Abstract
Introduction
Dose-dense therapies have had a major impact on reducing toxicity and improving outcomes in breast cancer. A combination of docetaxel plus cyclophosphamide (TC) every 3 weeks has emerged as a common chemotherapy regimen used for treatment of node-negative or lower-risk node-positive breast cancer. We tested whether it is feasible to deliver TC on a dose-dense schedule, with therapy completed within 10 weeks.
Methods
We enrolled women with early stage breast cancer on a single-arm phase II study of adjuvant dose-dense TC (ddTC) through a regional oncology network. All women completed primary surgery prior to accrual and subsequent therapy with TC was deemed appropriate by the treating physician. Planned treatment was docetaxel 75 mg/m2 plus cyclophosphamide 600 mg/m2 every 2 weeks for 4 cycles with subcutaneous pegfilgrastim 6 mg administered 24-48 hours after the administration of each chemotherapy cycle.
Results
Of 42 women enrolled, 41 were evaluable by prespecified criteria. Of these, 37 (90.2%) completed therapy within 10 weeks and 34 (83%) completed therapy at 8 weeks without dose modification. Rates of neuropathy were similar to that reported previously. The rate of neutropenic fever was low (2.5%). Rash and plantar/palmar erythrodythesia were common and reached grade 3 in four subjects (9.8%).
Conclusion
Dose-dense TC is feasible with tolerability profiles similar to standard TC and a low likelihood of neutropenic fever. This study supports further clinical development of this 8-week adjuvant chemotherapy regimen.
Keywords: chemotherapy, granulocyte-colony stimulating factor, pegfilgrastim
Introduction
Despite advances in hormonal and targeted therapies, chemotherapy remains a cornerstone of the adjuvant treatment of breast cancer. Polychemotherapy improves disease-free and overall survival for breast cancer1. Over the past decades, chemotherapy has been refined through incorporation of highly effective agents, including adriamycin2 and taxanes3,4. A second attempted refinement was dose intensification. Escalations of chemotherapy doses offered no significant improvements in outcomes, even when these were high enough to necessitate stem-cell rescue3,5. In contrast to escalations, increases in dose density have yielded promising results. Here we extend this approach to the ‘TC’ chemotherapy regimen consisting of four cycles of docetaxel and cyclophosphamide.
Dose density is increased through more frequent administration of standard chemotherapy doses to allow less time for tumor recovery and growth6. Increases in dose density were enabled through the cloning and production of recombinant granulocyte-colony stimulating factor (GCSF)7,8. GCSF accelerates recovery from hematologic effects without affecting tumor cell recovery, allowing dosing at shortened intervals. Short-interval dosing enhances the therapeutic index for treatment of tumors that grow according to Gompertzian kinetics9. Dose-dense anthracycline-plus-taxane based therapy improves survival and reduces toxicity compared with the same treatment given every 3 weeks10. Emerging data suggests that the increases in dose density of taxanes may be more important than that of anthracyclines; therapeutic benefit has not been detected from increasing density of taxane-free regimens11, 12.
Non-anthracycline chemotherapy regimens are often selected for adjuvant treatment of node-negative breast cancers. These regimens avoid the risk of secondary cardiomyopathy associated with anthracyclines and include TC and CMF (cyclophosphamide, methotrexate and 5-fluorouracil). Moreover, the TC regimen, given every 3 weeks for 4 cycles, is superior to doxorubicin plus cyclophosphamide (AC) in disease-free and overall survival13, 14. For this reason, TC has emerged as a common therapeutic choice for adjuvant therapy of node-negative or low-risk node positive breast cancer. Ongoing studies are evaluating whether TC for six cycles is an effective alternative to anthracycline-plus-taxane adjuvant therapy15. If so, TC may emerge as the most commonly prescribed adjuvant chemotherapy for breast cancer.
The optimal adjuvant chemotherapy would not only be highly effective, but would also minimize the impact on patient's quality of life through rapid completion with minimal toxicity. Toward this goal, we hypothesized that dose-dense TC (ddTC) delivered every 2 weeks with pegfilgrastim for 4 cycles is a feasible adjuvant chemotherapy regimen. Here, we report the results of a single-arm phase II study that tests this hypothesis.
Patients and Methods
We performed an open-label single-arm regional phase II study to assess the feasibility of dose-dense TC therapy. The prespecified primary objective was feasibility as defined by at least 60% of patients receiving 90% of the total dose of therapy within 10 weeks. Secondary objectives were to estimate incidence of febrile neutropenia and frequency and grade of neuropathy; and to establish the distribution of achievable density of docetaxel and cyclophosphamide administration. The study was performed through the Wisconsin Oncology Network at a combination of academic and community practice sites. The study was approved by the IRB at the University of Wisconsin (Protocol CO10104; IRB submission ID 2011-0062-CP001), by the IRB at each site, and registered on clinicaltrials.gov (NCT01671319). Written informed consent was obtained from each participant prior to study enrollment. A total of 42 patients were enrolled between June 2011 and June 2012.
Patient Population
Patients aged 18 or older were allowed to enroll if they had completed definitive surgery for histologically confirmed early-stage invasive breast cancer within 84 days. Final margins were required to be negative. Subjects were eligible if they had node-negative or node-positive breast cancer for which TC × 4 cycles was recommended by the treating physician as standard of care. Patients with metastatic or inflammatory breast cancer were excluded. All other stages and types of cancer were allowed, although guidance was provided in the protocol that subjects with pNO or pNX disease should have sufficient risk to warrant chemotherapy, whereas for those with node-positive disease, there should be compelling reasons to select TC rather than standard anthracycline-plus-taxane therapy. This was enforced for subjects with pN2-3 disease, in which explicit agreement of the PI or study chair was required prior to entry (ultimately no pN2-3 subjects were enrolled). Prior chemotherapy for a previous cancer was allowed only if at least 5 years had elapsed. Adequate bone marrow, liver, and kidney function were required and subjects could have no clinical evidence of pre-existing persistent neuropathy. For women of childbearing potential, a negative pregnancy test was required. Adjuvant trastuzumab, radiotherapy and endocrine therapy were recommended per standard of care under the direction of the treating oncologist, but were not allowed before or concurrent with protocol therapy.
Treatment
Intravenous docetaxel 75 mg/m2 and cyclophosphamide 600 mg/m2 were delivered sequentially, each over 30-60 minutes on day 1 of a 14-day cycle for four total cycles. Standard premedications and antiemetics were recommended, which included dexamethasone 8 mg by mouth twice daily for 3 days starting on the day before chemotherapy. Treatment was held if day 1 blood counts were inadequate to proceed with chemotherapy (required absolute neutrophil count > 1000/μL, hemoglobin ≥ 8 g/dL, and platelets > 75,000/μL.) Chemotherapy was allowed as soon as hematologic parameters were met. Similarly, grade 3 non-hematologic toxicities required treatment delay but could resume as soon as these resolved to grade 1. Treatment delays of greater than 35 days between cycles required termination of study therapy. All participants received study-supplied pegfilgrastim 6 mg subcutaneously 24-48 hours after each cycle of therapy.
Adverse events and dose modifications
Adverse events were assessed using the National Cancer Institute Common Terminology Criteria for Adverse Events Version 4.0. Toxicity assessments were made on day 1 of each cycle, and as indicated clinically. All subjects had 4-6 week and 5-7 month follow up visits for a toxicity assessment. Due to the curative intent of therapy, dose reductions were discouraged when dose delays were sufficient to allow adequate recovery of myelosuppression, neuropathy or other adverse events. For a first case of febrile neutropenia, prophylactic antibiotics were recommended in lieu of dose reductions. However, the protocol specified dose reductions after resolution of a second episode of febrile neutropenia; for 2 episodes of grade 3 neuropathy; or for a single episode of another type of grade 3 toxicity.
Statistical considerations
A Simon Optimal 2-Stage design was employed. This study was designed to distinguish between the null hypothesis (ddTC is tolerable in ≤ 60% of participants) and the alternative hypothesis (therapy is tolerable in ≥ 80% of participants). The primary endpoint was the binary outcome of administering therapy within 10 weeks. The probability to conclude that the ddTC regimen is deserving of future investigation when, in fact, tolerability is no better than under the null hypothesis was set at 0.05 (significance level). The probability to conclude that the ddTC regimen is worth further consideration, when the true tolerability rate is at least 80%, is at least 0.85 (power). Given this, total sample size was 41, with 16 evaluable subjects required for stage I. In the first stage, the study was to be terminated if ddTC was tolerable in 10 or fewer of the 16 evaluable subjects. Evaluable subjects were defined as those who completed 4 cycles of therapy or withdrew due to an adverse event, intercurrent illness, or disease progression. Those that did not complete therapy for any other reason were deemed unevaulable. The protocol specified that unevaluable subjects were to be replaced. Secondary endpoints included incidence and grades of neutropenia and neuropathy, and the delivered dose density of each chemotherapy agent. Baseline patient characteristics, chemotherapy delivered and adverse events were summarized with median and range for continuous outcomes and with frequency and percentage for discrete outcomes.
Results
Demographics
A total of forty-two patients enrolled in this study at 5 sites between June 2011 and June 2012; all initiated protocol therapy. One subject electively withdrew from the study due to grade 1-2 side effects. She discontinued chemotherapy after one cycle, was replaced and excluded from subsequent analysis. The other 41 participants were considered evaluable (Figure 1). Trial participants were representative of a population seen in routine practice. The majority of participants were postmenopausal with node-negative, hormone receptor-positive breast cancer. However, sizable fractions of participants were premenopausal (n=15; 37%), had node-positive cancer (n=10; 24%), or had triple-negative disease (n=11; 27%). Surgical therapy was equally divided between mastectomy and breast conservation. Ten participants with node-positive disease had fewer than 4 involved nodes and nine of these had estrogen-receptor positive disease. One subject had stage IIB (T2N1M0) triple-negative breast cancer; non-anthracycline therapy was recommended due to prior therapy with doxorubicin for a breast cancer that occurred over 5 years earlier. Although participants with HER2-positive disease were eligible, only one was enrolled.
Figure 1.
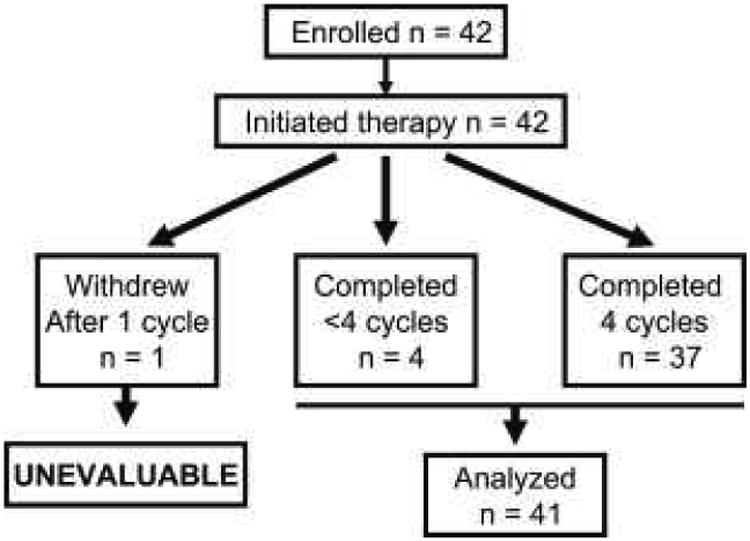
CONSORT diagram demonstrating the number of subjects enrolled, initiating and completing therapy with dose-dense TC.
Treatment
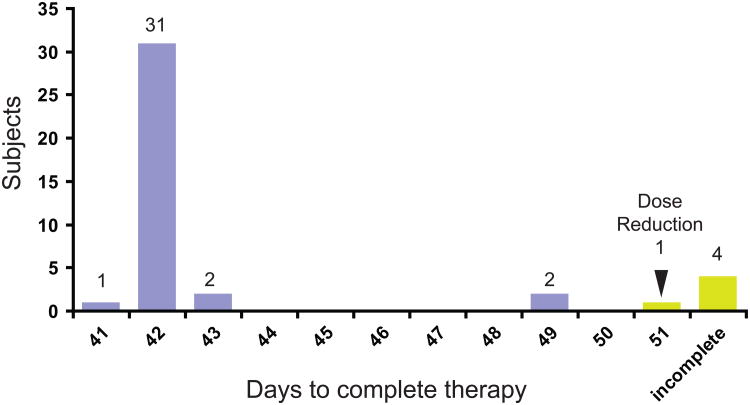
The primary endpoint of the study was the binary outcome of whether patients completed protocol therapy within 10 weeks. Thirty-seven subjects (90.2%) met this criterion, allowing us to conclude that the true rate of tolerability of this regimen exceeds 80% (Table 2). Most participants (78%) completed therapy as planned, with 41-43 days elapsing between the first and last infusions (Figure 2). Two participants had therapy delayed 1 week and completed therapy in 49 days. One participant had grade 3 hand pain, requiring a one-week delay and protocol-mandated dose reduction, completing therapy in 51 days. Four participants did not complete therapy, for a variety of reasons. One participant developed acute diverticulitis on Cycle 3 day 9, and was found to be afebrile with an absolute neutrophil count of 800/μL. After intravenous antibiotics, symptoms were improved, but protocol therapy was discontinued. Other participants discontinued therapy for grade 3 rash (n=2) and grade 3 palmar erythrodysesthesia (n=1)
Table 2. Chemotherapy delivered.
| Median Cycles received (range) | 4 (2-4) |
| Completed within 10 weeks (%) | 37 (90.2%) |
| Dose reduced (%) | 1 (2.4%) |
| Did not complete (%) | 4 (9.8%) |
| Density of docetaxel, mg/m2/weeka (n=37) | |
| Expected q3-week TC | 33.3 |
| Planned q2-week TC | 50.0 |
| Median Achieved (range) | 50 (38-51) |
| Density of cyclophosphamide, mg/m2/weeka (n=37) | |
| Expected q3week TC | 267 |
| Planned (q2-week TC) | 400 |
| Median Achieved (range) | 400 (296 – 410) |
Among subjects who completed 4 cycles of therapy
Figure 2.
Days to complete therapy. Most subjects completed 4 cycles of therapy within 43 days. Two subjects had a 1-week delay but completed all therapy. Subjects with dose reduction or incomplete therapy indicated in yellow. The protocol allowed chemotherapy dates to be rescheduled +/− 1 day for convenience, explaining completion in 41 and 43 days.
The ddTC regimen also met the secondary objective of increasing density of therapy (Table 2). Preplanned density was 50 mg/m2/week for docetaxel and 400 mg/m2/week for cyclophosphamide from first infusion to last. For most subjects, this density was achieved for both agents.
Adverse events
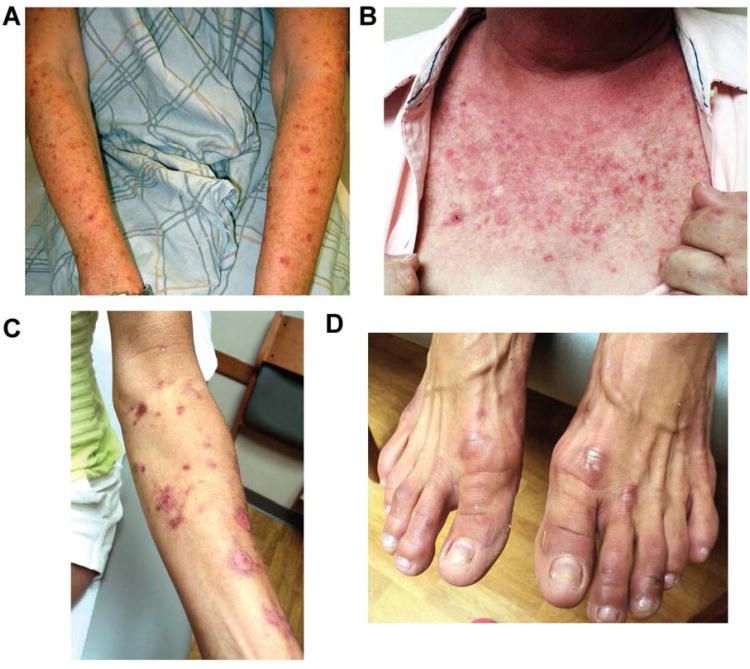
We anticipated that neutropenic fever and neuropathy might be clinically significant toxicities that limit the maximal density of TC therapy. However, these expectations were not realized, as only one subject experienced neutropenic fever (in addition to the neutropenic infection—diverticulitis—described above). Moreover, grade 2 neuropathy was observed in only 6 subjects (15%; 5 sensory, 1 motor) and no subject had grade 3 or higher neuropathy. We did observe an incidence of rash that exceeded expectations. Rashes were of various types including maculopapular, desquamating, and palmar/plantar erythrodysesthesia (Figure 3). In three cases the rash was severe and persistent enough that therapy was ultimately discontinued (two desquamating rashes, one palmar/plantar erythrodysesthesia). Other toxicities at grade 2 or higher were infrequent (Table 3). These results demonstrate that dose-dense TC therapy can be delivered safely.
Figure 3.
Rashes observed with protocol therapy A. Grade 1 maculopapular rash (subject 7, cycle 3 day 1). B. Grade 2 maculopapular rash (subject 42, cycle 3 day 7). C. Grade 3 desquamating rash (subject 41, cycle 3 day 1). D. Resolving acral erythrodysesthesia (subject 41).
Table 3. Adverse Events ≥ Grade 2a.
| Grade 2 | Grade 3 | ||||
|---|---|---|---|---|---|
| Type | Event | n | % | n | % |
| General | |||||
| Hypersensitivity/immune | 4 | 10% | 1 | 2% | |
| Fatigue | 11 | 27% | 1 | 2% | |
| Dermatologic | |||||
| Extravasation/skin reaction | 4 | 10% | - | - | |
| Palmar-plantar Erythrodysesthesia | 7 | 17% | 2 | 5% | |
| Rash | 8 | 20% | 2 | 5% | |
| Gastrointestinal | |||||
| Diarrhea | 6 | 15% | - | - | |
| Dehydration | 2 | 5% | - | - | |
| Anorexia/dysgeusea | 3 | 7% | - | - | |
| Stomatitis | 1 | 2% | - | - | |
| Gastritis | 2 | 5% | - | - | |
| Neurologic/Psychiatric | |||||
| Syncope | - | - | 2 | 5% | |
| Neuropathy | 6 | 15% | - | - | |
| Suicidal ideation | 1 | 2% | - | - | |
| HEENT/Pulmonary | |||||
| Tearing or dry eyes | 3 | 7% | - | - | |
| Sinus congestion | 1 | 2% | - | - | |
| Dyspnea | 2 | 5% | - | - | |
| Pain | |||||
| Headache | 4 | 10% | - | - | |
| Other pain | 13 | 32% | - | - | |
| Hematology | |||||
| Anemia | 9 | 22% | 1 | 2% | |
| Neutropenia at day 1b | - | - | - | - | |
| Neutropenic fever | - | - | 1 | 2% | |
| Neutropenic infection | - | - | 1 | 2% | |
| Other infection | 3 | 7% | 0 | - | |
No grade 4-5 events were reported.
Inter-cycle cell counts were not routinely monitored. A total of four episodes of neutropenia were reported between cycles, but only one was associated with infection.
Discussion
Increasing dose density of chemotherapy with GCSF has been a successful paradigm for increasing the safety and efficacy of standard adjuvant therapies for breast cancer10. Non-anthracycline regimens are increasingly being selected by oncologists because of efficacy and decreased risk of cardiotoxicity. TC is one commonly selected regimen for patients who have node-negative or lower-risk node-positive cancer, or for whom anthracycline therapy is contraindicated. Indeed, when compared with 4 cycles of AC chemotherapy every 3 weeks, TC provides a survival advantage among subjects including those with node-positive cancers14. A dose-dense regimen may improve the convenience and efficacy of the TC regimen.
In this phase II trial, we demonstrated the safety and tolerability of dose-dense TC therapy. We found that 90% of subjects completed ddTC therapy within 10 weeks. This compares favorably with the 93% of patients who completed standard q3-week TC therapy.13 Moreover, safety and adverse event rates of ddTC is comparable to TC given every 3 weeks (Table 4). Neuropathy had the greatest difference—15% experienced at least grade 2 with ddTC versus only 4% with standard TC in one reported study. Over 90% of subjects complete therapy with both q2-week and q3-week regimens. As seen with other dose-dense regimens, the rate of febrile neutropenia is significantly reduced due to the routine addition of GCSF. However, we did observe modestly increased rates of grade 2 or higher pain and neuropathy compared with what has been reported for q3-week therapy.
Table 4. Comparison of toxicities among studies of q3-week and q2-week TC.
| Completed | Grade ≥2 Rash | Grade ≥2 Neuropathy | Febrile Neutropenia | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Study | Na | n | % | n | % | n | % | cycle 1 GCSF | n | % |
| q3 week TC | ||||||||||
| Jones et al.13 | 506 | 469 | 92.7% | NRb | NR | NR | NR | NR | 25 | 4.9% |
| Takabatake et al.16 | 53 | 50 | 94.3% | 11 | 20.8% | 2 | 3.8% | no | 15 | 28.3% |
| Soong et al.25 | 12 | NR | NR | NR | NR | NR | NR | no | 6 | 50.0% |
| Chan et al.24 | 159 | 140 | 88.1% | NR | NR | NR | NR | no yes |
8/32 8/127 |
25% 6% |
| q2 week TC | ||||||||||
| This study | 41 | 37 | 90.2% | 11 | 26.8% | 6 | 14.6% | yes | 1 | 2.4% |
N a total sample size
NR, not reported
Grade 2 and 3 rashes were seen with dose-dense administration of TC (27%). However, these have been reported at a similar rate with q3-week therapy (21%) (Table 4).16 Palmar-plantar erythrodysesthesia is commonly associated with fluorouracil derivatives. Nevertheless, this has been reported with docetaxel use and in one study occurred in 11% of patients who received this medication.17 Another adjuvant study demonstrated dose-dense docetaxel was associated with up to 29% incidence of grade 2 or 3 palmar-plantar erythrodysesthesia.18 We are unable to determine whether this rash was potentiated by the dose-dense schedule or pegfilgrastim use. A case-report has suggested association between pegfilgrastim and palmar-plantar erythrodysesthesia.19
Duration of therapy
For the early adjuvant polychemotherapy for breast cancer, CMF, the optimal duration is 6 months20, 21. The dose dense AC-T (paclitaxel) regimen takes 4 months. Even the shortest regimens, AC and TC chemotherapy, are typically delivered in 4 cycles 3 weeks apart and thus completed in 64 days. Here, we demonstrate the safety of a 43-day adjuvant chemotherapy regimen, making this one of the briefest adjuvant regimens to our knowledge. Given the large number of women diagnosed with node-negative breast cancers, this brief regimen may have the advantage of minimizing interruption of work and family obligations and maximizing quality of life.
One potential concern with ddTC is the cost-effectiveness of this approach. However, this may not be a major concern because standard TC is cost-effective compared with AC22, 23, and, moreover, GCSF is often used with standard TC to reduce the significant risk of febrile neutropenia16, 24, 25. Given these considerations, we believe that ddTC is likely cost effective.
Strengths of this study include the multi-institutional network, including both academic and community practices and the rapid accrual. The study population reflected patients commonly encountered in the community practice setting, for whom TC is often recommended. However, this was a single-arm phase II study, limiting direct comparisons for efficacy or safety for q2- versus q3-week TC therapy. Furthermore, this study does not address the feasibility of administering a longer course of q2-week TC (i.e. 6 cycles).
Conclusion
This study demonstrates the safety and feasibility of 4 cycles of TC therapy given on a q2-week schedule. Randomized studies are warranted to determine whether this is equal or superior in efficacy to conventional q3-week TC; if so, this could represent a safe and rapid adjuvant chemotherapy regimen for low-risk breast cancer.
Table 1. Baseline Patient Characteristics.
| Demographics | n | % |
|---|---|---|
| Age median (range) | 54 (28-73) | |
| Premenopausal | 15 | 37 |
| Postmenopausal | 26 | 63 |
| Race | ||
| White | 39 | 95 |
| African-American | 2 | 5 |
| Ethnicity | ||
| Non-hispanic | 39 | 95 |
| Hispanic or Latino | 2 | 5 |
| Staging Criteria | ||
| Node negative | 30 | 73 |
| Node positive | 10 | 24 |
| Unknown | 1 | 2 |
| T1 | 21 | 51 |
| T2 | 20 | 49 |
| Receptor Status | ||
| Hormone receptor+ | 29 | 71 |
| HER2+ | 1 | 2 |
| Triple negative | 11 | 27 |
| Final Surgery | ||
| Mastectomy | 20 | 49 |
| Breast conservation | 21 | 51 |
Clinical Practice Points.
Increasing dose-density of adjuvant chemotherapy regimens with growth factors can improve effectiveness and reduce risk of infections
Docetaxel-cyclophosphamide (TC) every 3 weeks for 4 cycles is a commonly used adjuvant treatment of breast cancer.
This trial demonstrates that it is safe and feasible to deliver dose-dense TC every 2 weeks with pegfilgrastim support.
Additional studies are required to determine whether dose-dense TC improves efficacy of treatment.
Acknowledgments
Funding: We thank our patients and A-C. Andrei for assistance with statistical design. Funding was provided by National Cancer Institute Cancer Center Support Grant P30 CA014520. M.E.B. received salary support from Flight Attendant Medical Research Institute award 062541_YCSA. M.E.B and A.J.T received salary and training support from the Clinical and Translational Science Award (CTSA) program, through the NIH National Center for Advancing Translational Sciences (NCATS), grant UL1TR000427. Amgen provided pegfilgrastim for this study.
Footnotes
Conflict of Interest: The pegfilgrastim used in this study was provided by Amgen. The authors declare no other conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Polychemotherapy for early breast cancer: an overview of the randomised trials. Early Breast Cancer Trialists' Collaborative Group. Lancet. 1998;352:930–942. [PubMed] [Google Scholar]
- 2.Bonadonna G, Zambetti M, Valagussa P. Sequential or alternating doxorubicin and CMF regimens in breast cancer with more than three positive nodes. Ten-year results. JAMA. 1995;273:542–547. [PubMed] [Google Scholar]
- 3.Henderson IC, Berry DA, Demetri GD, et al. Improved outcomes from adding sequential Paclitaxel but not from escalating Doxorubicin dose in an adjuvant chemotherapy regimen for patients with node-positive primary breast cancer. Journal of Clinical Oncology. 2003;21:976–983. doi: 10.1200/JCO.2003.02.063. [DOI] [PubMed] [Google Scholar]
- 4.Martin M, Pienkowski T, Mackey J, et al. Adjuvant docetaxel for node-positive breast cancer. New England Journal of Medicine. 2005;352:2302–2313. doi: 10.1056/NEJMoa043681. [DOI] [PubMed] [Google Scholar]
- 5.Berry DA, Ueno NT, Johnson MM, et al. High-dose chemotherapy with autologous stem-cell support as adjuvant therapy in breast cancer: overview of 15 randomized trials. Journal of Clinical Oncology. 2011;29:3214–3223. doi: 10.1200/JCO.2010.32.5910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Norton L. Evolving concepts in the systemic drug therapy of breast cancer. Seminars in Oncology. 1997;24:S10-13–S10-10. [PubMed] [Google Scholar]
- 7.Welte K, Platzer E, Lu L, et al. Purification and biochemical characterization of human pluripotent hematopoietic colony-stimulating factor. Proceedings of the National Academy of Sciences of the United States of America. 1985;82:1526–1530. doi: 10.1073/pnas.82.5.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nagata S, Tsuchiya M, Asano S, et al. Molecular cloning and expression of cDNA for human granulocyte colony-stimulating factor. Nature. 1986;319:415–418. doi: 10.1038/319415a0. [DOI] [PubMed] [Google Scholar]
- 9.Norton L. Adjuvant breast cancer therapy: current status and future strategies--growth kinetics and the improved drug therapy of breast cancer. Seminars in Oncology. 1999;26:1–4. [PubMed] [Google Scholar]
- 10.Citron ML, Berry DA, Cirrincione C, et al. Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: first report of Intergroup Trial C9741/Cancer and Leukemia Group B Trial 9741. Journal of Clinical Oncology. 2003;21:1431–1439. doi: 10.1200/JCO.2003.09.081. [DOI] [PubMed] [Google Scholar]
- 11.Venturini M, Del Mastro L, Aitini E, et al. Dose-dense adjuvant chemotherapy in early breast cancer patients: results from a randomized trial. Journal of the National Cancer Institute. 2005;97:1724–1733. doi: 10.1093/jnci/dji398. [DOI] [PubMed] [Google Scholar]
- 12.Cameron D, Barrett-Lee P, Canney P, et al. The UK TACT2 Trial: comparison of standard vs accelerated epirubicin in patients requiring chemotherapy for early breast cancer (EBC) (CRUK/05/019) Cancer Research. 2012;72:S3–3. [Google Scholar]
- 13.Jones SE, Savin MA, Holmes FA, et al. Phase III trial comparing doxorubicin plus cyclophosphamide with docetaxel plus cyclophosphamide as adjuvant therapy for operable breast cancer. Journal of Clinical Oncology. 2006;24:5381–5387. doi: 10.1200/JCO.2006.06.5391. [DOI] [PubMed] [Google Scholar]
- 14.Jones S, Holmes FA, O'Shaughnessy J, et al. Docetaxel With Cyclophosphamide Is Associated With an Overall Survival Benefit Compared With Doxorubicin and Cyclophosphamide: 7-Year Follow-Up of US Oncology Research Trial 9735. Journal of Clinical Oncology. 2009;27:1177–1183. doi: 10.1200/JCO.2008.18.4028. [DOI] [PubMed] [Google Scholar]
- 15.NSABP B-49: Docetaxel and cyclophosphamide compared to anthracycline-based chemotherapy in treating women with HER2-negative breast cancer. http://clinicaltrials.gov/show/NCT01547741.
- 16.Takabatake D, Taira N, Hara F, et al. Feasibility study of docetaxel with cyclophosphamide as adjuvant chemotherapy for Japanese breast cancer patients. Japanese Journal of Clinical Oncology. 2009;39:478–483. doi: 10.1093/jjco/hyp050. [DOI] [PubMed] [Google Scholar]
- 17.Kawaguchi K, Ishiguro H, Morita S, et al. Correlation between docetaxel-induced skin toxicity and the use of steroids and H(2) blockers: a multi-institution survey. Breast Cancer Research and Treatment. 2011;130:627–634. doi: 10.1007/s10549-011-1641-9. [DOI] [PubMed] [Google Scholar]
- 18.Puhalla S, Mrozek E, Young D, et al. Randomized phase II adjuvant trial of dose-dense docetaxel before or after doxorubicin plus cyclophosphamide in axillary node-positive breast cancer. Journal of Clinical Oncology. 2008;26:1691–1697. doi: 10.1200/JCO.2007.14.3941. [DOI] [PubMed] [Google Scholar]
- 19.Bardia A, Loprinzi CL, Goetz MP. Hand-foot syndrome after dose-dense adjuvant chemotherapy for breast cancer: a case series. Journal of Clinical Oncology. 2006;24:e18–19. doi: 10.1200/JCO.2006.06.1143. [DOI] [PubMed] [Google Scholar]
- 20.Tancini G, Bonadonna G, Valagussa P, Marchini S, Veronesi U. Adjuvant CMF in breast cancer: comparative 5-year results of 12 versus 6 cycles. Journal of Clinical Oncology. 1983;1:2–10. doi: 10.1200/JCO.1983.1.1.2. [DOI] [PubMed] [Google Scholar]
- 21.Group IBCS. Duration and reintroduction of adjuvant chemotherapy for node-positive premenopausal breast cancer patients. Journal of Clinical Oncology. 1996;14:1885–1894. doi: 10.1200/JCO.1996.14.6.1885. [DOI] [PubMed] [Google Scholar]
- 22.Liubao P, Xiaomin W, Chongqing T, et al. Cost-effectiveness analysis of adjuvant therapy for operable breast cancer from a Chinese perspective: doxorubicin plus cyclophosphamide versus docetaxel plus cyclophosphamide. Pharmacoeconomics. 2009;27:873–886. doi: 10.2165/11314750-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 23.Younis T, Rayson D, Skedgel C. The cost-utility of adjuvant chemotherapy using docetaxel and cyclophosphamide compared with doxorubicin and cyclophosphamide in breast cancer. Curr Oncol. 2011;18:e288–296. doi: 10.3747/co.v18i6.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chan A, Fu WH, Shih V, Coyuco JC, Tan SH, Ng R. Impact of colony-stimulating factors to reduce febrile neutropenic events in breast cancer patients receiving docetaxel plus cyclophosphamide chemotherapy. Supportive Care in Cancer. 2011;19:497–504. doi: 10.1007/s00520-010-0843-8. [DOI] [PubMed] [Google Scholar]
- 25.Soong D, Haj R, Leung MG, et al. High rate of febrile neutropenia in patients with operable breast cancer receiving docetaxel and cyclophosphamide. Journal of Clinical Oncology. 2009;27:e101–102. doi: 10.1200/JCO.2009.23.0508. [DOI] [PubMed] [Google Scholar]