Abstract
Early hospital readmission (EHR) after kidney transplantation (KT) is associated with increased morbidity and higher costs. Registry-based recipient, transplant, and center-level predictors of EHR are limited, and novel predictors are needed. We hypothesized that frailty, a measure of physiologic reserve initially described and validated in geriatrics and recently associated with early KT outcomes, might serve as a novel, independent predictor of EHR in KT recipients of all ages. We measured frailty in 383 KT recipients at Johns Hopkins Hospital. EHR was ascertained from medical records as ≥1 hospitalization within 30 days of initial post-KT discharge. Frail KT recipients were much more likely to experience EHR (45.8% vs. 28.0%, P=0.005), regardless of age. After adjusting for previously described registry-based risk factors, frailty independently predicted 61% higher risk of EHR (adjusted RR=1.61, 95% CI: 1.18–2.19, P=0.002). In addition, frailty improved EHR risk prediction by improving the area under the receiver operating characteristic curve (P=0.01) as well as the net reclassification index (P=0.04). Identifying frail KT recipients for targeted outpatient monitoring and intervention may reduce EHR rates.
Keywords: frailty, readmission, transplantation
INTRODUCTION
Nearly one third of kidney transplantation (KT) recipients are readmitted to the hospital within 30 days of discharge (1). Early hospital readmission (EHR) is associated with increased avoidable morbidity, costs, and transition-of-care errors (2–4), and of great interest in healthcare (5–7). We previously developed a registry-based model to predict EHR in KT recipients and identified recipient and transplant factors as important predictors of EHR in this population (1). However, registry-based prediction, while informative and hypothesis-generating, is insufficient for EHR risk prediction (1), so novel predictors are needed.
We hypothesized that frailty, a measure of physiologic reserve initially described and validated in geriatric populations (8), is not only applicable to patients of all ages with end stage renal disease (ESRD) but also may capture the type of risk in this population that leads to EHR. We have previously demonstrated that frailty is an important predictor of mortality and hospitalization in adults of all ages undergoing hemodialysis (9); this supports our hypothesis that ESRD patients experience accelerated aging and thus are similar to older adults. In a cohort of KT recipients of all ages, we have recently identified an association between frailty and delayed graft function (DGF) (10). Furthermore, in general surgery we showed that frailty improved risk prediction for postoperative complications, length of stay, and the need for a skilled nursing facility at discharge (11). Others have identified frailty (using alternative definitions of frailty) as a predictor of 30 day EHR in older adults undergoing colorectal surgery (12) as well as 60 day EHR in adults of all ages with chronic diseases or chronic impairment (13).
The goal of this study was to explore frailty, as defined and validated by Fried et al, as a potential risk factor for 30 day EHR in KT recipients of all ages and to test whether frailty could improve EHR risk prediction above and beyond our previously published registry-based model.
METHODS
Study Design
This was a prospective, longitudinal study of 383 KT recipients at Johns Hopkins Hospital, Baltimore, Maryland, between December 2008 and December 2012. Frailty was measured at the time of KT as described below. EHR was ascertained from medical records and defined as one or more hospitalizations within 30 days of initial post-KT discharge. Only 2 participants died within 30 days of KT and both experienced an EHR prior to death, so both were included in the study. Recipient and transplant factors (sex, age, race, BMI, recipient diabetes, recipient heart disease, time on dialysis, donor type, donor age, use of induction therapy, HLA mismatches, and KT length of stay) were ascertained from medical records. The Johns Hopkins Institutional Review Board approved the study.
Frailty
At admission for KT, frailty was measured as defined and validated by Fried et al (8, 14–23) as a phenotype based on 5 components: shrinking (self-report of unintentional weight loss of more than 10 lbs in the past year based on dry weight); weakness (grip-strength below an established cutoff based on gender and BMI); exhaustion (self-report); low activity (Kcals/week below an established cutoff); and slowed walking speed (walking time of 15 feet below an established cutoff by gender and height) (8). A score of 1 was given to those with the presence of each measured component. The aggregate frailty score was calculated as the sum of the component scores (range 0–5) and frailty was defined as a score of 3 or higher, as we previously have published (10).
Quantifying the Association of Frailty and EHR
The association between frailty and EHR was evaluated using modified Poisson regression (24) adjusted for recipient and transplant factors based on our previously published registry-based model (1). For the sake of parsimony, only recipient and transplant factors that were statistically significant predictors of EHR in the original registry-based model were included in this analysis. As a sensitivity analysis, the registry-based model was additionally adjusted for DGF to account for the association between frailty and DGF (10). We tested for effect modification of the association of frailty and EHR by age and race using a Wald test.
Assessing Statistical Prediction Improvement: Nested AUC
Two methods were used to assess and quantify the improvement in risk prediction of EHR by frailty. The overall statistical difference in predictive power of the registry model with and without frailty was measured by the area under the receiver operating characteristic curve (AUC) (25).
Assessing Clinically Relevant Prediction Improvement: Net Reclassification Index
Additionally, we tested the clinically relevant improvement in prediction using the net reclassification index (NRI) (26). In other words, the NRI was used to quantify the relative ability of the two models (registry-based alone versus registry-based plus frailty) in classifying the patients as low, intermediate, or high risk for EHR as selected a priori (<20%, 20–50%, and >50%). First, the patients were classified as above based on predicted probability derived from the model without frailty. Then, patients were reclassified in the same way using the model with frailty, and “movement” from the non-frailty classification to the frailty-based classification was quantified. Finally, the NRI quantified “correct movement” in risk classification: for participants who experienced EHR, correct movement was an upgrade in classification (low to intermediate, low to high, or intermediate to high); for participants who did not experience EHR, correct movement was a downgrade in classification (high to intermediate, high to low, or intermediate to low). NRI was calculated using the NRI package in Stata12, based on methods described by Pencina et al (26).
Statistical Analysis
For all analyses, a P value <0.05 was considered significant. All analyses were performed using STATA 12.0 (College Station, Texas).
RESULTS
Study Population
Among 383 study participants, the mean age was 53.5 years (SD=13.9), 39.7% were female, 38.9% were African American, and 18.8% were frail at KT. Consistent with previous findings of frailty as an independent domain, no recipient factors were associated with frailty except sex (Table 1). Similar to our findings from the national registry, 31.3% of KT recipients at our center were readmitted within 30 days of initial discharge.
Table 1.
Study Population of Kidney Transplant Recipients, Stratified by Frailty.
| No frailty (n=311) | Frailty (n=72) | P value | |
|---|---|---|---|
|
| |||
| Recipient factors | |||
| Age (Mean [SD]) | 53.1 [13.8] | 54.9 [14.3] | 0.3 |
| African American race (%) | 39.2 | 37.5 | 0.9 |
| Female sex (%) | 42.1 | 29.2 | 0.046 |
| Body mass index (kg/m2) (Mean [SD]) | 27.4 [5.4] | 27.4 [5.6] | 0.9 |
| Diabetes (%) | 15.8 | 23.6 | 0.12 |
| Heart disease (%) | 14.8 | 13.9 | 1.0 |
| Time on dialysis (years) (Mean [SD]) | 4.4 [5.7] | 4.5 [5.7] | 0.9 |
| Transplant factors | |||
| Live donor (%) | 48.6 | 38.9 | 0.2 |
| Donor age (Mean [SD]) | 41.0 [15.4] | 42.7 [14.6] | 0.4 |
| Received induction therapy (%) | 92.9 | 90.3 | 0.5 |
| 0 HLA mismatches (%) | 4.8 | 5.6 | 0.8 |
| Length of stay for the transplant (in days) (Median [IQR]) | 9.0 [7.0] | 9.5 [7.5] | 0.9 |
SD=Standard Deviation; IQR=Interquartile Range
Frailty and Early Hospital Readmission
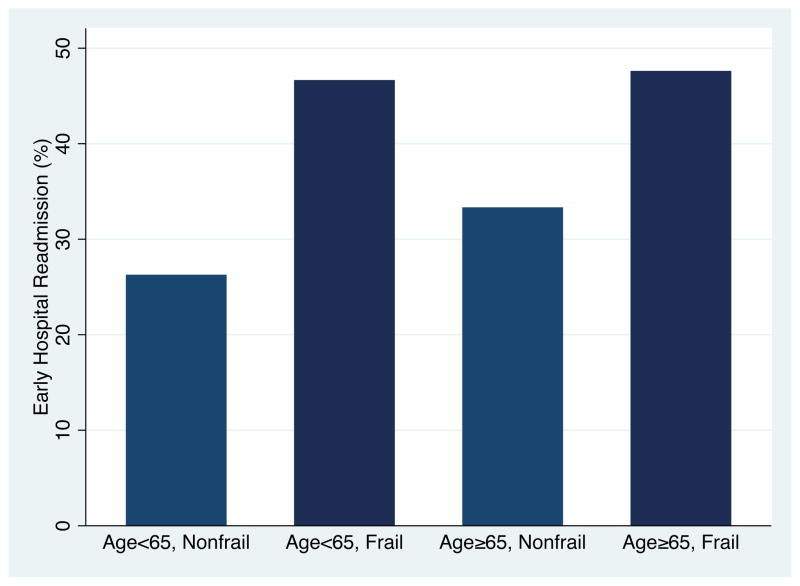
Frail KT recipients were much more likely to experience EHR (45.8% vs. 28.0%, P=0.005), regardless of age (Figure 1). Interestingly, frail younger recipients had the highest EHR rate (46.2%). In an unadjusted regression model, frail KT recipients were 1.64-fold (95% CI: 1.20–2.23, P=0.002) more likely to experience EHR than their non-frail counterparts. After adjusting for sex, age, race, BMI, recipient diabetes, recipient heart disease, time on dialysis, donor type, donor age, use of induction therapy, and HLA mismatches, frailty independently predicted 61% higher risk of EHR (adjusted RR=1.61, 95% CI: 1.18–2.19, P=0.002) in KT recipients. Adjusting for DGF did not alter the association of frailty and EHR (adjusted RR=1.59, 95% CI: 1.17–2.17, P=0.003). Consistent with our previous findings in KT, the frailty effect did not differ between older and younger KT recipients (interaction P=0.39) or African American and white KT recipients (interaction P=0.81).
Figure 1.
Early Hospital Readmission, by Age and Frailty.
Improved Prediction with Frailty
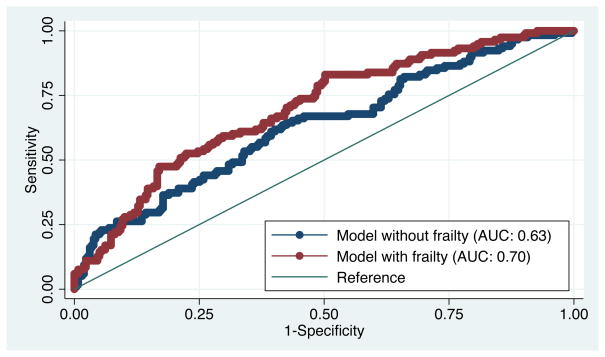
Importantly, frailty improved the ability to predict EHR, above and beyond our previously published registry-based model. The area under the ROC curve was statistically higher with the addition of frailty (0.70 vs. 0.63, P=0.008) to the 11 factors in the registry-based model (Figure 2). Frailty also improved the classification of KT recipients (Table 2); 10% (13/120) of those with EHR were correctly reclassified as being at higher risk for EHR by frailty and 10% (25/263) of those without EHR were correctly reclassified as being at lower risk of EHR by frailty (P=0.04).
Figure 2.
Receiver Operating Characteristic Curve for Early Hospital Readmission Prediction Models with and without Frailty.
Note: AUC is the area under the receiver operating characteristic curve. The registry-based model with frailty had statistically significant improvement in EHR prediction (P=0.01). The P value was obtained from a chi-squared test of the difference in the AUC for the registry-based models with and without frailty.
Table 2.
Reclassification Among Kidney Transplant Recipients Who Experienced Early Hospital Readmission (EHR) and Those Who Did Not.
| Model without frailty | Model with frailty | |||
|---|---|---|---|---|
| <20% | 20–50% | >50% | Total | |
| Participants who experience EHR | ||||
| <20% | 5 | 3 | 0 | 8 |
| 20–50% | 6 | 84 | 10 | 100 |
| >50% | 0 | 1 | 11 | 12 |
| Total | 11 | 88 | 21 | 120 |
| Participants who do not experience EHR | ||||
|
| ||||
| <20% | 33 | 5 | 0 | 60 |
| 20–50% | 24 | 187 | 8 | 219 |
| >50% | 0 | 1 | 5 | 6 |
| Total | 57 | 193 | 13 | 263 |
Note: This reclassification table, stratified by whether or not the participant had the outcome of EHR, was used to calculate the net reclassification index (NRI) (26) for frailty. The table quantifies the correct movement in categories (upward for those with EHR and downward for those without). For those with EHR 13/120 (10%) were correctly reclassified by including frailty in the model; for those without EHR, 10% (25/263) were correctly reclassified. Overall, the improvement was 10% (P=0.04).
DISCUSSION
In this single-center cohort of 383 patients, frail KT recipients were 1.6-times more likely to experience EHR than their non-frail counterparts, even after accounting for registry-based risk factors. Furthermore, frailty significantly improved EHR risk prediction when added to the registry-based model.
These results are consistent with previous findings that frailty is an important risk factor in surgical patients, patients undergoing hemodialysis, and transplant recipients. First, we previously showed that frailty predicted operative risk in older general surgery patients beyond that which is predicted by the Lee and Eagle or ASA scores (28). Furthermore, we identified frailty as a predictor of mortality and hospitalization in patients undergoing hemodialysis (9). Finally, we found that frailty doubled the risk of DGF in KT recipients (10). Additionally, Englesbe and colleagues studied sarcopenia as an objective and reproducible measure of frailty in liver transplant recipients, showing an association with 1-year mortality in these patients (27).
Previous studies have identified an association between frailty and EHR in older adults and patients of all ages with chronic conditions (12, 13). However, these studies did not define frailty using the 5 criteria established and validated by Fried et al; instead they used a combination of geriatric factors and comorbidities to define frailty. In one study of older adults undergoing elective colorectal surgery, EHR rates were higher for patients who they identified as frail (using an alternative definition) (32%) than for intermediately frail (15%) and non-frail (4%) patients (12). Our study extends the findings that frailty is a predictor of EHR to ESRD patients of all ages who are undergoing KT. Furthermore, our findings demonstrated that frailty is an independent predictor of EHR that improves risk prediction and is not just associated with a higher rate of EHR.
Strengths of this study were the prospective measurement of a validated, objective frailty instrument and granular ascertainment of the recipient and transplant factors that were identified as predictors of EHR in the registry-model using medical records abstraction. Additionally, frailty was ascertained immediately prior to KT and thus prior to EHR, so its predictive value could be assessed. The main limitation was the single-center study design so direct inferences must be interpreted in the context of the demographics of our study population, which is somewhat older and more represented by African Americans. That said, we did not identify any interactions between frailty and age or race so the likelihood that this novel association is specific only to our population is low.
Frailty is an independent predictor for EHR following KT in recipients; this novel measure of physiologic reserve, originally described in the geriatric literature, is predictive of EHR regardless of recipient age. Risk prediction of EHR is improved by frailty, which will allow high-risk recipients to be identified at the time of transplantation. Identifying frail KT recipients for prehabilitation prior to KT, improved transition-of-care at the time of KT, and targeted outpatient monitoring and interventions after KT may reduce the very high EHR rates seen in KT.
Acknowledgments
We thank the study participants, as well as the research staff at Johns Hopkins, especially Amanda Brennan and Erika Jones for their dedication to this study. This study was supported by a Clinical Scientist Development Award from the Doris Duke Charitable Foundation (Segev, PI). K23AG032885 (co-funded by the American Federation of Aging Research) and R21DK085409. Megan Salter was supported by T32AG000247 from the National Institute on Aging.
ABBREVIATIONS
- KT
Kidney transplantation
- EHR
Early hospital readmission
- ESRD
End stage renal disease
- DGF
Delayed graft function
- AUC
Area under the receiver operating characteristic curve
- NRI
Net reclassification index
Footnotes
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.McAdams-Demarco MA, Grams ME, Hall EC, Coresh J, Segev DL. Early hospital readmission after kidney transplantation: patient and center-level associations. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2012;12(12):3283–3288. doi: 10.1111/j.1600-6143.2012.04285.x. [DOI] [PubMed] [Google Scholar]
- 2.Lum HD, Studenski SA, Degenholtz HB, Hardy SE. Early hospital readmission is a predictor of one-year mortality in community-dwelling older Medicare beneficiaries. Journal of general internal medicine. 2012;27(11):1467–1474. doi: 10.1007/s11606-012-2116-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Medicare Payment Policy. Washington DC: Medicare Payment Advisory Committee; 2007. Report to Congress. [Google Scholar]
- 4.Hunter T, Nelson JR, Birmingham J. Preventing Readmissions Through Comprehensive Discharge Planning. Professional case management. 2013;18(2):56–63. doi: 10.1097/NCM.0b013e31827de1ce. [DOI] [PubMed] [Google Scholar]
- 5.QualityNet. Readmission measures overview: publicly reporting risk standardized, 30-day readmission for AMI, HF, and Ph. 2011 [cited 2011 October 25, 2011]; Available from: qualitynet.org.
- 6.Medicare Program, Hospital Inpatient Prospective Payment Systems for Acute Care Hospitals and the Long-Term Care Hospital Prospective Payment System and Fiscal Year 2012 Rates; Hospitals’ FTE Resident Caps for Graduate Medical Education Payment. Services. CfMaM, editor. Federal Registrar. 2011 Aug 18;2011 [PubMed] [Google Scholar]
- 7.Brock J, Mitchell J, Irby K, Stevens B, Archibald T, Goroski A, et al. Association between quality improvement for care transitions in communities and rehospitalizations among Medicare beneficiaries. JAMA. 2013;309(4):381–391. doi: 10.1001/jama.2012.216607. [DOI] [PubMed] [Google Scholar]
- 8.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 9.McAdams-Demarco MA, Law A, Salter M, Boyarsky B, Gimenez L, Jaar BG, et al. Frailty as a Novel Predictor of Mortality and Hospitalization in Hemodialysis Patients of All Ages. J Am Geriatr Soc. 2013 doi: 10.1111/jgs.12266. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garonzik-Wang JM, Govindan P, Grinnan JW, Liu M, Ali HM, Chakraborty A, et al. Frailty and delayed graft function in kidney transplant recipients. Arch Surg. 2012;147(2):190–193. doi: 10.1001/archsurg.2011.1229. [DOI] [PubMed] [Google Scholar]
- 11.Makary MA, Segev DL, Pronovost PJ, Syin D, Bandeen-Roche K, Patel P, et al. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg. 2010;210(6):901–908. doi: 10.1016/j.jamcollsurg.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 12.Robinson TN, Wu DS, Stiegmann GV, Moss M. Frailty predicts increased hospital and six-month healthcare cost following colorectal surgery in older adults. American journal of surgery. 2011;202(5):511–514. doi: 10.1016/j.amjsurg.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dai YT, Wu SC, Weng R. Unplanned hospital readmission and its predictors in patients with chronic conditions. Journal of the Formosan Medical Association = Taiwan yi zhi. 2002;101(11):779–785. [PubMed] [Google Scholar]
- 14.Bandeen-Roche K, Xue QL, Ferrucci L, Walston J, Guralnik JM, Chaves P, et al. Phenotype of frailty: characterization in the women’s health and aging studies. J Gerontol A Biol Sci Med Sci. 2006;61(3):262–266. doi: 10.1093/gerona/61.3.262. [DOI] [PubMed] [Google Scholar]
- 15.Barzilay JI, Blaum C, Moore T, Xue QL, Hirsch CH, Walston JD, et al. Insulin resistance and inflammation as precursors of frailty: the Cardiovascular Health Study. Arch Intern Med. 2007;167(7):635–641. doi: 10.1001/archinte.167.7.635. [DOI] [PubMed] [Google Scholar]
- 16.Cappola AR, Xue QL, Fried LP. Multiple hormonal deficiencies in anabolic hormones are found in frail older women: the Women’s Health and Aging studies. J Gerontol A Biol Sci Med Sci. 2009;64(2):243–248. doi: 10.1093/gerona/gln026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leng SX, Hung W, Cappola AR, Yu Q, Xue QL, Fried LP. White blood cell counts, insulin-like growth factor-1 levels, and frailty in community-dwelling older women. J Gerontol A Biol Sci Med Sci. 2009;64(4):499–502. doi: 10.1093/gerona/gln047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leng SX, Xue QL, Tian J, Walston JD, Fried LP. Inflammation and frailty in older women. J Am Geriatr Soc. 2007;55(6):864–871. doi: 10.1111/j.1532-5415.2007.01186.x. [DOI] [PubMed] [Google Scholar]
- 19.Newman AB, Gottdiener JS, McBurnie MA, Hirsch CH, Kop WJ, Tracy R, et al. Associations of subclinical cardiovascular disease with frailty. J Gerontol A Biol Sci Med Sci. 2001;56(3):M158–166. doi: 10.1093/gerona/56.3.m158. [DOI] [PubMed] [Google Scholar]
- 20.Walston J, McBurnie MA, Newman A, Tracy RP, Kop WJ, Hirsch CH, et al. Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities: results from the Cardiovascular Health Study. Arch Intern Med. 2002;162(20):2333–2341. doi: 10.1001/archinte.162.20.2333. [DOI] [PubMed] [Google Scholar]
- 21.Xue QL, Bandeen-Roche K, Varadhan R, Zhou J, Fried LP. Initial manifestations of frailty criteria and the development of frailty phenotype in the Women’s Health and Aging Study II. J Gerontol A Biol Sci Med Sci. 2008;63(9):984–990. doi: 10.1093/gerona/63.9.984. [DOI] [PubMed] [Google Scholar]
- 22.Chang SS, Weiss CO, Xue QL, Fried LP. Association between inflammatory-related disease burden and frailty: results from the Women’s Health and Aging Studies (WHAS) I and II. Archives of gerontology and geriatrics. 2012;54(1):9–15. doi: 10.1016/j.archger.2011.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang SS, Weiss CO, Xue QL, Fried LP. Patterns of comorbid inflammatory diseases in frail older women: the Women’s Health and Aging Studies I and II. J Gerontol A Biol Sci Med Sci. 2010;65(4):407–413. doi: 10.1093/gerona/glp181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 25.Pepe MS. The Statistical Evaluation of Medical Tests for Classification and Prediction. New York: Oxford University Press; 2003. [Google Scholar]
- 26.Pencina MJ, D’Agostino RB, Sr, D’Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Statistics in medicine. 2008;27(2):157–172. doi: 10.1002/sim.2929. discussion 207–112. [DOI] [PubMed] [Google Scholar]
- 27.Englesbe MJ, Patel SP, He K, Lynch RJ, Schaubel DE, Harbaugh C, et al. Sarcopenia and mortality after liver transplantation. Journal of the American College of Surgeons. 2010;211(2):271–278. doi: 10.1016/j.jamcollsurg.2010.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Makary MA, Segev DL, Pronovost PJ, Syin D, Bandeen-Roche K, Patel P, et al. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg. 2010;210(6):901–908. doi: 10.1016/j.jamcollsurg.2010.01.028. [DOI] [PubMed] [Google Scholar]