Abstract
Digital skin vasoconstriction on local cooling is exaggerated in primary Raynaud’s phenomenon (RP) compared to controls. A significant part of such vasoconstriction relies on the nitric oxide (NO) pathway inhibition. We tested the effect of PDE5 inhibitor sildenafil, which potentiates the effect of NO, on skin blood flow. We recruited 15 patients with primary RP, performing local cooling without sildenafil (day 1), after a single 50 mg oral dose (day 2), and 100 mg (day 3). Skin blood flow, skin temperature and arterial pressure were recorded, and data were expressed as cutaneous vascular conductance (CVC). Sildenafil at 100 mg, but not 50 mg, significantly lessened the cooling-induced decrease in CVC. It also increased resting CVC and skin temperature. These data suggest that 100 mg sildenafil improves digital skin blood flow to local cooling in primary RP. The benefit of sildenafil “as required” should be confirmed in a randomized controlled trial.
Keywords: Adult, Aged, Female, Humans, Male, Middle Aged, Phosphodiesterase 5 Inhibitors, adverse effects, pharmacology, Piperazines, adverse effects, pharmacology, Purines, adverse effects, pharmacology, Raynaud Disease, drug therapy, physiopathology, Regional Blood Flow, drug effects, Skin, blood supply, Skin Temperature, drug effects, Sulfones, adverse effects, pharmacology
Introduction
Raynaud’s phenomenon (RP) is defined as episodic vasospasm of the extremities in response to cold or emotional stimuli, often accompanied by pain. RP can be primary (i.e. idiopathic) or secondary to a connective tissue disease. The pathophysiology of primary RP is multifactorial and complex, with both vascular and neural abnormalities (1, 2). Although the morphology of skin capillaries is normal in primary RP, available evidence suggests that skin microvascular function is impaired (3–5). By using a local cooling test we recently showed increased digital skin vasoconstriction in participants with primary RP compared to healthy controls (6).
Local cooling of the skin induces an initial vasoconstriction followed by a transient vasodilation and finally, a prolonged vasoconstriction (7). Cooling exerts a significant portion of its vasoconstrictor effects through inhibition of the nitric oxide (NO) system (7). Indeed, NO is a potent vasodilator as it generates cyclic guanosine-5-monophosphate (cGMP), which induces vascular smooth muscle relaxation leading to vasodilation. cGMP is hydrolyzed by phosphodiesterases, and particularly by cGMP-specific phosphodiesterase 5 (PDE5).
PDE5 inhibitors have been mostly studied in RP secondary to systemic sclerosis as continuous therapy (8, 9). In less severe primary RP, single dose PDE5 inhibitors (e.g. before exposure to cold) could be an interesting therapy in the prevention of vasospasm, thus avoiding continuous exposure to vasodilators.
Although local cooling cannot be used as a surrogate outcome for RP, it could be a safe and reproducible experimental test to explore the effect of drugs on the different phases of microvascular reactivity to local cooling in primary RP (10).
We hypothesized that sildenafil, a PDE5 inhibitor, may reverse the exaggerated microvascular response to local cooling in primary RP. The primary objective of this study was to assess the effect of a single oral dose of sildenafil (50 mg and 100 mg) on cutaneous vascular conductance (CVC) in the fingers of patients with primary RP, while cooling locally at 15°C or 24°C. We also assessed the effect of sildenafil on resting skin blood flux, CVC and skin temperature, as well as on the perfusion gradient between the different phalanges and the hand. The safety of sildenafil in primary RP patients was also assessed.
Results
Population characteristics
The demographic and clinical characteristics of the study population are summarized in Table 1. Antinuclear antibodies were negative and the nailfold videocapillaroscopy pattern was normal in all subjects. One postmenopausal woman was taking hormonal substitutive treatment, and three of the premenopausal women were taking oral contraceptives. Among the premenopausal women, 4 were enrolled during the follicular phase and 4 during the luteal phase of the cycle.
Table 1.
Demographic and clinical characteristics of the study population (N=15).
| Age (years) | 48.5 (17.9) |
|
| |
| Female | 11 (73) |
| Postmenopausal | 3 (27) |
| Premenopausal | 8 (73) |
| Follicular phase | 4 (50) |
| Luteal phase | 4 (50) |
|
| |
| BMI (kg/m2) | 21.8 (3.4) |
|
| |
| Arterial blood pressure | |
| Systolic (mm Hg) | 124.5 (14.4) |
| Diastolic (mm Hg) | 75.3 (8.5) |
|
| |
| Glycemia (mmol/L) | 4.8 (0.5) |
| Serum creatinine (μmol/L) | 76.9 (14.6) |
|
| |
| Primary Raynaud’s phenomenon | |
| Duration (years) | 18.8 (16.2) |
| Number of fingers involved | 8.1 (1.4) |
| Thumb involved | 5 (33) |
| Feet involved | 10 (67) |
| Raynaud’s Condition Score | 4.1 (1.8) |
Quantitative data are expressed as mean (standard deviation). Qualitative data (female, thumb involvement and feet involvement) are expressed as number (percentage). BMI: body mass index.
Effect of sildenafil on resting cutaneous vascular conductance and skin temperature
Oral 50 mg sildenafil significantly increased skin temperature from 28 ± 3.3°C before administration to 29.3 ± 3.1°C 50 min later (P=0.02, Wilcoxon rank test). In the same way, 100 mg sildenafil significantly increased skin temperature from 28.9 ± 3.3°C to 30.7 ± 2.7°C (P=0.008, Wilcoxon rank test). There was a significant difference in skin temperature over time between 50 mg sildenafil and 100 mg sildenafil (P=0.048, two-way ANOVA) (Figure 1A).
Figure 1.
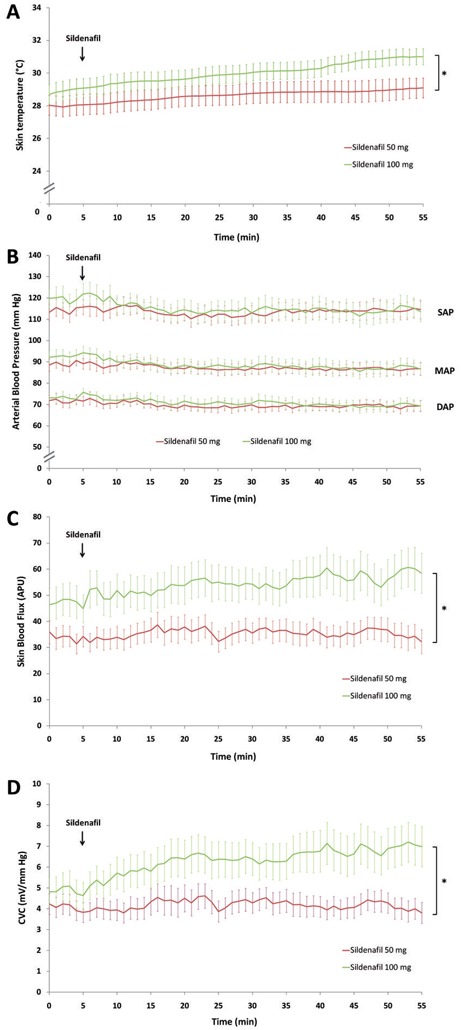
Effect of a single dose of 50 mg (red line) and 100 mg (green line) of sildenafil given orally on resting skin temperature in a temperature-controlled room (A); systolic (SAP), mean (MAP) and diastolic (DAP) arterial blood pressure (B); skin blood flux (C) and cutaneous vascular conductance (CVC) (D). APU: arbitrary perfusion units. * P<0.05
Resting skin blood flux was 32.1 ± 19.8 PU before and 32.3 ± 25.1 PU 50 min after administration of 50 mg sildenafil (P=0.92, Wilcoxon rank test), and 44.9 ± 30 PU before and 58.7 ± 37.9 PU after 100 mg sildenafil (P=0.007, Wilcoxon rank test) (Figure 1C). Resting CVC was 4.1 ± 2.7 mV/mm Hg before and 4 ± 2.8 mV/mm Hg 50 min after administration of 50 mg sildenafil (P=0.34, Wilcoxon rank test). Alternatively, CVC was 4.8 ± 3.9 mV/mm Hg before and 7 ± 5.3 mV/mm Hg 50 min after administration of 100 mg sildenafil (P=0.001, Wilcoxon rank test). There was a significant difference in skin blood flux and CVC over time between 50 mg sildenafil and 100 mg sildenafil (P=0.012 and P=0.035, respectively; two-way ANOVA) (Figure 1D).
Effect of sildenafil on cutaneous vascular conductance during local cooling
Sildenafil at 100 mg, but not 50 mg, significantly lessened the 15°C local cooling-induced decrease in skin blood flux compared to control (Table 2, Figure 2A). This difference is not uniquely due to the change in baseline, as the AUC5-35 (i.e. excluding baseline CVC) were also significantly different (6856.7 ± 5977.4 and 2248.7 ± 1778.6, respectively; P=0.04).
Table 2.
Effect of 50 mg and 100 mg sildenafil on cutaneous vascular conductance during local cooling on the finger of patients with primary RP.
|
|
|||||
|---|---|---|---|---|---|
| Sildenafil | P-value | ||||
| Control | 50 mg | 100 mg | |||
| 15°C | AUC0-35 (mV.s/mm Hg) | 3581.41 (2688.12) | 5326.08 (3349.08) | 9360.25 (6681.89)* | 0.02 |
|
|
|||||
| Baseline (mV/mm Hg) | 3.54 (2.61) | 6.33 (3.46)* | 8.35 (4.07)* | 0.001 | |
| Initial VC (mV/mm Hg) | 1.16 (0.71) | 2.49 (2.16) | 4.04 (3.46)* | 0.031 | |
| Transient VD (mV/mm Hg) | 1.86 (1.75) | 3.44 (2.33) | 5.05 (3.99)* | 0.026 | |
| Prolonged VC (mV/mm Hg) | 1.1 (1.06) | 1.16 (0.78) | 2.93 (2.44)* | 0.017 | |
|
|
|||||
| Initial VC (%BL) | −68.21 (10.85) | −63.13 (18.28) | −51.91 (26.44) | 0.077 | |
| Transient VD (%BL) | −50.17 (26.08) | −43.82 (27.86) | −39.35 (29.44) | 0.45 | |
| Prolonged VC (%BL) | −68.78 (27.34) | −80.46 (10.38) | −63.72 (24.03) | 0.069 | |
|
| |||||
| 24°C | AUC0-35 (mV.s/mm Hg) | 3609.76 (1507.41) | 5559.08 (3349.08) | 7546.98 (3976)* | <0.001 |
|
|
|||||
| Baseline (mV/mm Hg) | 3.64 (2.1) | 5.34 (2.4) | 6.52 (2.81)* | 0.001 | |
| Initial VC (mV/mm Hg) | 1.51 (1.16) | 2.08 (1.15) | 3.28 (2.14)* | 0.003 | |
| Transient VD (mV/mm Hg) | 2.08 (1.75) | 2.85 (1.83) | 4.09 (2.42)* | 0.007 | |
| Prolonged VC (mV/mm Hg) | 1.21 (0.37) | 2.1 (1.63) | 2.55 (1.67)* | 0.009 | |
|
| |||||
| Initial VC (%BL) | −54.39 (19.55) | −59.51 (14.65) | −49.14 (23.21) | 0.29 | |
| Transient VD (%BL) | −35.9 (27.23) | −44. 1 (23.56) | −34.43 (29.6) | 0.94 | |
| Prolonged VC (%BL) | −59.68 (15.5) | −59.77 (18.82) | −58.33 (21.56) | 0.92 | |
Data are expressed as mean (SD) cutaneous vascular conductance in mV/mm Hg or as a percentage of baseline CVC (%BL). Area under the curve (AUC0-35) includes the 5-min baseline and the 30-min cooling, and is expressed as mV.s/mm Hg.
VC: vasoconstriction; VD: vasodilation.
P<0.025 vs control.
Figure 2.
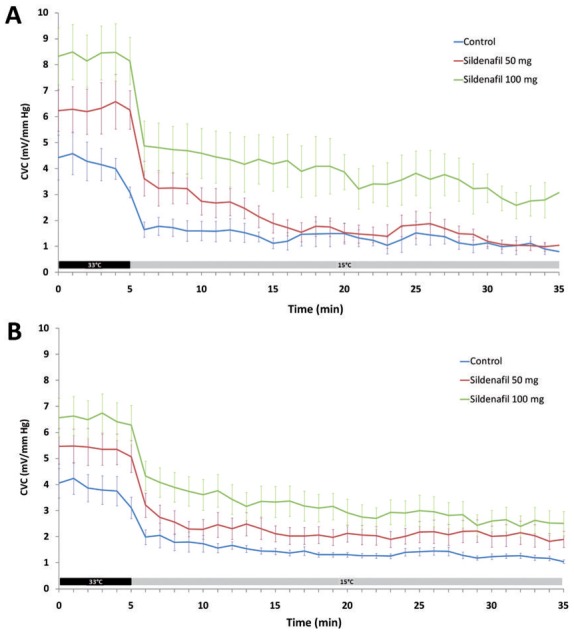
Effect of a single dose of 50 mg sildenafil given orally (red line) and 100 mg (green line) compared to control (blue line) on cutaneous vascular conductance (CVC). Local cooling was started one hour after oral administration. Local cooling (grey bar) was set at 15°C (A) and at 24°C (B) after a baseline at 33°C (black bar).
This difference is observed at baseline and for all phases of local cooling, i.e. initial vasoconstriction, transient vasodilation and prolonged vasoconstriction (Table 2). When data were expressed as a percent change from baseline however, there was no significant difference between sildenafil at 100 mg, 50 mg and control (Table 2).
Similar results were observed when the cooling temperature was set at 24°C (Table 2, Figure 2B). Again, the difference in skin blood flux between 100 mg sildenafil and control was not uniquely due to the change in baseline (AUC5-35 were 5592.1 ± 3434.2 and 2518.5 ± 920.9, respectively; P=0.008). The difference between 100 mg sildenafil and control was significant at baseline and for all phases of local cooling when expressed as CVC (Table 2), but not as a function of baseline.
Effect of sildenafil on the skin perfusion gradient
At each visit we observed a perfusion gradient between the distal, middle and proximal phalanges and the dorsum of the hand: 15.9 ±13.4, 11.5 ± 8.3, 10.2 ± 6.9 and 7.9 ± 4.5 mV/mm Hg respectively for control (P=0.008); 13 ± 9.9, 10.6 ± 7.1, 9.4 ± 6.1 and 8.1 ± 4.7 mV/mm Hg respectively for 50 mg sildenafil (P=0.004); and 22.3 ± 13.5, 18 ± 13.4, 16.2 ± 13.8 and 10.3 ± 5.7 respectively for 100 mg sildenafil (P=0.001). The difference between 100 mg sildenafil, but not 50 mg, and control is significant for each phalange and for the hand (Figure 3).
Figure 3.
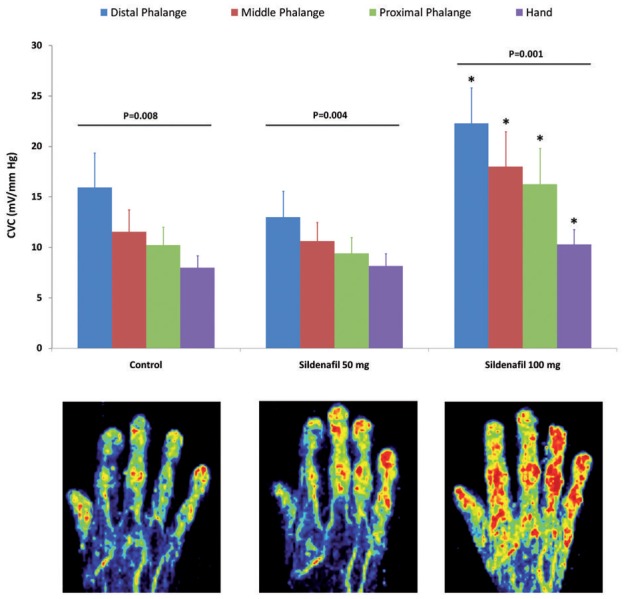
Effect of sildenafil on the gradient of skin perfusion between the distal, middle and proximal phalanges and the dorsum of the hand. * P<0.05 vs control. Below are examples of skin perfusion assessed with laser Doppler imaging in the same patient without sildenafil (left), and after sildenafil at 50 mg (middle) and at 100 mg (right).
Safety
None of the subjects complained of pain associated with the local cooling and it did not induce any symptoms of RP. No adverse event was reported during visit 1 (no treatment). At visit 2 (50 mg sildenafil), four patients experienced headache: 3 were mild and regressed spontaneously, the fourth one was moderate and ceased after taking 1 g acetaminophen. Mild flushing was observed in 6 patients. At visit 3 (100 mg sildenafil), the same patient complained of moderate headache and was given 1 g acetaminophen. Mild flushing was observed in 4 patients, one of them also reporting nasal congestion.
Mean arterial blood pressure before 50 mg sildenafil (visit 2) and 100 mg (visit 3) were 90 ± 10.9 and 93 ± 10.8 mm Hg, respectively. The nadir were reached 37 and 43 min after sildenafil administration, and were 85.9 ± 9.6 (P=0.07 vs baseline) and 83.5 ± 14.6 mm Hg (P=0.01 vs baseline), respectively (Figure 1B).
Discussion
Our data show that a single dose of 100 mg sildenafil significantly increases cutaneous vascular conductance during the three phases of localized digital cooling in patients with primary RP. It also increased resting CVC and skin temperature. The effect was observed within 1 hour after sildenafil intake, at 15°C and 24°C. Finally, we observed a non significant trend towards increased CVC while cooling with 50 mg sildenafil. None of the patients experienced a serious adverse event.
Microvascular reactivity impairment in primary RP is not yet fully understood. A loss of CGRP containing nerve fibers in the digits has been suggested (11), as well as increased endothelin-1 dependent vasoconstriction (1). Another mechanism could involve postjunctional α2c-adrenoreceptors, which are clustered distally, whereby their translocation from cytosol to cell surface is enhanced by cooling, thus leading to vasoconstriction (2). In vitro, cooling-induced α2-adrenergic constriction of arterioles isolated from patients with primary RP was increased compared to controls, which was suppressed by protein tyrosine kinase inhibitors (5).
Local cooling is a reproducible test to assess skin microvascular reactivity (10). Most of its vasoconstrictor effect depends on inhibition of the NO system and on adrenergic function (12), particularly through the translocation of α2c-adrenoreceptors mediated by RhoA-Rho kinase (13). We recently showed that local cooling-induced vasoconstriction on the fingers of patients with primary RP was increased compared to healthy controls (6).
This study further demonstrates that sildenafil, a selective PDE5 inhibitor, increases digital skin blood flow both at baseline and during local cooling in primary RP. However, sildenafil did not restore the typical pattern of skin blood flow during local cooling described in healthy subjects, i.e. initial vasoconstriction followed by a transient vasodilation and finally, a prolonged vasoconstriction (7). Indeed, in most cases the transient vasodilation is blunted in patients with RP compared to controls, and partially restored by local anesthesia, suggesting an abnormal neural response in primary RP depending on cold sensitive nerves (6). Sildenafil induced a shift towards higher flux values at all phases, explaining why its effect was not significant when data were expressed as a percentage of baseline.
Moreover, assessment of resting perfusion on the contralateral arm shows an increase in CVC on all phalanges and on the dorsum of the hand with sildenafil at 100 mg, but not 50 mg. All together, our data suggest that 100 mg sildenafil increases skin blood flow at baseline and in response to local cooling, one hour after oral administration. This delay was chosen according to previous work by our group showing that the effect of 100 mg oral sildenafil on the microcirculation was maximal one hour after administration (14). In that study, we showed that sildenafil at 100 mg, but not 50 mg, significantly increases forearm CVC (14), which is consistent with the present findings.
Sildenafil was well tolerated; none of the participants experienced a serious adverse event. The mild adverse drug reactions observed in this study are similar to those observed with PDE5 inhibitors in the treatment of erectile dysfunction. The frequencies of headache and flushing were comparable to those previously reported (15). The decrease in mean arterial pressure was maximal at around 40 min, and was −10% and −16% from baseline after administration of 50 mg sildenafil and 100 mg sildenafil, respectively.
Several controlled studies have shown improved outcomes (i.e. RP duration, frequency of attacks, RCS or a visual analogue scale score) in secondary RP when treated with PDE5 inhibitors (16, 17). While these studies enrolled patients with secondary RP (particularly patients with systemic sclerosis) and assessed the continuous use of PDE5 inhibitors, little data is available for primary RP.
Friedman et al have studied the effect of another PDE5 inhibitor, tadalafil, on cold-induced skin vasoconstriction in 18 patients with primary RP and 2 with secondary RP (18). However, they did not show any significant change in skin blood flow at baseline and during local cooling between a single 10mg dose of tadalafil and placebo. The discrepancy between their findings and ours might be explained by an insufficient skin concentration of tadalafil after a 10 mg dose. Indeed, sildenafil shows a higher volume of distribution than tadalafil, and the maximal concentration obtained with 100 mg sildenafil is approximately that obtained with 20 mg tadalafil (19). This is also consistent with the lack of response we observed with 50 mg sildenafil.
Another explanation could be the difference in methods between the studies. Indeed, in the study of Friedman et al cooling was regional (whole hand) and flux was recorded on the finger pad (18), whereas in the present work cooling and measurements were restricted to the dorsum of the finger. Importantly, in the study by Friedman et al, some blood flow measurements in response to cooling were paradoxically higher in patients with RP than in controls enrolled in preliminary studies (18). Therefore, the methods might be inappropriate to detect impaired microvascular reactivity to cooling in primary RP, thus making it more difficult to show a difference after tadalafil administration. The test we used in the present study allowed us to demonstrate microvascular dysfunction to local cooling in primary RP compared to healthy controls (6).
An open-label study has assessed the effect of vardenafil (10 mg bid) on skin blood flow in basal conditions and during exposure to cold, in 7 patients with primary RP and 33 with secondary RP (20). Caglayan et al showed a significant increase in skin blood flow after vardenafil administration during exposure to cold at 4°C, but not at room temperature. The effect was visible after a single dose and after 2 weeks (20). Another small open-label study has reported significant improvement in digital temperature in response to a mild cold challenge (immersion in water at 15°C) after 50 mg sildenafil in 3 out of 5 patients with secondary RP (21). We did not assess the effect of local cooling on skin temperature (as the temperature was set at 24°C or 15°C), but we observed a dose-dependent effect of sildenafil on resting skin temperature and cutaneous blood flow.
In order to allow for the variability of baseline skin blood flow (i.e. after sildenafil administration but before cooling) we homogenized baseline skin temperature to 33°C, as previously described (22). Homogenizing skin temperature considerably decreased inter-individual variability (data not shown for clarity) although baseline CVC remained significantly increased by sildenafil. This increase in baseline may explain the trend towards greater vasoconstriction when data are expressed as a percentage of baseline while cooling at 15°C after 50 mg sildenafil. This is probably the consequence of a greater elevation in baseline CVC when homogenizing skin temperature at 33°C at 50 mg than 100 mg. This could be explained by the potentiating effect of sildenafil on the vasodilation induced by local heating (which is partly NO-dependent). Indeed, after 50 mg sildenafil skin temperature increased from 29.3 to 33°C, whereas the difference was smaller (30.7 to 33°C) after 100 mg sildenafil.
Gender is another concern when studying microvascular function. The effect of the phase of the menstrual cycle or of oral contraception on microvascular function has been explored, but with conflicting results. Resting cutaneous blood flux and conductance are affected by sex, females having lower values than males (23). Finally, healthy females show greater vasoconstriction on local cooling than males, the response being more pronounced during the luteal phase than the follicular phase of the menstrual cycle (24). As our study design (7-day intervals between visits) implied differing hormonal status, we took care to include as many premenopausal women in the follicular phase as those in the luteal phase. We deliberately chose to shorten the inclusion period to limit seasonal differences in microvascular reactivity, which have been previously described in patients with primary RP (25, 26)
The main limitation of this study is that it was not randomized and double-blind. Indeed, we were unable to obtain a placebo for sildenafil from the manufacturer. We therefore chose objective measurements as the primary outcome (i.e. cutaneous vascular conductance). Moreover, given the lack of safety data on single doses of 50 or 100 mg in young and low body weight women, as well as an adverse drug event in a previous study by our group (14), we sequentially tested 50 mg sildenafil followed by 100 mg rather than randomize the visits.
In conclusion, this study shows that a single dose of 100 mg sildenafil significantly increases skin blood flow within one hour in patients with primary RP, both at baseline and during all phases of digital local cooling. Sildenafil administration did not induce any serious adverse drug event. This pilot pharmacology study suggests that 100 mg sildenafil could be used “as required” before exposure to cold in primary RP. This should be confirmed in a randomized double-blind controlled trial. Another interesting approach would be to determine in a laboratory study whether the higher skin blood flow decreases the pain or the frequency of vasospasm to whole body cooling in a laboratory study.
Methods
Study population
All the participants enrolled in this study were recruited through local newspaper advertisements and included between February 2011 and June 2011. All subjects were 18 years of age or older. Primary RP was diagnosed according to the criteria of LeRoy and Medsger (27), and had to affect more than 2 fingers on a hand. Subjects taking calcium-channel blockers were instructed to stop medication one week before enrolment in the study. The Raynaud’s Condition Score (RCS) was assessed as previously described (28).
Non-inclusion criteria included pregnancy (urine pregnancy tests were performed at the beginning of each visit), cigarette smoking, any associated chronic disease, and an abnormal capillaroscopic pattern (29). Antinuclear autoantibodies were determined for all participants. In cases with positive antinuclear autoantibodies (>80 UI/mL), specific autoantibodies against topoisomerase I (Scl-70) or centromere-associated proteins were sought. Positive autoantibodies against topoisomerase I (Scl-70) or centromere-associated proteins were exclusion criteria.
The investigation conforms to the principles outlined in the Declaration of Helsinki. Grenoble Institutional Review Board (IRB n°6705) approval was obtained and each subject gave written informed consent before participation.
Study design
This was an open-label pharmacology study. Three consecutive visits were planned for each volunteer, separated by 7 days +/− 3 at the same time of the day. Upon arrival at the center subjects were placed in a temperature-controlled room (23+/−1°C). They remained supine for the whole experiment.
Visit 1 included a clinical examination, followed by a local cooling test during which digital skin blood flux was continuously recorded. Blood pressure was also recorded continuously by using digital photoplethysmography (Nexfin monitor, Bmeye B.V., Amsterdam, The Netherlands). Before recording started, the arm was immobilized with a vacuum cushion to ensure stable positioning, as previously described (6). The procedure was repeated at visit 2 and visit 3, one hour after the oral administration of sildenafil citrate (purchased from Pfizer France, Paris, France). Patients were being given 50 mg sildenafil on visit 2 and 100 mg sildenafil on visit 3 (Figure 4).
Figure 4.
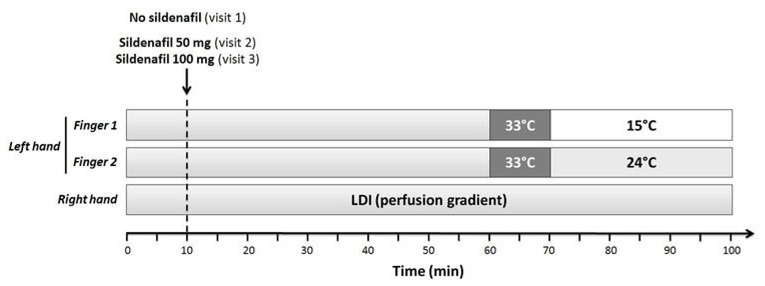
Study day schema. LDI: laser Doppler imaging.
Local cooling and skin blood flow measurements
Cutaneous blood flow was assessed by LDF (Periflux System 5000, Perimed, Järfälla, Sweden). Two skin sites were equipped with custom-designed LDF cooling probes (Probe 415-317, Perimed, Järfälla, Sweden) (10). Risk analysis was performed and was fully compatible with human use. The skin sites were chosen on the dorsum of two fingers affected by RP between the index, the middle and the ring finger (left hand). Maricq color charts (30) were used to confirm the diagnosis of RP and to specify its topography. When the three fingers were equally affected by RP, two of them were randomly chosen.
After a 30-min acclimation at room temperature (23°C +/− 1), resting skin blood flux was recorded for 10 minutes. Skin temperature was then homogenized at 33°C and skin blood flux was recorded during the following 10 minutes to obtain baseline flux (visit 1). In visits 2 and 3, the 10-min baseline (skin temperature homogenized at 33°C) was done 50 min after taking sildenafil. Indeed, we previously showed that the effect of sildenafil on microvascular reactivity was maximal 60 min after oral administration (14). After baseline, local cooling was maintained over 30 min at 15°C on one skin site and at 24°C on the other, simultaneously. Blood flux measurements were 50 min (visit 1) to 100 min (visits 2 and 3).
Perfusion gradient measurements
Skin perfusion of the dorsal surface of the hand (right hand) was recorded before cooling with laser Doppler imaging (PeriScan PIM 3, Perimed, Järfälla, Sweden). Regions of interest (ROI) were defined on the distal, middle and proximal phalanges of three fingers (index, middle and ring fingers), as well as on the dorsal surface of the hand (without the fingers). Mean CVC was calculated for all ROIs. Data from the 3 fingers were averaged.
Data analysis
Data were digitized, stored on a computer, and analyzed off-line with signal processing software (PeriSoft 2.5.5, Perimed, Järfälla, Sweden). Skin blood flow was expressed as cutaneous vascular conductance (CVC) in mV/mmHg (i.e. flux in millivolts divided by mean arterial pressure). Expressing data as conductance is a more physiological approach, as it takes into account differences and variations in blood pressure (31). Then, a minute by minute analysis of CVC was performed to assess the kinetics of the response and data were expressed as area under the curve over a 5-min baseline and the 30-min cooling period (AUC0-35, in mV.s/mm Hg) as the primary outcome. We subsequently analyzed the 3 phases of the response as previously described: 1. initial vasoconstriction: CVC was averaged over 1 min around the lowest flux value within the first five minutes; 2. transient vasodilation: CVC was averaged over 1 min around the highest flux value within the first fifteen minutes; 3. late prolonged vasoconstriction: CVC was averaged over the last 3 minutes of the measurement (6). In order to take into account baseline (BL) flux variations, data were expressed as a percentage change from baseline CVC as a secondary outcome.
Statistical analysis
Categorical data were reported as frequency and percentage, and continuous data as mean and standard deviation. They were analyzed by repeated measures ANOVA, and paired t tests for 2×2 comparisons. Mauchly’s test of sphericity was used to assess equality of variance in the data. When significant (i.e. inequality of variance cannot be excluded) a Greenhouse-Geisser adjustment was used. When the conditions of application of parametric tests were not respected, nonparametric tests were used (Friedman test, and Wilcoxon test for paired comparisons). Two-sided significance tests were used throughout. We considered p values <0.05 as significant, corrected by Bonferroni’s method for multiple comparisons. Statistical analysis was performed with SPSS 13.0 for Windows (SPSS Inc, Chicago IL, USA).
Acknowledgments
We thank Claire Sors and Florence Gaillard-Bigot for their assistance in data collection, as well as Dr Alison Foote for correction of English language usage.
Financial support
Association des Sclérodermiques de France; Groupe Français de Recherche sur la Sclérodermie; Délégation à la Recherche Clinique et à l’Innovation, Grenoble University Hospital. Marcin Hellmann received a French government scholarship delivered by the French embassy in Poland.
Footnotes
Conflicts of interest
JLC and MR have received research grants from Pfizer for other studies.
References
- 1.Herrick AL. Pathogenesis of Raynaud’s phenomenon. Rheumatology (Oxford) 2005;44:587–96. doi: 10.1093/rheumatology/keh552. [DOI] [PubMed] [Google Scholar]
- 2.Cooke JP, Marshall JM. Mechanisms of Raynaud’s disease. Vasc Med. 2005;10:293–307. doi: 10.1191/1358863x05vm639ra. [DOI] [PubMed] [Google Scholar]
- 3.Bunker CB, Goldsmith PC, Leslie TA, Hayes N, Foreman JC, Dowd PM. Calcitonin gene-related peptide, endothelin-1, the cutaneous microvasculature and Raynaud’s phenomenon. Br J Dermatol. 1996;134:399–406. [PubMed] [Google Scholar]
- 4.Roustit M, Simmons GH, Baguet JP, Carpentier P, Cracowski JL. Discrepancy between simultaneous digital skin microvascular and brachial artery macrovascular post-occlusive hyperemia in systemic sclerosis. J Rheumatol. 2008;35:1576–83. [PMC free article] [PubMed] [Google Scholar]
- 5.Furspan PB, Chatterjee S, Freedman RR. Increased tyrosine phosphorylation mediates the cooling-induced contraction and increased vascular reactivity of Raynaud’s disease. Arthritis Rheum. 2004;50:1578–85. doi: 10.1002/art.20214. [DOI] [PubMed] [Google Scholar]
- 6.Roustit M, Blaise S, Millet C, Cracowski JL. Impaired transient vasodilation and increased vasoconstriction to digital local cooling in primary Raynaud’s phenomenon. Am J Physiol Heart Circ Physiol. 2011;301:H324–30. doi: 10.1152/ajpheart.00246.2011. [DOI] [PubMed] [Google Scholar]
- 7.Johnson JM, Kellogg DL., Jr Local thermal control of the human cutaneous circulation. J Appl Physiol. 2010;109:1229–38. doi: 10.1152/japplphysiol.00407.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levien TL. Advances in the treatment of Raynaud’s phenomenon. Vasc Health Risk Manag. 2010;6:167–77. doi: 10.2147/vhrm.s4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De LaVega AJ, Derk CT. Phosphodiesterase-5 inhibitors for the treatment of Raynaud’s: a novel indication. Expert Opin Investig Drugs. 2009;18:23–9. doi: 10.1517/13543780802525100. [DOI] [PubMed] [Google Scholar]
- 10.Roustit M, Maggi F, Isnard S, Hellmann M, Bakken B, Cracowski JL. Reproducibility of a local cooling test to assess microvascular function in human skin. Microvasc Res. 2010;79:34–9. doi: 10.1016/j.mvr.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 11.Bunker CB, Terenghi G, Springall DR, Polak JM, Dowd PM. Deficiency of calcitonin gene-related peptide in Raynaud’s phenomenon. Lancet. 1990;336:1530–3. doi: 10.1016/0140-6736(90)93307-b. [DOI] [PubMed] [Google Scholar]
- 12.Hodges GJ, Johnson JM. Adrenergic control of the human cutaneous circulation. Appl Physiol Nutr Metab. 2009;34:829–39. doi: 10.1139/H09-076. [DOI] [PubMed] [Google Scholar]
- 13.Thompson-Torgerson CS, Holowatz LA, Flavahan NA, Kenney WL. Cold-induced cutaneous vasoconstriction is mediated by Rho kinase in vivo in human skin. Am J Physiol Heart Circ Physiol. 2007;292:H1700–5. doi: 10.1152/ajpheart.01078.2006. [DOI] [PubMed] [Google Scholar]
- 14.Blaise S, Hellmann M, Roustit M, Isnard S, Cracowski JL. Oral sildenafil increases skin hyperaemia induced by iontophoresis of sodium nitroprusside in healthy volunteers. Br J Pharmacol. 2010;160:1128–34. doi: 10.1111/j.1476-5381.2010.00778.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldstein I, Lue TF, Padma-Nathan H, Rosen RC, Steers WD, Wicker PA. Oral sildenafil in the treatment of erectile dysfunction. Sildenafil Study Group. N Engl J Med. 1998;338:1397–404. doi: 10.1056/NEJM199805143382001. [DOI] [PubMed] [Google Scholar]
- 16.Fries R, Shariat K, von Wilmowsky H, Bohm M. Sildenafil in the treatment of Raynaud’s phenomenon resistant to vasodilatory therapy. Circulation. 2005;112:2980–5. doi: 10.1161/CIRCULATIONAHA.104.523324. [DOI] [PubMed] [Google Scholar]
- 17.Herrick AL, et al. Modified-release sildenafil reduces Raynaud’s phenomenon attack frequency in limited cutaneous systemic sclerosis. Arthritis Rheum. 2011;63:775–82. doi: 10.1002/art.30195. [DOI] [PubMed] [Google Scholar]
- 18.Friedman EA, Harris PA, Wood AJ, Stein CM, Kurnik D. The effects of tadalafil on cold-induced vasoconstriction in patients with Raynaud’s phenomenon. Clin Pharmacol Ther. 2007;81:503–9. doi: 10.1038/sj.clpt.6100103. [DOI] [PubMed] [Google Scholar]
- 19.Wright PJ. Comparison of phosphodiesterase type 5 (PDE5) inhibitors. Int J Clin Pract. 2006;60:967–75. doi: 10.1111/j.1742-1241.2006.01049.x. [DOI] [PubMed] [Google Scholar]
- 20.Caglayan E, et al. Phosphodiesterase type 5 inhibition is a novel therapeutic option in Raynaud disease. Arch Intern Med. 2006;166:231–3. doi: 10.1001/archinte.166.2.231. [DOI] [PubMed] [Google Scholar]
- 21.Kumar N, Griffiths B, Allen J. Thermographic and symptomatic effect of a single dose of sildenafil citrate on Raynaud’s phenomenon in patients with systemic sclerosis: a potential treatment. J Rheumatol. 2006;33:1918–9. [PubMed] [Google Scholar]
- 22.Minson CT, Berry LT, Joyner MJ. Nitric oxide and neurally mediated regulation of skin blood flow during local heating. J Appl Physiol. 2001;91:1619–26. doi: 10.1152/jappl.2001.91.4.1619. [DOI] [PubMed] [Google Scholar]
- 23.Hodges GJ, Sharp L, Clements RE, Goldspink DF, George KP, Cable NT. Influence of age, sex, and aerobic capacity on forearm and skin blood flow and vascular conductance. Eur J Appl Physiol. 2010;109:1009–15. doi: 10.1007/s00421-010-1441-7. [DOI] [PubMed] [Google Scholar]
- 24.Cankar K, Finderle Z, Strucl M. Gender differences in cutaneous laser doppler flow response to local direct and contralateral cooling. J Vasc Res. 2000;37:183–8. doi: 10.1159/000025729. [DOI] [PubMed] [Google Scholar]
- 25.Gardner-Medwin JM, Macdonald IA, Taylor JY, Riley PH, Powell RJ. Seasonal differences in finger skin temperature and microvascular blood flow in healthy men and women are exaggerated in women with primary Raynaud’s phenomenon. Br J Clin Pharmacol. 2001;52:17–23. doi: 10.1046/j.0306-5251.2001.01405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leppert J, et al. Seasonal variations in cyclic GMP response on whole-body cooling in women with primary Raynaud’s phenomenon. Clin Sci (Lond) 1997;93:175–9. doi: 10.1042/cs0930175. [DOI] [PubMed] [Google Scholar]
- 27.LeRoy EC, Medsger TA., Jr Raynaud’s phenomenon: a proposal for classification. Clin Exp Rheumatol. 1992;10:485–8. [PubMed] [Google Scholar]
- 28.Merkel PA, et al. Measuring disease activity and functional status in patients with scleroderma and Raynaud’s phenomenon. Arthritis Rheum. 2002;46:2410–20. doi: 10.1002/art.10486. [DOI] [PubMed] [Google Scholar]
- 29.Cutolo M, Grassi W, Matucci Cerinic M. Raynaud’s phenomenon and the role of capillaroscopy. Arthritis Rheum. 2003;48:3023–30. doi: 10.1002/art.11310. [DOI] [PubMed] [Google Scholar]
- 30.Maricq HR, Weinrich MC. Diagnosis of Raynaud’s phenomenon assisted by color charts. J Rheumatol. 1988;15:454–9. [PubMed] [Google Scholar]
- 31.O’Leary DS. Regional vascular resistance vs. conductance: which index for baroreflex responses? Am J Physiol. 1991;260:H632–7. doi: 10.1152/ajpheart.1991.260.2.H632. [DOI] [PubMed] [Google Scholar]


