Abstract
A dose-dependent combination of environmental exposures, estrogenic hormones and genetic predisposition is thought to be required for lupus to develop and flare, but how the environment modifies the immune system in genetically predisposed people is unclear. Current evidence indicates that environmental agents that inhibit DNA methylation can convert normal antigen-specific CD4+ T lymphocytes into autoreactive, cytotoxic, pro-inflammatory cells that are sufficient to cause lupus-like autoimmunity in animal models, and that the same changes in DNA methylation characterize CD4+ T cells from patients with active lupus. Environmental agents implicated in inhibiting T cell DNA methylation include the lupus-inducing drugs procainamide and hydralazine, as well as diet, and agents causing oxidative stress, such as smoking, UV light exposure, and infections, which have been associated with lupus onset or disease activity. Other studies demonstrate that demethylated T cells cause only anti-DNA antibodies in mice lacking a genetic predisposition to lupus, but are sufficient to cause lupus-like autoimmunity in genetically predisposed mice and likely people, and that estrogens augment the disease. Collectively, these studies suggest that environmental agents that inhibit DNA methylation, together with lupus genes and estrogens or endocrine disruptors, combine in a dose-dependent fashion to cause lupus flares.
Keywords: Lupus, epigenetics, environment, oxidative stress, mercury, endocrine disruptors, genetics
Introduction
Lupus develops and flares when genetically predisposed people encounter environmental agents that trigger the disease. The genetic predisposition is evidenced by the validation of at least 38 lupus susceptibility loci (reviewed by Sawalha et al.),1 as well as epidemiologic data quantifying strong familial aggregation, with parental history of SLE being associated with >14-fold increased risk of SLE among offspring.2 An environmental contribution is confirmed by reports that certain drugs cause a lupus-like disease in genetically predisposed people,3 and that environmental agents causing oxidative stress, such as UV light,4 infections,5 and smoking6,7 are associated with lupus onset and flares. Estrogen and environmental endocrine disruptors may also contribute.8 Evidence acquired over more than 25 years indicates that oxidative stress and other environmental exposures, including diet, can contribute to lupus onset and flares through epigenetic mechanisms that modify CD4+ T cell gene expression. The epigenetic changes convert normal antigen-specific “helper” T cells into autoreactive, cytotoxic, pro-inflammatory T cells that are sufficient to cause lupus in animal models, and similar epigenetic changes characterize T cells from patients with active lupus. Understanding how environmental agents cause these changes in T cells first requires understanding how epigenetic mechanisms regulate gene expression.
Background
Chromatin structure, gene expression and the environment
Most nucleated cells in the human body contain approximately 3 meters of DNA, packaged into the nucleus as chromatin. The basic chromatin subunit is the nucleosome, consisting of two turns of DNA wrapped around a core containing 8 histone proteins. The nucleosomes are then arranged into higher ordered structures to form chromatin. This tightly packaged DNA is inaccessible to the transcription initiation complexes that drive mRNA synthesis and gene expression, so genes required for the function of any given cell type typically have an open chromatin configuration that is accessible to the transcription complexes, while those genes that are unnecessary or potentially detrimental to the cell’s function remain tightly packaged. Importantly, every time a cell divides these structural modifications must be replicated, and thus are heritable.9 The mechanisms responsible for heritable changes in gene expression are termed “epigenetic”, and include DNA methylation and histone modifications. DNA methylation refers to the methylation of cytosine bases occurring in CpG pairs to form deoxymethylcytosine (dmC) and is a repressive modification. The covalent bond between the methyl group and cytosine is very stable, and can only be removed by excision repair mechanisms or by inhibiting the replication of methylation patterns during mitosis. DNA methylation patterns are established during differentiation by the de novo DNA methyltransferases Dnmt3a and Dnmt3b, then replicated each time a cell divides by Dnmt1, the maintenance methyltransferase. A family of methylcytosine binding proteins binds the methylated sequences and tethers chromatin inactivation complexes that promote a locally compact, transcriptionally repressive configuration. DNA methylation serves not only to help stabilize chromatin in a tightly packed configuration, but also to silence genes inappropriate for the function of any given cell, but for which the cell expresses transcription factors that might otherwise drive gene expression.9
Histone protein “tails” also protrude from the nucleosome, and amino acids in these tails can be covalently modified with a number of moieties such as methylation, acetylation, phosphorylation, ubiquitination, citrullination, SUMOylation, poly(AdP-ribosyl)ation and others. These modifications serve a number of functions including regulation of gene expression. In contrast to DNA methylation, histone modifications can be enzymatically removed and are thus more dynamic.10 However, the enzymatic reactions responsible for maintaining these epigenetic marks are sensitive to the environment, and drugs, chemicals, and other agents which inhibit enzymatic activity of the DNA methyltransferases or histone modification enzymes, or dietary deficiencies that decrease bioavailability of epigenetic modifiers like the methyl donor S-adenosylmethionine, will prevent replication of the epigenetic patterns during mitosis, causing changes in gene expression. Further, if not repaired, the epigenetic changes may accumulate over time, causing age-dependent changes in gene expression.9 A partial list of environmental agents inhibiting DNA methylation, and the proposed mechanisms, is shown in Table 1.
Table 1.
Environmental Agents and DNA Demethylation
| Agent | Mechanism |
|---|---|
| Procainamide | Dnmt1 inhibitor 9 |
| Hydralazine | Decreased Dnmt1 levels 9 |
| Methyl-poor diet | Impaired DNA methylation 9 |
| UV light exposure | Oxidative stress 9 |
| Infections | Oxidative stress 23 |
| Silica exposure | Oxidative stress 23 |
| Heavy metals | Oxidative stress 23 |
| Pesticides | Oxidative stress 23 |
| Smoking | Oxidative stress 23 |
| Air pollution | Oxidative stress 23 |
T cells and DNA methylation
T cells are particularly dependent on DNA methylation to regulate gene expression. T cells differentiate throughout life into various subsets, each defined by a unique repertoire of surface molecules and effector functions. While subset differentiation is determined by transcription factors such as T-bet, GATA-3, FoxP3 and others,11 expression of the subset-specific effector functions is driven by an overlapping repertoire of transcription factors, and effector genes unnecessary or detrimental to the function of any given subset are silenced by mechanisms that include DNA methylation. Data from murine models and in vitro studies demonstrate that inhibiting DNA methylation in dividing CD4+ T cells, either with direct DNA methyltransferase inhibitors, by decreasing Dnmt1 upregulation during mitosis, or by restricting dietary methyl donors,9 is sufficient to activate expression of normally silenced immune genes. These include the cytotoxic molecule perforin in CD4+ “helper” cells,9 the killer cell immunoglobulin-like receptor (KIR) gene family, normally expressed clonally on NK cells but not on T cells,12 IFN-γ in Th2 cells, IL-4, -5 and -13 in Th1 cells, 13 and overexpression of the B cell costimulatory molecules CD70 and CD40L.9,14 Inhibiting DNA methylation also converts cloned and polyclonal, antigen-specific CD4+ T cells into autoreactive cells that respond to self class II MHC molecules without added antigen. The autoreactivity is caused by increasing LFA-1 (CD11a/CD18) levels through effects on the methylation of ITGAL (CD11a) regulatory regions, and increasing T cell LFA-1 levels by transfection causes a similar autoreactivity. These demethylated, autoreactive CD4+ T cells kill syngeneic or autologous macrophages by inducing apoptosis through mechanisms including LFA-1 and perforin overexpression, and overstimulate syngeneic or autologous B cell antibody production through CD70, CD40L and cytokine overexpression.9,14
Importantly, semi-allogeneic CD4+ T cells responding to host class II MHC molecules cause a lupus-like disease in the chronic graft-vs-host disease model,9 suggesting that demethylated, autoreactive CD4+ T cells might cause a similar lupus-like disease in mice or people. To test this, CD4+ T cells from normal mice were treated with the Dnmt1 inhibitor 5-azacytidine (5-azaC) then injected into genetically identical recipients. Mice receiving the epigenetically modified T cells developed a disease closely resembling human lupus with anti-nuclear antibodies and an immune complex glomerulonephritis,9 similar to the graft-vs-host disease model.
DNA Methylation and Lupus
T cell DNA methylation and drug-induced lupus
The observation that CD4+ T cells treated with a drug that inhibits DNA methylation could cause a lupus-like disease suggested that drugs that cause a lupus-like disease might be DNA methylation inhibitors. In the context of genetic predisposition, hydralazine and procainamide are drugs known to induce a lupus-like autoimmunity.15 In a United Kingdom-based pharmacoepidemiology study (including 875 incident lupus cases and 3632 matched controls) designed to quantify risk of lupus associated with these drugs in the general population, no cases or controls were exposed to procainamide; hydralazine was prescribed to 4 cases and 2 controls, and was associated with a 6-fold increase in odds of lupus after adjustment for confounders (OR 6.62, 95% CI 1.03, 42.74).3 Both drugs have been shown to inhibit T cell DNA methylation in vitro. Procainamide was found to be a competitive Dnmt1 inhibitor,16 while hydralazine decreased T cell Dnmt1 mRNA and protein levels, and CD4+ T cells treated with hydralazine, procainamide or 5-azaC all caused a similar lupus-like disease when injected into genetically identical mice. 9 Together these results indicate that drugs or other environmental agents that inhibit CD4+ T cell DNA methylation could play an important role in the development of lupuslike diseases.
T cell DNA demethylation and idiopathic lupus
The same T cell DNA methylation abnormalities contribute to idiopathic human lupus. Early studies demonstrated that T cells from patients with active lupus had lower total DNA dmC content than healthy controls, as well as decreased Dnmt1 levels.9 Genes affected by DNA demethylation were identified by treating normal human T cells with 5-azaC and comparing gene expression patterns in untreated and treated T cells using arrays. These studies identified the cytotoxic molecule perforin, the B cell costimulatory molecule CD70, the KIR gene family, and the B cell costimulatory molecule CD40L as genes upregulated by 5-azaC treatment.9,12,14 Like CD11a, CD4+ T cells from patients with active lupus were also found to overexpress perforin, CD70, CD40L and KIR, and the same regulatory elements affected by 5-azaC were demethylated in the lupus T cells.9,12,14 Interestingly, CD40L is encoded on the X chromosome, so T cells from women have one methylated and one unmethylated CD40L gene, while T cells from men have only one, unmethylated gene. 5-azacytidine treated CD4+ T cells from women, as well as CD4+ T cells from women with active lupus, were found to overexpress CD40L, while CD4+ T cells from men did not, because the methylated gene on the normally silenced female X chromosome demethylated.14
T cell signaling and DNA demethylation in lupus
The decreased Dnmt activity in CD4+ lupus T cells was traced to low Dnmt1 mRNA levels, caused by impaired ERK pathway signaling and failure to adequately upregulate Dnmt1 as T cells entered mitosis, resulting in DNA demethylation of the daughter cells. Hydralazine was also found to inhibit ERK pathway signaling.9 The importance of decreased ERK pathway signaling in autoimmunity was confirmed by treating CD4+ T cells with MEK inhibitors in vitro. The treated cells demethylated and overexpressed the same genes as 5-azaC treated T cells, and injecting murine CD4+ T cells treated either with MEK inhibitors, hydralazine or 5-azaC into syngeneic mice caused similar lupus-like autoimmunity.9,17 The importance of decreased T cell ERK pathway signaling was further confirmed by creating a double transgenic mouse strain, in which expression of a dominant negative MEK could be induced in T cells by adding doxycycline to their drinking water. C57BL/6 double transgenic mice receiving doxycycline developed anti-DNA antibodies and an “interferon signature” in their leukocytes but no tissue damage.18 However, C57BL/6 mice lack genes predisposing to lupus. The transgenic mice were therefore crossed with lupus-prone SJL mice. In contrast to the C57BL/6 mice, the C57BL/6 X SJL mice developed higher titer anti-DNA antibodies and an immune complex glomerulonephritis.8 This experiment indicates that inhibiting T cell DNA methylation is sufficient to cause anti-DNA antibodies in mice without lupus genes, and anti-DNA antibodies plus lupus-like disease in mice with lupus genes, consistent with a gene-environment interaction similar to idiopathic human lupus and drug-induced lupus.9
T cell PKCδ inactivation, DNA methylation and lupus
The mechanism causing the decreased T cell ERK pathway signaling in lupus was sought by treating T cells from lupus patients with phorbolmyristate acetate (PMA), which stimulates protein kinase C (PKC) autophosphorylation, then measuring activation of the ERK pathway signaling cascade (PKC→ras→raf→MEK→ERK). PMA-stimulated PKCδ failed to autophosphorylate in T cells from patients with active lupus. Pharmacologic inhibition of PKCδ, or transfection with a dominant negative PKCδ, decreased ERK pathway signaling and Dnmt1 levels, and caused demethylation of the CD70 promoter and CD70 overexpression in T cells from healthy controls, similar to lupus and hydralazine-treated T cells.19 This indicates a critical role for PKCδ in maintaining T cell DNA methylation patterns. Interestingly, PKCδ “knockout” mice develop a lupus-like disease,20,21 suggesting that this signaling abnormality could contribute to the pathogenesis of lupus. However, the reason why PKCδ is catalytically inactive in lupus patients, and whether PKCδ deficiency only in T cells was sufficient to cause lupus, was unclear.
Oxidative stress, PKCδ inactivation and lupus
As noted above, environmental agents causing oxidative stress, including UV light, infections, and smoking, have been associated with risk of lupus or lupus flares. Other causes of oxidative stress include air pollution and traffic exhaust, 22 and mercury, which is discussed in more detail below. The oxidative damage is mediated by highly reactive molecules such as superoxide (O2−) oxidizing proteins and other molecules in vivo, or combining with nitric oxide (NO), an intracellular signaling molecule, to form peroxynitrite (ONOO−) which nitrates serines and tyrosines in proteins.23
Patients with active lupus have evidence for ongoing oxidative stress. Oates et al. reported that serum proteins from patients with active lupus are modified by nitration, and proposed nitration as a biomarker of lupus activity and renal disease.24 Similarly, Perl et al. reported that T cells from patients with active lupus generate significant amounts of reactive oxygen species (ROS), which may play a role in lupus pathogenesis. His group gave the antioxidant N-acetylcysteine to patients with active lupus, and demonstrated a dose-dependent therapeutic effect of this drug on disease activity, as measured by a decrease in SLEDAI score,25 supporting this hypothesis.
These observations suggest that oxidative damage may contribute to the signaling and possibly other abnormalities characterizing lupus T cells and contributing to disease pathogenesis. Gorelik et al. used antibodies to 3-nitro-Tyr to separate nitrated and unmodified proteins in CD4+ T cells from patients with active lupus, and demonstrated that PKCδ in the nitrated fraction was catalytically inert, while the non-nitrated fraction retained normal function. Further, the amount of nitrated PKCδ was directly proportional to disease activity.23 This suggests that oxidative damage to PKCδ may be responsible for the T cell ERK pathway signaling defect in lupus patients. The same group has now generated another transgenic mouse in which doxycycline administration activates expression of a dominant negative PKCδ selectively in T cells, and found that activating expression of the dominant negative PKCδ also causes a lupus-like disease.26
Mercury, oxidative stress and lupus
Mercury is a ubiquitous toxicant, currently ranked as a top three priority pollutant by the US Environmental Protection Agency.27 Exposure to organic (methyl) mercury is predominantly from consumption of contaminated seafood, while sources of inorganic/elemental mercury exposure include dental amalgams, products such as thermometers and fluorescent light bulbs, and occupational exposures.28 Immunotoxic effects, including association with development of antinuclear antibodies (ANAs) and lupus-like disease in murine models, have become increasingly recognized in association with both methyl and inorganic mercury.29 A case-control study of 265 SLE patient and 355 controls in the southeastern US found that SLE was associated with self-reported occupational mercury exposure (OR 3.6, 95% CI 1.3, 10.0) and dental profession (OR 7.1, 95% CI 2.2, 23.4).30 An ecologic study in New Mexico found that residence in a community with elevated exposure to mercury and petroleum products from an oil field waste site, compared to residence in a control community, was associated with SLE (OR 19.3, 95% CI 2.0, 190.7).31 Several studies in human populations have found ANA positivity to be associated with mercury exposure both from occupational mining settings in South America, as well as among residents of proximate riverine communities with high levels of consumption of contaminated fish.32,33
Mercury exposure in Brazilian Amazon communities has further been linked to markers of oxidative stress, with the effects being most robust among women.34 Mercury, as a redox-inactive metal, induces oxidative stress by sulphydryl reactivity, including depletion of thiol-containing and other cellular antioxidants.35 In human T lymphocytes, both methylmercury and inorganic mercury have been shown to induce alterations in mitochondrial function and glutathione (GSH) depletion,36 resulting in ROS generation and activation of apoptotic signaling pathways,37 potentially contributing to PKCδ inactivation as well. As recently reviewed by Shah et al, an imbalanced redox state and apoptosis are also hallmarks of SLE, and interactions of apoptotic macromolecules in the milieu of ROS might also lead to neoepitopes involved in autoimmunity.38
Diet, DNA Methylation and lupus
Diet also plays an essential role in the homeostasis of epigenetic marks, and is another environmental agent potentially contributing to lupus onset and flares. DNA methylation patterns are maintained through mitosis by Dnmt1, which binds the replication fork in dividing cells and “reads” CpG pairs. Where the parent strand is methylated, Dnmt1 transfers the methyl group from S-adenosylmethionine (SAM), the methyl donor, to dC bases in the daughter strand, producing dmC and S-adenosylhomocysteine (SAH), an inhibitor of transmethylation reactions.39 This reaction can be written as:
The velocity (V) of this reaction is directly dependent on the level or activity of Dnmt1 and intracellular SAM pools, and inversely to intracellular SAH pools. This may be written as:
where V is the forward velocity of the reaction and k is a constant to correct for units. SAM levels are dependent on dietary micronutrients including folate, methionine (Met), Zn, choline and vitamins B2, B6 and B12,40 and Dnmt1 levels are decreased by oxidative stress.23 Thus, when Dnmt1 levels are low, for example when T cells undergo oxidative stress, SAM levels will need to be increased, or SAH levels decreased, to maintain DNA methylation patterns during mitosis. This equation also implies that lupus patients with low Dnmt1 levels, due to environmental exposures that cause oxidative stress like UV light, smoking or infections, may be particularly sensitive to a diet poor in methyl donors, and the combination of oxidative stress and a poor diet may synergize to increase disease activity. Further, SAH levels are often increased in lupus patients,41 and notably transmethylation micronutrient levels are decreased in patients with active lupus.42
The relationship between T cell Dnmt1 levels, transmethylation micronutrient levels and methylation sensitive gene expression was tested in a study of the effect of aging on T cells in vitro. T cell Dnmt1 levels normally decrease with aging. Thus, Li et al. cultured PHA stimulated CD4+ T cells from healthy subjects ages 23–76 years in custom media containing folate or Met at normal or low serum levels, and measured expression of KIR2DL2 and CD70, two genes normally suppressed by DNA methylation. They found that the custom media had no effect on expression of these genes in T cells from people less than 50 years of age, but that gene expression increased and promoter methylation decreased progressively with age greater than 50 as Dnmt1 levels declined, 39 indicating an interaction between Dnmt1 levels, transmethylation micronutrient bioavailability, and age. A similar effect of a diet poor in transmethylation micronutrients on lupus severity has now been shown in the tet-on dominant negative MEK model described above. In this model, supplementing dietary methyl donors suppressed anti-DNA antibodies and renal disease, while restricting methyl donors increased anti-DNA antibodies and kidney disease.43 Finally, a population-based study in the United Kingdom of over 1600 incident SLE patients found that age-specific incidence rates of lupus among females in this predominantly white population increased steadily with age until peaking at approximately the age of menopause, and among males incidence increased with age up to 74 years of age.44 It can be hypothesized from these age-specific incidence patterns that age dependent decline in T cell Dnmt1 and DNA dmC content may also contribute to age of lupus onset.
Hormones and Lupus
Estrogens and endocrine disruptors also play a role in determining lupus flare severity.45 The SELENA trial demonstrated that hormonal replacement therapy contributes to a mild to moderate increase in lupus flares.46 Estrogen levels also increase during pregnancy, and may contribute to flares and increased disease severity in pregnant women.47 Further, a recent study using mice with an inducible T cell ERK pathway signaling defect, previously shown to cause lupus-like autoimmunity in genetically predisposed female mice,8,18 reported that the development of lupus required an environmental signal inhibiting ERK pathway signaling to cause T cell DNA demethylation plus estrogen and two X chromosomes. This study thus suggests that an environmental signal inhibiting T cell ERK pathway signaling to inhibit DNA methylation, such as oxidative stress,23 together with estrogen and 2 X chromosomes, are required to develop lupus-like autoimmunity in this model.
Given the role of natural estrogens (e.g., endogenous 17-β estradiol and its metabolites) in immune regulation, it has also been proposed that endocrine disruptors may be involved in promoting autoimmunity.48 The prototypic endocrine disruptor diethylstilbestrol (DES) illustrates the potential for immune dysregulation that may stem even from prenatal exposure to environmental estrogenic compounds. DES was synthesized in 1938 and widely used from the 1940–1970s in pregnant women and as a livestock feed supplement. In 1971, a now well-recognized association between maternal DES use during pregnancy and vaginal adenocarcinoma in offspring was reported.49 Although less well-characterized, there have also been reports of lupus and other immune-mediated diseases in association with in utero DES exposure,50 though not all studies investigating lupus have detected an association.51,52 A study in C57BL/6 mice demonstrated that in utero exposure to DES ‘programmed’ T lymphocytes so that subsequent postnatal DES exposure, at as late as 1.5 years of age, led to upregulation of IFN-γ.53 Increased lymphocyte apoptosis has been observed in C57BL/6 and CD-1 mice in association with DES treatment,54,55 as well as increased mitogen-induced proliferation in female, but not male, CD-1 mice at low dose DES.54 Both DES and another endocrine disruptor, bisphenol-A (BPA), were shown in BWF1 mice to be associated with enhanced autoantibody production, and among the mice that developed lupus nephritis, upregulation of estrogen receptor expression was observed.56 Conversely, another study found that low dose BPA administration to 5-week-old NZB × NZW F1 mice for 1 week was associated with reduced IFN-γ production and increased lupus-free period, demonstrating the complexities of immunomodulation related to endocrine disruptors.57
Some pesticides also act as endocrine disruptors, and further, have been linked to oxidative stress.58,59 Occupational studies have indicated increased risk of SLE associated with mixing pesticides for agricultural work (OR 7.4, 95% CI 1.4, 40).30 Likewise, personal mixing or application of insecticides has been associated with increased risk of rheumatoid arthritis (RA) or SLE (HR 2.04, 95% CI 1.17–3.56 for >6 applications/year), as well as indirect exposure through commercial or other residential applications (HR 1.85, 95% CI 1.07–3.20 for ≥20 years).60 Data from mouse models are consistent with these findings. In NZB × NZW F1 ovariectomized female mice, three organochlorine pesticides with estrogenic properties [chlordecone, methoxychlor, and o,p´-dichlorodiphenyl-trichloroethane (DDT)], as well as 17β-estradiol, were each shown to significantly accelerate time to development of renal involvement.61 However, another study in NZB × NZW F1 female (non-ovariectomized) mice found that while DDT treatment accelerated development of albuminuria, the xenoestrogen 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), a dioxin herbicidal, had immunosuppressive effects, including decreased incidence of anti-DNA antibodies.62
Gene/Environment Interactions in Lupus
Both genes and environmental exposures are required for lupus to develop and flare, and the studies cited above similarly confirm that experimentally demethylated T cells cause only a positive ANA and “interferon signature” in mice lacking lupus genes, but are sufficient to cause lupus-like autoimmunity in genetically predisposed mice.8,18 The example of drug induced lupus similarly indicates that both an environmentally-mediated epigenetic effect and predisposing genes are required to develop idiopathic and drug-induced lupus. Estrogens and possibly endocrine disruptors also contribute to the development of lupus-like autoimmunity in genetically predisposed individuals.45 Importantly, the degree of T cell DNA demethylation, endogenous estrogen levels, and endocrine disruptor levels are all variable, and T cell DNA methylation levels and endocrine disruptor effects depend on exposure levels while estrogen levels vary with conditions like pregnancy and hormonal supplementation.
Genetic predisposition is also variable. At least 38 genes have been identified that predispose to lupus, each contributing its own relative risk, and these genes largely assort independently.63 Thus, any given person may inherit 0 to76 recognized lupus genes, approximating a continuous variable. This suggests that the degree of T cell DNA demethylation and the total lupus genetic risk lupus may interact to determine disease manifestations and flare severity.
The interaction between total genetic risk, degree of T cell DNA demethylation, and flare severity was tested in a recent study in which men and women with active and inactive lupus were genotyped for 32 validated lupus susceptibility loci, and genetic risk for each person approximated by multiplying the number of alleles for each gene (0, 1 or 2) by its relative risk and adding the results. The methylation level of two genetic loci known to demethylate with increasing disease activity (KIR2DL4 and PRF1) was also measured in each subject. Combining the degree of DNA demethylation with the total genetic risk score for each patient demonstrated that the (genetic risk)/(DNA methylation) ratio increased directly with disease activity in both men and women with lupus. However, males required a greater (genetic risk)/(DNA methylation) ratio to achieve a SLEDAI score equivalent to females (P=0.01 for KIR2DL4; P=0.005 for PRF1). This difference was not explained by a difference in the genetic risk or T cell DNA demethylation alone, suggesting a different genetic-epigenetic interaction between men and women, likely due to differences in X chromosome number and hormones.1,8,14 These results support the hypothesis that genetic risk and T cell DNA demethylation interact in lupus patients to influence the severity of lupus flares, and that men require a higher genetic risk and/or greater degree of environmentally induced T cell DNA demethylation to achieve a lupus flare equal in severity to women.1
Aging, DNA methylation and lupus
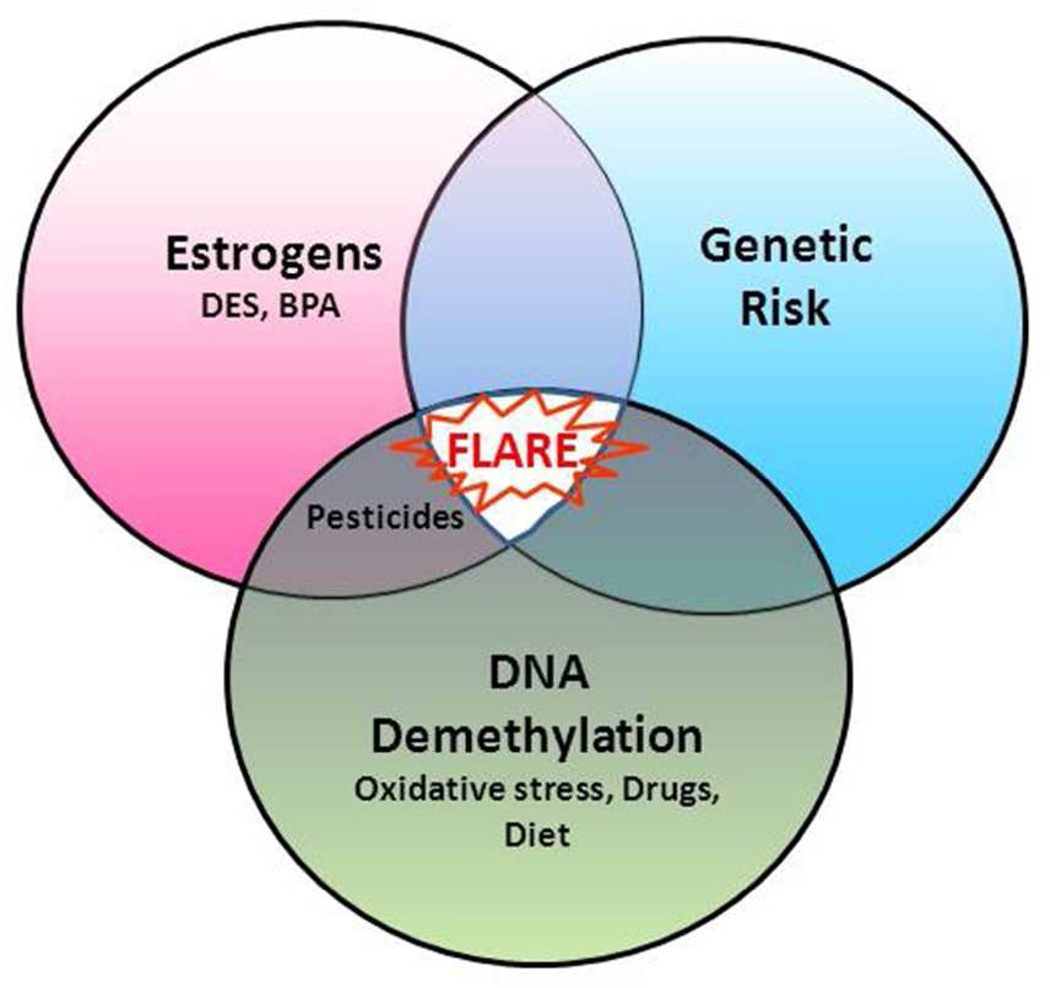
Two other studies compared age of lupus onset with total genetic risk, and found that people with lupus onset early in life tend to have a stronger genetic predisposition to lupus than those developing lupus later in life.64,65 Since T cell DNA demethylates progressively with age,66 this is consistent with a model in which a greater genetic risk is required for lupus to develop and flare at a young age when T cell DNA methylation levels are still high, but progressive DNA demethylation will eventually cause a flare later in life in those with a lesser total genetic risk. Together, these studies suggest that the degree of T cell DNA demethylation, caused by environmental exposures and over time, may interact with inherited genetic susceptibility as well as estrogen and/or endocrine disruptors to determine age of lupus onset and flare severity. The interactions between total genetic risk, DNA demethylation and hormonal influences are summarized in Figure 1.
Figure 1. Genetic, epigenetic and hormonal interactions in lupus flares.
Lupus develops when genetically predisposed people encounter environmental agents that trigger the flares. Any person can inherit from 0 to more than 76 lupus genes, making the genetic contribution variable from person to person (light shading indicates low genetic predisposition; dark shading indicates high genetic predisposition). Endogenous estrogen levels vary in women, as do exposure levels to endocrine disruptors (e.g., diethylstilbesterol, bisphenol-A, and some pesticides), which may provide another variable contribution to flares (low to high also indicated by shading). CD4+ T cell DNA demethylation, caused by certain drugs, diet and oxidative stress, provides a third variable contribution (similarly indicated by shading).
Conclusion
Accumulating evidence supports the premise that a dose-dependent combination of lupus susceptibility genes, environmental agents that inhibit DNA methylation, and estrogenic hormones, are involved in the etiology of lupus. This framework can be used to guide further investigations into gene-environment interactions that may underlie risk and progression of lupus.
Acknowledgements
The authors thank Ms. Patricia Bergeron for her excellent administrative support for this manuscript, and Dr. Faith Strickland for review of the manuscript. This report was supported by PHS grants AR42525, P30ES017885, and K01ES019909, a grant from the Lupus Foundation of America, an Arthritis Foundation New Investigator Award (to ECS) and a Merit grant from the Department of Veterans Affairs.
References
- 1.Sawalha AH, Wang L, Nadig A, et al. Sex-specific differences in the relationship between genetic susceptibility, T cell DNA demethylation and lupus flare severity. J Autoimmun. 2012;38:J216–J222. doi: 10.1016/j.jaut.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Somers EC, Antonsen S, Pedersen L, Sorensen HT. Parental history of lupus and rheumatoid arthritis and risk in offspring in a nationwide cohort study: does sex matter? Ann Rheum Dis. 2013;72:525–529. doi: 10.1136/annrheumdis-2011-201165. [DOI] [PubMed] [Google Scholar]
- 3.Schoonen WM, Thomas SL, Somers EC, et al. Do selected drugs increase the risk of lupus? A matched case-control study. Br J Clin Pharmacol. 2010;70:588–596. doi: 10.1111/j.1365-2125.2010.03733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lehmann P, Holzle E, Kind P, Goerz G, Plewig G. Experimental reproduction of skin lesions in lupus erythematosus by UVA and UVB radiation. J Am Acad Dermatol. 1990;22:181–187. doi: 10.1016/0190-9622(90)70020-i. [DOI] [PubMed] [Google Scholar]
- 5.James JA, Harley JB, Scofield RH. Epstein-Barr virus and systemic lupus erythematosus. Curr Opin Rheumatol. 2006;18:462–467. doi: 10.1097/01.bor.0000240355.37927.94. [DOI] [PubMed] [Google Scholar]
- 6.Ghaussy NO, Sibbitt W, Jr, Bankhurst AD, Qualls CR. Cigarette smoking and disease activity in systemic lupus erythematosus. J Rheumatol. 2003;30:1215–1221. [PubMed] [Google Scholar]
- 7.Costenbader KH, Kim DJ, Peerzada J, et al. Cigarette smoking and the risk of systemic lupus erythematosus: a meta-analysis. Arthritis Rheum. 2004;50:849–857. doi: 10.1002/art.20049. [DOI] [PubMed] [Google Scholar]
- 8.Strickland FM, Hewagama A, Lu Q, et al. Environmental exposure, estrogen and two X chromosomes are required for disease development in an epigenetic model of lupus. J Autoimmun. 2012;38:J135–J143. doi: 10.1016/j.jaut.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Richardson B. Primer: epigenetics of autoimmunity. Nat Clin Pract Rheumatol. 2007;3:521–527. doi: 10.1038/ncprheum0573. [DOI] [PubMed] [Google Scholar]
- 10.Wang Z, Patel DJ. Combinatorial readout of dual histone modifications by paired chromatin-associated modules. J Biol Chem. 2011;286:18363–18368. doi: 10.1074/jbc.R111.219139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakayamada S, Takahashi H, Kanno Y, O’Shea JJ. Helper T cell diversity and plasticity. Curr Opin Immunol. 2012;24:297–302. doi: 10.1016/j.coi.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Basu D, Liu Y, Wu A, et al. Stimulatory and inhibitory killer Ig-like receptor molecules are expressed and functional on lupus T cells. J Immunol. 2009;183:3481–3487. doi: 10.4049/jimmunol.0900034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilson CB, Rowell E, Sekimata M. Epigenetic control of T-helper-cell differentiation. Nat Rev Immunol. 2009;9:91–105. doi: 10.1038/nri2487. [DOI] [PubMed] [Google Scholar]
- 14.Lu Q, Wu A, Tesmer L, Ray D, Yousif N, Richardson B. Demethylation of CD40LG on the inactive X in T cells from women with lupus. J Immunol. 2007;179:6352–6358. doi: 10.4049/jimmunol.179.9.6352. [DOI] [PubMed] [Google Scholar]
- 15.Yung RL, Quddus J, Chrisp CE, Johnson KJ, Richardson BC. Mechanism of drug-induced lupus I Cloned Th2 cells modified with DNA methylation inhibitors in vitro cause autoimmunity in vivo. J Immunol. 1995;154:3025–3035. [PubMed] [Google Scholar]
- 16.Scheinbart LS, Johnson MA, Gross LA, Edelstein SR, Richardson BC. Procainamide inhibits DNA methyltransferase in a human T cell line. J Rheumatol. 1991;18:530–534. [PubMed] [Google Scholar]
- 17.Deng C, Lu Q, Zhang Z, et al. Hydralazine may induce autoimmunity by inhibiting extracellular signal-regulated kinase pathway signaling. Arthritis Rheum. 2003;48:746–756. doi: 10.1002/art.10833. [DOI] [PubMed] [Google Scholar]
- 18.Sawalha AH, Jeffries M, Webb R, et al. Defective T-cell ERK signaling induces interferon-regulated gene expression and overexpression of methylation-sensitive genes similar to lupus patients. Genes Immun. 2008;9:368–378. doi: 10.1038/gene.2008.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gorelik G, Fang JY, Wu A, Sawalha AH, Richardson B. Impaired T cell protein kinase C delta activation decreases ERK pathway signaling in idiopathic and hydralazine-induced lupus. J Immunol. 2007;179:5553–5563. doi: 10.4049/jimmunol.179.8.5553. [DOI] [PubMed] [Google Scholar]
- 20.Miyamoto A, Nakayama K, Imaki H, et al. Increased proliferation of B cells and auto-immunity in mice lacking protein kinase Cdelta. Nature. 2002;416:865–869. doi: 10.1038/416865a. [DOI] [PubMed] [Google Scholar]
- 21.Mecklenbrauker I, Saijo K, Zheng NY, Leitges M, Tarakhovsky A. Protein kinase Cdelta controls self-antigen-induced B-cell tolerance. Nature. 2002;416:860–865. doi: 10.1038/416860a. [DOI] [PubMed] [Google Scholar]
- 22.Kelly F, Anderson HR, Armstrong B, et al. The impact of the congestion charging scheme on air quality in London. Part 2 Analysis of the oxidative potential of particulate matter. Res Rep Health Eff Inst. 2011:73–144. [PubMed] [Google Scholar]
- 23.Gorelik GJ, Yarlagadda S, Richardson BC. Protein kinase Cdelta oxidation contributes to ERK inactivation in lupus T cells. Arthritis Rheum. 2012;64:2964–2974. doi: 10.1002/art.34503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oates JC, Shaftman SR, Self SE, Gilkeson GS. Association of serum nitrate and nitrite levels with longitudinal assessments of disease activity and damage in systemic lupus erythematosus and lupus nephritis. Arthritis Rheum. 2008;58:263–272. doi: 10.1002/art.23153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lai ZW, Hanczko R, Bonilla E, et al. N-acetylcysteine reduces disease activity by blocking mammalian target of rapamycin in T cells from systemic lupus erythematosus patients: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2012;64:2937–2946. doi: 10.1002/art.34502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gorelik G, Sawalha A, Richardson B. Lack of PKC δ kinase activity in T cells induces a lupus-like disease. Lupus. 2010;19:7. [Google Scholar]
- 27.Agency for Toxic Substances and Diseases Registry (ATSDR) Priority List of Hazardous Substances. Center for Disease Control and Prevention; 2012. updated August 31, 2012 ed. [Google Scholar]
- 28.Clarkson TW, Magos L. The toxicology of mercury and its chemical compounds. Crit Rev Toxicol. 2006;36:609–662. doi: 10.1080/10408440600845619. [DOI] [PubMed] [Google Scholar]
- 29.Vas J, Monestier M. Immunology of mercury. Ann N Y Acad Sci. 2008;1143:240–267. doi: 10.1196/annals.1443.022. [DOI] [PubMed] [Google Scholar]
- 30.Cooper GS, Parks CG, Treadwell EL, St Clair EW, Gilkeson GS, Dooley MA. Occupational risk factors for the development of systemic lupus erythematosus. J Rheumatol. 2004;31:1928–1933. [PubMed] [Google Scholar]
- 31.Dahlgren J, Takhar H, Anderson-Mahoney P, Kotlerman J, Tarr J, Warshaw R. Cluster of systemic lupus erythematosus (SLE) associated with an oil field waste site: a cross sectional study. Environ Health. 2007;6:8. doi: 10.1186/1476-069X-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gardner RM, Nyland JF, Silva IA, Ventura AM, de Souza JM, Silbergeld EK. Mercury exposure, serum antinuclear/antinucleolar antibodies, and serum cytokine levels in mining populations in Amazonian Brazil: a cross-sectional study. Environ Res. 2010;110:345–354. doi: 10.1016/j.envres.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Silva IA, Nyland JF, Gorman A, et al. Mercury exposure, malaria, and serum antinuclear/antinucleolar antibodies in Amazon populations in Brazil: a cross-sectional study. Environ Health. 2004;3:11. doi: 10.1186/1476-069X-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grotto D, Valentini J, Fillion M, et al. Mercury exposure and oxidative stress in communities of the Brazilian Amazon. Sci Total Environ. 2010;408:806–811. doi: 10.1016/j.scitotenv.2009.10.053. [DOI] [PubMed] [Google Scholar]
- 35.Ercal N, Gurer-Orhan H, Aykin-Burns N. Toxic metals and oxidative stress part I: mechanisms involved in metal-induced oxidative damage. Curr Top Med Chem. 2001;1:529–539. doi: 10.2174/1568026013394831. [DOI] [PubMed] [Google Scholar]
- 36.Shenker BJ, Mayro JS, Rooney C, Vitale L, Shapiro IM. Immunotoxic effects of mercuric compounds on human lymphocytes, monocytes. IV Alterations in cellular glutathione content. Immunopharmacol Immunotoxicol. 1993;15:273–290. doi: 10.3109/08923979309025999. [DOI] [PubMed] [Google Scholar]
- 37.Guo TL, Miller MA, Shapiro IM, Shenker BJ. Mercuric chloride induces apoptosis in human T lymphocytes: evidence of mitochondrial dysfunction. Toxicol Appl Pharmacol. 1998;153:250–257. doi: 10.1006/taap.1998.8549. [DOI] [PubMed] [Google Scholar]
- 38.Shah D, Sah S, Nath SK. Interaction between glutathione and apoptosis in systemic lupus erythematosus. Autoimmun Rev. 2013;12:741–751. doi: 10.1016/j.autrev.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li Y, Liu Y, Strickland FM, Richardson B. Age-dependent decreases in DNA methyltransferase levels and low transmethylation micronutrient levels synergize to promote overexpression of genes implicated in autoimmunity and acute coronary syndromes. Exp Gerontol. 2010;45:312–322. doi: 10.1016/j.exger.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ross SA, Poirier L. Proceedings of the Trans-HHS Workshop: diet, DNA methylation processes and health. J Nutr. 2002;132:2329S–2332S. doi: 10.1093/jn/132.8.2329S. [DOI] [PubMed] [Google Scholar]
- 41.Lazzerini PE, Capecchi PL, Selvi E, et al. Hyperhomocysteinemia: a cardiovascular risk factor in autoimmune diseases? Lupus. 2007;16:852–862. doi: 10.1177/0961203307084176. [DOI] [PubMed] [Google Scholar]
- 42.Wu T, Xie C, Han J, et al. Metabolic disturbances associated with systemic lupus erythematosus. PLoS One. 2012;7:37210. doi: 10.1371/journal.pone.0037210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Strickland FM, Hewagama A, Wu A, et al. Diet influences expression of autoimmune associated genes and disease severity by epigenetic mechanisms in a transgenic lupus model. Arthritis Rheum. 2013 doi: 10.1002/art.37967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Somers EC, Thomas SL, Smeeth L, Schoonen WM, Hall AJ. Incidence of systemic lupus erythematosus in the United Kingdom, 1990–1999. Arthritis Rheum. 2007;57:612–618. doi: 10.1002/art.22683. [DOI] [PubMed] [Google Scholar]
- 45.Peeva E, Zouali M. Spotlight on the role of hormonal factors in the emergence of autoreactive B-lymphocytes. Immunol Lett. 2005;101:123–143. doi: 10.1016/j.imlet.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 46.Buyon JP, Petri MA, Kim MY, et al. The effect of combined estrogen and progesterone hormone replacement therapy on disease activity in systemic lupus erythematosus: a randomized trial. Ann Intern Med. 2005;142:953–962. doi: 10.7326/0003-4819-142-12_part_1-200506210-00004. [DOI] [PubMed] [Google Scholar]
- 47.Walker SE. Estrogen and autoimmune disease. Clin Rev Allergy Immunol. 2011;40:60–65. doi: 10.1007/s12016-010-8199-x. [DOI] [PubMed] [Google Scholar]
- 48.Ahmed SA, Hissong BD, Verthelyi D, Donner K, Becker K, Karpuzoglu-Sahin E. Gender and risk of autoimmune diseases: possible role of estrogenic compounds. Environ Health Perspect. 1999;107(Suppl 5):681–686. doi: 10.1289/ehp.99107s5681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Herbst AL, Ulfelder H, Poskanzer DC. Adenocarcinoma of the vagina Association of maternal stilbestrol therapy with tumor appearance in young women. N Engl J Med. 1971;284:878–881. doi: 10.1056/NEJM197104222841604. [DOI] [PubMed] [Google Scholar]
- 50.Wingard DL, Turiel J. Long-term effects of exposure to diethylstilbestrol. West J Med. 1988;149:551–554. [PMC free article] [PubMed] [Google Scholar]
- 51.Noller KL, Blair PB, O’Brien PC, et al. Increased occurrence of autoimmune disease among women exposed in utero to diethylstilbestrol. Fertil Steril. 1988;49:1080–1082. doi: 10.1016/s0015-0282(16)59965-8. [DOI] [PubMed] [Google Scholar]
- 52.Strohsnitter WC, Noller KL, Troisi R, et al. Autoimmune disease incidence among women prenatally exposed to diethylstilbestrol. J Rheumatol. 2010;37:2167–2173. doi: 10.3899/jrheum.091092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Karpuzoglu-Sahin E, Hissong BD, Ansar Ahmed S. Interferon-gamma levels are upregulated by 17-beta-estradiol and diethylstilbestrol. J Reprod Immunol. 2001;52:113–127. doi: 10.1016/s0165-0378(01)00117-6. [DOI] [PubMed] [Google Scholar]
- 54.Calemine JB, Gogal RM, Jr, Lengi A, Sponenberg P, Ahmed SA. Immunomodulation by diethylstilbestrol is dose and gender related: effects on thymocyte apoptosis and mitogen-induced proliferation. Toxicology. 2002;178:101–118. doi: 10.1016/s0300-483x(02)00201-9. [DOI] [PubMed] [Google Scholar]
- 55.Fenaux JB, Gogal RM, Jr, Ahmed SA. Diethylstilbestrol exposure during fetal development affects thymus: studies in fourteen-month-old mice. J Reprod Immunol. 2004;64:75–90. doi: 10.1016/j.jri.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 56.Yurino H, Ishikawa S, Sato T, et al. Endocrine disruptors (environmental estrogens) enhance autoantibody production by B1 cells. Toxicol Sci. 2004;81:139–147. doi: 10.1093/toxsci/kfh179. [DOI] [PubMed] [Google Scholar]
- 57.Sawai C, Anderson K, Walser-Kuntz D. Effect of bisphenol A on murine immune function: modulation of interferon-gamma, IgG2a, and disease symptoms in NZB X NZW F1 mice. Environ Health Perspect. 2003;111:1883–1887. doi: 10.1289/ehp.6359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Abdollahi M, Ranjbar A, Shadnia S, Nikfar S, Rezaie A. Pesticides and oxidative stress: a review. Med Sci Monit. 2004;10:RA141–RA147. [PubMed] [Google Scholar]
- 59.Mostafalou S, Abdollahi M. Pesticides and human chronic diseases: evidences, mechanisms, and perspectives. Toxicol Appl Pharmacol. 2013;268:157–177. doi: 10.1016/j.taap.2013.01.025. [DOI] [PubMed] [Google Scholar]
- 60.Parks CG, Walitt BT, Pettinger M, et al. Insecticide use and risk of rheumatoid arthritis and systemic lupus erythematosus in the Women’s Health Initiative Observational Study. Arthritis Care Res (Hoboken) 2011;63:184–194. doi: 10.1002/acr.20335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sobel ES, Gianini J, Butfiloski EJ, Croker BP, Schiffenbauer J, Roberts SM. Acceleration of autoimmunity by organochlorine pesticides in (NZB x NZW)F1 mice. Environ Health Perspect. 2005;113:323–328. doi: 10.1289/ehp.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li J, McMurray RW. Effects of chronic exposure to DDT and TCDD on disease activity in murine systemic lupus erythematosus. Lupus. 2009;18:941–949. doi: 10.1177/0961203309104431. [DOI] [PubMed] [Google Scholar]
- 63.Lessard CJ, Adrianto I, Ice JA, et al. Identification of IRF8, TMEM39A, and IKZF3-ZPBP2 as susceptibility loci for systemic lupus erythematosus in a large-scale multiracial replication study. Am J Hum Genet. 2012;90:648–660. doi: 10.1016/j.ajhg.2012.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Webb R, Kelly JA, Somers EC, et al. Early disease onset is predicted by a higher genetic risk for lupus and is associated with a more severe phenotype in lupus patients. Ann Rheum Dis. 2011;70:151–156. doi: 10.1136/ard.2010.141697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Taylor KE, Chung SA, Graham RR, et al. Risk alleles for systemic lupus erythematosus in a large case-control collection and associations with clinical subphenotypes. PLoS Genet. 2011;7:e1001311. doi: 10.1371/journal.pgen.1001311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Golbus J, Palella TD, Richardson BC. Quantitative changes in T cell DNA methylation occur during differentiation and ageing. Eur J Immunol. 1990;20:1869–1872. doi: 10.1002/eji.1830200836. [DOI] [PubMed] [Google Scholar]