more thorough understanding of putative differences between the brain functions of men and women has implications for education, clinical neuropsychiatry and many other fields. For example, research into sex differences in the brain and cognitive functioning has already fueled education policy-making (e.g., same sex classrooms; Eliot, 2011) and in theory could be used to leverage unique strengths and learning styles of girls and boys. Further, given large sex differences in the incidence and severity of many psychiatric illnesses (e.g., schizophrenia and attention deficit hyperactivity disorder) and evidence for sex differences in pathophysiological mechanisms of disease, an understanding of sex differences in brain and behavior could impact both theory and clinical decision-making.
With respect to cognitive functioning, most previous studies have examined sex differences in visuospatial and verbal abilities, with women typically outperforming men on verbal tasks and men performing better on spatial tasks (Hyde, 1988; Voyer, Voyer, & Bryden, 1995). Although these differences are relatively small and might have some basis in social biases (Baenninger & Newcombe, 1989) and experience, correlations of these differences with both prenatal sex hormone exposure (see Hampson, 1995 for review) and circulating levels in adulthood suggest a biological basis. For example, variability in testosterone and estradiol levels during the menstrual cycle has been found to predict changes in cognitive ability, particularly spatial skills (Broverman, et al., 1981; Hampson, 1990; Hampson, 1995; Hausmann, et al., 2000). Interestingly, there is also evidence that sex hormones mediate the effect of social biases and stereotypes on spatial abilities (Hausmann, et al., 2009).
Surprisingly neglected in studies of sex differences in cognition is executive functioning, which refers to the diverse cognitive abilities, largely subserved by frontal cortex, involved in the control of thought and action. Despite clear sex differences in psychiatric disorders that are associated with pronounced impairments in executive functions and putative effects of sex hormones on executive functioning via their contribution to prenatal brain development and modulation of dopamine, few studies have examined sex differences in executive functioning in healthy populations. Executive dysfunction has been posited to be a central feature of both schizophrenia and Attention Deficit/Hyperactivity Disorder (ADHD; Barkley, 1997; Goldman-Rakic & Selemon, 1997). As previously noted, sex differences in the epidemiology and illness severity of both schizophrenia and ADHD have been well documented. Although there is evidence for androgenic modulation of neurodevelopment (Wisniewski, 1998) and behavior, which may help explain sex differences in the epidemiology of these diseases, these differences still remain poorly understood. Importantly, circulating estrogen is a significant neuromodulator of dopamine (Becker, 1990; Pasqualini, Olivier, Guibert, Frain, & Leviel, 1995; Xiao & Becker, 1994), a neurotransmitter that has been implicated both in executive control (Robbins & Arnsten, 2009) and pathophysiological mechanisms of both ADHD and schizophrenia (Iversen & Iversen, 2007).
Results from the few studies that have investigated sex differences in executive functioning using standard neuropsychological measures have been mixed. A handful of studies have reported a female advantage (Baroun & Alansari, 2006; Kalkut, Han, Lansing, Holdnack, & Delis, 2009; Mekarski, Cutmore, & Suboski, 1996; Sarmany, 1977; von Kluge, 1992), others reporting a male advantage (Clayson, Clawson, & Larson, 2011), and most reporting no difference across the sexes (Brocki & Bohlin, 2004; Daniel, Pelotte, & Lewis, 2000; Houx & Jolles, 1993; Schirmer & Kotz, 2003; Silveri et al., 2006; Swerdlow, Filion, Geyer, & Braff, 1995). There are a few factors that contribute to the lack of consensus on sex differences in executive functioning. First, there is significant variability in both the putative executive function constructs examined and the specific paradigms used to measure these. Although there is no clear consensus about the most valid subdivisions of executive function (e.g. Sabb et al., 2008), some studies suggest that discrete constructs can be identified at the behavioral level (Miyake et al., 2000) or as defined by distinctive neural circuitry (Rushworth et al., 2011; Bilder, 2012). Second, many measures of executive functioning place considerable demands on multiple cognitive processes, which might obscure sex differences in specific components of executive functioning. Third, most previous studies used relatively small samples and therefore lacked statistical power to detect subtle sex differences.
Sex differences in response monitoring and response inhibition, two key components of executive functioning, have been examined with the Stop-signal task. Response inhibition refers to the ability to deliberately suppress inappropriate motor responses and response monitoring involves the ability to evaluate actions and use feedback signaling success or failure to guide future performance. The Stop-signal task has emerged as one of the leading methods to examine these two constructs. In this task, subjects are presented with a stimulus that requires executing a speeded motor response unless a signal is presented following initial stimulus presentation. In this case, subjects are instructed to inhibit their response. A measure of the latency of the covert inhibitory process, stop-signal reaction time (SSRT), can be estimated (Logan, Cowan, & Davis, 1984). Along with measures of inhibition, well-characterized reaction time (RT) adjustments as a function of trial history have been described in this task. Participants tend to slow down following errors and correctly inhibited responses, and to speed up with consecutive trials that do not require inhibition (Emeric et al., 2007; Rieger & Gauggel, 1999; Verbruggen, Logan, Liefooghe, & Vandierendonck, 2008). An additional advantage of this task is the relatively low demands it places on other cognitive processes, for example, working memory. Further, using a “tracking” version of the task, in which task difficulty is manipulated to ensure equal inhibition success across subjects, allows measures of response inhibition and response monitoring that are not confounded by differences in success rate.
Although most studies have failed to observe sex differences in Stop-signal task performance, reduced functional asymmetry of inhibition-related ERPs in women (Huster, Westerhausen, & Herrmann, 2011) and different networks of fMRI activity in men and women during inhibition and error processing (Li, Huang, Constable, & Sinha, 2006; Li et al., 2009) have been reported. Colzato, et al. (2011) reported less efficient inhibition in women, but only in the follicular stage of their menstrual cycle. In the current study, sex differences in response inhibition and response monitoring were examined in a large sample of healthy adults performing the Stop-signal task.
2. Methods
2.1 Participants
Participants were drawn from the first 1000 enrollees in a project of the Consortium for Neuropsychiatric Phenomics at UCLA (www.phenomics.ucla.edu), approved by the UCLA Institutional Review Board. The participants, ages 21–50, were recruited by community advertisements from the Los Angeles area and completed the Stop-signal task. To be included individuals had to be either “White, Not of Hispanic or Latino Origin” or “Hispanic or Latino, of Any Race” following NIH designations of racial and ethnic minority groups, and have completed at least 8 years of education (other racial and ethnic minority groups were excluded because this was thought to increase risk of confounding planned genetic studies). For participants who spoke both English and Spanish, language for testing was determined by a verbal fluency test. Participants were screened for neurological disease, history of head injury with loss of consciousness or cognitive sequelae, use of psychoactive medications, substance dependence within past 6 months, history of major mental illness or ADHD, and current mood or anxiety disorder. Self-reported history of psychopathology was verified with the SCID-IV (First, Spitzer, Gibbon, & Williams, 1995). Urinalysis was used to screen for drugs of abuse (cannabis, amphetamine, opioids, cocaine, benzodiazepines) on the day of testing, and participants were excluded if results were positive. These primary inclusion/exclusion criteria yielded 640 participants who completed the study. We then applied additional exclusion criteria based on Stop-signal task performance (see Statistical Analyses), yielding 285 males and 346 females in the final analysis. Demographic data are outlined in Table 1. Males and females were similar on age, race, handedness, and ethnicity. Compared to Hispanic or Latino individuals who were examined in English, Hispanic or Latino individuals who were examined in Spanish were slower on both Go trials (t(270)=3.36, p=0.0009) and Stop-error trials (F(270)=2.48, p=0.01) and had a higher proportion of Go trials in which they failed to respond (t(270)=2.21, p=0.03). However, men and women were matched on the language in which the test was administered. Women averaged about 0.5 more years of education than men. Written informed consent was obtained from all subjects prior to testing. Subjects were paid $15/hour and received compensation for transportation and parking. After receiving a thorough explanation, all participants gave written informed consent according to procedures approved by the University of California Los Angeles Institutional Review Board, and a certificate of confidentiality was obtained from the NIH.
Table 1.
Demographic data.
| Males (n=285) | Females (n=346) | Analysis
|
||||
|---|---|---|---|---|---|---|
| Test Statistic | p-value | |||||
| n | % | n | % | |||
|
|
||||||
| Race | χ2=4.0 | 0.55 | ||||
| White | 212 | 74.7 | 246 | 71.1 | ||
| Native Americana | 66 | 23.2 | 84 | 24.3 | ||
| Multi-racial | 4 | 1.4 | 10 | 2.9 | ||
| African American | 1 | 0.4 | 4 | 1.2 | ||
| Asian | 0 | 0 | 1 | 0.3 | ||
| Not reported | 1 | 0.4 | 1 | 0.3 | ||
| Ethnicity | ||||||
| Hispanic Origin | 122 | 43% | 150 | 43% | χ2=0.02 | 0.89 |
| Not of Hispanic Origin | 163 | 57% | 196 | 57% | ||
| Language Testing Administered | ||||||
| English | 244 | 86% | 290 | 84% | χ2=0.39 | 0.53 |
| Spanish | 41 | 14% | 56 | 16% | ||
|
|
||||||
| Mean | SD | Mean | SD | |||
|
|
||||||
| Age | 31.2 | 8.9 | 31.3 | 8.5 | t=1.32 | 0.19 |
| Edinburgh Handedness Quotientb | 0.73 | 0.54 | 0.72 | 0.57 | T=0.40 | 0.69 |
| Education (years) | 14.7 | 2.0 | 15.2 | 2.1 | t=2.71 | 0.007 |
A high proportion of these individuals who identified themselves as Native Americans were of Mexican origin
Higher scores indicate greater right-handedness
2.2. Data acquisition
E-Prime software was used for task presentation and response collection; the E-prime programs used are available on request. Data were collected on Dell workstations with Intel Core Duo processors, 2.4 or 2.2 GHz processing speed, 1 GB memory, and Dell 17″ LCD monitors. Responses were indicated using a standard Dell keyboard. Subjects were positioned so that their eyes were approximately 20″ from the monitor.
2.3 Task Design
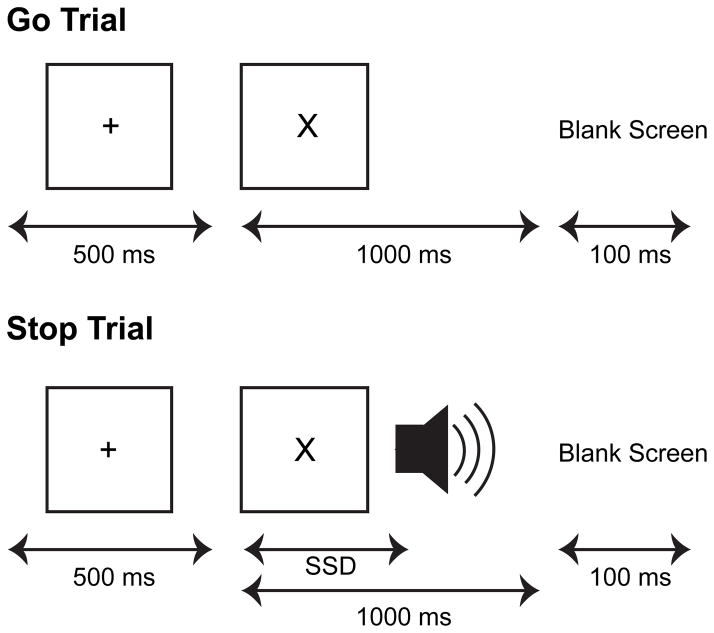
Subjects performed the Stop-signal task (Figure 1; Lappin & Eriksen, 1966). On each trial, participants were shown a visual stimulus (“X” or “O”) in the center of the screen. On 75% of the trials (Go trials), they were instructed to press the left arrow button on the keyboard when they saw an “X” and to press the right arrow button on the keyboard when they saw an “O”, as quickly and accurately as possible. On the remaining 25% of trials (Stop trials), a 500 Hz tone lasting 250 ms was presented through headphones at a variable delay following onset of the visual stimulus (stop-signal delay; SSD). On these trials, participants were instructed to withhold their response to the visual stimulus. Trials in which the participant successfully inhibited the response were labeled Stop-correct, and trials in which the participant erroneously responded to the visual stimulus were labeled Stop-error. Participants were instructed that stopping and going were equally important. All trials began with a 500 ms fixation cross in the center of the screen. Then, the Go stimulus was presented for a 1000 ms fixed-response interval. Participants were allowed to respond at the start of stimulus presentation until the end of the 1000 ms fixed-response interval. Each trial was separated by a fixed, 100 ms delay.
Figure 1.
Stop signal task. All trials began with the presentation of a central fixation spot. After 500 ms, the fixation spot disappeared, and, simultaneously, an ‘X’ or ‘O’ appeared at a central location. On 75% of the trials (Go trials), they were instructed to press the left arrow button on the keyboard when they saw an “X” and to press the right arrow button on the keyboard when they saw an “O”, as quickly and accurately as possible. On the remaining 25% of trials (Stop trials), a 500 Hz tone lasting 250 ms was presented through headphones at a variable delay following onset of the visual stimulus (stop-signal delay; SSD). On these trials, subjects were instructed to withhold their response to the visual stimulus.
Response inhibition becomes more difficult as the SSD increases. SSDs were dynamically adjusted using two independent 1-up/1-down tracking procedures (or “staircases”), thereby ensuring successful inhibition on approximately 50% of the Stop trials. The initial SSD for each staircase was based upon performance in a practice block. SSD was increased or decreased by 50 ms when the participant succeeded or failed to inhibit, respectively. The testing session included at least one practice block of 22 Go trials and 10 Stop trials. Participants were required to inhibit on at least 20% of Stop trials and have a mean Go RT of less than 750 ms in order to continue. Two experimental blocks of 128 trials each then followed.
2.4 Performance measures
Behavioral performance was evaluated through measurements of RT on Go and Stop-error trials, and of mean SSD. Performance in the Stop-signal task can be accounted for by a mathematical model that assumes a race between independent processes that generate (GO) and inhibit (STOP) the movement (Logan & Cowan, 1984). The response is executed if the GO process finishes first and inhibited if the STOP process finishes first. The latency of the GO process can be measured directly from the observable RTs, but the latency of the STOP process can only be estimated from performance (considering both proportion of successful inhibition on Stop trials and SSD). The estimate of time needed to respond to the stop signal and to cancel the movement is referred to as the stop-signal reaction time (SSRT; Figure 2). SSRT was calculated using a quantile method (for more detailed description of this method see Band et al., 2003; Congdon et al., 2012). First, the proportion of failed inhibition trials, which are the proportion of Stop trials in which the participants responded, was calculated across both of the two independent 1-up/1-down staircases used to manipulate task difficulty. Then correct RTs from Go trials were sorted in ascending order. The quantile RT was determined by finding the RT corresponding to the proportion of failed inhibition. For example, if the proportion of inhibition for a particular subject was 0.6, the quantile RT would be the RT for which 60% of trials were faster and 40% of trials were slower. The average SSD (across both staircases) was then subtracted from the quantile RT in order to calculate SSRT.
Figure 2.
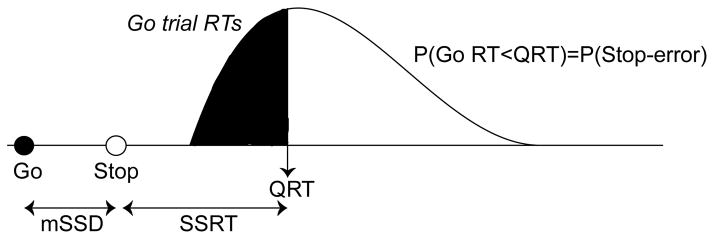
Stop-signal reaction time (SSRT) calculation. The proportion of failed inhibition trials, P(Stop-error), was calculated for each participant. The mean stop-signal delay (mSSD), represented by the delay between the stimulus to respond (filled circle) and the stop signal (empty circle), was calculated for the same participant. Then RTs from correct Go trials were sorted in ascending order, represented by the probability distribution in the figure. The quantile RT (QRT) was determined by finding the RT corresponding to P(Stop-error). Under race model assumptions, those Go trials that were left of the QRT (represented by the shaded area under the curve), would have been too fast to be inhibited, provided a stop signal was presented at mSSD following the Go signal. Likewise, the Go trials to the right of the QRT (represented by the empty area under the curve) would have been slow enough to be inhibited. The average SSD (mSSD) was then subtracted from the QRT in order to calculate SSRT.
To index response monitoring, RT was examined as a function of trial history (Figure 3). Triads of trials were identified for each of our trials of interest (Stop-correct, Stop-error, Go) such that the trial of interest was preceded and followed by a correct Go trial. The middle trial of the triad is referred to as the critical trial. Post-error and post-inhibit slowing were calculated by subtracting the RT preceding a Stop-error or Stop-correct trial, respectively, from the trial following it. Likewise, post-Go speeding was calculated by subtracting the RT following a Go trial from the RT preceding it. For each critical trial type, change in RT between the preceding and following Go trial were averaged to arrive at one aggregate measure of post-error slowing, post-inhibit slowing, and post-Go speeding for each subject.
Figure 3.
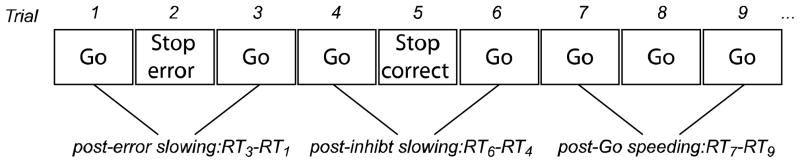
Representation of how trial history effects were calculated using an example trial sequence. For each critical trial type (Stop-correct, Stop-error, and Go), triads of trials were identified such that the critical trial was preceded and followed by a correct Go trial. Post-error and post-inhibit slowing were calculated by subtracting the RT preceding a Stop-error or Stop-correct trial, respectively, from the trial following it. Likewise, post-Go speeding was calculated by subtracting the RT following a Go trial from the RT preceding it. For each critical trial, change in RT between the preceding and following Go trial were averaged to arrive at one aggregate measure of post-error slowing, post-inhibit slowing, and post-Go speeding for each subject.
2.5 Statistical Analyses
The following rules were used to exclude subjects on the basis of performance: 1) Ineffective tracking procedure as defined by percent inhibition greater than 75% or less than 25%; 2) Go trial accuracy less than 60% (see Congdon et al., 2012 for justification of these criteria). These criteria resulted in the exclusion of 2 males and 7 females.
Analysis of covariance was conducted to measure the effect of sex on directional accuracy, omission errors, Stop trial accuracy, and SSRT. To measure the effect of sex and current trial type (Go or Stop-error) on current trial RT, a mixed-model analysis was conducted, using sex as a between-subject variable and trial type as a within-subject variable. To assess effects of trial history on Go trials, a mixed model analysis was conducted on Go RTs with sex as a between-subject variable and critical trial (Go, Stop-correct, Stop-error) and history (n−1 or n+1, relative to critical trial) entered as within-subject variables.. Additionally, sex differences in post-inhibit slowing, post-error slowing, and post-Go speeding, as defined in the previous section, were examined using analysis of covariance. In all analyses that included sex, we included years of education as a covariate, as this measure differed significantly between men and women. Levene’s test indicated no significant difference in within-group variability between sexes on any of the dependent measures (all p’s > 0.09). All tests were two-tailed except as otherwise specified. Eta-squared (η2), Cohen’s d, and correlation coefficients (r) were used where appropriate to report effect sizes. Generalized eta-squared (η2G) measures of effect size are reported for repeated measures analyses, as they provide comparability across within- and between-subjects designs and have been recommended for such analyses (Bakeman, 2005).
3. Results
Table 2 shows Stop-signal task performance and RT adjustments for men and women.
Table 2.
Stop-signal task performance.
| Males (n=285) | Females (n=346) | |||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
|
|
||||
| Go trial directional errors (%) | 0.39 | 0.58 | 0.32 | 0.81 |
| Go trial no response (%) | 1.03 | 1.52 | 1.02 | 2.06 |
| Probability of inhibition (%) | 52.95 | 5.43 | 53.53 | 5.26 |
| No-stop signal RT (ms) | 588.94 | 108.19 | 595.71 | 104.89 |
| Signal-respond RT (ms) | 490.57 | 88.18 | 491.68 | 87.34 |
| SSRT (ms) | 210.13 | 39.51 | 213.00 | 41.12 |
| Post-Go speeding (ms) | 11.62 | 20.90 | 17.63 | 21.91 |
| Post-inhibit slowing (ms) | −2.82 | 44.73 | 0.51 | 44.22 |
| Post-error slowing (ms) | 49.89 | 47.00 | 63.70 | 52.81 |
3.1. Accuracy
Directional accuracy on Go trials was high and did not differ between sexes (F(1,628)=0.10, p=0.75, d<0.10). The proportion of Go trials in which participants did not respond within the deadline was low and also did not differ between groups (F(1,628)=0.56, p=0.45, d<0.10). On Stop trials, the dynamic tracking procedure was successful, and the mean proportion of Stop-correct trials was 53%. The two groups did not differ significantly in the proportion of Stop-correct trials (F(1,628)=1.65, p=0.20, d=0.11).
3.2. No-stop signal and signal-respond RTs
Consistent with race model logic, there was a significant effect of trial type (F(1,628) = 4099.88, p < 0.0001, η2G=0.21), with Go trial responses being slower than Stop-errors. There was no significant main effect of sex (F(1,628) = 0.43, p = 0.51, η2G=0.0007) nor sex-by-trial type interaction (F(1,628) = 3.16, p = 0.08, η2G=0.0002).
3.3. SSRT
There was no sex difference in SSRT (F(1, 628)=1.31, p=0.25, d<0.1).
3.4. Trial history effects
See Figure 2. There was a significant effect of history (F(1, 628) = 318.09, p < 0.0001, η2G=0.005), and critical trial (F(2, 1256) = 128.79, p < 0.0001, η2G=0.013) on Go RT. Notably, there was a significant history-by-critical trial interaction (F(2,1256) = 468.61, p < 0.0001, η2G=0.024). Significance of post-hoc tests were Bonferroni-corrected for the 7 comparisons planned to disentangle this three-way interaction, which included: (1) Go RT when preceded by Stop-error versus Go trial, (2) Go RT when preceded by Stop-correct versus Go trial, (3) Go RT when preceded by Stop-error versus Stop-correct trial, (4) Go RT preceding a Stop-error versus Stop-correct trial, (5–7) Go RT preceding versus following either a Stop-error, Stop-correct, or Go trial. Comparisons revealed that Go trials following Stop-correct (t(630)=13.2, adjusted p<0.0001) and Stop-error (t(630)=14.4, adjusted p<0.0001) trials were slower than those following another Go trial. There was no difference in RT for trials following Stop-correct versus Stop-error (t(630)=1.8, adjusted p=0.49). There were also differences in Go trials preceding Stop-correct and Stop-error trials. Go trials preceding a Stop-correct trial were slower than those preceding Stop-error trials (t(630)=25.1, adjusted p<0.0001) That is, when subjects are responding more slowly, they are more likely to inhibit. Relative to the immediately preceding trial, participants slowed following a Stop-error trial (t(630)=28.5, adjusted p<0.0001) and sped up following a Go trial (t(630)=17.3, adjusted p<0.0001). However, no difference between RTs for Go trials preceding and following a Stop-correct trial was observed (t(630)=0.54, adjusted p>0.99). To summarize, when compared to Go trials following Go trials, significant slowing following both Stop-correct trials and Stop-error trials was observed. When compared to the immediately preceding trial, speeding following consecutive Go trials and slowing following Stop-errors was observed; no change in RT was observed between the trial preceding and following Stop-correct trials.
In examining the effect of sex on history-based adjustments, there was no main effect of sex (F(1,628)=1.0, p=0.32, η2G=0.001). However, the sex-by-history (F(1,628)=5.64, p=0.02 η2G=0.00009), sex-by-critical trial (F(2, 1256)=5.32, p=0.0005, η2G=0.0004) and three-way sex-by-history-by-critical trial interaction effects (F(2,1256)=6.2, p=0.002, η2G=0.0004) were significant. To investigate the differences in means giving rise to this three way interaction, the following comparisons 5 were planned: (1–3) sex differences between Go RT when preceded by either a Go, Stop-error, or Stop-correct trial; (4–5) sex differences between Go RT when followed by either a Stop-error or Stop-correct trial. Planned comparisons revealed no sex difference in RT for Go trials preceding either Stop-correct or Stop-error trials (all adjusted p-values > 0.99); however, Go trials following a Stop-error were slower in women (F(1,628)=6.62, adjusted p=0.05). No sex differences in trials following Go trials (F(1,628)=0.01, adjusted p>0.99) or Stop-correct trials (F(1,628)=0.56, adjusted p=0.99) were observed.
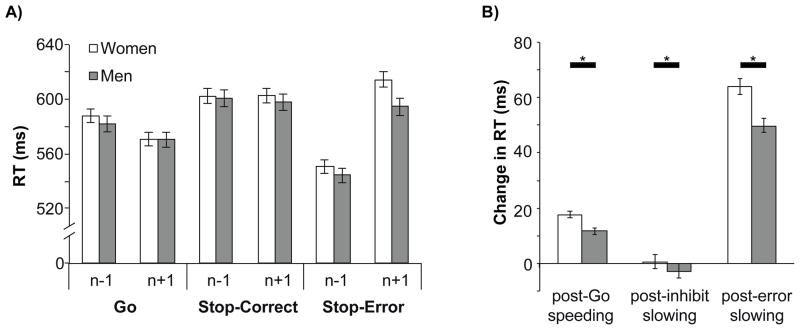
Lastly, sex differences in the magnitude of change between RTs following and preceding each trial of interest were also examined. Compared to the immediately preceding trial, females slowed down significantly more than males following Stop-errors (F(1,628)=14.55, p=0.0001, d=0.28) and sped up significantly more following consecutive Go trials (F(1,628)=13.20, p=0.0003, d=0.28). There was no significant sex difference in post-inhibit slowing (F(1,628)=0.75, p=0.39, d<0.10).
3.5 Within-subject variability
As greater trial history-based adjustments might give rise to greater within-subject variability in RT, we also examined the within-subject standard deviation of Go RT and its relationship to the magnitude of post-error slowing and post-Go speeding as a post-hoc analysis. Controlling for years of education, women showed greater inter-individual variability in RT (F(1, 628)=11.3, p=0.0008, d=0.25). Further, the magnitude of post-error slowing was significantly related to within-subject variability in Go RT in both men (r=0.27, r<0.0001) and women (r=0.20, p=0.0001). A small relationship was also observed between within-subject variability and post-Go speeding in men (r=0.14, p=0.01), but not women (r=0.01, p=0.87). Finally, we examined differences between men and women in within-subject variability of the magnitude of post-Go speeding and post-error slowing. Controlling for education, women showed greater variability in both post-error slowing (F(1,628)=14.6, p=0.0001, d=0.39) and post-Go speeding (F(1,628)=13.2, p=0.0003, d=0.28).
3.6. Age Effects
As an exploratory analysis, we investigated sex differences in the relations between age and Stop-signal task measures of response inhibition and response monitoring in men and women. After controlling for years of education, only post-Go speeding showed a differential relationship with age between men and women, evidenced by an age-by-sex interaction effect (F(1, 626)=7.46, p=0.007 η2=0.01). In men, age was associated with greater speeding following Go trials (F(1,283)=13.31, p=0.0003, r=0.21). There was no significant relationship between age and post-Go speeding in women (F(1,344)=0.04, p=0.84, r=0.01).
4. Discussion
This study found differences between men and women on a measure of response monitoring derived from the Stop-signal task, even though the sexes did not differ in overall accuracy, response speed or the efficiency of inhibitory control. Data from this study of a large group of healthy individuals performing the Stop-signal task indicated no sex differences in overall response speed or in the efficiency of inhibitory control. Men and women differed, however, on a more subtle aspect of task performance. Specifically, women made greater adjustments to their performance based on the outcome of the preceding trial. Both men and women sped up following consecutive trials in which they were not required to inhibit a response, and slowed down on the trial following an error, consistent with prior reports. But compared to men, women showed a larger response to trial history: they sped up more following consecutive Go trials and slowed down more following Stop-error commission. Combined, these findings support the hypothesis that women manifest more flexible adjustments to speed-accuracy trade-offs in response to recent experience of success and failure.
We are not aware of prior studies that explicitly examined sex differences in speed-accuracy adjustments. Several prior studies of sex differences in post-error slowing reported no difference (Larson, South, & Clayson, 2011; Li, et al., 2006; Li, et al., 2009), but these studies were underpowered to detect subtle differences1. Increased speeding in women following successive Go trials has also not been previously reported in the literature but may be consistent with findings from studies showing greater RT variability in women. In a large sample of individuals performing a choice RT task, Der and Deary (2006) found that the most robust sex difference was increased RT variability in women, which was present even after controlling for mean RT. In a follow-up study, Reimers and Maylor (2006) replicated these findings and observed that increased RT variability in women was systematic in nature and due to women being slower initially, but speeding up with experience. In the current study, the variability in Go RT was greater in women than men and significantly related to post-error slowing. Thus, in this study, larger performance-based adjustments appear to contribute to increased overall RT variability in women.
There are several theories about the origin and significance of speed-accuracy trade-off (SATO; Schouten & Bekker, 1967) and post-error slowing. Computational accounts of SATO posit that evidence accumulates until a decision threshold is crossed, and that shifts in SATO reflect changes in decision threshold, baseline information, or rate of information accrual (e.g. Reddi & Carpenter, 2000). Post-error slowing is a robust phenomenon (Rabbitt & Rodgers, 1977) widely interpreted as reflecting bias towards a more cautious response strategy (Dutilh et al., 2012). Recent work shows that slowing is not specific to errors, and may generally follow rare events due to attention being oriented away from current task demands (Notebaert et al., 2009). Another theory posits that errors delay the start of information accrual on the following trial due to task-irrelevant factors like affective responses (Rabbitt & Rodgers, 1977). Recent computational work using diffusion models supported the idea that increased response caution gives rise to post-error adjustments (Dutilh, et al., 2012), but other task-related factors and individual differences can also affect the psychological process that result in post-error slowing, including error-related distraction away from task demands and delayed accumulation of sensory information (Dutilh, et al., in press). Although not explicitly accounted for in drift diffusion models, it remains plausible that affective response to errors underlies response caution. Indeed, it has been observed that individuals high in negative affect show more post-error slowing (Robinson, Meier, Wilkowski, & Ode, 2007), and individuals high in anxiety were more cautious after committing an error, as indexed by diffusion model parameters (White, Ratcliff, Vasey, & McKoon, 2010), possibly to avoid further negative affect associated with error commission.
Although it is possible that more cautious adjustment in women is related to emotional reactions to errors, given that women tend to report more negative affect (Feingold, 1994), our data do not indicate that sex differences in affect can fully account for the present results as women also sped up more than men following consecutive correct Go trials. Further, post-error slowing was significantly related to post-Go speeding in both men (r=0.48, p<0.0001) and women (r=0.50, p<0.0001). Rather, we favor the explanation that women show greater flexibility than men in their cognitive control adjustments.
With regards to the mechanisms underlying trial history adjustments, extensive research suggests that error processing involves anterior cingulate cortex (ACC) in an integrated circuit involving prefrontal cortex, thalamus, and basal ganglia (see van Veen & Carter, 2006 for review). The error related negativity (ERN; Falkenstein, Hohnsbein, Hoormann, & Blanke, 1991; Gehring, Goss, Coles, Meyer, & Donchin, 1993), which peaks after an error, is localized to the ACC (Dehaene, Posner, & Tucker, 1994; Van Veen & Carter, 2002), and the error positivity (Pe), which also follows errors and peaks later than the ERN, has been linked to post-error slowing (Hajcak, McDonald, & Simons, 2003; Nieuwenhuis, Ridderinkhof, Blom, Band, & Kok, 2001) and error awareness (Endrass, Franke, & Kathmann, 2005; Endrass, Reuter, & Kathmann, 2007; Nieuwenhuis, et al., 2001). Theories about error-related ACC activity emphasize comparisons of motor output and representations of the correct response (Falkenstein, et al., 1991; Gehring, et al., 1993), conflict between incorrect and correct responses (Botvinick, Cohen, & Carter, 2004; Carter et al., 1998), phasic decreases in dopaminergic activity resulting from violations in expectations based on prior reinforcements (Holroyd & Coles, 2002), and affective responses to error commission (Luu, Flaisch, & Tucker, 2000; Luu, Tucker, Derryberry, Reed, & Poulsen, 2003). It has been suggested that monitoring-related activity in the ACC signals the need for additional control processes, which are implemented in lateral PFC (see Ridderinkhof, van den Wildenberg, Segalowitz, & Carter, 2004 for review); this argument is supported by findings that ACC activity on error trials is associated with post-error slowing and greater activity in lateral PFC on the subsequent trial (Garavan, Ross, Murphy, Roche, & Stein, 2002; Kerns et al., 2004; Li et al., 2008). A role of the lateral PFC has also been highlighted in a recent study of speed-accuracy trade-off (Ivanoff, Branning, & Marois, 2008). While so far there is insufficient evidence to falsify any of these theories, further tests might consider sex-related moderating factors associated with neuroanatomical or neurochemical mechanisms.
Despite the absence of prior evidence for sex differences in error-related behavioral measures, some studies found sex differences in the magnitude of error-related brain activity. A larger ERN and Pe in men has been reported (Larson, et al., 2011), which would be contrary to the current results, to the extent that these ERPs are related to post-error slowing. Li and colleagues report different networks of error-related fMRI activation in men and women in a Stop-signal task (2006, 2009). Unfortunately methodological limitations obscure the significance of these findings as the authors contrasted successful versus unsuccessful Stop trials, which differ in the presence of a motor response. Thus, it is unclear whether sex differences are related to error processing or motor output. Li et al (2009) also compared trials following Stop-error trials in which participants did and did not show post-error slowing, and found that women exhibited greater posterior cingulate cortex activation than men. While we currently lack compelling evidence, further study of sex differences in the ERN and Pe appears warranted, specifically as these relate to post-error adjustments.
Interestingly, neuroanatomical differences between men and women in the midcingulate region have been reported, with men having greater left-asymmetric doubling of the midcingulate gyri (Yucel et al., 2001). With regards to the functional significance of variability in cingulate morphology, greater leftward folding has been associated with more accurate Stroop performance (Huster, Enriquez-Geppert, Pantey, & Bruchmann, 2012; Huster, et al., 2009), spatial working memory (Fornito, et al., 2004), and verbal fluency (Fornito, et al., 2004), as well as augmented conflict-related ERPs (Huster, Enriquez-Geppert, Pantey, & Bruchmann, 2012; Huster, et al., 2009). Thus, the observed sex differences in history-based RT adjustments might have some basis in differences in cingulate morphology. Further, sex differences in morphology should also be considered when interpreting sex differences in ERP amplitude, as they might reflect differences in brain structure rather than differences in neural activity.
Other possible neuroanatomical distinctions that might help explain the response monitoring sex differences reported here include: differences in interhemispheric connections and functional asymmetries (Davatzikos & Resnick, 1998; Voyer, 1996); and sex differences in functions mediated by archicortical and paleocortical systems (Bilder, 2012) as suggested by sex differences in dorsal and ventral visual stream functions (Alexander, 2003; Lewin & Herlitz, 2002; Voyer, et al., 1995), evidence for a greater bias towards novelty-detection in women (Colzato, et al., 2011), and larger volumes of frontoorbital cortex volume in women but frontomedial cortex in men (Cosgrove, Mazure, & Staley, 2007). The paleocortical system has been hypothesized to play a special role assigning emotional salience to external stimuli (Christensen & Bilder, 2000), thus increased paleocortical activity in women might promote bonding and empathy—processes for which women are argued to be more specialized (De Vries & Panzica, 2006; Hoffman, 1977).
Levels of circulating estrogen may also help explain sex differences in response monitoring. Estradiol enhances dopaminergic activity via effects on dopamine synthesis, release, and turnover (Becker, 1990; Pasqualini, et al., 1995; Xiao & Becker, 1994). Further, one of the more compelling theories of error-related ACC activity posits that it is related to phasic dopamine signaling (Holroyd & Coles, 2002), and dopamine antagonists decrease the ERN amplitude and post-error slowing in healthy volunteers (de Bruijn, Sabbe, Hulstijn, Ruigt, & Verkes, 2006; Zirnheld et al., 2004). Thus, a relationship between estrogen and reactive behavioral adjustments via estrogen’s neuromodular effects on dopamine is possible, and exaggerated trial history effects in women might be dependent on menstrual cycle stage and circulating estradiol levels. Unfortunately, the current study cannot speak to this hypothesis, as hormonal status of participants was not controlled.
In terms of implications, the current findings are relevant for understanding the nature of sex differences in executive function and their underlying neural mechanisms. They also highlight the advantage of using tasks from the cognitive neuroscience literature over standard neuropsychological measures of executive function to understanding sex differences in cognition, as they are better suited to isolate specific functions. These findings of larger RT adjustments as a function of trial history in women also have potential implications for understanding cognitive dysfunction in psychiatric diseases, particularly those with robust sex differences in incidence and severity. For example, intra-individual differences in RT across different tasks have been reported in both ADHD and schizophrenia. We are not aware of any studies that have explored the extent to which increased variability in RT is related to exaggerated performance-based adjustments. Although most studies have failed to find differences in post-error slowing between individuals with schizophrenia and ADHD compared to healthy controls (O’Connell, Bellgrove, Dockree, & Robertson, 2004; van Meel, Heslenfeld, Oosterlaan, & Sergeant, 2007; but see Schachar, et al., 2004), patients with schizophrenia have been found to show exaggerated adjustments in RT following correctly inhibited responses during oculomotor tasks (Barton, Cherkasova, Lindgren, Goff, & Manoach, 2005; Thakkar, Schall, Boucher, Logan, & Park, 2011). To our knowledge, changes in reaction time following correctly performed trials in ADHD have not been investigated.
In interpreting the significance of the current findings, it should be noted that, like with most findings of sex differences in cognitive functions, men and women are more alike than different. The current findings reflect a small (Cohen’s d=0.25–0.3) difference in magnitude of trial-history adjustments, rather than qualitative differences in how men and women are performing the task. Nevertheless, these novel findings of enhanced adjustments in reaction time based on trial history in women, in the context of otherwise similar performance on the Stop-signal task, help clarify the specific aspects of executive functioning that differ between men and women and suggest an alternate framework for interpreting sex differences in cognition that may provide compelling new insights for future studies.
Figure 4.
A) Mean Go RT (with standard error) for trials following (n+1) and preceding (n−1) Go, Stop-correct and Stop-error trials for women (empty bars) and men (filled bars). B) Mean post-Go speeding, post-inhibit slowing, and post-error slowing.
Acknowledgments
The authors would like to thank Jill Goldstein for her helpful comments on this manuscript.
Footnotes
The statistical powers of Li, et al. (2006) and Li, et al. (2009) to detect a difference with alpha of .05 (two-tailed) were 23% and 17%, respectively. Statistical power could not be calculated for Larson, et al. 2010. In contrast, the statistical power for the current study was 91%.
References
- Alexander GM. An evolutionary perspective of sex-typed toy preferences: pink, blue, and the brain. Archives of Sexual Behavior. 2003;32(1):7–14. doi: 10.1023/a:1021833110722. [DOI] [PubMed] [Google Scholar]
- Baenninger M, Newcombe N. The role of experience in spatial test performance: A meta-analysis. Sex Roles. 1989;20:327–344. [Google Scholar]
- Bakeman R. Recommended effect size measures for repeated measures designs. Behavior Research Methods. 2005;37:379–384. doi: 10.3758/bf03192707. [DOI] [PubMed] [Google Scholar]
- Barkley RA. Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychological Bulletin. 1997;121(1):65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Baroun K, Alansari B. Gender differences in performance on the Stroop test. Social Behavior and Personality. 2006;34:309–318. [Google Scholar]
- Barton JJ, Cherkasova MV, Lindgren KA, Goff DC, Manoach DS. What is perseverated in schizophrenia? Evidence of abnormal response plasticity in the saccadic system. Journal of Abnormal Psychology. 2005;114(1):75–84. doi: 10.1037/0021-843X.114.1.75. [DOI] [PubMed] [Google Scholar]
- Becker JB. Estrogen rapidly potentiates amphetamine-induced striatal dopamine release and rotational behavior during microdialysis. Neuroscience Letters. 1990;118(2):169–171. doi: 10.1016/0304-3940(90)90618-j. [DOI] [PubMed] [Google Scholar]
- Bilder RM. Executive control: balancing stability and flexibility via evoultionary cytoarchitectonic trends. Dialogues in Clinical Neuroscience. 2012;14(1):39–47. doi: 10.31887/DCNS.2012.14.1/rbilder. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends in Cognitive Science. 2004;8(12):539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Brocki KC, Bohlin G. Executive functions in children aged 6 to 13: a dimensional and developmental study. Developmental Neuropsychology. 2004;26(2):571–593. doi: 10.1207/s15326942dn2602_3. [DOI] [PubMed] [Google Scholar]
- Broverman DM, Vogel W, Klaiber EL, Majcher D, Shea D, Paul V. Changes in cognitive task performance across the menstrual cycle. J Comp Physiol Psychol. 1981;95(4):646–654. doi: 10.1037/h0077796. [DOI] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280(5364):747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Christensen BK, Bilder RM. Dual cytoarchitectonic trends: an evolutionary model of frontal lobe functioning and its application to psychopathology. Canadian Journal of Psychiatry. 2000;45(3):247–256. doi: 10.1177/070674370004500303. [DOI] [PubMed] [Google Scholar]
- Clayson PE, Clawson A, Larson MJ. Sex differences in electrophysiological indices of conflict monitoring. Biological Psychology. 2011;87(2):282–289. doi: 10.1016/j.biopsycho.2011.03.011. [DOI] [PubMed] [Google Scholar]
- Colzato LS, Pratt J, Hommel B. Estrogen modulates inhibition of return in healthy human females. Neuropsychologia. 2011 doi: 10.1016/j.neuropsychologia.2011.11.003. [DOI] [PubMed] [Google Scholar]
- Congdon E, Mumford JA, Cohen JR, Galvan A, Canli T, Poldrack RA. Measurement and reliability of response inhibition. Frontiers in Psychology. 2012;3:Article 37. doi: 10.3389/fpsyg.2012.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove KP, Mazure CM, Staley JK. Evolving knowledge of sex differences in brain structure, function, and chemistry. Biological Psychiatry. 2007;62(8):847–855. doi: 10.1016/j.biopsych.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel DB, Pelotte M, Lewis J. Lack of sex differences on the Stroop Color-Word Test across three age groups. Perceptual and Motor Skills. 2000;90(2):483–484. doi: 10.2466/pms.2000.90.2.483. [DOI] [PubMed] [Google Scholar]
- Davatzikos C, Resnick SM. Sex differences in anatomic measures of interhemispheric connectivity: correlations with cognition in women but not men. Cerebral Cortex. 1998;8(7):635–640. doi: 10.1093/cercor/8.7.635. [DOI] [PubMed] [Google Scholar]
- de Bruijn ER, Sabbe BG, Hulstijn W, Ruigt GS, Verkes RJ. Effects of antipsychotic and antidepressant drugs on action monitoring in healthy volunteers. Brain Research. 2006;1105(1):122–129. doi: 10.1016/j.brainres.2006.01.006. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Panzica GC. Sexual differentiation of central vasopressin and vasotocin systems in vertebrates: different mechanisms, similar endpoints. Neuroscience. 2006;138(3):947–955. doi: 10.1016/j.neuroscience.2005.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene S, Posner MI, Tucker DM. Localization of a neural system for error detection and compensation. Psychol Science. 1994;5:303–305. [Google Scholar]
- Der G, Deary IJ. Age and sex differences in reaction time in adulthood: results from the United Kingdom Health and Lifestyle Survey. Psychology and Aging. 2006;21(1):62–73. doi: 10.1037/0882-7974.21.1.62. [DOI] [PubMed] [Google Scholar]
- Dutilh G, Forstmann BU, Vandekerckhove J, Wagenmakers EJ. A diffusion model account of age differences in post-error slowing. Psychology and Aging. doi: 10.1037/a0029875. (in press) [DOI] [PubMed] [Google Scholar]
- Dutilh G, Vandekerckhove J, Forstmann BU, Keuleers E, Brysbaert M, Wagenmakers EJ. Testing theories of post-error slowing. Attention, Perception & Psychophysics. 2012;74:454–465. doi: 10.3758/s13414-011-0243-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliot L. The trouble with sex differences. Neuron. 2011;72(6):895–898. doi: 10.1016/j.neuron.2011.12.001. [DOI] [PubMed] [Google Scholar]
- Emeric EE, Brown JW, Boucher L, Carpenter RH, Hanes DP, Harris R, et al. Influence of history on saccade countermanding performance in humans and macaque monkeys. Vision Research. 2007;47(1):35–49. doi: 10.1016/j.visres.2006.08.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endrass T, Franke C, Kathmann N. Error awareness in a saccade countermanding task. Journal of Psychophysiology. 2005;19(4):275–280. [Google Scholar]
- Endrass T, Reuter B, Kathmann N. ERP correlates of conscious error recognition: aware and unaware errors in an antisaccade task. European Journal of Neuroscience. 2007;26(6):1714–1720. doi: 10.1111/j.1460-9568.2007.05785.x. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Hohnsbein J, Hoormann J, Blanke L. Effects of crossmodal divided attention on late ERP components. II. Error processing in choice reaction tasks. Electroencephalography and Clinical Neurophysiology. 1991;78(6):447–455. doi: 10.1016/0013-4694(91)90062-9. [DOI] [PubMed] [Google Scholar]
- Feingold A. Gender differences in personality: a meta-analysis. Psychological Bulletin. 1994;116(3):429–456. doi: 10.1037/0033-2909.116.3.429. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I disorders. New York: Biometrics Research Department; 1995. [Google Scholar]
- Garavan H, Ross TJ, Murphy K, Roche RA, Stein EA. Dissociable executive functions in the dynamic control of behavior: inhibition, error detection, and correction. Neuroimage. 2002;17(4):1820–1829. doi: 10.1006/nimg.2002.1326. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Goss B, Coles MGH, Meyer DE, Donchin E. A neural system for error detection and compensation. Psychological Science. 1993;4(6):385–390. [Google Scholar]
- Goldman-Rakic PS, Selemon LD. Functional and anatomical aspects of prefrontal pathology in schizophrenia. Schizophrenia Bulletin. 1997;23(3):437–458. doi: 10.1093/schbul/23.3.437. [DOI] [PubMed] [Google Scholar]
- Hampson E. Estrogen-related variations in human spatial and articulatory-motor skills. Psychoneuroendocrinology. 1990;15(2):97–111. doi: 10.1016/0306-4530(90)90018-5. [DOI] [PubMed] [Google Scholar]
- Hampson E. Spatial cognition in humans: possible modulation by androgens and estrogens. J Psychiatry Neurosci. 1995;20(5):397–404. [PMC free article] [PubMed] [Google Scholar]
- Hausmann M, Slabbekoorn D, Van Goozen SH, Cohen-Kettenis PT, Güntürkün O. Sex hormones affect spatial abilities during the menstrual cycle. Behavioral Neuroscience. 2000;114(6):1245–50. doi: 10.1037//0735-7044.114.6.1245. [DOI] [PubMed] [Google Scholar]
- Hausmann M, Schoofs D, Rosenthal HE, Jordan K. Interactive effects of sex hormones and gender stereotypes on cognitive sex differences—a psychobiological approach. Psychoneuroendocrinology. 2009;34(3):389–401. doi: 10.1016/j.psyneuen.2008.09.019. [DOI] [PubMed] [Google Scholar]
- Hajcak G, McDonald N, Simons RF. To err is autonomic: error- related brain potentials, ANS activity, and post-error compensatory behavior. Psychophysiology. 2003;40(6):895–903. doi: 10.1111/1469-8986.00107. [DOI] [PubMed] [Google Scholar]
- Hoffman ML. Sex differences in empathy and related behaviors. Psychological Bulletin. 1977;84(4):712–722. [PubMed] [Google Scholar]
- Holroyd CB, Coles MG. The neural basis of human error processing: reinforcement learning, dopamine, and the error-related negativity. Psychological Review. 2002;109(4):679–709. doi: 10.1037/0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- Houx PJ, Jolles J. Age-related decline of psychomotor speed: effects of age, brain health, sex, and education. Perceptual and Motor Skills. 1993;76(1):195–211. doi: 10.2466/pms.1993.76.1.195. [DOI] [PubMed] [Google Scholar]
- Huster RJ, Westerhausen R, Kreuder F, Schweiger E, Wittling W. Morphologic asymmetry of the human anterior cingulate cortex. Neuroimage. 2007;34:888–895. doi: 10.1016/j.neuroimage.2006.10.023. [DOI] [PubMed] [Google Scholar]
- Huster RJ, Westerhausen R, Herrmann CS. Sex differences in cognitive control are associated with midcingulate and callosal morphology. Brain Structure & Function. 2011;215(3–4):225–235. doi: 10.1007/s00429-010-0289-2. [DOI] [PubMed] [Google Scholar]
- Huster RJ, Enriquez-Geppert S, Pantev C, Bruchmann M. Variations in midcingulate morphology are replated to ERP indices of cognitive control. Brain Structure and Function. doi: 10.1007/s00429-012-0483-5. (in press) [DOI] [PubMed] [Google Scholar]
- Hyde JS. Gender differences in verbal ability: A meta-analysis. Psychological Bulletin. 1988;104:53–69. [Google Scholar]
- Ivanoff J, Branning P, Marois R. fMRI evidence for a dual process account of the speed-accuracy tradeoff in decision-making. PloS ONE. 2008;3(7):e2635. doi: 10.1371/journal.pone.0002635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen SD, Iversen LL. Dopamine: 50 years in perspective. Trends in Neuroscience. 2007;30(5):188–193. doi: 10.1016/j.tins.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Kalkut EL, Han SD, Lansing AE, Holdnack JA, Delis DC. Development of set-shifting ability from late childhood through early adulthood. Archives of Clinical Neuropsychology. 2009;24(6):565–574. doi: 10.1093/arclin/acp048. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, 3rd, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303(5660):1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- Lappin J, Eriksen CW. Use of a delayed signal to stop a visual reaction-time response. Journal of Experimental Psychology: General. 1966;72(6):805–811. [Google Scholar]
- Larson MJ, South M, Clayson PE. Sex differences in error-related performance monitoring. Neuroreport. 2011;22(1):44–48. doi: 10.1097/WNR.0b013e3283427403. [DOI] [PubMed] [Google Scholar]
- Lewin C, Herlitz A. Sex differences in face recognition--women’s faces make the difference. Brain and Cognition. 2002;50(1):121–128. doi: 10.1016/s0278-2626(02)00016-7. [DOI] [PubMed] [Google Scholar]
- Li CS, Huang C, Constable RT, Sinha R. Gender differences in the neural correlates of response inhibition during a stop signal task. NeuroImage. 2006;32(4):1918–1929. doi: 10.1016/j.neuroimage.2006.05.017. [DOI] [PubMed] [Google Scholar]
- Li CS, Huang C, Yan P, Paliwal P, Constable RT, Sinha R. Neural correlates of post-error slowing during a stop signal task: a functional magnetic resonance imaging study. Journal of Cognitive Neuroscience. 2008;20(6):1021–1029. doi: 10.1162/jocn.2008.20071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CS, Zhang S, Duann JR, Yan P, Sinha R, Mazure CM. Gender Differences in Cognitive Control: an Extended Investigation of the Stop Signal Task. Brain Imaging and Behavior. 2009;3(3):262–276. doi: 10.1007/s11682-009-9068-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan GD, Cowan WB. On the ability to inhibit thought and action: A theory of an act of control. Psychological Review. 1984;91:295–327. doi: 10.1037/a0035230. [DOI] [PubMed] [Google Scholar]
- Logan GD, Cowan WB, Davis KA. On the ability to inhibit simple and choice reaction time responses: a model and a method. Journal of Experimental Psycholology: Human Perception and Performance. 1984;10(2):276–291. doi: 10.1037//0096-1523.10.2.276. [DOI] [PubMed] [Google Scholar]
- Luck SJ. An Introduction to the Event-Related Potential Technique. 1. Cambridge, MA: The MIT Press; 2005. [Google Scholar]
- Luu P, Flaisch T, Tucker DM. Medial frontal cortex in action monitoring. Journal of Neuroscience. 2000;20(1):464–469. doi: 10.1523/JNEUROSCI.20-01-00464.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luu P, Tucker DM, Derryberry D, Reed M, Poulsen C. Electrophysiological responses to errors and feedback in the process of action regulation. Psychological Science. 2003;14(1):47–53. doi: 10.1111/1467-9280.01417. [DOI] [PubMed] [Google Scholar]
- Mekarski JE, Cutmore TR, Suboski W. Gender differences during processing of the Stroop task. Perceptual and Motor Skills. 1996;83(2):563–568. doi: 10.2466/pms.1996.83.2.563. [DOI] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex “Frontal Lobe” tasks: a latent variable analysis. Cognitive Psychology. 2000;41(1):49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Ridderinkhof KR, Blom J, Band GP, Kok A. Error-related brain potentials are differentially related to awareness of response errors: evidence from an antisaccade task. Psychophysiology. 2001;38(5):752–760. [PubMed] [Google Scholar]
- Notebaert W, Houtman F, Opstal FV, Gevers W, Fias W, Verguts T. Post-error slowing: an orienting account. Cognition. 2009;111(2):275–279. doi: 10.1016/j.cognition.2009.02.002. [DOI] [PubMed] [Google Scholar]
- O’Connell RG, Bellgrove MA, Dockree PM, Robertson IH. Reduced electrodermal response to errors predicts poor sustained attention performance in attention deficit hyperactivity disorder. Neuroreport. 2004;15(16):2535–2538. doi: 10.1097/00001756-200411150-00021. [DOI] [PubMed] [Google Scholar]
- Pasqualini C, Olivier V, Guibert B, Frain O, Leviel V. Acute stimulatory effect of estradiol on striatal dopamine synthesis. Journal of Neurochemistry. 1995;65(4):1651–1657. doi: 10.1046/j.1471-4159.1995.65041651.x. [DOI] [PubMed] [Google Scholar]
- Rabbitt PM, Rodgers B. What does a man do after he makes an error? An analysis of response programming. Quarterly Journal of Experimental Psychology. 1977;29:727–743. [Google Scholar]
- Reddi BA, Carpenter RH. The influence of urgency on decision time. Nature Neuroscience. 2000;3(8):827–830. doi: 10.1038/77739. [DOI] [PubMed] [Google Scholar]
- Reimers S, Maylor EA. Gender effects on reaction time variability and trial-to-trial performance: reply to Deary and Der (2005) Neuropsychology, Development, and Cognition Section B, Aging, neuropsychology and cognition. 2006;13(3–4):479–489. doi: 10.1080/138255890969375. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, van den Wildenberg WP, Segalowitz SJ, Carter CS. Neurocognitive mechanisms of cognitive control: the role of prefrontal cortex in action selection, response inhibition, performance monitoring, and reward-based learning. Brain and Cognition. 2004;56(2):129–140. doi: 10.1016/j.bandc.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Rieger M, Gauggel S. Inhibitory after-effects in the stop signal paradigm. British Journal of Psychology. 1999;90:509–518. [Google Scholar]
- Robbins TW, Arnsten AF. The neuropsychopharmacology of fronto-executive function: monoaminergic modulation. Annual Review of Neuroscience. 2009;32:267–287. doi: 10.1146/annurev.neuro.051508.135535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MD, Meier BP, Wilkowski BM, Ode S. Introversion, inhibition, and displayed anxiety: The role of error reactivity processes. Journal of Research in Personality. 2007;41(3):558–578. [Google Scholar]
- Rushworth MFS, Noonan MP, Boorman ED, Walton ME, Behrens TE. Frontal cortex and reward-guided learning and decision-making. Neuron. 2011;70(6):1054–1069. doi: 10.1016/j.neuron.2011.05.014. [DOI] [PubMed] [Google Scholar]
- Sabb FW, Bearden CE, Glahn DC, Parker DS, Freimer N, Bilder RM. A collaborative knowledge base for cognitive phenomics. Molecular Psychiatry. 2008;13(4):350–360. doi: 10.1038/sj.mp.4002124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarmany I. Different performance in Stroop’s interference test from the aspect of personality and sex. Studies Psychologia. 1977;19:60–67. [Google Scholar]
- Schachar RJ, Chen S, Logan GD, Ornstein TJ, Crosbie J, Ickowicz A, et al. Evidence for an error monitoring deficit in attention deficit hyperactivity disorder. Journal of Abnormal Child Psychology. 2004;32(3):285–293. doi: 10.1023/b:jacp.0000026142.11217.f2. [DOI] [PubMed] [Google Scholar]
- Schirmer A, Kotz SA. ERP evidence for a sex-specific Stroop effect in emotional speech. Journal of Cognitive Neuroscience. 2003;15(8):1135–1148. doi: 10.1162/089892903322598102. [DOI] [PubMed] [Google Scholar]
- Schouten JF, Bekker JA. Reaction time and accuracy. Acta Psychologica. 1967;27:143–153. doi: 10.1016/0001-6918(67)90054-6. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Rohan ML, Pimentel PJ, Gruber SA, Rosso IM, Yurgelun-Todd DA. Sex differences in the relationship between white matter microstructure and impulsivity in adolescents. Magnetic Resonance Imaging. 2006;24(7):833–841. doi: 10.1016/j.mri.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Filion D, Geyer MA, Braff DL. “Normal” personality correlates of sensorimotor, cognitive, and visuospatial gating. Biological Psychiatry. 1995;37(5):286–299. doi: 10.1016/0006-3223(94)00138-S. [DOI] [PubMed] [Google Scholar]
- Thakkar KN, Schall JD, Boucher L, Logan G, Park S. Response inhibition and response monitoring in a saccadic countermanding task in schizophrenia. Biological Psychiatry. 2011;69(1):55–62. doi: 10.1016/j.biopsych.2010.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Meel CS, Heslenfeld DJ, Oosterlaan J, Sergeant JA. Adaptive control deficits in attention-deficit/hyperactivity disorder (ADHD): the role of error processing. Psychiatry Research. 2007;151(3):211–220. doi: 10.1016/j.psychres.2006.05.011. [DOI] [PubMed] [Google Scholar]
- Van Veen V, Carter CS. The timing of action-monitoring processes in the anterior cingulate cortex. Journal of Cognitive Neuroscience. 2002;14(4):593–602. doi: 10.1162/08989290260045837. [DOI] [PubMed] [Google Scholar]
- van Veen V, Carter CS. Error detection, correction, and prevention in the brain: a brief review of data and theories. Clinical EEG and Neuroscience. 2006;37(4):330–335. doi: 10.1177/155005940603700411. [DOI] [PubMed] [Google Scholar]
- Verbruggen F, Logan GD, Liefooghe B, Vandierendonck A. Short-term aftereffects of response inhibition: repetition priming or between-trial control adjustments? Journal of Experimental Psychology: Human Perception and Performance. 2008;34(2):413–426. doi: 10.1037/0096-1523.34.2.413. [DOI] [PubMed] [Google Scholar]
- von Kluge S. Trading accuracy for speed: gender differences on a Stroop task under mild performance anxiety. Perceptual and Motor Skills. 1992 doi: 10.2466/pms.1992.75.2.651. [DOI] [PubMed] [Google Scholar]
- Voyer D, Voyer S, Bryden MP. Magnitude of sex differences in spatial abilities: a meta-analysis and consideration of critical variables. Psychological Bulletin. 1995;117(2):250–270. doi: 10.1037/0033-2909.117.2.250. [DOI] [PubMed] [Google Scholar]
- Voyer D. On the magnitude of laterality effects and sex differences in functional lateralities. Laterality. 1996;1(1):51–83. doi: 10.1080/713754209. [DOI] [PubMed] [Google Scholar]
- White CN, Ratcliff R, Vasey MW, McKoon G. Anxiety enhances threat processing without competition among multiple inputs: a diffusion model analysis. Emotion. 2010;10(5):662–677. doi: 10.1037/a0019474. [DOI] [PubMed] [Google Scholar]
- Wisniewski AB. Sexually-dimorphic patterns of cortical asymmetry, and the role for sex steroid hormones in determining cortical patterns of lateralization. Psychoneuroendocrinology. 1998;23(5):519–547. doi: 10.1016/s0306-4530(98)00019-5. [DOI] [PubMed] [Google Scholar]
- Xiao L, Becker JB. Quantitative microdialysis determination of extracellular striatal dopamine concentration in male and female rats: effects of estrous cycle and gonadectomy. Neuroscience Letters. 1994;180(2):155–158. doi: 10.1016/0304-3940(94)90510-x. [DOI] [PubMed] [Google Scholar]
- Yucel M, Stuart GW, Maruff P, Velakoulis D, Crowe SF, Savage G, et al. Hemispheric and gender-related differences in the gross morphology of the anterior cingulate/paracingulate cortex in normal volunteers: an MRI morphometric study. Cerebral Cortex. 2001;11(1):17–25. doi: 10.1093/cercor/11.1.17. [DOI] [PubMed] [Google Scholar]
- Zirnheld PJ, Carroll CA, Kieffaber PD, O’Donnell BF, Shekhar A, Hetrick WP. Haloperidol impairs learning and error-related negativity in humans. Journal of Cognitive Neuroscience. 2004;16(6):1098–1112. doi: 10.1162/0898929041502779. [DOI] [PubMed] [Google Scholar]