Abstract
Purpose
We assessed the efficacy of supervised toothbrushing with xylitol toothpaste to prevent early childhood caries (ECC) and to reduce mutans streptococci (MS).
Methods
In this cluster-randomized efficacy trial, 4 Head Start classrooms in the Marshall Islands were randomly assigned to supervised toothbrushing with 1,400ppm/31% fluoride-xylitol (Epic Dental, Provo, UT) or 1,450ppm fluoride-sorbitol toothpaste (Colgate-Palmolive, New York, NY) (N=196 children, ages 4–5 yrs). We hypothesized no difference in efficacy between the two types of toothpaste. The primary outcome was primary molar d2-3mfs increment after 6 mos. A single examiner was blinded to classroom assignments. Two classrooms were assigned to the fluoride-xylitol group (85 children) and 2 classrooms to the fluoride-sorbitol group (83 children). The child-level analyses accounted for clustering.
Results
There was no difference between the two groups in baseline or end-of-trial mean d2-3mfs. The mean d2-3mfs increment was greater in the fluoride-xylitol group compared to the fluoride-sorbitol group (2.5 and 1.4 d2-3mfs, respectively), but the difference was not significant (95% CI:−0.17, 2.37;P=0.07). No adverse effects were reported.
Conclusion
After 6 mos, brushing with a low strength xylitol/fluoride toothpaste is no more efficacious in reducing ECC than a fluoride only toothpaste in a high caries risk child population.
INTRODUCTION
Early childhood caries (ECC) is on the rise among U.S. preschool-aged children, especially preschoolers from low-income households.1 This trend has heightened interest in population-based interventions aimed at prevention.2
The American Academy of Pediatric Dentistry (AAPD) endorses the use of xylitol as part of a comprehensive strategy to prevent caries but does not recommend xylitol toothpaste use because the research evidence is inconclusive.3 There are several xylitol delivery modalities that have been tested in young children including gummy bears, syrup, and gum.4–6 While a single in vitro study suggests that xylitol toothpastes have the potential to prevent tooth decay7, clinical evidence is conflicting.8–13 Clinical studies on xylitol toothpastes have focused on permanent teeth in older children and no studies have tested xylitol toothpastes to prevent early childhood caries.
The purpose of this study was to test the efficacy of supervised toothbrushing with xylitol toothpaste among Head Start children. A cluster-randomized design was adopted to avoid contamination of the two interventions and to optimize feasibility of this population-based trial. We hypothesized no difference in efficacy at preventing ECC and reducing MS levels between xylitol toothpaste and an over-the-counter fluoride toothpaste.
METHODS
Design
This was a 6 mos prospective cluster-randomized efficacy trial with 2 arms and 1:1 allocation. During the initial 2 mos of the trial, the children brushed twice per day. After 2 mos, toothbrushing frequency was changed from twice to once daily because Head Start classroom hours of operation were reduced in January 2011.
Participants and Human Subjects
This population-based study included all children in 4 Head Start classrooms on Majuro Atoll in the Republic of the Marshall Islands. We focused on all children ages 4 to 5 yrs enrolled in Head Start during the 2010–2011 school year. This public health evaluation was conducted by the Ministry of Health. A study staff member enrolled subjects and obtained informed consent from the parents. The University of Washington Institutional Review Board approved this study.
Intervention
Children in the experimental arm brushed once-per-day under teacher supervision with a pea-sized amount of 1,400 ppm fluoride/31% xylitol toothpaste (fluoride 0.14 w/v; Epic Dental, Provo, UT). Children in the control group brushed their teeth once-per-day with a pea-sized amount of 1,450 ppm fluoride-sorbitol toothpaste (Colgate Cavity Protection, sodium monofluorophosphate [MFP] 0.76% w/v and sodium fluoride 0.10% w/v; Colgate-Palmolive, New York, NY). Head Start classroom teachers were trained on how to administer a classroom toothbrushing program (e.g., proper toothpaste dose, toothbrushing technique, how long to brush, rinsing, hygienic storage of toothbrushes). Children in both groups also received topical 5% sodium fluoride varnish treatment every 3 mos.
Outcomes
The primary end point was the surface-level primary molar caries increment (d2-3mfs). A single dental examiner was trained according to the NIDCR Early Childhood Caries Collaborative (EC4) Criteria, which is based on the World Health Organization caries classification system, and examined each child visually using a disposable mouth mirror and artificial light. No explorers were used. The examiner demonstrated excellent reliability (intrarater correlation coefficient, 1.00 at baseline dental examination and 0.96 at the end-of-trial examination). Caries data were collected at baseline (post-allocation) and at the end of the 6 mos trial.
A secondary end point was the intraoral MS level at the end of the 6 month trial. MS levels were assessed using the Dentocult SM System (Orion Diagnostica, Espoo, Finland). Plaque and saliva samples were incubated at 37°C for 48 h and dried at room temperature prior to reading. Using the Dentocult SM criteria, each strip was independently read by 2 examiners and assigned a score ranging from 0 to 3. Scores 0 or 1 indicate <100,000 colony forming units (CFU)/mL, 2 indicates 100,000–1,000,000 CFU/mL, and 3 indicates >1,000,000 CFU/mL. Scoring discrepancies were resolved by consensus.
Sample Size
We did not derive sample size estimates because this population-based trial included all children in the 4 Head Start classrooms on Majuro Atoll.
Allocation Sequence Generation and Concealment
We used block randomization to assign the 4 classrooms into 1 of 2 arms. It took 4 attempts to reach balanced groups. Allocation to the experimental or treatment group was based on clusters and was completed by a study coordinator. All examiners assessing primary and secondary outcomes were blinded to study group assignment.
Statistical Methods
The unit of analysis was the child. Any surface-level reversals (e.g., tooth surfaces noted as decayed at baseline but sound at the end of the trial) were classified as decayed. To adjust for clustering by classroom, we calculated revised t critical values using 2-way mixed models, consistency agreement, and intracluster correlation coefficient (ICC) estimates for each measure based on single-rating reliability.14–15 ICC estimates are used to derive test statistics and p-values that account for clustering. For baseline and end-of-trial d2-3mfs, we compared means using the 2-tailed two-sample t-test (α=0.05). The primary end point, d2-3mfs increment, was estimated by subtracting baseline d2-3mfs from end-of-trial d2-3mfs at 6 mo and compared across the 2 groups using the 2-tailed t-test. For the secondary outcome, the mean MS score at 6 mo was compared using the 2-tailed t- test (for both plaque and saliva). The statistician was blind to group assignment until after the analyses were complete. All data were analyzed using SPSS Version 19.0 for Windows.
RESULTS
Participant Flow
Across the 4 clusters, there were 196 children who were enrolled at the start of the school year and received a baseline caries exam. Of these, 168 children (85.7%) received an end-of-trial caries exam (primary end point) and 145 children (74.0%) were tested for MS (secondary end point) (Figure 1). We were unable to collect data from children who were absent from school on exam and bacterial testing days. The trial ended after 6 mos, corresponding to the end of the school year.
FIGURE 1.
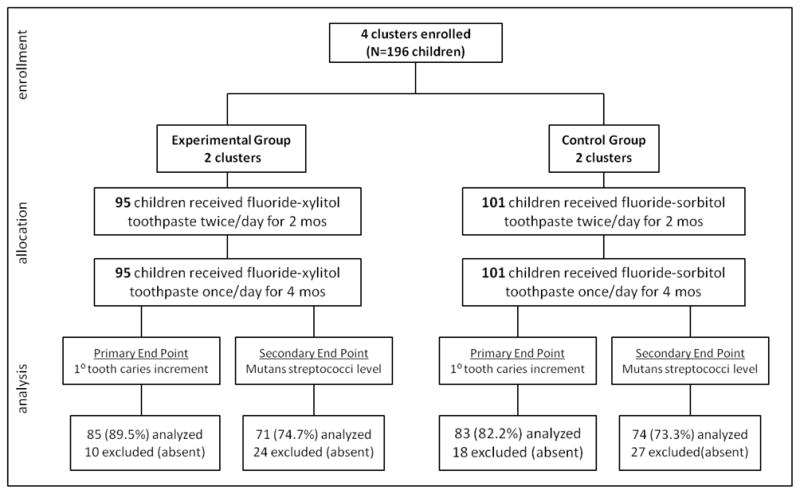
CONSORT flowchart for cluster-randomized trial of fluoride-xylitol toothpaste among 4–5 year old Head Start enrollees in the Marshall Islands.
Baseline Data
Table 1 summarizes the characteristics of the children in the final analyses for the primary end point (d2-3mfs increment) by study group. Baseline demographic characteristics were similar for the secondary end points (MS score) (Table 2). There were no significant differences in age or sex between children who remained in the study and those who were absent for the final clinical examination or bacterial sampling. No children switched classrooms. Nearly all children had at least one d2-3mfs at baseline.
TABLE 1.
Characteristics of subjects included in the final analyses for primary molar caries increment (N=168)
| Characteristic | Control Group Over-the-counter fluoride toothpaste n=83 |
Experimental Group Xylitol toothpaste n=85 |
Overall N=168 |
|---|---|---|---|
| Female sex, N (%) | 40 (48.2) | 44 (51.8) | 84 (50.0) |
| Age, mean (SD), years | 5.4 (0.41) | 5.5 (0.61) | 5.4 (0.52) |
| Proportion caries free at baseline (%) | 2.4 | 1.2 | 1.8 |
TABLE 2.
Characteristics of subjects included in the final analyses for mutans streptococci level (N=145)
| Characteristic | Control Group Over-the-counter fluoride toothpaste n=74 |
Experimental Group Xylitol toothpaste n=71 |
Overall N=145 |
|---|---|---|---|
| Female sex, N (%) | 34 (45.9) | 35 (49.3) | 69 (47.6) |
| Age, mean (SD), years | 5.4 (0.39) | 5.5 (0.63) | 5.4 (0.53) |
| Proportion caries free at baseline (%) | 2.7 | 1.4 | 2.1 |
Primary End Points
All analyses were conducted by original assigned group. There was no significant difference in mean d2-3mfs at baseline between the xylitol and over-the-counter fluoride toothpaste groups (ICC=0.05; 20.3±9.7 and 23.2±10.4, respectively; tα/2=−1.46; df=2; P=0.28) (Table 3) nor was there a difference in mean end-of-trial d2-3mfs between the 2 groups (ICC=0.04; 22.8±9.5 and 24.6±10.1, respectively; tα/2=−1.03; df=2; P=0.41). The mean d2-3mfs increment, after adjusting for clustering, was higher in the xylitol group than in the over-the-counter fluoride group (ICC=0; 2.5±2.8 and 1.4±2.5, respectively), but the difference was not statistically significant (95% CI: −0.17, 2.37; tα/2=3.72; df=2; P=0.07).
TABLE 3.
Mean baseline caries, end-of-trial caries, and caries increment in primary molars (primary end points) for children in the final analyses (N=168)
| Group | Mean baseline caries (SD) | P-value | Mean end-of-trial caries (SD) | P-value | Mean caries increment (SD) | P-value |
|---|---|---|---|---|---|---|
| Control Group Over-the-counter fluoride toothpaste (n=83) |
23.2 (10.4) | P=0.28 | 24.6 (10.1) | P=0.41 | 1.4 (2.5) | P=0.07 |
| Experimental Group Xylitol toothpaste (n=85) |
20.3 (9.7) | 22.8 (9.5) | 2.5 (2.8) |
Secondary End Points
There was no significant differences across the xylitol and the over-the-counter fluoride groups in the mean MS level for both plaque (ICC=0.07; 1.6±0.9 and 1.7±1.0, respectively; 95% CI: −1.71, 1.47; tα/2=−0.32; df=2; P=0.78) and saliva (ICC=0.02; 0.9±0.8 and 0.9±0.9, respectively; 95% CI: −1.13, 0.99; tα/2=−0.28; df=2; P=0.80) (Table 4).
TABLE 4.
Mean plaque and salivary mutans streptococci levels (secondary end points) for children in the final analyses (N=145)
| Group | Mean plaque mutans streptococci levels (SD) | P-value | Mean salivary mutans streptococci levels (SD) | P-value |
|---|---|---|---|---|
| Control Group Over-the-counter fluoride toothpaste (n=74) |
1.7 (1.0) | P=0.78 | 0.9 (0.9) | P=0.80 |
| Experimental Group Xylitol toothpaste (n=71) |
1.6 (0.9) | 0.9 (0.8) |
Harms
There were no adverse outcomes or unintended effects.
DISCUSSION
The trial focused on Head Start children in the Republic of the Marshall Islands – a population that has high caries rates, which makes our findings generalizable to other high-risk pediatric populations.16 The trial lasted 6 mo and involved 2 mos of twice daily exposure to about 0.16 g xylitol (assuming 0.25 g toothpaste/dose) and 4 mos of once daily exposure to 0.08 g xylitol, both of which were below the recommended daily xylitol dose and frequency.5 Toothbrushing with a xylitol toothpaste resulted in no therapeutic benefit compared to an over-the-counter fluoride toothpaste, both in terms of early childhood caries prevention or MS reduction. While statistically not significant, there was a tendency for children in the xylitol toothpaste group to have a higher d2-3mfs increment. There are 2 potential explanations. First, the surfactants used in toothpaste may interfere with xylitol uptake.17 Second, the particular surfactant in the xylitol toothpaste – sodium lauroyl sarcosinate – may interfere with adsorption of fluoride by tooth enamel18 whereas fluoride adsorption may not have been affected by the sodium lauryl sulfate surfactant in the control toothpaste. Thus, the surfactant used in the xylitol toothpaste may have rendered the xylitol toothpaste chemotherapeutically less active. Consumer safety concerns associated with sodium lauryl sulfate19 may prompt toothpaste manufacturers to switch to other surfactants like sodium lauroyl sarcosinate without considering the deleterious health effects. Future studies should continue to investigate how surfactants and other ingredients added to toothpastes can modify toothpaste efficacy.
Our findings of no difference in caries prevention between xylitol and over-the-counter fluoride toothpastes are consistent with a previous 3-y trial that found no difference in permanent tooth decay when 15 mg/g xylitol was added to high fluoride toothpaste (2,500 or 5,000 ppm) and used once daily in a classroom setting.9 Assuming that each child brushed with 0.25 g of toothpaste, this is less than 0.004 g of daily xylitol exposure, which is one-twentieth the maximum dose to which children in our study were exposed. Contrary to our findings, 2 school-based trials of twice-daily use of toothpastes containing 10% xylitol added to 0.243% sodium fluoride or 0.836% sodium monofluorophosphate reported significant reductions in caries after 3 y.10,13 Based on 0.25 g of toothpaste use twice daily, children were exposed to 0.05 g xylitol. These findings conflict with a 3-y effectiveness trial comparing twice daily use of 0.8% sodium monofluorophosphate (MFP)+6% sorbitol+3% xylitol toothpaste and 0.8% MFP+6% sorbitol toothpaste that detected no difference in permanent tooth caries.8 Children would have been exposed to a maximum of 0.015 g xylitol per day. Because the setting was home-based, xylitol exposure may have been even lower because of poor adherence. In the context of previous studies, our findings suggest that once-a-day, short-term exposure to xylitol toothpastes containing low concentrations of xylitol does not reduce caries in the primary teeth.
Our finding of no difference between the two types of toothpaste in regards to controlling mutans streptococci (MS) levels is consistent with a previous 3-mos trial11 as well as a 3-y xylitol toothpaste trial8. In another study, children who brushed twice daily with xylitol toothpaste (0.2 g xylitol/day) had significantly lower levels of salivary and plaque MS after 6 mo12. These findings were confounded by the presence of triclosan in the xylitol toothpaste. Extrapolating from other xylitol lozenge and gum studies suggest that higher xylitol doses beyond that available through the fluoride-xylitol toothpaste in our study are needed to reduce mutans streptococci levels.20–21
The current community-based public health evaluation had a number of strengths including randomization into 1 of 2 treatment arms, supervised toothbrushing to ensure high treatment fidelity, and 2 clinically relevant end points (caries increment and MS level). However, there are 4 main study limitations. First, the control and experimental toothpastes contained different ionic fluoride concentrations and types. It is unlikely that there is a clinically relevant difference in caries prevention between 1,400 ppm and 1,450 ppm toothpastes.22 Regarding fluoride type, our over-the-counter fluoride toothpaste contained sodium monofluorophosphate whereas our xylitol toothpaste contained sodium fluoride. Previous studies suggest that sodium fluoride is slightly more effective at preventing caries in children than sodium monofluorophosphate.23–26 This should have biased our findings in favor of the xylitol toothpaste, but the caries increment was higher in the xylitol toothpaste group, making this unlikely. Future work should ensure identical toothpaste formulations that differ only in the main exposure of interest. Second, because this was a population-based study that included all Head Start classrooms on the Majuro Atoll, we did not calculate sample sizes. Retrospective power analyses were not conducted because this approach does not provide useful information.27 Of value are the estimated intracluster correlation coefficients that can be used to help guide sample size calculation in future studies. Third, we were unable to obtain attendance information and to assess how often children missed toothbrushing. The randomization protocol is likely to have addressed this potential problem. Finally, a 6 mos trial length is short. A Consensus Statement by the International Consensus Workshop on Caries Clinical Trials (ICW-CCT) indicates that clinical trials involving anti-caries oral care products should last 2 to 3 years.28 This allows adequate time for caries to develop in lower-risk populations. In very high-risk populations, however, carious lesions develop quickly, enabling shorter duration trials.5,29 The caries rate in the population was high (98% of participants had at least one d2-3mfs at baseline), which allowed for a short evaluation time, particularly because d2-3mfs increment was the primary outcome measure. Shorter trials are arguably the most ethical approach in evaluating interventions in populations with high caries rates.
More broadly, our study findings indicate that adding xylitol to fluoride toothpaste does not lead to additional health benefits for children at high risk for caries. Given the small amount of toothpaste that is recommended for daily toothbrushing, it is unlikely that xylitol toothpastes alone can deliver the requisite 8 g of xylitol per day, especially when most high-risk children do not brush regularly. A Cochrane review on fluoride toothpastes reported a 14% increase in the prevented fraction when brushing twice daily with fluoride toothpaste compared to once daily30, which highlights the importance of implementing a minimum of twice daily brushing in future interventions to optimize outcomes. An additional consideration is cost. The commercially available xylitol toothpaste used in the current study costs 3 times as much per ounce as over-the-counter fluoride toothpastes. A more promising approach is alternative xylitol delivery modalities with known efficacy such as gummy bears, syrups, and gum. In fact, our findings raise questions about all such toothpastes currently on the market. Toothpastes in the U.S. are regulated by the Food and Drug Administration’s monograph on anticaries products.31 Because of the increased availability and marketing of xylitol toothpastes in recent years, future regulation of toothpastes containing additional chemotherapeutic agents such as xylitol may require additional steps to ensure that therapeutic levels of xylitol are present in these products, which will protect consumer safety and health.
CONCLUSION
Brushing with a low strength xylitol/fluoride toothpaste is no more efficacious in reducing caries increment or MS levels than a fluoride only toothpaste in a high caries risk child population. Additional studies are needed to identify alternative modalities of delivering therapeutic xylitol doses to children at high risk for early childhood caries.
Acknowledgments
This study was funded by the Health Resources and Services Administration (HRSA), Targeted State Maternal and Child Oral Health Service Systems Grant Number H47MC08647 and National Institute of Dental and Craniofacial Research (NIDCR) Grant Numbers K08DE020856 and U54DE019346. We would like to thank Dr. Brian Leroux for providing statistical advice. All toothpastes used in this study were purchased directly from the manufacturers. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Trial Registration: Controlled-Trials.com; URL: http://www.controlled-trials.com/ISRCTN51682476/icrctn51682476; No. ISRCTN51682476.
References
- 1.Dye BA, Arevalo O, Vargas CM. Trends in paediatric dental caries by poverty status in the United States, 1988–1994 and 1999–2004. Int J Paediatr Dent. 2010;20:132–143. doi: 10.1111/j.1365-263X.2009.01029.x. [DOI] [PubMed] [Google Scholar]
- 2.Milgrom P, Chi DL. Prevention-centered caries management strategies during critical periods in early childhood. J Calif Dent Assoc. 2011;39:735–741. [PubMed] [Google Scholar]
- 3.American Academy on Pediatric Dentistry. Guideline on xylitol use in caries prevention. Pediatr Dent. 2011;33:157–160. [Google Scholar]
- 4.Ly KA, Riedy CA, Milgrom P, Rothen M, Roberts MC, Zhou L. Xylitol gummy bear snacks: a school-based randomized clinical trial. BMC Oral Health. 2008;8:20. doi: 10.1186/1472-6831-8-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Milgrom P, Ly KA, Tut OK, et al. Xylitol pediatric topical oral syrup to prevent dental caries: a double-blind randomized clinical trial of efficacy. Arch Pediatr Adolesc Med. 2009;7:601–607. doi: 10.1001/archpediatrics.2009.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seki M, Karakama F, Kawato T, Tanaka H, Saeki Y, Yamashita Y. Effect of xylitol gum on the level of oral mutans streptococci of preschoolers: block-randomised trial. Int Dent J. 2011;61:274–280. doi: 10.1111/j.1875-595X.2011.00073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sano H, Nakashima S, Songpaisan Y, Phantumvanit P. Effect of a xylitol and fluoride containing toothpaste on the remineralization of human enamel in vitro. J Oral Sci. 2007;49:67–73. doi: 10.2334/josnusd.49.67. [DOI] [PubMed] [Google Scholar]
- 8.Petersson LG, Birkhed D, Gleerup A, Johansson M, Jönsson G. Caries-preventive effect of dentifrices containing various types and concentrations of fluorides and sugar alcohols. Caries Res. 1991;25:74–79. doi: 10.1159/000261346. [DOI] [PubMed] [Google Scholar]
- 9.Cutress T, Howell PT, Finidori C, Abdullah F. Caries preventive effect of high fluoride and xylitol containing dentifrices. ASDC J Dent Child. 1992;59:313–318. [PubMed] [Google Scholar]
- 10.Sintes JL, Escalante C, Stewart B, et al. Enhanced anticaries efficacy of a 0. 243% sodium fluoride/10% xylitol/silica dentifrice: 3-year clinical results. Am J Dent. 1995;8:231–235. [PubMed] [Google Scholar]
- 11.Twetman S, Petersson LG. Influence of xylitol in dentifrice on salivary microflora of preschool children at caries risk. Swed Dent J. 1995;19:103–108. [PubMed] [Google Scholar]
- 12.Jannesson L, Renvert S, Kjellsdotter P, Gaffar A, Nabi N, Birkhed D. Effect of a triclosan-containing toothpaste supplemented with 10% xylitol on mutans streptococci in saliva and dental plaque. A 6-month clinical study. Caries Res. 2002;36:36–39. doi: 10.1159/000057588. [DOI] [PubMed] [Google Scholar]
- 13.Sintes JL, Elias-Boneta A, Stewart B, Volpe AR, Lovett J. Anticaries efficacy of a sodium monofluorophosphate dentifrice containing xylitol in a dicalcium phosphate dihydrate base. A 30-month caries clinical study in Costa Rica. Am J Dent. 2002;15:215–219. [PubMed] [Google Scholar]
- 14.Campbell MK, Mollison J, Steen N, Grimshaw JM, Eccles M. Analysis of cluster randomized trials in primary care: a practical approach. Fam Pract. 2000;17:192–196. doi: 10.1093/fampra/17.2.192. [DOI] [PubMed] [Google Scholar]
- 15.Donner A, Klar N. Design and analysis of cluster randomization trials in health research. Hodder & Stoughton Educational; London: 2000. pp. 79–110. [Google Scholar]
- 16.Tut OK, Greer MH, Milgrom P. Republic of the Marshall Islands: planning and implementation of a dental caries prevention program for an island nation. Pac Health Dialog. 2005;12:118–123. [PubMed] [Google Scholar]
- 17.Assev S, Wåler SM, Rølla G. Are sodium lauryl sulfate-containing toothpastes suitable vehicles for xylitol? Eur J Oral Sci. 1997;105:178–182. doi: 10.1111/j.1600-0722.1997.tb00197.x. [DOI] [PubMed] [Google Scholar]
- 18.Lanigan RS. Final report on the safety assessment of Cocoyl Sarcosine, Lauroyl Sarcosine, Myristoyl Sarcosine, Oleoyl Sarcosine, Stearoyl Sarcosine, Sodium Cocoyl Sarcosinate, Sodium Lauroyl Sarcosinate, Sodium Myristoyl Sarcosinate, Ammonium Cocoyl Sarcosinate, and Ammonium Lauroyl Sarcosinate. Int J Toxicol. 2001;20:1–14. [PubMed] [Google Scholar]
- 19.Robinson VC, Bergfeld WF, Belsito DV, et al. Final report of the amended safety assessment of sodium laureth sulfate and related salts of sulfated ethoxylated alcohols. Int J Toxicol. 2010:151S–61S. doi: 10.1177/1091581810373151. [DOI] [PubMed] [Google Scholar]
- 20.Oscarson P, Lif Holgerson P, Sjöström I, Twetman S, Stecksén-Blicks C. Influence of a low xylitol-dose on mutans streptococci colonisation and caries development in preschool children. Eur Arch Paediatr Dent. 2006;7:142–147. doi: 10.1007/BF03262555. [DOI] [PubMed] [Google Scholar]
- 21.Campus G, Cagetti MG, Sacco G, Solinas G, Mastroberardino S, Lingström P. Six months of daily high-dose xylitol in high-risk schoolchildren: a randomized clinical trial on plaque pH and salivary mutans streptococci. Caries Res. 2009;43:455–461. doi: 10.1159/000264682. [DOI] [PubMed] [Google Scholar]
- 22.Walsh T, Worthington HV, Glenny AM, Appelbe P, Marinho VC, Shi X. Fluoride toothpastes of different concentrations for preventing dental caries in children and adolescents. Cochrane Database Syst Rev. 2010;20:CD007868. doi: 10.1002/14651858.CD007868.pub2. [DOI] [PubMed] [Google Scholar]
- 23.DePaola PF, Soparkar PM, Triol C, et al. The relative anticaries effectiveness of sodium monofluorophosphate and sodium fluoride as contained in currently available dentifrice formulations. Am J Dent. 1993;6:S7–12. [PubMed] [Google Scholar]
- 24.Stookey GK, DePaola PF, Featherstone JD, et al. A critical review of the relative anticaries efficacy of sodium fluoride and sodium monofluorophosphate dentifrices. Caries Res. 1993;27:337–60. doi: 10.1159/000261563. [DOI] [PubMed] [Google Scholar]
- 25.Marks RG, Conti AJ, Moorhead JE, Cancro L, D’Agostino RB. Results from a three-year caries clinical trial comparing NaF and SMFP fluoride formulations. Int Dent J. 1994;44:275–85. [PubMed] [Google Scholar]
- 26.Stephen KW, Chestnutt IG, Jacobson AP, et al. The effect of NaF and SMFP toothpastes on three-year caries increments in adolescents. Int Dent J. 1994;44:287–95. [PubMed] [Google Scholar]
- 27.Lenth RV. Statistical power calculations. J Anim Sci. 2007;85:E24–29. doi: 10.2527/jas.2006-449. [DOI] [PubMed] [Google Scholar]
- 28.Pitts NB, Stamm JW. International Consensus Workshop on Caries Clinical Trials (ICW-CCT)--final consensus statements: agreeing where the evidence leads. J Dent Res. 2004;83:C125–8. doi: 10.1177/154405910408301s27. [DOI] [PubMed] [Google Scholar]
- 29.Sitthisettapong T, Phantumvanit P, Huebner C, Derouen T. Effect of CPP-ACP paste on dental caries in primary teeth: a randomized trial. J Dent Res. 2012;91:847–52. doi: 10.1177/0022034512454296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marinho VC, Higgins JP, Sheiham A, Logan S. Fluoride toothpastes for preventing dental caries in children and adolescents. Cochrane Database Syst Rev. 2003;1:CD002278. doi: 10.1002/14651858.CD002278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Food and Drug Administration. Anticaries drug products for over-the-counter human use; final rule. Federal Register. 1995;60:52473–52510. [Google Scholar]


