Abstract
Previous research has shown that healthy individuals who fail to differentiate among emotional states (i.e., those with low emotional granularity; EG) have poorer social functioning (SF) than those with high EG. It is unknown, however, whether these associations extend to clinical disorders characterized by impaired SF, such as schizophrenia. In the present study, we compared SF and EG in individuals with schizophrenia and healthy controls, and then, within the schizophrenia group, we examined the links between EG and SF. Employing an Experience Sampling Method approach, 77 individuals with schizophrenia and 27 healthy controls rated their momentary emotions (sadness, anxiety, anger, and happiness) up to 10 times/day over a two-day period using mobile electronic devices. For each participant, we then calculated the within-subject average correlations among the momentary emotion ratings, producing two EG indices – EGIall for all emotions and EGIneg for negative ones. A subsample of participants with schizophrenia also completed self-report, interview, and ability-based measures of SF. Compared to healthy controls, individuals with schizophrenia displayed significantly poorer SF and lower EGIall, but comparable EGIneg. Within the schizophrenia group, hierarchical multiple regression analyses indicated that EGIall, but not EGIneg, significantly predicted social dysfunction after controlling for emotional awareness, symptoms, and emotional intensity and variability. Our findings indicate that individuals with schizophrenia have a relatively intact ability to differentiate among negative emotions in everyday life. However, they experience significant difficulties differentiating between positive and negative emotions, and this may contribute to their social difficulties.
1. INTRODUCTION
Emotions have long been thought to play a crucial role in guiding social interactions. For example, Ekman defined the primary function of emotions as mobilizing the individual “to deal quickly with important interpersonal encounters” (p.171; Ekman, 1992). Emotions are said to do this by providing information about the significance of various social situations (Frijda & Mesquita, 1994; Gross, 2002; Keltner & Haidt, 1999), as well as by guiding potential actions to address such situations (Barrett et al., 2001a; Campos et al., 1989). For emotions to be maximally useful in a social context, they must be represented in a differentiated fashion, not just to others, but also to the self. However, individuals differ considerably in their experience of emotions. Some individuals experience emotions in a highly differentiated manner, making clear distinctions between discrete emotions (i.e., “sad” is distinguished from “anxious”). In contrast, others experience emotions in a relatively undifferentiated manner, making interchangeable use of similarly-valenced emotion labels (i.e., all negatively-valenced states are represented as “I feel bad”). Such individuals tend to characterize their emotions more globally based on the overall pleasantness or unpleasantness of their experience (Barrett et al., 2001a, 2001b; Carstensen et al., 1999, 2000; Lane & Schwartz, 1987; Lane et al., 1990, 1996; Larsen et al., 1996).
The degree to which individuals differentiate among emotional states and represent them with precision and specificity has been termed Emotional Granularity (EG; Barrett, 2004), where high granularity represents description of emotional experiences with a high precision, whereas low granularity represents description of emotional experiences in more global terms. Because emotional experiences convey important information to an individual, EG plays an important role in shaping how social interactions unfold (Barrett et al., 2001a, 2001b; Clore & Tamir, 2002). Specifically, reduced EG may make it difficult for individuals to select appropriate response strategies for dealing with the social situation, potentially resulting in social dysfunction (Barrett et al., 2001a). Thus, successful adaptation to ever-changing social environments is contingent upon having clear representations of one’s own emotional state. Among healthy individuals, a number of studies support the association between EG and social functioning (SF). In one experience-sampling study, greater EG was associated with larger repertoires of emotion-regulation strategies (Barrett et al., 2001b), which is suggestive of enhanced SF. In another study, the precision with which individuals represented their emotional experiences was linked to the management of emotions during stressful situations (Barrett et al., 2001a). Taken together, these findings suggest a link between higher EG and better SF, at least in non-clinical populations.
One important application of this line of reasoning regarding EG and SF is to clinical disorders that are characterized by difficulties with SF. Individuals with schizophrenia display substantial difficulties processing emotions (Blanchard et al., 1998; Hooker & Park, 2002; Kee et al., 2003; Kring & Caponigro, 2010), as well as navigating social situations as manifested by difficulties in multiple domains including impaired social skills (Patterson et al., 2001), infrequent achievement of social milestones (Harvey et al., 2009), and smaller social networks (Patterson et al., 1997). Such impairments show no evidence of improvement over time (Green et al., 2012) and often result in employment difficulties and disability (Harvey et al., 2012), thus representing a serious public health concern. Despite its potential relevance, the link between EG and social dysfunction in schizophrenia has not been examined.
Thus, the goals of the present study were: 1) To confirm previously reported differences in SF between individuals with schizophrenia and healthy controls; 2) To compare EG in individuals with schizophrenia and healthy controls; and 3) To examine the links between EG and SF in the schizophrenia group and assess whether EG would predict SF even when previously identified predictors and confounding factors were taken into account. We hypothesize that individuals with schizophrenia would display significantly poorer SF and lower EG compared to healthy controls. We further hypothesized that among individuals with schizophrenia, higher EG would predict better SF.
2. METHOD
2.1. Participants
Seventy-seven individuals with schizophrenia and 26 healthy controls were recruited at the New York State Psychiatric Institute (NYSPI) at Columbia University Medical Center. Individuals with schizophrenia were recruited from patients treated at the NYSPI. Healthy participants were recruited via advertisements. Inclusion and exclusion criteria for all participants included: age 18–55, English speaking, and no diagnosis of mental retardation. For the schizophrenia group, the inclusion criteria also included a DSM-IV diagnosis of schizophrenia spectrum disorder. For the control group, exclusion criteria included a history of psychosis, a diagnosis of any DSM-IV Axis-II Cluster A personality disorder, a first degree relative with history of psychosis, and being adopted. The DSM-IV diagnoses in the schizophrenia group were: 59 Schizophrenia, 11 Schizoaffective Disorder, 1 Delusional Disorder, and 6 Psychosis NOS. The study was approved by the NYSPI’s Institutional Review Board and all subjects provided written informed consent.
2.2. Procedure
After signing the informed consent form, participants typically completed the experience sampling protocol and clinical research assessments during weekdays within 2–4 weeks of study entry. The diagnoses and neurocognitive functioning assessment were typically completed within 1–2 months from study entry. Diagnoses were determined using the Diagnostic Interview for Genetic Studies (DIGS).
2.3. Primary Measures
Emotional Granularity was assessed over a two-day period using Experience Sampling Method (ESM) with mobile devices (Kimhy & Corcoran, 2008; Kimhy et al., 2006, 2010). As the data were originally collected as part of studies assessing concurrent ambulatory autonomic regulation, a relatively shorter assessment period was scheduled (vs. the typical 6 days ESM assessment). Participants were provided with a Palm Tungsten T3 computer with the iESP software to present questions and collect responses. The devices were preprogrammed to beep randomly 10 times/day (10am→10pm) to elicit 20 experience samples over a two-day period. Upon hearing the beeps, participants were instructed to complete a brief questionnaire, which also included four questions about current emotions - sadness, anger, anxiety, and happiness. For each question, participants were asked to rate their current experience using a graphical slider. Responses were represented in the output as a value between 1 (“not at all”) and 100 (“very much”). To determine EG, we calculated for each participant two Emotional Granularity Indices (EGI):
The overall EGI was determined by calculating for each participant the six correlations among the four ESM-rated emotions (sadness, anger, anxiety, and happiness), averaging the six correlations, and multiplying the outcome by −100. The second index for negative emotions, EGIneg, was determined by calculating for each subject the three correlations among the three ESM-rated negative emotions (sadness, anger, anxiety), averaging them, and multiplying the outcome by −100. The indices had a range from −100 to 100, with higher EG ratings reflecting lower average correlations between emotions within a participant. All ESM within-subject correlations of emotional states where transformed using Fisher’s r-to-z transformation before the EG indices were calculated.
Social Functioning was assessed using three measures reflecting distinct assessment methods – self-report, interview-based, and an ability task:
Self-Report: The Provision of Social Relations Scale (PSRS; Turner et al., 1983) is a 15-item scale measuring quality of social relationships with family and friends. Items are scored on a 5-point scale, with higher scores indicating lower quality of social relationships. The PSRS was found to have alpha coefficients of .85, .80, and .78 among individuals with schizophrenia, bipolar disorder and healthy controls, respectively (Horan et al., 2007) as well as good test-retest reliability (.75–.87; Huprich et al., 2002).
Interview: Item #20 (“Ability to Feel Intimacy and Closeness”) from The Scale for Assessment of Negative Symptoms (SANS; Andreasen, 1982) assesses the ability to form close or intimate relationships, especially with opposite sex and family. This item was administered as part of a standard SANS administration. Responses are rated by the interviewer from 0 (no difficulties) to 5 (severe).
Ability Task: The Social Management Task of the Mayer-Salovey-Caruso Emotional Intelligence Test (MSCEIT, Section H; Nuechterlein & Green, 2006) is an ability task in which the respondent rates the effectiveness of potential actions in managing various social situations depicted in short vignettes. Respondents are asked to indicate the effectiveness of each action. The MSCEIT has been shown to be a reliable and valid measure of social management in schizophrenia (r=.73; ICC=.73).
2.4. Control Measures
We included in our analyses a number control measures including previously identified predictors along with potential confounding factors. Our group has previously identified emotion awareness as a strong predictor of SF in individuals with schizophrenia (Kimhy et al., 2012b). We also controlled for the potential impact of emotion intensity and variability on EG (Demiralp et al., 2012), along with demographic, clinical and neurocognitive covariates.
Emotion Awareness was assessed using the Toronto Alexithymia Scale (TAS-20; Bagby et al., 1994), a 20-item self-report questionnaire, with higher score indicating poorer functioning. We used the Difficulty Identifying Feelings (DIF; 7 items) and Difficulty Describing Feelings (DDF; 5 items) subscales. Participants are asked to indicate on a 5-point scale to what extent they agreed with each statement. The total score TAS-20 has a good internal consistency (≥.80) with the DIF and DDF subscales demonstrating solid reliability (r=.79–.83).
Emotion Intensity was indexed by averaging the momentary emotion ratings for each of the four emotions across the entire sampling period. Then, we calculated the mean overall emotion and negative emotion intensity scores for each participant.
Emotion Variability was indexed by calculating the variance of the intensity of each emotion over the entire sampling period, again separately for overall and negative emotions.
Demographic, Clinical and Neurocognitive Covariates were collected including demographic variables, reading ability (Wechsler Test of Adult Reading; WTAR), depression (Beck Depression Inventory), Anxiety (Beck Anxiety Inventory), psychotic symptoms (Scale for Assessment of Positive symptoms; SAPS), and use of antipsychotic medication (as indexed by chlorpromazine equivalence). Neurocognition was assessed using The MATRICS Consensus Cognitive Battery (MCCB; Nuechterlein et al., 2006).
2.5. Data Analyses
Significance levels were set at p<.05. Consistent with previous ESM studies in schizophrenia, participants had to complete >30% of the experience samples for their data to be included in the analyses. For group comparisons, we first evaluated the SF and EG variables alone using two-tailed t-tests, followed by ANCOVAs controlling for covariates. Associations between SF and EG indices were examined first using Pearson correlations, followed by partial correlations controlling for covariates within the schizophrenia group. Finally, assessment of whether EG would predict SF was tested using hierarchical regression analyses, with the SF indices entered as dependent variables and EG variables as predictors. Next, we repeated the analyses to ascertain whether EG would predict SF above and beyond previously identified predictors and covariates. The SF indices were entered as dependent variables, previously identified predictors were entered in block 1, the covariates were entered in block 2, and the EG variables entered in block 3.
3. RESULTS
Demographic and clinical characteristics are presented in Table 1 for individuals with schizophrenia and healthy controls. There were no significant differences between the individuals with schizophrenia and healthy controls with regard to sex, race, ethnicity, marital status, reading ability or number of completed ESM responses. However, the schizophrenia group was significantly older. The number of ESM responses was not associated with EGIall or EGIneg. There were no significant differences between individuals with schizophrenia and healthy controls with regard to their emotional variability for all emotions and negative ones. However, individuals with schizophrenia reported significantly higher emotional intensity for overall and negative emotions (t=3.15, p=.002 and t=3.51, p=.001, respectively).
Table 1.
Demographic and Clinical Information
| Schizophrenia (n=77) | Healthy Controls (n=27) | t/X2/rho | p | |
|---|---|---|---|---|
|
| ||||
| Age | 32.51 (9.19) | 23.95 (5.01) | 5.35 | <.001 |
|
| ||||
| Sex (% female) | 32 (42%) | 17 (63%) | 2.98 | .08 |
|
| ||||
| Marital Status (% Never Married) | 86% | 89% | .21 | .90 |
|
| ||||
| Ethnicity (% Hispanic) | 21% | 18% | .00 | .97 |
|
| ||||
| Race: | .43 | |||
| Asian | 11 (14%) | 4 (15%) | ||
| Black/African-American | 11 (14%) | 3 (11%) | 3.82 | |
| White | 46 (60%) | 14 (52%) | ||
| More than one race | 9 (12%) | 6 (22%) | ||
|
| ||||
| Education: | .04 | |||
| Less than High School | 2 (3%) | 0 (0%) | ||
| Completed High School | 11 (14%) | 0 (0%) | 9.72 | |
| Some College | 26 (34%) | 5 (18%) | ||
| Completed College | 27 (35%) | 16 (59%) | ||
| Some Graduate School | 11 (14%) | 6 (22%) | ||
|
| ||||
| Reading Ability (WTAR Total Score) | 38.55 (9.53) | 41.62 (8.69) | 1.37 | .17 |
|
| ||||
| Number of ESM Responses | 15.83 (3.28) | 17.30 (2.89) | 1.62 | .12 |
| Schizophrenia | Mean | SD |
|---|---|---|
|
| ||
| Positive Symptoms (SAPS Global Ratings) | ||
| Hallucinations | 3.11 | 1.96 |
| Delusions | 3.17 | 1.37 |
| Bizarre Behavior | .65 | 1.08 |
| Positive Formal Thought Disorder | 1.15 | 1.32 |
|
| ||
| Negative Symptoms (SANS Global Ratings) | ||
| Affective Flattening | 2.18 | 1.27 |
| Alogia | 1.10 | 1.34 |
| Avolition-Apathy | 2.40 | 1.41 |
| Anhedonia-Asociality | 2.88 | 1.33 |
| Attention | 1.74 | 1.64 |
|
| ||
| Mood Symptoms (Total Scores) | ||
| Depression (BDI) | 14.71 | 10.92 |
| Anxiety (BAI) | 13.88 | 11.23 |
|
| ||
| Neurocognition (Standardized MCCB Domain Scores) | ||
| Speed of Processing | 32.86 | 11.63 |
| Attention/Vigilance | 43.65 | 11.20 |
| Working Memory | 40.67 | 10.16 |
| Verbal Learning | 38.57 | 9.51 |
| Visual Learning | 38.98 | 10.51 |
| Reasoning & Problem Solving | 36.77 | 10.57 |
| Social Cognition | 38.85 | 14.26 |
WTAR–Wechsler Test of Adult Reading; ESM – Experience Sampling Method; SAPS–Scale for Assessment of Positive Symptoms; SANS–Scale for Assessment of Negative Symptoms; BDI–Beck Depression Inventory; BAI–Beck Anxiety Inventory; MCCB–MATRICS Consensus Cognitive Battery.
3.1. Social Functioning - Comparison of Individuals with Schizophrenia and Healthy Controls
Our first aim was to confirm previously reported differences in SF between individuals with schizophrenia and healthy controls. Only a subsample (n=48) of participants with schizophrenia had data on all three social functioning measures. There were no significant differences between the individuals with schizophrenia with or without the social functioning data with regard to sex, race, ethnicity, marital status, reading ability or number of completed ESM responses. As hypothesized, individuals with schizophrenia displayed significantly lower SF than the healthy controls as assesses by all three indices: self-report, interview, and ability-based (see Table 2). These differences remained after controlling for age, and emotional intensity and variability. Calculations of Cohen’s d indicated large effect sizes for group differences.
Table 2.
Comparison of Emotional Granularity and Social Functioning between Individuals with Schizophrenia and Healthy Controls
| Schizophr. | Healthy Controls | t-test | ANCOVA | Cohen’s d | ||||
|---|---|---|---|---|---|---|---|---|
| t | p | F | p | |||||
|
| ||||||||
| Social Functioning | Provision of Social Relations Scale (PSRS) | 37.11 (12.34) | 22.00 (4.81) | 5.58 | <.001 | 28.19 | *<.001 | 1.61 |
|
| ||||||||
| Ability to Feel Intimacy & Closeness (SANS Item #20) | 2.29 (1.49) | .21 (0.05) | 6.68 | <.001 | 27.87 | *<.001 | 1.97 | |
|
| ||||||||
| Social Management Task (MSCEIT) | 89.23 (12.12) | 101.24 (13.28) | 3.34 | .001 | 6.78 | *.012 | .95 | |
|
| ||||||||
| Emotion Granularity | All Emotions (EGIall) | −5.39 (22.95) | 9.68 (14.90) | 3.18 | .002 | 5.39 | **.022 | .78 |
|
| ||||||||
| Negative Emotions (EGIneg) | −48.54 (49.75) | −36.81 (41.22) | 1.10 | .274 | .15 | **.696 | .26 | |
EGI–Emotional Granularity Index; PSRS–Provision of Social Relations Scale; SANS–Scale for Assessment of Negative Symptoms; MSCEIT– Mayer-Salovey-Caruso Emotional Intelligence Test;
controlling for age;
controlling for age, emotional intensity and variability.
3.2. Emotional Granularity - Comparison of Individuals with Schizophrenia and Healthy Controls
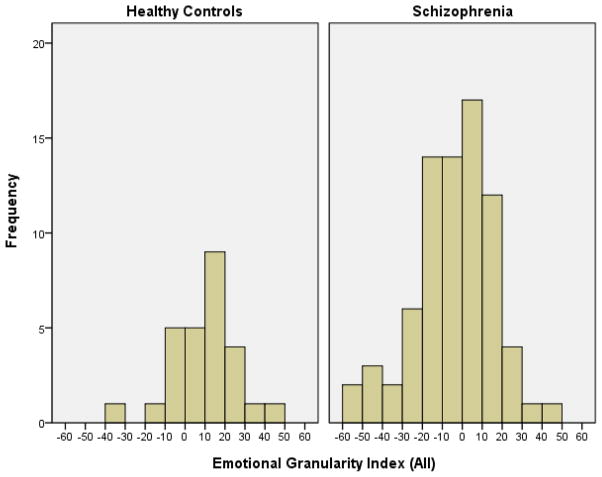
Our second aim was to evaluate whether individuals with schizophrenia differed from healthy individuals in their ability to differentiate among emotional states and represent them in a distinct manner, with higher EG ratings reflecting lower average correlations between emotions within a participant. The distribution of EGIall for individuals with schizophrenia and for healthy controls are presented in Figure 1. The Shapiro-Wilk test of normality indicate normal EGIall distribution among both the schizophrenia and healthy controls groups (W=.98, p=.30 and W=.97, p=.69, respectively). The results of the comparison are presented in Table 2 - consistent with our hypothesis, individuals with schizophrenia had significantly lower EGIall, with Cohen’s d calculation indicating a moderate to large effect size. These differences remained significant after controlling for age, emotion variability and intensity. There were no significant EGIneg group differences.
Figure 1.
Distribution of Emotional Granularity Index (all) Scores in People with Schizophrenia and Healthy Individuals
Note: N=104 (Healthy Controls=27, Schizophrenia=77)
3.3. Emotional Granularity and Social Functioning in Schizophrenia – Links to Social Functioning
Our third aim was to examine the links between EG and SF in the schizophrenia group and assess whether EG would predict SF in in this group even when previously identified predictors and confounding factors are taken into account. These associations were evaluated in a subsample of participants with schizophrenia (n=48) that completed SF ratings. There were no significant differences in EGIall and EGIneg scores between participants with and without SF ratings, as well as age, sex, race, ethnicity, marital status, reading ability or number of completed ESM responses. Consistent with our hypothesis, EGIall was significantly correlated with all three SF indices. These associations remained significant after controlling for delusions, emotional intensity and variability (see Table 3). However, contrary to our hypothesis, EGIneg was not associated with SF.
Table 3.
Zero-Order and Partial Correlations of Emotional Granularity and Social Functioning in Individuals with Schizophrenia
| Emotion Granularity - All Emotions (EGIall) | Emotion Granularity – Neg. Emotions (EGIneg) | Provision of Social Relations Scale (PSRS) | Ability to Feel Intimacy & Closeness (SANS Item #20) | Social Management Task (MSCEIT) | |
|---|---|---|---|---|---|
| Emotion Granularity – All Emotions (EGIall) | --- | **.37 | *** −.57 | *** −.50 | ** .44 |
| Emotion Granularity - Negative Emotions (EGIneg) | * .32 | --- | −.11 | −.15 | −.01 |
| Provision of Social Relations Scale (PSRS) | *** −.48 | −.13 | --- | *** .49 | ** −.45 |
| Ability to Feel Intimacy & Closeness (SANS Item #20) | ** −.49 | −.19 | ** .42 | --- | −.19 |
| Social Management Task (MSCEIT) | *.34 | −.10 | −.25 | −.05 | --- |
N=48;
<.05,
<.01,
<.001; Zero-order correlations are presented above the diagonal. Partial correlations are presented below the diagonal (controlling for delusions and emotional intensity & variability); EGI–Emotional Granularity Index; PSRS– Provision of Social Relations Scale; MSCEIT– Mayer-Salovey-Caruso Emotional Intelligence Test.
Next, we evaluated whether EG would predict SF in individuals with schizophrenia. As a first step, we conducted regression analyses with the SF indices entered as dependent variables and EG variables as predictors. For the self-report SF measure, the model accounted for 58% of the variance (F2,45=11.57, p<0.001), with EGIall contributing uniquely to the model’s validity (β=−.62, t=4.73, p<.001). Similarly, the models indexed by the interview and ability-based measures accounted for 50% and 48% of the SF variance (F2,37=6.22, p=0.005 and F2,35=5.23, p=0.010, respectively). EGIall contributed uniquely to both models’ variability (β=−.52, t=3.37, p=.002 and β=.52, t=3.23, p=.003, respectively). In contrast, in all three models EGIneg did not account for a significant proportion of the SF variance. We repeated the analyses to evaluate whether EG would predict SF above and beyond previously identified predictors and covariates. We first evaluated the associations of EG indices with potential covariates. EGIall and EGIneg were not associated with age, sex, antipsychotic dosage, reading ability, or neurocognitive functioning. EGIall, but not EGIneg, was significantly associated with depression (r=−.38, p=.008), anxiety (r=−.38, p=.007), and delusions (SAPS item #20; r=−.34, p=.020). Additionally, our group has previously demonstrated that emotion awareness difficulties are predictive of SF in schizophrenia (Kimhy et al., 2012b). Thus, we included these variables in the regression analyses, along with emotional intensity and variability (from the ESM data).
Three sets of hierarchical multiple regression analyses were conducted with self-report, interview, and ability-based measures of SF entered as the dependent variable. We entered difficulties identifying and describing emotions in block 1; covariates including depression, anxiety, and delusions along with emotional intensity and variability were entered in block 2; and the EG indices were entered in block 3. We elected not to include negative symptoms as predictors given such symptoms largely represent measures of emotion and social dysfunction, just with different labels (Hunter & Barry, 2012).
The first hierarchical multiple regression analysis indicated that the model accounted for 79% of the self-report SF variance. After controlling for emotion awareness, depression, anxiety, delusions, and emotional intensity and variability, EGIall accounted for 13% of the variance (F11,35=12.08, p<0.001), contributing uniquely to the model’s validity (β=−.46, t=4.60, p<.001). Likewise, the second hierarchical multiple regression analysis indicated that the model accounted for 46% of the interview-based measure of SF variance. After controlling for emotion awareness, depression, anxiety, delusions, and emotional intensity and variability, EGIall accounted for 23% of the variance (F9,30=2.85, p=0.015) and contributed uniquely to the model’s validity (β=−.58, t=3.38, p=0.002). Finally, the third hierarchical multiple regression analysis indicated that the model accounted for 50% of the ability-based measure of SF variance. After controlling for emotion awareness, depression, anxiety, delusions, and emotional intensity and variability, EGIall accounted for 9% of the variance (F9,28=3.12, p=0.010), contributing uniquely to the model’s validity (β=.38, t=2.20, p=0.036).
4. DISCUSSION
Our group has previously examined the impact of emotional awareness and regulation on social functioning in schizophrenia (Kimhy et al., 2012b). The present study extends these findings by examining EG - an aspect of emotion processing that has not been investigated previously in schizophrenia. Our findings indicate that compared to healthy individuals, people with schizophrenia display significantly more difficulties differentiating between positive and negative emotions, and such difficulties are associated with poorer SF. In contrast, their ability to differentiate between negative emotions appears to be relatively intact.
4.1. Emotional Granularity in Individuals with Schizophrenia
As hypothesized, individuals with schizophrenia displayed significantly lower EGall compared to the healthy individuals. These findings are consistent with a meta-analysis of laboratory studies that found that individuals with schizophrenia experience co-activation of positive and negative emotions characterized by increased negative emotions following exposure to positive and neutral stimulus (Cohen & Minor, 2010). Similar difficulties have been also reported among schizotypal individuals (Kwapil et al., 2000; 2002; Mann et al., 2008). Thus, our findings are particularly significant as they expand on previous laboratory studies by demonstrating that lower EGall is also experienced during the course of “real world” daily functioning. The co-activation of positive and negative emotions in schizophrenia may potentially reflect limbic dysfunction resulting in over-activation of negative emotion-related circuitry. Consistent with this view, individuals with schizophrenia show decreased amygdala volume compared to controls (Velakoulis et al., 2006), increased tonic activity in brain regions related to negative emotions (Taylor et al., 2002, 2005), as well as decreased amygdala activation in response to social stimuli (Kosaka et al., 2002). Alternatively, such co-activation may represent impairments in high-order structures known to be involved in inhibiting limbic activity (e.g., orbitofrontal brain regions; Taylor et al., 2002; Cohen et al., 2011).
While the schizophrenia literature has generally viewed the experience of simultaneous emotions as reflecting dysfunction, it may be argued that such experience may in fact represent a more sophisticated emotion processing (e.g., a parent feeling sad about their child departing to college while also feeling proud about their accomplishment). Supporting this view, Carstensen and colleagues (2011) used ESM ratings of emotional experiences in 553 healthy adults. They found that aging was associated with improved emotional well-being along with higher co-occurrence of positive and negative emotions, which they interpreted as reflecting greater emotional complexity. Also, consistent with this view, Lane and Schwartz developed the Levels of Emotional Awareness Scale (LEAS) that conceptualized emotional awareness as a phenomena ranging from no emotional awareness (lowest level), to responses describing undifferentiated emotions (i.e., “bad”), to use of well-differentiated single emotion (i.e., “sad”), to responses involving simultaneous experiences of distinct emotions (Lane et al., 1987). The LEAS consists of brief descriptions of twenty evocative interpersonal situations to which participants are asked “How would you feel?” However, Baslet et al. (2009) used the LEAS and found no difference in emotional awareness for self between individuals with schizophrenia and healthy controls.
The differences between our findings and Baslet and colleagues (2009) may reflect different assessment methods, with the LEAS asking participants to imagine their emotional response to hypothetical scenarios vs. our ESM-based results reflecting emotional responses to actual first-hand experiences. Alternatively, the conflicting perspectives may be reconciled by taking into consideration the individual’s clarity about the causes of their emotions. Specifically, the ability to attribute causes for experienced emotions may help generate adaptive responses and contribute to effective SF. In contrast, experiencing multiple emotions simultaneously without a clear sense of their origin may result in confusion, leading to social dysfunction. Supporting this view, Russell and Barrett (1999) found that specific emotions are linked to causal objects, whereas undifferentiated global affective states are not. Consequently, specific emotional states may have more adaptive value than global ones due to reduced misattribution errors (Schwarz & Clore, 1996). Thus, within the context of schizophrenia, the SF difficulties may potentially reflect difficulties attributing emotions, resulting in poor emotional coherence.
It may be argued that our findings regarding EG and social functioning may represent a change in the factor-structure of emotions due to more severe psychopathology (i.e., depression; Elhai et al., 2013). Accordingly, when people get more ill, their emotions become more intense, and therefore more difficult to differentiate. However, our results remained significant after controlling for depression, anxiety, delusions, and emotional intensity and variability, negating this view. Likewise, previous reports indicate no significant differences between people with schizophrenia and healthy controls in their ability to use mobile devices as part of ESM studies (Kimhy et al., 2012a), suggesting that our findings are not due to participant response characteristics.
4.2. Emotional Granularity and Social Functioning in Individuals with Schizophrenia
Our results are consistent with an extensive literature indicting substantial difficulties in emotion regulation and SF among individuals with schizophrenia. Barrett (2004) argued that for emotions to be maximally useful in a social context, it is essential that they be represented in a differentiated fashion, not just to others, but also to the self. Supporting Barrett’s view and our hypothesis, higher EGall predict better SF among individuals with schizophrenia. The results are particularly meaningful given that SF was indexed by three distinct measures. While all individuals may experience momentary co-occurrence of positive and negative emotions, EGall differences between the individuals with schizophrenia and healthy individuals suggest that the former may experience a more chronic pattern characterized by a higher frequency of emotional co-activation. Such chronic experiences, may lead to increased sense of confusion, more frequent displays of dissonant verbal and non-verbal communications, as well as negatively influence interpersonal decision-making – all potentially contributing to social dysfunction.
Another implication of our findings is to the ongoing debate regarding hedonic deficits among individuals with schizophrenia. Specifically, our results provide further evidence that people with schizophrenia appear to have intact “consummatory pleasure”, supporting the view proposed by Kring and colleagues (Gard et al., 2007).
4.3. Limitations and Future Directions
Several limitations of our study should be acknowledged. One limitation is the relatively small range of emotions assessed as part of the current study, which was not designed to assess EG. Thus, future studies should assess a wider range of emotions. Alternatively, the results may have also been influenced by the relatively short assessment period. Potentially related to this issue, the lack of differences in EGneg may be associated with these limited ranges. A second limitation is the lack of a clinical control group, leaving the possibility that the findings may apply to other clinical populations rather than just schizophrenia. Demiralp and colleagues (2012) found that individuals with MDD had less differentiated emotional experiences than did healthy participants, but only for negative emotions, suggesting an EG profile distinct from schizophrenia. However, they assessed a broader range of emotions. Thus, future studies may want to replicate our assessment in other clinical populations, as well as evaluate a larger number of emotions, especially positive ones. Additionally, providing participants with specific emotion labels could alter their emotional experience or misrepresent their true EG. This could be assessed in future studies by evaluating emotional responses in an open-ended way, and/or comparing open-ended labeling to fixed dimensions in ESM evaluations. Future studies will also be needed to determine the underlying circuitry associated with co-activations of positive and negative emotions in schizophrenia, as well as the potential impact of emotional coherence on SF. Finally, while the SANS has been validated extensively, our use of item #20 as an interview-based index of social functioning is novel.
Acknowledgments
This work was supported by grants R21 MH096132 (DK) and K23 MH077653 (DK) from the National Institute of Mental Health, Bethesda, MD.
Role of Funding Source:
The sponsors had no role in the design and conduct of the study (collection, management, analysis), nor in the interpretation of the data. The sponsors have not seen the manuscript.
Footnotes
Contributors
Dr. Kimhy designed the study, recruited participants, administered the ambulatory assessments, managed the literature searches, conducted the statistical analyses, and wrote the first draft of the manuscript.
Ms. Vakhrusheva contributed to the study design, conducted the diagnostic, social functioning, neurocognitive and clinical assessments, coordinated the data processing, and provided feedback for the final draft of the manuscript.
Ms. Khan, Chang, and Hansen administered some of the social functioning measures along with the clinical questionnaires, served as liaison to study participants, and provided feedback for the final draft of the manuscript.
Dr. Ballon conducted the initial eligibility screens of participants for the study and provided feedback for the final draft of the manuscript.
Drs. Malaspina and Gross contributed to the study design, interpretation of the findings for the manuscript, and provided feedback for the final draft of the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andreasen NC. Negative symptoms in schizophrenia. Definition and reliability. Arch Gen Psychiatry. 1982;39:784–788. doi: 10.1001/archpsyc.1982.04290070020005. [DOI] [PubMed] [Google Scholar]
- Bagby RM, Parker JDA, Taylor GJ. The twenty-item Toronto Alexithymia scale--I. Item selection and cross-validation of the factor structure. Journal of Psychosomatic Research. 1994;38:23–32. doi: 10.1016/0022-3999(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Barrett LF. Feelings or words? Understanding the content in self-report ratings of experienced emotion. J Pers Soc Psychol. 2004;87:266–281. doi: 10.1037/0022-3514.87.2.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF, Gross J, Christensen TC, Benvenuto M. Knowing what you’re feeling and knowing what to do about it: Mapping the relation between emotion differentiation and emotion regulation. Cognition & Emotion. 2001a;15:713–724. [Google Scholar]
- Barrett LF, Gross JJ. Emotion: Current Issues and Future Directions. New York: Guilford; 2001b. Emotion representation and regulation: A process model of emotional intelligence. [Google Scholar]
- Baslet G, Termini L, Herbener E. Deficits in Emotional Awareness in Schizophrenia and Their Relationship With Other Measures of Functioning. Journal of Nervous and Mental Disease. 2009;197:655–660. doi: 10.1097/NMD.0b013e3181b3b20f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard JJ, Mueser KT, Bellack AS. Anhedonia, positive and negative affect, and social functioning in schizophrenia. Schizophr Bull. 1998;24:413–424. doi: 10.1093/oxfordjournals.schbul.a033336. [DOI] [PubMed] [Google Scholar]
- Campos JJ, Campos RG, Barrett KC. Emergent Themes in the Study of Emotional Development and Emotion Regulation. Developmental Psychology. 1989;25:394–402. [Google Scholar]
- Carstensen LL, Isaacowitz DM, Charles ST. Taking time seriously. A theory of socioemotional selectivity. Am Psychol. 1999;54:165–181. doi: 10.1037//0003-066x.54.3.165. [DOI] [PubMed] [Google Scholar]
- Carstensen LL, Pasupathi M, Mayr U, Nesselroade JR. Emotional experience in everyday life across the adult life span. J Pers Soc Psychol. 2000;79:644–655. [PubMed] [Google Scholar]
- Carstensen LL, Turan B, Scheibe S, Ram N, Ersner-Hershfield H, Samanez-Larkin GR, Brooks KP, Nesselroade JR. Emotional experience improves with age: evidence based on over 10 years of experience sampling. Psychol Aging. 2011;26:21–33. doi: 10.1037/a0021285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clore GL, Tamir M. Affect as embodied information. Psychological Inquiry. 2002;13:37–45. [Google Scholar]
- Cohen AS, Beck MR, Najolia GM, Brown LA. Affective disturbances in psychometrically defined schizotypy across direct, but not indirect assessment modes. Schizophr Res. 2011;128:136–142. doi: 10.1016/j.schres.2011.02.004. [DOI] [PubMed] [Google Scholar]
- Cohen AS, Minor KS. Emotional experience in patients with schizophrenia revisited: meta-analysis of laboratory studies. Schizophr Bull. 2010;36:143–150. doi: 10.1093/schbul/sbn061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demiralp E, Thompson RJ, Mata J, Jaeggi SM, Buschkuehl M, Barrett LF, Ellsworth PC, Demiralp M, Hernandez-Garcia L, Deldin PJ, Gotlib IH, Jonides J. Feeling blue or turquoise? Emotional differentiation in major depressive disorder. Psychol Sci. 2012;23:1410–1416. doi: 10.1177/0956797612444903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekman P. An Argument for Basic Emotions. Cognition & Emotion. 1992;6:169–200. [Google Scholar]
- Elhai JD, Contractor AA, Biehn TL, Allen JG, Oldham J, Ford JD, Grubaugh AL, Frueh BC. Changes in the Beck Depression Inventory-II’s underlying symptom structure over 1 month of inpatient treatment. J Nerv Ment Dis. 2013;201:371–376. doi: 10.1097/NMD.0b013e31828e1004. [DOI] [PubMed] [Google Scholar]
- Frijda NH, Mesquita B. The social roles and functions of emotions. In: Kitayama S, Markus HR, editors. Emotion and culture: Emprical studies of mutual influence. Washington, DC: American Psychological Association; 1994. [Google Scholar]
- Gard DE, Kring AM, Gard MG, Horan WP, Green MF. Anhedonia in schizophrenia: distinctions between anticipatory and consummatory pleasure. Schizophr Res. 2007;93:253–60. doi: 10.1016/j.schres.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MF, Bearden CE, Cannon TD, Fiske AP, Hellemann GS, Horan WP, Kee K, Kern RS, Lee J, Sergi MJ, Subotnik KL, Sugar CA, Ventura J, Yee CM, Nuechterlein KH. Social cognition in schizophrenia, Part 1: performance across phase of illness. Schizophr Bull. 2012;38:854–864. doi: 10.1093/schbul/sbq171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JJ. Emotion regulation: affective, cognitive, and social consequences. Psychophysiology. 2002;39:281–291. doi: 10.1017/s0048577201393198. [DOI] [PubMed] [Google Scholar]
- Harvey PD, Heaton RK, Carpenter WT, Jr, Green MF, Gold JM, Schoenbaum M. Functional impairment in people with schizophrenia: focus on employability and eligibility for disability compensation. Schizophr Res. 2012;140:1–8. doi: 10.1016/j.schres.2012.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey PD, Helldin L, Bowie CR, Heaton RK, Olsson AK, Hjarthag F, Norlander T, Patterson TL. Performance-based measurement of functional disability in schizophrenia: a cross-national study in the United States and Sweden. Am J Psychiatry. 2009;166:821–827. doi: 10.1176/appi.ajp.2009.09010106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooker C, Park S. Emotion processing and its relationship to social functioning in schizophrenia patients. Psychiatry Res. 2002;112:41–50. doi: 10.1016/s0165-1781(02)00177-4. [DOI] [PubMed] [Google Scholar]
- Horan WP, Ventura J, Mintz J, Kopelowicz A, Wirshing D, Christian-Herman J, Foy D, Liberman RP. Stress and coping responses to a natural disaster in people with schizophrenia. Psychiatry Res. 2007;151:77–86. doi: 10.1016/j.psychres.2006.10.009. [DOI] [PubMed] [Google Scholar]
- Hunter R, Barry S. Negative symptoms and psychosocial functioning in schizophrenia: neglected but important targets for treatment. Eur Psychiatry. 2012;27:432–436. doi: 10.1016/j.eurpsy.2011.02.015. [DOI] [PubMed] [Google Scholar]
- Huprich SK, Sanford K, Smith M. Psychometric evaluation of the depressive personality disorder inventory. J Pers Disord. 2002;16:255–269. doi: 10.1521/pedi.16.3.255.22539. [DOI] [PubMed] [Google Scholar]
- Kee KS, Green MF, Mintz J, Brekke JS. Is emotion processing a predictor of functional outcome in schizophrenia? Schizophr Bull. 2003;29:487–497. doi: 10.1093/oxfordjournals.schbul.a007021. [DOI] [PubMed] [Google Scholar]
- Keltner D, Haidt J. Social functions of emotions at four levels of analysis. Cognition & Emotion. 1999;13:505–521. [Google Scholar]
- Kimhy D, Corcoran C. Use of Palm computer as an adjunct to cognitive-behavioural therapy with an ultra-high-risk patient: a case report. Early Intervention in Psychiatry. 2008;2:234–241. doi: 10.1111/j.1751-7893.2008.00083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimhy D, Delespaul P, Ahn H, Cai S, Shikhman M, Lieberman JA, Malaspina D, Sloan RP. Concurrent measurement of “real-world” stress and arousal in individuals with psychosis: assessing the feasibility and validity of a novel methodology. Schizophr Bull. 2010;36:1131–1139. doi: 10.1093/schbul/sbp028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimhy D, Delespaul P, Corcoran C, Ahn H, Yale S, Malaspina D. Computerized experience sampling method (ESMc): assessing feasibility and validity among individuals with schizophrenia. Journal of psychiatric research. 2006;40:221–230. doi: 10.1016/j.jpsychires.2005.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimhy D, Myin-Germeys I, Palmier-Claus J, Swendsen J. Mobile assessment guide for research in schizophrenia and severe mental disorders. Schizophr Bull. 2012a;38:386–395. doi: 10.1093/schbul/sbr186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimhy D, Vakhrusheva J, Jobson-Ahmed L, Tarrier N, Malaspina D, Gross JJ. Emotion awareness and regulation in individuals with schizophrenia: Implications for social functioning. Psychiatry Res. 2012b;200:193–201. doi: 10.1016/j.psychres.2012.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosaka H, Omori M, Murata T, Iidaka T, Yamada H, Okada T, Takahashi T, Sadato N, Itoh H, Yonekura Y, Wada Y. Differential amygdala response during facial recognition in patients with schizophrenia: an fMRI study. Schizophr Res. 2002;57:87–95. doi: 10.1016/s0920-9964(01)00324-3. [DOI] [PubMed] [Google Scholar]
- Kring AM, Caponigro JM. Emotion in Schizophrenia. Current Directions in Psychological Science. 2010;19:255–259. doi: 10.1177/0963721410377599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwapil TR, Mann MC, Raulin ML. Psychometric properties and concurrent validity of the schizotypal ambivalence scale. J Nerv Ment Dis. 2002;190:290–295. doi: 10.1097/00005053-200205000-00004. [DOI] [PubMed] [Google Scholar]
- Kwapil TR, Raulin M, Midthun J. A ten-year longitudinal study of intense ambivalence as a predictor of risk for psychopathology. J Nerv Ment Dis. 2000;188:402–408. doi: 10.1097/00005053-200007000-00002. [DOI] [PubMed] [Google Scholar]
- Lane RD, Quinlan DM, Schwartz GE, Walker PA, Zeitlin SB. The Levels of Emotional Awareness Scale: a cognitive-developmental measure of emotion. J Pers Assess. 1990;55:124–134. doi: 10.1080/00223891.1990.9674052. [DOI] [PubMed] [Google Scholar]
- Lane RD, Schwartz GE. Levels of emotional awareness: a cognitive-developmental theory and its application to psychopathology. Am J Psychiatry. 1987;144:133–143. doi: 10.1176/ajp.144.2.133. [DOI] [PubMed] [Google Scholar]
- Lane RD, Sechrest L, Reidel R, Weldon V, Kaszniak A, Schwartz GE. Impaired verbal and nonverbal emotion recognition in alexithymia. Psychosom Med. 1996;58:203–210. doi: 10.1097/00006842-199605000-00002. [DOI] [PubMed] [Google Scholar]
- Larsen RJ, Billings DW, Cutler SE. Affect intensity and individual differences in informational style. J Pers. 1996;64:185–207. doi: 10.1111/j.1467-6494.1996.tb00819.x. [DOI] [PubMed] [Google Scholar]
- Mann MC, Vaughn AG, Barrantes-Vidal N, Raulin ML, Kwapil TR. The schizotypal ambivalence scale as a marker of schizotypy. J Nerv Ment Dis. 2008;196:399–404. doi: 10.1097/NMD.0b013e3181710900. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Green MF. MATRICS Consensus Battery Manual. Los Angeles: MATRICS Assessment Inc; 2006. [Google Scholar]
- Patterson TL, Moscona S, McKibbin CL, Davidson K, Jeste DV. Social skills performance assessment among older patients with schizophrenia. Schizophr Res. 2001;48:351–360. doi: 10.1016/s0920-9964(00)00109-2. [DOI] [PubMed] [Google Scholar]
- Patterson TL, Semple SJ, Shaw WS, Halpain M, Moscona S, Grant I, Jeste DV. Self-reported social functioning among older patients with schizophrenia. Schizophr Res. 1997;27:199–210. doi: 10.1016/S0920-9964(97)00078-9. [DOI] [PubMed] [Google Scholar]
- Russell JA, Barrett LF. Core affect, prototypical emotional episodes, and other things called emotion: dissecting the elephant. J Pers Soc Psychol. 1999;76:805–819. doi: 10.1037//0022-3514.76.5.805. [DOI] [PubMed] [Google Scholar]
- Schwarz N, Clore GL. Social psychology: Handbook of basic principles. New York: Guilford; 1996. Feelings and phenomenal experiences. [Google Scholar]
- Taylor SF, Liberzon I, Decker LR, Koeppe RA. A functional anatomic study of emotion in schizophrenia. Schizophr Res. 2002;58:159–172. doi: 10.1016/s0920-9964(01)00403-0. [DOI] [PubMed] [Google Scholar]
- Taylor SF, Phan KL, Britton JC, Liberzon I. Neural response to emotional salience in schizophrenia. Neuropsychopharmacology. 2005;30:984–995. doi: 10.1038/sj.npp.1300679. [DOI] [PubMed] [Google Scholar]
- Turner RJ, Frankel BG, Levin D. Research in community mental health. Greenwich, CT: JAI Press; 1983. Social support: Conceptualization, measurement, and implications for mental health. In Greenley JR editor. [Google Scholar]
- Velakoulis D, Wood SJ, Wong MT, McGorry PD, Yung A, Phillips L, Smith D, Brewer W, Proffitt T, Desmond P, Pantelis C. Hippocampal and amygdala volumes according to psychosis stage and diagnosis: a magnetic resonance imaging study of chronic schizophrenia, first-episode psychosis, and ultra-high-risk individuals. Arch Gen Psychiatry. 2006;63:139–149. doi: 10.1001/archpsyc.63.2.139. [DOI] [PubMed] [Google Scholar]