Abstract
Early phase trials targeting the T-cell inhibitory molecule PD-L1 have shown clinical efficacy in cancer. This study was undertaken to determine whether PD-L1 is overexpressed in triple-negative breast cancer (TNBC) and to investigate the loss of the phosphatase and tensin homolog (PTEN) as a mechanism of PD-L1 regulation. The Cancer Genome Atlas (TCGA) RNA sequencing data showed significantly greater expression of the PD-L1 gene in TNBC (n=120) compared to non-TNBC (n=716) (P<0.001). Breast tumor tissue microarrays were evaluated for PD-L1 expression which was present in 19% (20 of 105) TNBC specimens. PD-L1+ tumors had greater CD8+ T-cell infiltrate than PD-L1− tumors (688 cells/mm versus 263 cells/mm; P<0.0001). To determine the effect of PTEN loss on PD-L1 expression, stable cell lines were generated using PTEN shRNA. PTEN knockdown led to significantly higher cell-surface PD-L1 expression and PD-L1 transcripts, suggesting transcriptional regulation. Moreover, PI3K pathway inhibition using the AKT inhibitor MK-2206 or rapamycin resulted in decreased PD-L1 expression, further linking PTEN and PI3K signaling to PD-L1 regulation. Co-culture experiments were performed to determine the functional effect of altered PD-L1 expression. Increased PD-L1 cell surface expression by tumor cells induced by PTEN loss led to decreased T cell proliferation and increased apoptosis. PD-L1 is expressed in 20% of TNBC, suggesting PD-L1 as a therapeutic target in TNBC. Since PTEN loss is one mechanism regulating PD-L1 expression, agents targeting the PI3K pathway may increase the antitumor adaptive immune responses.
Keywords: PTEN, PD-L1, triple negative breast cancer, adaptive immunity
Introduction
Triple-negative breast cancer (TNBC), which constitutes 10–20% of all breast tumors, is characterized by a lack of expression of estrogen receptor (ER), progesterone receptor (PR) and HER2/neu (HER2) (1, 2). TNBCs are generally high grade, aggressive tumors with a high rate of distant metastasis and poorer disease-specific survival than other breast cancer subtypes (1, 3). The poor outcomes occur even though standard chemotherapy regimens have activity against these tumors. Studies evaluating chemotherapy in the neoadjuvant setting have demonstrated that TNBC has higher rates of pathologic complete response than other tumor types; however there is a paradoxical shortening of progression-free and overall survival (4). Therefore, novel therapeutic strategies are needed to improve the management of patients with TNBC.
There is significant heterogeneity within TNBC. A study analyzing gene expression profiles identified six TNBC subtypes, one of which was an immunomodulatory subtype enriched for genes involved in immune cell processes including immune cell signaling, cytokine signaling, antigen processing and presentation, and signaling through core immune signal transduction pathways (2). In a meta-analysis integrating published gene expression data with clinicopathologic data, investigators developed gene expression modules related to key biologic processes in breast cancer. For TNBC, only the immune response module was associated with clinical outcome (5). Loi et al. recently reported a prognostic role for tumor-infiltrating lymphocytes in TNBC in a large prospective clinical trial (6), and in a study looking specifically at CD8+ intratumoral lymphocytes, Liu et al. found that TNBC had higher rates of CD8+ T cell infiltration, which was an independent favorable prognostic factor (7). Taken together, these data suggest that there may be a role for immunotherapy in the management of patients with TNBC.
A promising approach to augmenting antitumor immunity is blockade of immune checkpoints. One example is cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4), a T cell inhibitory receptor that is expressed on activated CD8+ T cells. CTLA-4 attenuates the T cell immune response by counteracting the activity of the T cell co-stimulatory receptor CD28 (8, 9). Ipilimumab, a monoclonal antibody targeting CTLA-4, has received the US Food and Drug Administration approval for the treatment of metastatic or unresectable melanoma. Programmed cell death protein 1 (PD-1) is a second immune checkpoint receptor that limits T cell effector function within tissues (10). PD-1 has two known ligands, PD-L1 and PD-L2, which have distinct expression profiles with PD-L1 being expressed on several tumor types (11). In a small study of 44 breast tumors, PD-L1 was expressed in 34% of the tumors and the expression was associated with high-risk clinicopathologic features including high histologic grade and hormone receptor negative status (12). Recently reported phase I studies evaluating antibodies targeting either PD-1 or PD-L1 have shown that these agents elicited durable, objective responses in patients with melanoma, non-small-cell lung cancer and renal-cell carcinoma (13, 14).
PD-L1 regulation is an area of active investigation. One mechanism of regulation is the induction of tumor cell surface expression of PD-L1 in response to interferon gamma (IFNγ) (11). This is likely a mechanism whereby tumor cells evade the antitumor immune response of tumor-specific T cells. A second mechanism is through oncogenic signaling. Expression of PD-L1 on glioblastomas is increased by the deletion or silencing of the phosphatase and tensin homolog (PTEN) implicating involvement of the phosphatidylinositol 3-kinase (PI3K) pathway (15). The PTEN/PI3K pathway is important in breast cancer as PTEN loss and the phosphatidylinositol-4,5-bisphosphate 3-kinase (PIK3CA) mutations have been identified in approximately 30% and 40% of primary breast tumors, respectively (16). Furthermore, PTEN loss is correlated with ER/PR negative tumors (17). Recently published data from The Cancer Genome Atlas (TCGA) showed that basal-like tumors, the majority of which were TNBCs, showed PTEN mutation or loss in 35% of tumors, which also correlated with PI3K pathway activation (18).
In this study, we hypothesized that TNBC express PD-L1 and that PD-L1 expression may be regulated in part by PTEN loss. We show that 20% of TNBC express PD-L1 and PTEN is deficient in almost half of the PD-L1-expressing tumors. Furthermore, we show that PTEN knockdown results in increased cell surface PD-L1 expression, an effect that is in part transcriptionally regulated. Increased PD-L1 expression following PTEN knockdown has functional consequences as we show that activated T cells co-cultured with PTEN-silenced breast cancer cells have decreased proliferation and increased apoptosis. Taken together, these data provide evidence for PTEN loss as a mechanism regulating PD-L1 expression in TNBC and suggest that antibodies targeting PD-1 or PD-L1 may have utility as a novel therapeutic strategy in TNBC.
Methods
Study Patients, Tumors and Cell Lines
TCGA RNA Seq data were obtained from the Cancer Genomics Hub (CGHub, https://cghub.ucsc.edu) and the TCGA Data Portal (https://tcga-data.nci.nih.gov/tcga/). Tumor samples used to construct tissue microarrays (TMAs) and peripheral blood mononuclear cells (PBMC) were obtained through an Institutional Review Board-approved protocol. Tumors were considered hormone receptor (HR) negative if nuclear staining for both the estrogen and progesterone receptors was ≤5%. Tumors were considered HER2 negative if they were 0, 1+ by immunohistochemistry (IHC) or 2+ by IHC and negative for gene amplification by fluorescence in situ hybridization (FISH). Fresh frozen tumor samples used to isolate breast cancer cells by laser capture microdissection (LCM) were obtained from Origene. Cultured breast cancer cell lines were obtained from American Type Culture Collection. Cell lines were validated by STR DNA fingerprinting using the AmpF/STR Identifier kit according to manufacturer’s instructions (Applied Biosystems). Cells were cultured in Dulbecco’s modified Eagle’s medium with 10% fetal bovine serum, 100U/mL penicillin, and 100μg/mg streptomycin.
Immunohistochemistry
One millimeter cores from paraffin blocks of breast tumors were used to generate tissue microarrays. Prior to staining, microarrays were baked overnight after which they were deparaffinized and rehydrated. Nonspecific binding was blocked and then the sections were incubated with primary antibody. For PD-L1 staining, the primary antibody used was 5H1, a mouse anti-human PD-L1 monoclonal antibody previously reported by Dong et al. for human tumor staining (19, 20). The specificity of this antibody for PD-L1 was validated using a PD-L1 fusion protein and PD-L1-transduced melanoma cells (positive control) and non-transduced parental cells (negative control) (20). Slides were stained for 60 minutes with antibody diluted 1:300 with antibody diluent containing background-reducing components. Slides were washed and incubated in FITC- labeled anti-mouse immunoglobulins then anti-FITC horseradish peroxidase (HRP). Slides were visualized with diaminobenzidine (DAB). Consistent with previous reports of PD-L1 staining using the 5H1 antibody in renal cell carcinoma, cell surface membrane staining > 5% was considered positive (20). For PTEN staining, TMAs were incubated with primary anti-PTEN antibody (1:100; clone 6H2.1, Dako). After washing, slides were incubated with the secondary anti-mouse IgG conjugated with HRP, then visualized with chromogen DAB. Any staining of PTEN was considered positive. For CD8 staining, TMAs were incubated with primary anti-CD8 antibody (1:20; Labvision). Slides were incubated with the secondary anti-mouse IgG-biotin antibody (1:200; Vectastain Elite ABC kit; Vector laboratories), then with the avidin-biotin peroxidase complex (1:100; Vectastain Elite ABC kit), after which visualization was conducted with chromagen. The number of CD8+ T cells per 1 mm core was determined. Human tonsil tissue was used as a positive control for both PD-L1 and CD8 staining. For PD-L1 staining, irrelevant isotype-matched antibodies were used to control for nonspecific staining during protocol development. Specificity of staining was confirmed by pre-incubation of primary antibody with recombinant PD-L1 antibody. For CD8 staining, omission of primary antibodies was used as a negative staining control.
RNA Extraction and Amplification, cDNA Synthesis and Reverse Transcription Polymerase Chain Reaction
Breast cancer cells were isolated from fresh frozen tumor samples by LCM and RNA was extracted, purified and amplified as described previously (21). Prior to polymerase chain reaction (PCR), RNA was amplified using the Arcturus RiboAmp RNA Amplification Kit (Life Technologies, Applied Biosystems) to generate amplified antisense RNA (aRNA). cDNA was synthesized from 1μg of aRNA using the Roche Transcriptor First Strand cDNA Synthesis kit (Roche Applied Science). For cultured cell lines, total cellular RNA was extracted using the RNeasy mini kit (Qiagen). cDNA was synthesized from 2μg of RNA using the Roche Transcriptor First Strand cDNA Synthesis kit. RT-PCR reactions were performed on an iCycler iQ™ thermal cycler (Bio-Rad Laboratories). Quantitative RT-PCR was performed on a StepOnePlus instrument (Applied Biosystems). Data was analyzed as relative mRNA expression quantified with StepOnePlus software and normalized to actin transcription levels. Primer sequences used included: cytokeratin 7 (CK7) (forward primer 5′-TGTGGATGCTGCCTACATGAGC -3′, reverse primer 5′-AGCACCACAGATGTGTCGGAGA -3′), PD-L1 (forward primer 5′-TATGGTGGTGCCGACTACAA -3′, reverse primer 5′-TGGCTCCCAGAATTACCAAG-3′), and actin, an endogenous control, (forward primer 5′-TCCTGTGGCATCCACGAAAC-3′, reverse primer 5′-GAAGCATTTGCGGACGAT-3′) (oligonucleotides from Sigma Aldrich).
shRNA Constructs and Transduction
PTEN shRNA lentiviral transduction particles (TRCN0000002746 and TRCN0000002749) and non-targeting shRNA lentiviral transduction particles (pLKO.1-puro Non-Target Control [SHC016V]) were obtained from Sigma-Aldrich. Transductions were performed with 1x104 cells per well in 96-well plates. Lentiviral particles were added at a multiplicity of infection of 5. After 48 hours, media was changed to fresh media with 2 μg/ml puromycin. Media was replaced every third day with fresh puromycin-containing media until stable clones were identified. PTEN knockdown was confirmed using western blot analysis.
Drug Reagents
MK-2206 was provided (to FMB) by the SU2C PI3K Dream Team Consortium. Rapamycin was purchased from LC Laboratories, Inc.
Flow Cytometry Analysis
To assess cell surface PD-L1 expression, cells were stained with phycoerythrin (PE)-conjugated anti-PD-L1 antibody (ebioscience). Aqua live/dead stain (Invitrogen) was used to assess cell viability. Flow cytometry was performed using the Fortessa flow cytometer (BD Biosciences) and data were analyzed using FlowJo software (Tree Star Inc).
T Cell Proliferation Assay
CD4+ and CD8+ T cells were isolated from healthy donor PBMC using negative selection kits (Miltenyi Biotech). Cells were labeled with carboxyfluorescein succinimidyl ester (CFSE; Invitrogen) after which 3x105 CD4+ or CD8+ T cells were combined with 1x103 to 1x104 breast cancer cells and seeded into 96-well plates. T cells were stimulated by the addition of anti-CD3 and anti-CD28 antibodies (3 μg/ml) (BD Biosciences). After 72 hours, cells were stained with allophycocyanin (APC)-conjugated anti-CD4 antibody, PE-conjugated anti-CD8 antibody and aqua live/dead stain (BD Biosciences). Flow cytometry analyses were performed as described above.
Apoptosis Assay
CD4+ or CD8+ T cells isolated from PBMCs were plated with breast cancer cells at a 1:1 ratio (3x105) in 96-well plates. T cells were stimulated with anti-CD3 and anti-CD28 antibodies. Cells were co-cultured at 37° for 20 hours after which they were stained with APC-conjugated anti-CD4 antibody and PE-conjugated anti-CD8 antibody. Cells were washed in phosphate buffered saline then resuspended in Annexin binding buffer containing fluorescein isothiocyanate (FITC)-conjugated annexin V and 7-amino-actinomycin D (7-AAD) viability stain (BD Biosciences). Flow cytometry analyses were performed as described above.
Statistical Analysis
Comparison of PD-L1 between TNBC and non-TNBC samples in the TCGA data set was made using a two-sample t test performed in R (http://www.R-project.org). Remaining statistical analyses were performed using GraphPad Prism 5.0 software. P-values <0.05 were considered significant.
Results
Triple Negative Breast Cancer Expresses PD-L1
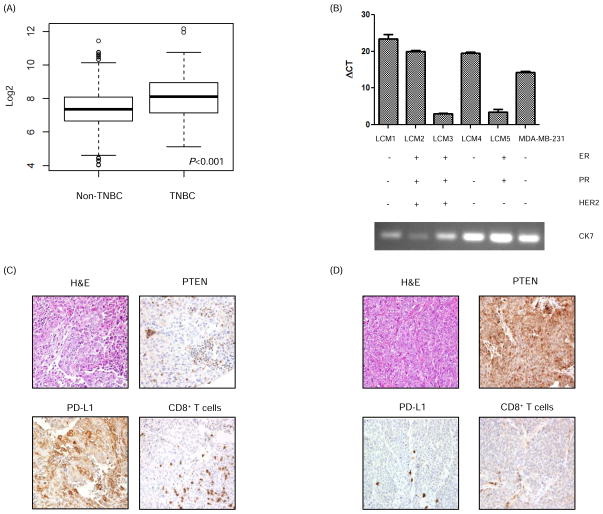
To evaluate for the presence of PD-L1 in breast cancer, we used TCGA RNA sequencing data to determine if the PD-L1 transcript was present. This analysis demonstrated differential PD-L1 expression with significantly higher levels in TNBC (n=120) versus non-TNBC (n=716) samples (P<0.001) (Fig. 1A). Because whole tumors such as those evaluated by TCGA contain cells of the microenvironment including inflammatory cells that might express PD-L1, we next performed laser capture microdissection to isolate breast cancer cells from primary tumors. Following RNA extraction, qRT-PCR confirmed the presence of PD-L1 mRNA in all five samples with transcript levels in three tumors being higher than in the MDA-MB-231 cell line, which has been shown to have baseline high PD-L1 expression (Fig. 1B) (12). Two of the three tumors (LCM1 and LCM 4) with the highest PD-L1 mRNA levels were TNBC.
Figure 1. PD-L1 is expressed in breast cancer.
(A) Analysis of The Cancer Genome Atlas data demonstrated higher PD-L1 mRNA expression in breast tissue specimens from patients with TNBC (n=120) in contrast to patients with non-TNBC (n=716). Data are mean ± SD of PD-L1 mRNA expression. Analysis was done using a t test. Log2 expression of PD-L1 is shown on the y-axis. (B) PD-L1 mRNA expression (mean ± SD) in breast cancer patient tumors was measured using RT-PCR. RNA was extracted from breast cancer cells that were isolated from tumors using laser capture microdissection (LCM 1–5). Cytokeratin 7 (CK7) was used as a marker of breast cancer to confirm the source of the extracted RNA. MDA-MB-231 was used as a positive control for PD-L1. (C) Representative TNBC patient tumor tissues showing loss of PTEN expression and high PD-L1 expression in tumor cells, and a significant CD8+ T cell infiltrate in contrast with another TNBC patient tissue (D) which shows high PTEN expression in breast tumor cells, no PD-L1 expression in tumor cells, minimal PD-L1 expression in associated inflammatory cells, and minimal intratumoral CD8+ T cell infiltrate. Magnification = 100x.
Having shown the presence of PD-L1 transcripts in breast tumors, we next evaluated PD-L1 expression at the protein level. Immunohistochemical staining for PD-L1 expression was performed on a tissue microarray comprising 105 tumors from early stage triple negative breast cancer patients that had not received neoadjuvant chemotherapy. PD-L1 expression, defined as > 5% membranous staining, was identified in 20 (19%) of the tumors. Furthermore, nine of 17 evaluable PD-L1+ tumors were negative for PTEN staining on immunohistochemistry. Staining for the presence of CD8+ T cells was available for 82 tumors. For tumors that were PD-L1+, the average number of CD8+ T cells per 1mm core was 668 compared to 263 for tumors that were PD-L1− (P < 0.0001) (Fig. 1 C and D).
Taken together, these data confirm that TNBC expresses PD-L1, and approximately half of the PD-L1+ tumors did not express PTEN.
PD-L1 Expression and PTEN Status
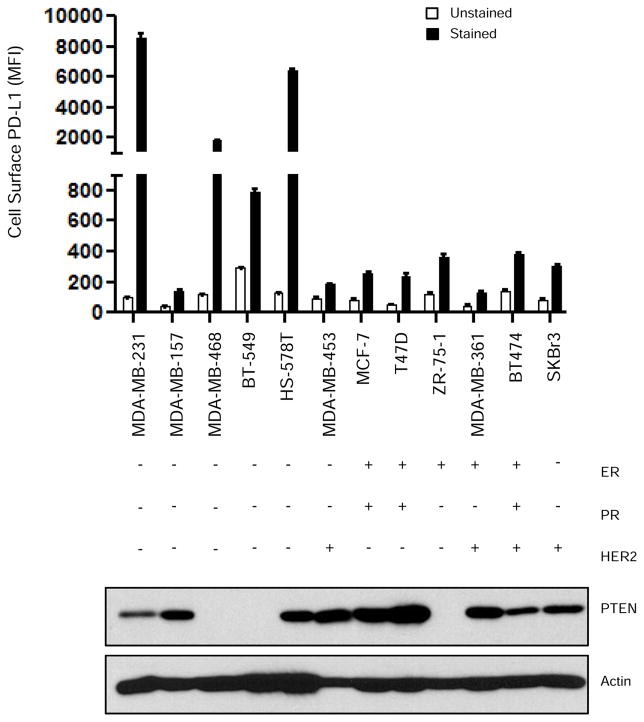
Having demonstrated PD-L1 expression in approximately 20% of TNBC specimens, we next screened a panel of cell lines, including 5 TNBC cell lines, for cell surface PD-L1 expression (Fig. 2). Four of the 5 TNBC cell lines expressed high levels of cell-surface PD-L1; two of these PD-L1-expressing TNBC cell lines (MDA-MB-468 and BT-549) are PTEN-deficient (22). Notably, the TNBC cell line MDA-MB-157, which has wild-type PTEN, had low levels of PD-L1 expression. Our data also show that PTEN expression in the MDA-MB-231 and HS-578 TNBC cell lines, which have high levels of cell surface PD-L1, suggesting that there are other mechanisms of PD-L1 regulation in addition to PTEN.
Figure 2. PD-L1 and PTEN expression in cultured breast cancer cell lines.
A panel of breast cancer cell lines was evaluated for cell-surface PD-L1 expression by flow cytometry (MFI mean ± SD) and for PTEN expression by western blot analysis. Actin was used as a loading control. Data shows higher PD-L1 expression in four of the five TNBC cell lines evaluated in comparison with non-TNBC cell lines. MFI, median fluorescence intensity; ER, estrogen receptor; PR, progesterone receptor; PTEN, phosphatase and tensin homolog
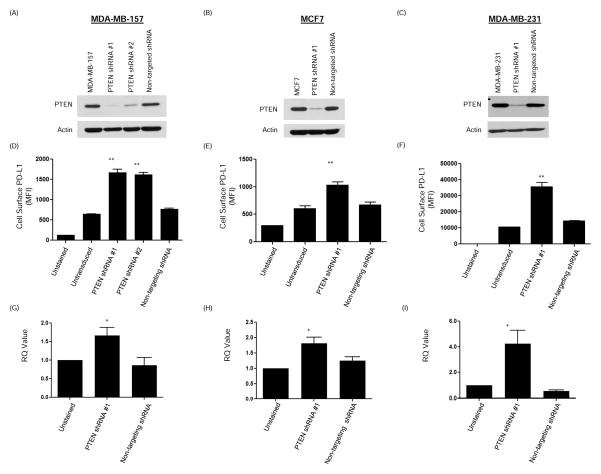
We investigated if PTEN regulates PD-L1 expression. The MDA-MB-157 cell line, which expresses PTEN and low levels of cell surface PD-L1, was transduced with two different PTEN lentiviral shRNA vectors to generate stable PTEN knockdown clones. Cell surface PD-L1 expression was assessed using flow cytometry. As shown (Fig. 3A), loss of PTEN led to a significant increase in PD-L1 cell surface expression (P<0.0001). Results were confirmed in the MCF-7 cell line (Fig. 3B), which also expresses PTEN and low levels of cell surface PD-L1. In addition, we showed that PTEN knockdown could further increase PD-L1 expression in the MDA-MB-231 cell line that expresses PTEN and high levels of cell surface PD-L1 (Fig. 3C). Interestingly, PTEN knockdown in the MDA-MB-231 cell line resulted in a greater increase in PD-L1 expression than the addition of IFNγ, which is known to enhance PD-L1 expression (Supplementary figure 1) (11). For all three cell lines, RNAs were extracted and qRT-PCR demonstrated a significant increase in the PD-L1 mRNA transcripts, suggesting that the regulation of PD-L1 by PTEN may be in part at the level of transcription (Fig. 3D–F).
Figure 3. PTEN downregulation increases PD-L1 cell surface expression.
MDA-MB-157, MCF7 and MDA-MB-231 breast cancer cell lines were transduced with PTEN shRNA lentiviral transduction particles. (A–C) Western blot analysis performed on lysates obtained from these cells confirmed decreased PTEN expression. Non-targeting lentiviral transduction particles were used as a control. Actin was used to show equal loading. (D–F) Cell surface PD-L1 expression was evaluated by flow cytometry staining and PD-L1 MFI (mean ± SD) demonstrated a significant increase in PD-L1 expression on cells, correlating with a decrease in PTEN expression. Decreased PTEN expression in MDA-MB-231 cell line, which has the highest baseline cell surface expression of PD-L1, resulted in a significant further increase in PD-L1 expression. (G–I) RNA was extracted from MDA-MB-157, MCF7 and MDA-MB-231 cells that were transduced with PTEN or non-targeting shRNA. Mean ± SD RQ values obtained from qRT-PCR demonstrated a significant increase in the PD-L1 transcript that coincided with decreased PTEN expression. All assays were performed in triplicate. ANOVA test was performed using Prism 5.0 software (*P<0.05); (**P<0.0001) MFI = median fluorescence intensity.
Effects of Inhibiting the PI3K Pathway on PD-L1 expression
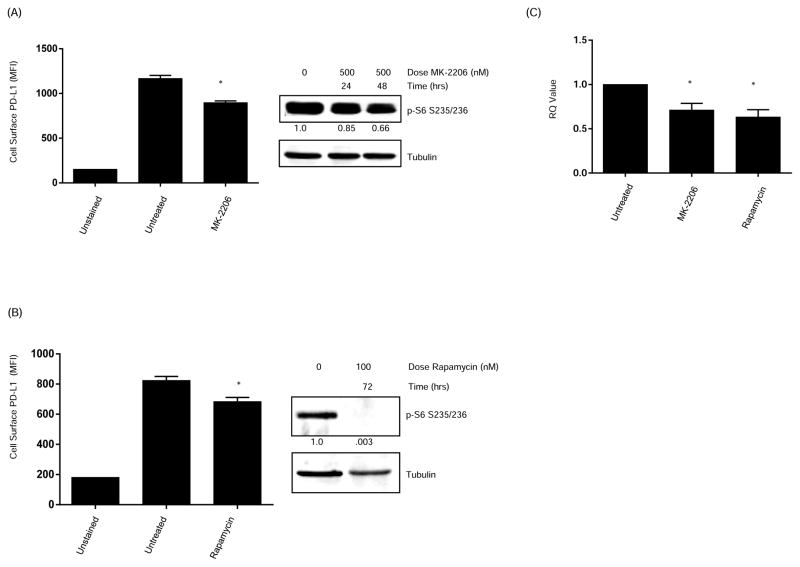
Cancer cells lacking PTEN have increased levels of PI3K activity. To determine if PTEN regulation of PD-L1 in TNBC is mediated by PI3K signaling, we treated MDA-MB-468 cells with either the Akt inhibitor MK-2206 or the mTOR inhibitor rapamycin. MDA-MB-468 was chosen because it has been rendered PTEN-deficient by a deletion mutation at codon 70, resulting in increased PI3K signaling as evidenced by higher levels of basal AKT phosphorylation (22). As shown in figures 4A and B, treatment with either agent resulted in a significant decrease in PD-L1 cell surface expression.
Figure 4. Inhibition of the Phosphatidylinositol 3-kinase pathway increases PD-L1 expression.
(A) MDA-MB-468 breast cancer cells were treated for 48 hours with the AKT inhibitor MK-2206 (500 nM) followed by PD-L1 cell surface expression assessed by flow cytometry. (B) MDA-MB-468 breast cancer cells were treated with the mTOR inhibitor rapamycin (100 nM). After 72 hours, cells were harvested and PD-L1 cell surface expression was assessed by flow cytometry. The addition of the PI3K pathway inhibitors significantly decreased the levels of surface PD-L1 expression. Data represent PD-L1 MFI (mean ± SD). Western blot showing decreased expression of p-S6 confirmed pathway inhibition by MK-2206 and rapamycin. (C) RNA was extracted from additional MDA-MB-468 breast cancer cells treated with MK-2206 or rapamycin. qRT-PCR showed a decrease in PD-L1 mRNA expression after addition of AKT and mTOR inhibitors. Data shown is representative of three separate experiments. All experiments were performed in triplicate. Data represent RQ value (mean ± SD). ANOVA test was performed using Prism 5.0 software (*P<0.01). MFI, median fluorescence intensity.
Because our data generated using PTEN shRNA suggested that PTEN regulation of PD-L1 is transcriptional, we next investigated the effect of treatment with MK-2206 or rapamycin on PD-L1 transcript levels. As shown in figure 4C, there was a significant decrease in the PD-L1 mRNA transcripts after treatment with either agent when compared to untreated controls. These data provide additional evidence that PD-L1 regulation by PTEN is in part transcriptional via PI3K signaling.
Functional Effects of PTEN Loss and PD-L1 Upregulation
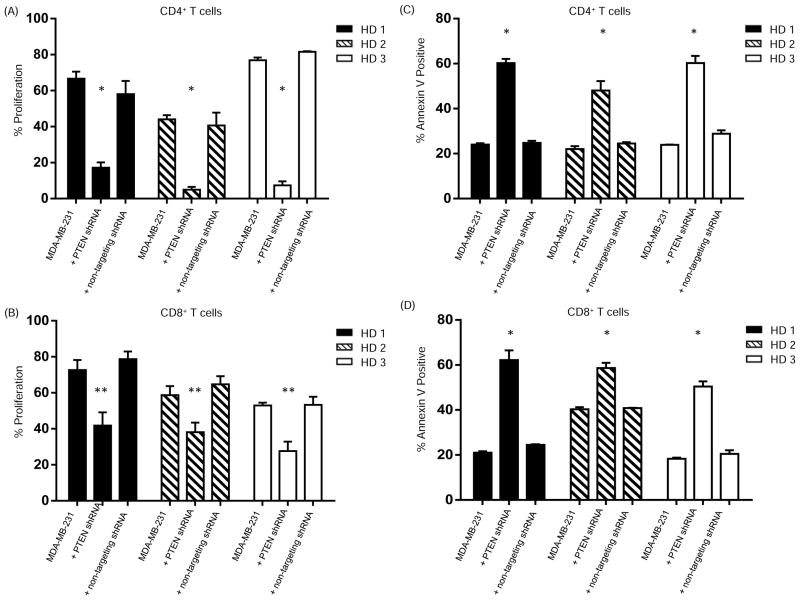
PD-L1 is the ligand for the T cell inhibitory receptor PD-1. Activation of the PD-1/PD-L1 pathway decreases T cell proliferation, survival and cytokine production (11, 23, 24). To address the functional consequences of increased PD-L1 cell surface expression that is mediated by PTEN knockdown, we first measured the proliferation of activated CD4+ and CD8+ T cells isolated from healthy donor PBMC and co-cultured with 1) MDA-MB-231 breast cancer cells transduced with a PTEN shRNA lentiviral vector, 2) parental cells or 3) cells transduced with a control shRNA lentiviral vector. In experiments using PBMCs from three separate healthy donors, co-culturing activated T cells with breast cancer cells with increased PD-L1 cell surface expression induced by PTEN-silencing resulted in a significant decrease in CD4+ T cell proliferation compared to PBMC co-cultured with parental cells (P<0.0001)(Fig. 5A). Similarly, there was a significant decrease in CD8+ T cell proliferation (P<0.005) (Fig. 5B). Similar results were seen using bulk PBMC from MDA-MB-231 (Supplementary figure 2) and MDA-MB-157 cells (Supplementary figure 3). Annexin V assays showed increased apoptosis of both CD4+ (Fig. 5C) and CD8+ (Fig. 5D) T cells that were enriched from healthy donor PBMC as well as CD4+ and CD8+ T cells in bulk PBMC (Supplementary figure 4) co-cultured with PTEN-silenced breast cancer cells. These data confirm a functional effect of PTEN loss that is mediated by increased PD-L1 expression.
Figure 5. Increased PD-L1 cell surface expression following PTEN knockdown inhibits T cell proliferation and increases apoptosis.
To determine the effect of the increased PD-L1 cell surface expression following PTEN knockdown on T cell proliferation, CD4+ (A) or CD8+ (B) T cells were isolated from peripheral blood mononuclear cells (PBMC) from healthy donors, were labeled with carboxyfluorescein succinimidyl ester (CFSE) and co-cultured with MDA-MB-231 breast cancer cells that were transduced with PTEN shRNA (i.e. increase surface PD-L1) or negative control groups including parental MDA-MB-231 cells or MDA-MB-231 cells transduced with non-targeting shRNA.. After stimulation with anti-CD3/CD28, proliferation was measured using flow cytometry. The experiment was performed three times in triplicate and the average percent proliferation ± SD for each experiment is shown. CD4+ (A) or CD8+ (B) T cells were also cultured alone (unstimulated, negative control) or stimulated with anti-CD3-CD28 in the absence of MDA-MB-231 cells (positive control). To determine the effect of increased PD-L1 cell surface expression on apoptosis, standard annexin V assays were performed. Anti-CD3/CD28-stimulated CD4+ (C) or CD8+ (D) T cells were co-cultured with breast cancer cells for 20 hours and then resuspended in Annexin binding buffer. Analysis was performed using flow cytometry. The experiment was repeated three times in triplicate and the average percent Annexin V positive CD4+ (D) and CD8+ (E) T cells ± SD for each experiment is shown. Significance was determined using an ANOVA test (**P<0.005) (*P<0.0001).
Discussion
Evading antitumor immunity is a hallmark for the development and progression of cancer (25). Tumors employ multiple mechanisms to avoid recognition by the host immune system, including expression of the negative T cell regulatory molecule PD-L1 (11). In this report, we identified PD-L1 expression in approximately 20% of TNBC. We also showed that PTEN loss is one mechanism regulating PD-L1 at the transcriptional level and that this effect occurs via signaling through the PI3K pathway. Importantly, increased PD-L1 expression on the surface of TNBC cells had functional consequences on T cells including decreasing their proliferation and increasing apoptosis. These observations provide the rationale for implementing therapeutic strategies targeting the PD-1/PD-L1 axis in TNBC and suggest a role for enhanced antitumor immunity when targeting the PI3K pathway in TNBC.
PD-L1 is not expressed on normal epithelial tissues but is expressed on many cancers including renal cell carcinoma (20, 26), pancreatic cancer (27), ovarian cancer (28), gastric cancer (29), esophageal cancer (30) and hepatocellular carcinoma (31). PD-L1 expression in breast cancer has previously been investigated by Ghebeh et al. who identified PD-L1 expression in 22 (50%) of 44 tumors evaluated; in 15 (34%) it was restricted to the tumor epithelium while in 18 (41%) it was identified in tumor-infiltrating lymphocytes (12). Furthermore, they found that intratumoral expression of PD-L1 was associated with high histologic grade and negative hormone receptor status. Consistent with the previous study, we found that approximately 20% of TNBC tumors express PD-L1. The majority (95%) of these TNBC tumors were grade 3.
The difference in the rates of PD-L1 expression in the current study (20%) and the study by Ghebeh et al. (34%) may be attributable to several factors. One important difference is that in our study, PD-L1 expression was assessed on tissue microarrays. Importantly, this allowed us to evaluate a greater number of tumors. However, there are limitations to using a TMA. One limitation that is relevant for the current study is that multiple cores were dislodged during the PTEN and CD8 staining therefore we only had PTEN data available for 17 of 20 PD-L1+ tumors and CD8+ T cell data on 82 specimens. A second limitation of using a TMA is the small size (1mm) of cores used to construct the TMA. However, because of the small size, and because tumor tissues are known to be heterogeneous, the rate of PD-L1 positivity identified in our study may be an underestimation of the true frequency of PD-L1 expression. Nonetheless, by showing PD-L1 expression in 20% of TNBC, we have provided a rationale for investigating PD-L1/PD-1 targeting therapies in TNBC, which is known to have few therapeutic options. The recently reported phase I study evaluating the anti-PD-1 antibody BMS-936588 included data from a nonrandom subset of patients enrolled on the trial in whom a pretreatment biopsy was available to assess PD-L1 expression (14). Although this analysis included pre-treatment biopsies from only 42 of 296 patients, investigators did make the observation that an objective response occurred in 9 (36%) of 25 patients with PD-L1-positive tumors. In contrast, none of the 17 patients with PD-L1-negative tumors responded. The investigators urge caution in interpreting these data due to the small sample size; nevertheless their findings suggest that PD-L1 expression in pre-treatment tumor samples predicts a greater likelihood of objective response. Future trials should incorporate biopsies to further test this hypothesis.
An interesting finding in our study was that in the 82 TNBC patients in whom staining was available, there was a significantly greater number of intratumoral CD8+ T cells in tumors that were PD-L1+ when compared to PD-L1− tumors. This is in contrast to a recent study of 45 oral squamous cell carcinoma cases in which 39 cases had PD-L1 expression which was associated with low peritumoral CD8+ T cell infiltrate (32). Our results however are consistent with a recent report from Taube et al, who identified a strong association between the expression of PD-L1 in melanoma and tumor-infiltrating lymphocytes (TILs) (33). In that study, 98% of PD-L1+ tumors had associated TIL versus 28% of PD-L1− tumors. Furthermore, IFNγ expression was detected at the interface of PD-L1-positive tumor cells and CD45-positive infiltrating immune cells. The results are also consistent with a study from Spranger et al. that showed CD8+ T cells in metastatic melanoma foci that also contained high levels of regulatory T cells and expressed the immunosuppressive factors indoleamine-2,3-dioxygenase (IDO) and PD-L1. Mouse models from that study further suggested that PD-L1 upregulation was dependent on IFNγ (34). Other studies evaluating PD-L1 regulation on antigen presenting cells have shown that interleukin (IL)-6, IL-10 and the common gamma-chain cytokines IL-2, IL-7, and IL-15 upregulate PD-L1 expression on tumor cells, suggesting that multiple factors present in the tumor microenvironment in addition to IFNγ may promote increased PD-L1 expression by tumors (35, 36). These data suggest a positive feedback loop whereby inflammatory factors produced by immune cells in the tumor microenvironment cause tumor cells to increase cell surface expression of PD-L1 (37), a possible mechanism whereby tumor cells evade the adaptive immune response.
A second mechanism by which tumors can drive PD-L1 expression is by oncogenic signaling pathways. This was first demonstrated in glioblastomas when Parsa et al. showed that PTEN loss was associated with increased PD-L1 expression, suggesting the involvement of the PI3K pathway (15). Because PTEN loss is commonly seen in TNBC, we investigated the relationship between PTEN and PD-L1 expression. In approximately 50% of TNBC tumors included in our TMA in which there was >5% PD-L1 expression, we identified a loss of PTEN staining. Similarly, in a panel of TNBC cell lines, we found that two cell lines with PTEN loss, MDA-MB-468 and BT-549, had high cell surface PD-L1 expression. Together these data suggest that there are likely multiple mechanisms of PD-L1 regulation in TNBC.
Using PTEN shRNA transduction particles, we generated stable cell lines and consistently found significantly increased PD-L1 expression after PTEN-silencing. In addition, in the MDA-MB-468 cell line, which has PTEN loss and increased PI3K signaling, treatment with the AKT inhibitor MK-2206 and the mTOR inhibitor rapamycin resulted in decreased cell surface PD-L1 expression, confirming a role for PTEN loss and PI3K signaling in PD-L1 regulation. Our results confirm findings from Crane et al, who reported increased PD-L1 protein using western blots of whole cell lysates in two PTEN mutant breast cancer cell lines when compared to two PTEN wild-type cell lines (38). Furthermore, their data demonstrated decreased PD-L1 in the BT549 cell line following treatment with multiple inhibitors of the PI3K pathway. However, in contrast to the findings by Crane et al showing regulation of PD-L1 by PTEN/PI3K signaling at the translational level, our data suggest transcriptional regulation of PD-L1 by PTEN/PI3K pathway. This discrepancy in our data and the previous report, along with our data showing high PD-L1 expression in two TNBC cell lines (MDA-MB-231 and HS-578T) with wild-type PTEN highlight that multiple mechanisms may be involved in PD-L1 regulation in tumors. These mechanisms may be influenced by the tumor type and other oncogenic signaling pathways that are activated by the tumor cell. Ongoing work in our laboratory is investigating the mechanisms by which PTEN loss and increased PI3K signaling regulate PD-L1 expression.
In conclusion, we have shown that approximately 20% of TNBC express PD-L1, suggesting a potential role for anti-PD-L1/anti-PD1 therapy in this patient population. Furthermore, we show that PTEN loss upregulates PD-L1 expression, indicating that therapeutic strategies targeting the PI3K pathway may enhance adaptive immune responses against TNBC. Additional investigations are required to fully elucidate the mechanisms regulating PD-L1 expression in TNBC and to determine the extent of regulation by PTEN/PI3K pathway.
Supplementary Material
Acknowledgments
Grant Support
This work was supported in part by grant R00CA133244 (EAM) and The State of Texas Grant for Rare and Aggressive Cancer through the Morgan Welch Inflammatory Breast Cancer Research Program (EAM) a Stand Up To Cancer Dream Team Translational Cancer Research Grant, a Program of the Entertainment Industry Foundation (SU2C-AACR-DT0209 01, FMB, AA, and AMGA), Susan G. Komen for the Cure SAC10006 (FMB), and NCRR Grant 3UL1RR024148 (FMB, XS, YW). The Flow Cytometry and Cellular Imaging core and Characterized Cell Line core are supported by Cancer Center Support Grant NCI #P30CA16672.
Footnotes
Conflict of Interest: The authors declare no conflict of interest.
Disclosure of Potential Conflicts of Interest
The authors report no potential conflicts of interest.
References
- 1.Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13:4429–34. doi: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- 2.Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011;121:2750–67. doi: 10.1172/JCI45014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haffty BG, Yang Q, Reiss M, Kearney T, Higgins SA, Weidhaas J, et al. Locoregional relapse and distant metastasis in conservatively managed triple negative early-stage breast cancer. J Clin Oncol. 2006;24:5652–7. doi: 10.1200/JCO.2006.06.5664. [DOI] [PubMed] [Google Scholar]
- 4.Liedtke C, Mazouni C, Hess KR, Andre F, Tordai A, Mejia JA, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol. 2008;26:1275–81. doi: 10.1200/JCO.2007.14.4147. [DOI] [PubMed] [Google Scholar]
- 5.Desmedt C, Haibe-Kains B, Wirapati P, Buyse M, Larsimont D, Bontempi G, et al. Biological processes associated with breast cancer clinical outcome depend on the molecular subtypes. Clin Cancer Res. 2008;14:5158–65. doi: 10.1158/1078-0432.CCR-07-4756. [DOI] [PubMed] [Google Scholar]
- 6.Loi S, Sirtaine N, Piette F, Salgado R, Viale G, Van Eenoo F, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J Clin Oncol. 2013;31:860–7. doi: 10.1200/JCO.2011.41.0902. [DOI] [PubMed] [Google Scholar]
- 7.Liu S, Lachapelle J, Leung S, Gao D, Foulkes WD, Nielsen TO. CD8+ lymphocyte infiltration is an independent favorable prognostic indicator in basal-like breast cancer. Breast Cancer Res. 2012;14:R48. doi: 10.1186/bcr3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brunet JF, Denizot F, Luciani MF, Roux-Dosseto M, Suzan M, Mattei MG, et al. A new member of the immunoglobulin superfamily--CTLA-4. Nature. 1987;328:267–70. doi: 10.1038/328267a0. [DOI] [PubMed] [Google Scholar]
- 9.Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med. 1995;182:459–65. doi: 10.1084/jem.182.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11:3887–95. doi: 10.1002/j.1460-2075.1992.tb05481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 12.Ghebeh H, Mohammed S, Al-Omair A, Qattan A, Lehe C, Al-Qudaihi G, et al. The B7-H1 (PD-L1) T lymphocyte-inhibitory molecule is expressed in breast cancer patients with infiltrating ductal carcinoma: correlation with important high-risk prognostic factors. Neoplasia. 2006;8:190–8. doi: 10.1593/neo.05733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–65. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parsa AT, Waldron JS, Panner A, Crane CA, Parney IF, Barry JJ, et al. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat Med. 2007;13:84–8. doi: 10.1038/nm1517. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez-Angulo AM, Ferrer-Lozano J, Stemke-Hale K, Sahin A, Liu S, Barrera JA, et al. PI3K pathway mutations and PTEN levels in primary and metastatic breast cancer. Mol Cancer Ther. 2011;10:1093–101. doi: 10.1158/1535-7163.MCT-10-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saal LH, Holm K, Maurer M, Memeo L, Su T, Wang X, et al. PIK3CA mutations correlate with hormone receptors, node metastasis, and ERBB2, and are mutually exclusive with PTEN loss in human breast carcinoma. Cancer Res. 2005;65:2554–9. doi: 10.1158/0008-5472-CAN-04-3913. [DOI] [PubMed] [Google Scholar]
- 18.Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5:1365–9. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- 20.Thompson RH, Kuntz SM, Leibovich BC, Dong H, Lohse CM, Webster WS, et al. Tumor B7-H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res. 2006;66:3381–5. doi: 10.1158/0008-5472.CAN-05-4303. [DOI] [PubMed] [Google Scholar]
- 21.Mittendorf EA, Alatrash G, Qiao N, Wu Y, Sukhumalchandra P, St John LS, et al. Breast cancer cell uptake of the inflammatory mediator neutrophil elastase triggers an anticancer adaptive immune response. Cancer Res. 2012;72:3153–62. doi: 10.1158/0008-5472.CAN-11-4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu Y, Lin YZ, LaPushin R, Cuevas B, Fang X, Yu SX, et al. The PTEN/MMAC1/TEP tumor suppressor gene decreases cell growth and induces apoptosis and anoikis in breast cancer cells. Oncogene. 1999;18:7034–45. doi: 10.1038/sj.onc.1203183. [DOI] [PubMed] [Google Scholar]
- 23.Mazanet MM, Hughes CC. B7-H1 is expressed by human endothelial cells and suppresses T cell cytokine synthesis. J Immunol. 2002;169:3581–8. doi: 10.4049/jimmunol.169.7.3581. [DOI] [PubMed] [Google Scholar]
- 24.Patsoukis N, Sari D, Boussiotis VA. PD-1 inhibits T cell proliferation by upregulating p27 and p15 and suppressing Cdc25A. Cell Cycle. 2012;11:4305–9. doi: 10.4161/cc.22135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 26.Thompson RH, Dong H, Kwon ED. Implications of B7-H1 expression in clear cell carcinoma of the kidney for prognostication and therapy. Clin Cancer Res. 2007;13:709s–15s. doi: 10.1158/1078-0432.CCR-06-1868. [DOI] [PubMed] [Google Scholar]
- 27.Nomi T, Sho M, Akahori T, Hamada K, Kubo A, Kanehiro H, et al. Clinical significance and therapeutic potential of the programmed death-1 ligand/programmed death-1 pathway in human pancreatic cancer. Clin Cancer Res. 2007;13:2151–7. doi: 10.1158/1078-0432.CCR-06-2746. [DOI] [PubMed] [Google Scholar]
- 28.Hamanishi J, Mandai M, Iwasaki M, Okazaki T, Tanaka Y, Yamaguchi K, et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci U S A. 2007;104:3360–5. doi: 10.1073/pnas.0611533104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu C, Zhu Y, Jiang J, Zhao J, Zhang XG, Xu N. Immunohistochemical localization of programmed death-1 ligand-1 (PD-L1) in gastric carcinoma and its clinical significance. Acta Histochem. 2006;108:19–24. doi: 10.1016/j.acthis.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 30.Ohigashi Y, Sho M, Yamada Y, Tsurui Y, Hamada K, Ikeda N, et al. Clinical significance of programmed death-1 ligand-1 and programmed death-1 ligand-2 expression in human esophageal cancer. Clin Cancer Res. 2005;11:2947–53. doi: 10.1158/1078-0432.CCR-04-1469. [DOI] [PubMed] [Google Scholar]
- 31.Gao Q, Wang XY, Qiu SJ, Yamato I, Sho M, Nakajima Y, et al. Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin Cancer Res. 2009;15:971–9. doi: 10.1158/1078-0432.CCR-08-1608. [DOI] [PubMed] [Google Scholar]
- 32.Cho YA, Yoon HJ, Lee JI, Hong SP, Hong SD. Relationship between the expressions of PD-L1 and tumor-infiltrating lymphocytes in oral squamous cell carcinoma. Oral Oncol. 2011;47:1148–53. doi: 10.1016/j.oraloncology.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 33.Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med. 2012;4:127ra37. doi: 10.1126/scitranslmed.3003689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spranger S, Spaapen RM, Zha Y, Williams J, Meng Y, Ha TT, et al. Up-regulation of PD-L1, IDO, and T(regs) in the melanoma tumor microenvironment is driven by CD8(+) T cells. Sci Transl Med. 2013;5:200ra116. doi: 10.1126/scitranslmed.3006504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kinter AL, Godbout EJ, McNally JP, Sereti I, Roby GA, O’Shea MA, et al. The common gamma-chain cytokines IL-2, IL-7, IL-15, and IL-21 induce the expression of programmed death-1 and its ligands. J Immunol. 2008;181:6738–46. doi: 10.4049/jimmunol.181.10.6738. [DOI] [PubMed] [Google Scholar]
- 36.Wolfle SJ, Strebovsky J, Bartz H, Sahr A, Arnold C, Kaiser C, et al. PD-L1 expression on tolerogenic APCs is controlled by STAT-3. Eur J Immunol. 2011;41:413–24. doi: 10.1002/eji.201040979. [DOI] [PubMed] [Google Scholar]
- 37.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–64. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crane CA, Panner A, Murray JC, Wilson SP, Xu H, Chen L, et al. PI(3) kinase is associated with a mechanism of immunoresistance in breast and prostate cancer. Oncogene. 2009;28:306–12. doi: 10.1038/onc.2008.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.