Abstract
Nonviral systems for nucleic acid delivery offer a host of potential advantages compared with viruses, including reduced toxicity and immunogenicity, increased ease of production and less stringent vector size limitations, but remain far less efficient than their viral counterparts. In this article we review recent advances in the delivery of nucleic acids using polymeric and inorganic vectors. We discuss the wide range of materials being designed and evaluated for these purposes while considering the physical requirements and barriers to entry that these agents face and reviewing recent novel approaches towards improving delivery with respect to each of these barriers. Furthermore, we provide a brief overview of past and ongoing nonviral gene therapy clinical trials. We conclude with a discussion of multifunctional nucleic acid carriers and future directions.
Nucleic acid therapies have enormous potential in the clinic, from treatment of specific genetic diseases such as cystic fibrosis [1], Leber hereditary optic neuropathy [2], hemoglobinopathies [3,4] and hemophilia [5], to the treatment of cancer [6,7], cardiovascular disease [8] and the use of genetic vaccines [9]. Additionally, nucleic acid delivery plays a crucial role in cellular engineering and basic biomedical research through the ability to knock-in and knockdown genes and proteins in the laboratory, as well as in the creation of induced pluripotent stem cells via viral methods [10,11] and investigations into the induction of induced pluripotent stem cells via nonviral [12] methods. The central challenge for effective therapy using nucleic acids is finding a safe and effective delivery system [13]. Since viral gene therapy can have serious safety concerns [14], recent efforts have focused on nonviral methods.
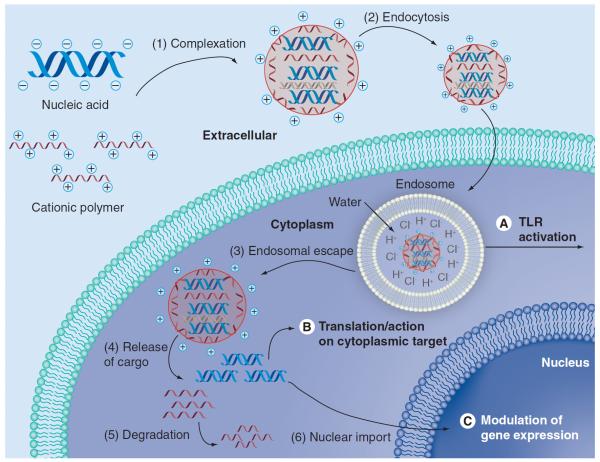
Nonviral methods can be used to deliver various nucleic acids (Table 1), including DNA [15], siRNA [16–18] for RNAi [19], isRNA [20], shRNA [21], agRNA and saRNA [22,23]. The choice of nucleic acid to deliver may influence where the nanocarrier needs to deliver its cargo (Figure 1). For example, to target Toll-like receptors (TLRs) such as TLR-3, -7 and -8, isRNA should be targeted to the endosome itself [20]. siRNA needs to get into the cytoplasm; therefore, vectors that carry these cargoes, if they are trafficked through the endosome, need some method to escape it. Finally, DNA, shRNA-encoding plasmids, agRNA and saRNA all need to be further transported from the cytoplasm into the nucleus to be expressed, to interfere with, or to promote gene expression.
Table 1.
Summary of results of various polymeric and inorganic vectors for delivering genes.
| Materials used & size | Cargo (DNA) | Target | Cell viability | Transfection efficacy | Ref. |
|---|---|---|---|---|---|
| Folate PLL, chloroquine | Luc | KB cells | Not reported in detail | 6-times higher than w/o folate at 24 hpt | [37] |
| Partially histidylated PLL | Luc | HepG2 | No cytotoxicity 4–24 h incubation | ~5 log orders of higher RLU than PLL at 48 hpt | [30] |
| Galactosylated PLL (Gal13-PLL13000; ~179 nm | CAAT | Human epitome cell line HepG2 | Not reported in detail | ~850 mU/mg at 48 hpt | [34] |
| 800 kDa PEI (nitrogen to DNA base ratio 9:1) | Luc | 3T3 cells | Only toxicity above concentration for optimal efficiency | 4 log orders more efficient than PLL (light units/mg protein) | [38] |
| Low molecular weight PEI at 11.9 kDa; high molecular weight PEI at 1620 kDa | Luc | ECV304 | MTT assay: low molecular weight, none up to 1 mg/ml; high molecular weight, IC50: 35 μg/ml | Low molecular weight PEI (N:P = 66.66) RLU was 100-fold higher than high molecular weight PEI (N:P = 13.33) | [40] |
| Fully deAc linear PEI: 25 kDA (PEI25) and 87 kDa (PEI87) | β-Gal in vitro; Luc in vivo (mouse) | A549 cells in vitro; lungs in vivo | >80% viability with N:P ratio <20; increasing toxicity with increasing N:P; deAc-PEI was more toxic than PEI | deAc-PEI25 21x higher than Ac-PEI25 in vitro; 115x-higher expression vs PEI25 seen by deAc-PEI87 in vitro; 10 N:P deAc-PEI25 showed 5 log orders of higher RLU and 1500-fold enhancement in lung specificity vs PEI25 in vivo | [44] |
| PBAE (C32); 71 nm; 1.2:1 amine:acrylate ratio | Luc | COS-7 | No cytotoxicity observable | Better than Lipofectamine™ 2000 | [50] |
| PBAE (C32–117); <200 nm | Luc | HUVEC | 2 orders of magnitude lower than 25 kDa PEI | ~adenovirus and lentivirus; 2 log orders greater than 25 kDa PEI | [51] |
| PBAE (C32–117); ~200 nm | GFP and RFP | hESC | 70% at 24 hpt | 50% at 24 hpt | [17] |
| (Mannose/galactose)-PEG-PAMAM linear-dendritic hybrid polymers (~150 nm) | Luc | P388D1 murine macrophages bearing man-receptor | 60–80% for G5.0 and 50–70% for G6.0 when transfecting P388D1 cells | Man-PEG-G6.0 transfected P388D1 1.6–1.8-fold more efficiently than PEI with no serum and fourfold more efficiently in the presence of serum | [63] |
| Chitosan (~150 nm) | Arah2 (peanut allergy gene) | Oral administration | Not reported in detail | Decreased IgE levels in response to anaphylaxis induction | [76] |
| Biomineral solution (CaCl2, KH2PO4, NaCl, KCl, MgSO4, MgCl2, NaHCO3) (Figure 3) | β-Gal | MG-63 | >90% at 24 h using concentrations ranging from 1 to 20 μg/ml | Transfection is greater than Transfast™ (1 μg/ml) using inorganic mineral solutions at 1, 10 and 20 μg/ml | [144] |
| Tetra(piperazino) fullerene epoxide | eGFP | C57/BL6 mice | No acute toxicity for liver or kidney | Increased plasma insulin levels and reduced blood glucose concentrations | [102] |
| ZnO quantum dots with poly(2-[dimethylamino] ethyl methacrylate) | Luc | COS-7 | 90% at 50 μg/ml; however, at 100 μg/ml (experimental levels) viability was 18%, which is most likely due to quantum dot vectors | ~1 log order lower than PEI(25 k) at 48 hpt | [265] |
| PLGA nanoparticles (slightly <200 nm) with spermidine or protamine used as a counter ion to the siRNA in the loading process | Anti-MAPK1 (ERK2) siRNA in vitro, anti-eGFP siRNA in vivo | Vaginal epithelium | In vitro: no observed decreased cell viability up to 10 mg/ml; in vivo: no histological changes (mice significant inflammation when treated with siRNA lipopolyplexes) | Spermidine improved loading by >40-fold; in vitro: ≥ gene silencing compared with Lipofectamine™ RNAiMax; in vivo: 50–60% knockdown in vaginal epithelium and submucosa | [48] |
| Mg2Al(OH)6NO3; layered double hydroxide nanoparticles; ~100 nm | Anti-MAPK1 (ERK2) siRNA | HEK293T | >80% over 3-day period; IC50: 0.125 mg ml−1 | RLU for nanoparticle alone and with siRNA: ~1.1 a.u. at 24 hpt and ~0.1 a.u. at 8 hpt | [121] |
| PBAE (C32–221)-siRNA-SS-PEG- AuNP; ~100 nm | Anti-Luc siRNA | HeLa | ~90% at 24 hpt | ~95% at 24 hpt | [93] |
| Mesoporous silica nanoparticles-PEI; ~100 nm | Anti-eGFP siRNA | PANC-1 | Not reported in detail | 61.7% at 72 hpt | [105] |
| AuNP, siRNA, MUA, 25 kDa PEI (LbL); 26.8 nm | Anti-eGFP siRNA | CHO-K1 | ~95% at 0.37 nM AuNP | ~72% at 0.37 nM AuNP | [203] |
| Lipospheres, cationic lipid metafectene, dioleoylphosphatidyl-ethanolamine functionalized with SPION | Anti-eGFP and Anti-Luc siRNA | eGFP-HeLa, firefly Luc-HeLa | Anti-eGFP siRNA: not significantly affected; anti-Luc: 50% viability; likely due to transfection | 90% at 48 hpt | [143] |
It is important to note that direct comparisons are difficult as the experimental setups are likely different.
β-Gal: β-galactosidase; a.u.: Absorbance units; CAAT: Chloramphenicol acetyltransferase; deAc: Deacetylated; (e)GFP: (Enhanced) green fluorescent protein; hESC: Human embryonic stem cell; hpt: Hours post-transfection; HUVEC: Human umbilical vein endothelial cell; Luc: Luciferase; MTT: 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; PBAE: Poly(β-amino esters); PEG: Polyethylene glycol; PEI: Poly(ethylene imine); PLGA: Poly(lactide-co-glycolide); PLL: Poly(l-lysine); RFP: Red fluorescent protein; RLU: Relative light units SPION: Superparamagnetic iron oxide nanoparticle.
Figure 1. Barriers to intracellular nucleic acid delivery.
(1) Nucleic acid must be complexed to the nanocarrier and protected from degradation as it makes its way to the target cell. (2) The nanocarrier and cargo must be internalized successfully. (A) TLR7 is localized to the endosome; for isRNA activity, endosomal escape is not required. For other nucleic acid, (3) endosomal escape is required. (B) (4) For cytoplasmic activity, nucleic acid must be released intracellularly. (5) Nanocarrier degradation is not required, but is useful for reduced toxicity. (C) (6) For DNA, shRNA-encoding plasmids, and agRNA, nuclear import is required for successful effect.
There are several barriers to cellular entry and delivery of the nucleic acid cargo that challenge the development of an effective delivery vehicle (Figure 1). The vehicle needs to form a stable complex with its nucleic acid cargo, protect it from degradation extracellularly, arrive at the cell of interest, get internalized (typically via either receptor-mediated endocytosis and/or nonspecific endocytic pathways), escape endolysosomal degradation, release its cargo and harmlessly degrade or otherwise be eliminated.
After escaping the endosomal compartment and making it into the cytoplasm, nucleic acids, such as DNA and agRNA, need to make it to the nucleus. This is among the largest challenges remaining for nonviral gene delivery. Simply getting the plasmid into the cytoplasm of the cell is not sufficient; in order to achieve the same level of transfection, delivery of up to 100-fold more DNA to the cytoplasm is required compared with direct delivery of DNA to the nucleus [24]. Dividing cells are more easily transfected due to the breakdown of the nuclear membrane that occurs during mitosis. While this breakdown can enhance localization of plasmids to the nucleus and transfection efficiency, cell division is not a requirement for successful transfection. Plasmids can also enter the nucleus through nuclear pore complexes (NPCs) when they are coupled to nuclear localization signals (NLSs; i.e., PKKKRKV), but this process is not as efficient [25].
Here we review current progress in nonviral nucleic acid delivery, with a focus on cationic polymers and inorganic nanoparticles (as well as their hybrids). Lipid-based materials for nucleic acid delivery are outside the scope of this article and are well-described elsewhere for siRNA [26] and gene delivery [27]. General properties and biomedical applications of polymeric and inorganic materials are described first. This is then followed by a discussion of new approaches to solve barriers to nonviral delivery when using these materials. Subsequently, an overview of past and present nonviral gene-therapy clinical trials is discussed.
Materials/general properties
Cationic polymers
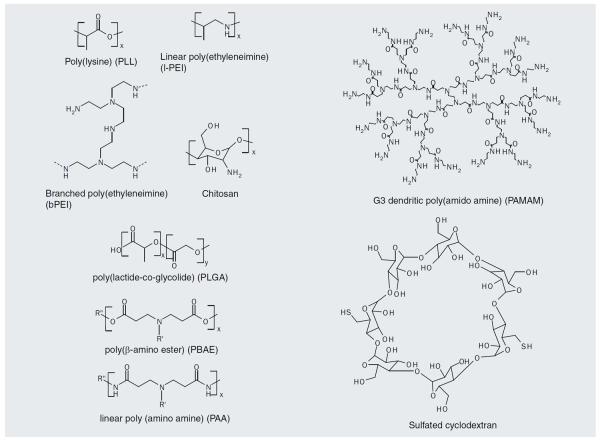
Various cationic polymer systems have been utilized for nucleic acid delivery. A wide range of structures have been explored, including linear and branched nondegradable polycations as well as biodegradable and bioreducible polycations and oligosacharides. Some of the most commonly used polymer structures are shown in Figure 2. All of the cationic polymers have primary amine groups that are protonated at neutral pH, which enables electrostatic interaction with the anionic nucleic acid.
Figure 2.
Commonly used cationic polymers and polysaccharides used in gene delivery.
Poly-l-lysine (PLL) was one of the first polymeric gene transfection agents developed, and was shown to condense DNA into small complexes with rod (25–50 nm) or toroidal (40–80 nm) structures [28]. PLL can be synthesized by several-step polymerization of ε,N-benzyloxycarbonyl-α,N-carboxy-l-lysine anhydride [29]. PLL is limited for intracellular delivery by its lack of an endosomal escape mechanism, and endosomolytic groups such as histidine [30], have been used to improve delivery. To reduce serum interaction and increase cell uptake, a variety of other molecules, such as poly(ethylene glycol) (PEG) [31], and targeting ligands such as asialoorosomucoid [32], transferrin [33], galactose [34,35], lactose [36] and folate [37], have been conjugated to PLL. Poly(ethylenimine) (PEI) was the second polymeric transfection agent developed [38]. Branched PEI (b-PEI) can be synthesized from aziridine monomers under acidic conditions, and linear PEI can be synthesized by the hydrolysis of poly(2-proplyl-2-oxazoline)[39], or by polymerization of aziridine monomers at lower temperatures [40]. Compared to later generation nucleic acid delivery agents, PEI is cytotoxic, leading to necrosis and apoptosis [41]. The high proportion of nitrogen atoms provides for a strong buffering effect (`the proton sponge effect'), which is advantageous for endosomal escape, as described later. 25 kDa b-PEI has been shown to be an efficient transfection reagent with reduced toxicity as compared with higher molecular weight b-PEI [42]. For delivery of shorter nucleic acids (e.g., mRNA) low molecular weight PEI (2 kDa or less) leads to enhanced biological effect, as complexes with higher molecular weight PEI are more stable and do not release the nucleic acid as efficiently into the cytoplasm [43]. Standard PEI has also been modified by deacylation to boost delivery of DNA and siRNA by orders of magnitude in vitro and in vivo [44].
Poly(lactide-co-glycolide) (PLGA) microspheres have been used in nucleic acid delivery for their relative biocompatibility and biodegradability. PLGA is synthesized by copolymerization of cyclic dimers of glycolic acid and lactic acid with various catalysts. Microparticles can be formed from premade polymers by emulsion evaporation, emulsion diffusion, solvent displacement and salting-out techniques, and particle size depends on the formulation conditions and molecular weight of the starting material [45]. Both the polymer and its degradation products are well tolerated in animal studies [46,47]. PLGA has recently been used to deliver siRNA in vivo and achieved sustained gene silencing when delivered to the vaginal mucosa [48].
Poly(β-amino ester)s (PBAE) are synthesized by Michael addition of either primary or bis(secondary) aliphatic amines to diacrylate compounds [49], and their simple chemistry leads them naturally to a combinatorial approach to synthesis and screening of polymer libraries [50–54]. They are hydrolytically degradable at the backbone ester linkages, which allows for release of nucleic acid cargoes and reduced cytotoxicity.
As opposed to mostly linear, crosslinked or other branched systems, dendrimers such as poly(amido amine) (PAMAM) are synthesized iteratively to produce nanoscale structures characterized by dendritic connectivity and radial symmetry. Advantages of dendrimeric systems include precise, nanoscale, structural control, dense and tunable surface chemistry (for addition of targeting ligands, modification of surface charge and so on), and high-charge density for complexation and buffering. PAMAM dendrimers were first synthesized in the mid-1980s [55]. Typically, ethylenediamine or ammonia are used as cores and allowed to undergo repeating two-step reactions whereby methyl acrylate is added by Michael addition to all the primary amines, and then the ester groups are amidated by a large excess of ethylenediamine to produce primary amine termini. They have been extensively studied for gene delivery [56,57] as well as oligonucleotide delivery [58–61]. Interestingly, thermal degradation of the dendrimers was shown to increase transfection efficacy [62]. Dendrons, rather than full dendrimers, have also been used for successful gene delivery [63]. Mannose–PEG–PAMAM linear-dendritic hybrid polymers successfully delivered the luciferase gene to P388D1 murine macrophages bearing the mannose receptor, and demonstrated a 1.6–1.8-fold more efficient transfection of these cells than PEI with no the presence of serum; this boosted transfection was shown to be targeting-ligand dependant [63].
Oligosaccharides
Sugars are crucial in a wide variety of biological applications. They are hydrophilic molecules composed predominantly of carbon, hydrogen and oxygen, and exist both in ring form as well as in extended conformations. Every extra cellular protein in the human body is glycosylated (addition of oligosaccharides to proteins). The ABO blood group antigens are oligosaccharides, and oligosaccharides play a crucial role in tethering and rolling via the interaction of selectins to sialyl-lewis X [64]. Glycosylation is a crucial consideration in the production of monoclonal antibodies (mAb) for therapeutics in terms of optimization of biological activity [65] and improved pharmacological profile [66].
Due to the hydrophilic nature of oligosaccharides and the fact that sugars are relatively well tolerated by the body, cationic polysaccharides have been explored for gene and nucleic acid delivery. Cyclodextrins (CD) are produced by the degradation of starch by the enzyme glucosyl transferase. This generates natural cyclic oligosaccharides composed of 6, 7 or 8 d(+)-glucose units known as α, β and γ CDs, respectively. CDs are of particular interest because, in addition to having low toxicity and good biocompatibility, they can form inclusion complexes with small, hydrophobic compounds. This ability allows for modification of the surface of the CD-based particles without interfering with polycation–nucleic acid interactions and particle morphology [67]. Polycationic CDs have been shown to transfect cells in serum in a comparable level to 1,2-dioleoyl-3-trimethylammonium propane (DOTAP) [68]. Grafting of CDs onto a PEI polymer leads to reduced transfection efficacy depending on the extent of modification (increased modification leads to further decreases in transfection efficacy), but also leads to significantly reduced toxicity [67]. Interestingly, however, grafting of CDs onto PAMAM dendrimers increased their transfection efficacy (100× that of the dendrimer alone and comparable to Lipofectin® and TransFast™); the optimal formulation used α-CD [69].
One excellent recent example of utilizing CDs for nucleic acid delivery currently in clinical trials is CALAA-01. CALAA-01 is a transferrin-targeted, PEGylated CD-containing polymer for siRNA delivery [70,71]. The CD-containing polymers are synthesized by condensing two difunctionalized comonomers. In addition, imidazole end-group modifications were added to enhance endosomal escape [72]. Interestingly, separate in vivo studies using earlier constructs for DNA and DNAzyme delivery demonstrated that nanoparticle localization to the tumors was independent of the targeting ligand, but addition of the targeting ligand increased tumor-cell uptake [73,74]. CALAA was later formulated for siRNA delivery, and after animal studies was used to treat the first patient in a Phase I clinical trial in May 2008 [72]; this trial was able to provide evidence of inducing an RNAi mechanism of action in a human from the delivered siRNA for the first time [71].
Another sugar used significantly in drug and nucleic acid delivery is chitosan. Chitosan is formed by the deacetylation of chitin. It is mucoadhesive and biodegradable, with a reasonable toxicity profile, and has been specifically useful in transmucosal drug delivery [75]. Oral administration of chitosan and Arah2 (a peanut allergy gene) was shown to significantly decrease IgE levels in response to anaphylaxis induction [76]. N-alkylated chitosan was investigated for gene delivery, and lengthening the side chains to eight carbons was found to improve gene delivery [77]. The optimal trimethyl chitosan–cysteine conjugate showed 1.5-fold higher in vitro and 4.1-fold higher in vivo transgene expression when compared with Lipofectamine 2000 [78]. Imidazole-modified chitosan siRNA delivery demonstrated good gene knockdown in the lungs and the liver [79]. Chitosan hydroxybenzotriazole showed approximately 60% knockdown of the control-enhanced green fluorescent (GFP) protein gene expression [80].
Inorganic materials
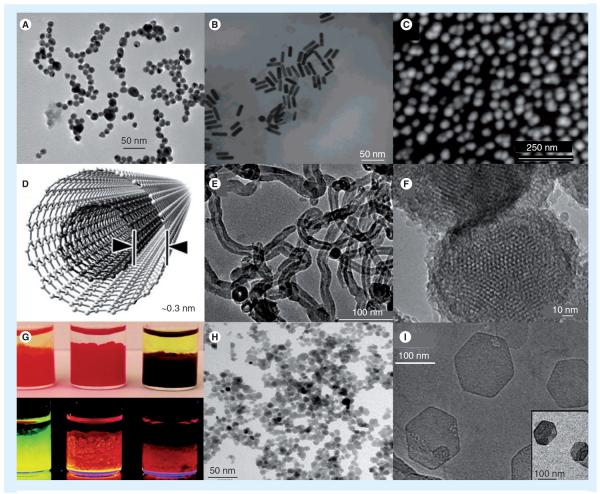
Many types of inorganic nanoparticles are in use today for gene therapy and have properties that can be exploited for multifunctional use (i.e., theranostics – therapy and diagnostics). A large portion of these are gold nanoparticles (AuNP; Figure 3A–C) [81–97], carbon nanotubes (CNTs; Figure 3D & E) [98–103], silica (Figure 3F) [98,104–108], quantum dots (QDs; Figure 3G) [109–116], superparamagnetic iron oxide nanoparticles (SPIONs; Figure 3H) [115,117–120], and layered double hydroxide nanoparticles (LDHNPs; Figure 3I) [121].
Figure 3. Typical inorganic nanoparticles being investigated for nucleic acid delivery.
(A) Transmission electron microscopy (TEM) of gold nanoparticle (AuNP) spheres, adapted from [81]; (B) TEM of AuNP rods, adapted from [82]; (C) AFM topography of AuNP shells coating platinum, adapted from [83]; (D) Multiwalled carbon nanotubes adapted from [276]. (E) Representative TEM of carbon samples produced by catalysis, adapted from [98]; (F) Mesoporous silica nanoparticles, adapted from [134]; (G) Quantum dots - top and bottom row are illuminated under visible and UV, respectively, adapted from [104]; (H) Doxorubicin-loaded superparamagnetic iron oxide nanoparticles with a diameter of 8 ± 2 nm, adapted from [115]; (I) TEM of pristine-layered, double-hydroxide NPs of Mg2Al(OH)6NO3 - inset NPs are associated with siRNA, adapted from [121].
Figures adapted with permission.
AuNPs
Gold nanoparticles' structures can be solid spheres (Figure 3A), rods (Figure 3B) or shells (Figure 3D). Many investigators synthesize AuNP spheres by dissolving tetrachloroauric acid (HAuCl4) in purified water and then adding a reducing agent (i.e., sodium borohydride or sodium citrate) converting Au(III) to its neutral and solid form [81,84–86,122]. To make gold nanorods (AuNR), a seed solution and a growth solution can be prepared separately and mixed. A possible seed solution uses cetyltrimethyl ammonium bromide (CTAB), HAuCl4 and NaBH4, and a possible growth solution uses CTAB, AgNO3, HAuCl4 and ascorbic acid [87]. When AuNP shells are synthesized, a shell forms around a core. Investigators have used the reverse-micelle method [88] or the Stöber method [89] to synthesize AuNP shells. AuNP shells have encapsulated SiO2 [90], Fe [88,91], Pt [83] and other materials. Other ways to synthesize AuNPs are by citrate reduction or the Brust-Schiffrin method as reported by Daniel et al. [92]. The timing and the relative amounts of reagents used can be varied to tune the size of the AuNPs [92]. AuNPs are known to have low cytotoxicity, can be synthesized with decent monodispersity, and can be conjugated at high densities with a wide range of organic molecules [93,94]. AuNPs can be functionalized with antibodies for the detection of molecules. The detection is made possible because AuNPs have surface plasmon resonance effects that scatter light at various intensities and absorb light at different wavelengths depending on their size and degree of aggregation [95]. For example, the high expression of a surface receptor (i.e., folic acid receptor [107], EGF receptor [95] or HER2 [89]) due to the presence of a cancer can cause an increase in AuNP–antibody local concentration, which causes a shift in optical properties and detection. In addition, AuNPs do not undergo photobleaching [95].
Furthermore, AuNPs can be used to localize photothermal cancer therapy. Spatially localizing the thermal therapy minimizes collateral damage. The AuNPs can be excited at near-infrared wavelengths to produce heat in an aspect ratio-dependent manner [82,89,96]. The near-infrared light can be applied externally as biological tissue does not attenuate the energy significantly, which allows for control over timing of the thermotherapy.
Fullerenes
Fullerenes are carbon-only nanostructures (i.e., spherical, cylindrical and ellipsoidal). The two most common nanostructures applied to nanomedicine are CNTs and spherical fullerenes (C-60 buckyballs) as in the following section.
CNTs
Carbon nanotubes are highly ordered and hollow [97]. They are single atom-thick cylinders of sp2-bonded carbon atoms [97]. CNTs can be synthesized by a variety of methods including laser ablation, arc discharge, thermal chemical deposition and plasma-enhanced chemical vapor deposition [99] (for an in depth discussion of methods (see [100]). Post-synthesis, CNTs can be sonicated to a desired length with some size restraints [101]. Single-walled and multiwalled CNT diameters can be 1–2 nm and 2–25 nm, respectively [101] (Figure 3D & E). Spherical, hydrophobic carbon–nanostructures can be cationically functionalized via amine groups to become soluble and to enable ionic complexation with nucleic acids [102] for gene-delivery applications. Carbon nanostructures can be conjugated to antibodies to increase specificity [123]. The single-walled CNTs have Raman signal to improve cancer detection and near-infrared absorption for photothermal applications [123] similar to AuNPs. CNTs have unprecedented high-tensile strength [99] and CNTs' electrical and thermal conductivities make them useful for biosensor applications [99]. CNTs also have a high surface area for dense loading of cargo [50,124]. Their needle-like shape can also enable the penetration of cell membranes with greater ease [124]; however, their structural similarity to asbestos warrants further research [125]. Nanotoxicology of these materials and other nanomedicines is important to consider prior to any clinical experimentation [116].
Carbon nanotubes are cytotoxic as there is lipid membrane peroxidation due to residual metal catalysts. Owing to this toxicity, CNTs are known to downregulate adhesive proteins and increase cell death (aspect ratio dependent), but can be nontoxic to primary immune cells when functionalized appropriately [126]. PAMAM dendrimers can be used to coat multi-walled CNTs to improve biocompatibility and cellular uptake [126]. Glycodendrimers can be used to coat single-walled CNTs to lower cytoxicity as well [127]. DNA-CNT complexes have been demonstrated to be nontoxic and nonmitogenic to activated or nonactivated lymphocytes [128]. Larger carbon nanomaterials (i.e., fibers and flakes) are less toxic than single-walled or multiwalled CNTs, possibly owing to their different interaction with the cellular membrane [129]. Aggregation of CNTs can influence their toxicity due to the alteration of their physical properties [130]. Fullerenes can be stabilized via functional groups to decrease cell death. For example, functionalization using single-walled nanotubes in 1% Pluronic® F108 at 2 μg/ml, di-carboxylation with a carbon:functional group ratio of 23:1 at 2 mg/ml, SO3H with a carbon functional group ratio of 80:1, 41:1 and 18:1 at 2 mg/ml resulted in approximately 65, 50, 40, 15 and nearly 0% cell death, respectively [131,132].
Spherical fullerenes
Carbon-60 spherical fullerenes (buckyballs) are approximately 1 nm in diameter. They have a propensity to aggregate, and are hydrophobic but can be made to be hydrophilic by the addition of functional groups (i.e., amine and carboxyl). Hydrophilic buckyballs mainly localize in the liver and have slow metabolism [133]. Buckyballs can function as an antioxidant for neuroprotective applications [103], an antiviral agent, such as HIV, a gene delivery carrier (particularly amine-derivatized fullerenes) and as a photosensitizer for photodynamic therapy applications [133]. Buckyballs can carry an unstable atom (i.e., Gd3+, 99mTc, which mainly localizes in macrophages of the bone marrow, liver and spleen), which is not released into the biological system and can then be used as an MRI contrast agent, x-ray imaging agent or radiopharmaceutical [133].
Silica nanoparticles
Mesoporous silica nanoparticles (Figure 3F) used for gene therapy are commonly synthesized using tetraethyl orthosilicate and CTAB under basic conditions using sodium hydroxide [105,106] or aqueous ammonia [134] at approximately 80°C. By varying the amount of CTAB added, the nanoparticles size can be modified [134]. Mesoporous silica nanoparticles are used in gene delivery because they have relatively large surface areas for dense conjugation, tunable pore sizes for cargo encapsulation and are surface modifiable [105]. A few nonexhaustive applications of silica nanoparticles include the delivery of nucleic acids (siRNA) by functionalization of PEI for ionic complexation [105], the delivery of GFP to osteoblasts by ionic complexation of conjugated Ca–silica [108] and the delivery of genes (luciferase) to the Achilles tendon [97]. Silica nanoparticles can activate macrophages and produce proinflammatory cytokines and reactive oxygen species [135]. Positively charged silica nanotubes are significantly more toxic than their bare counterparts. Toxicity was also significantly greater for positively charged silica nanotubes that were 200 versus 500 nm for a given mass in both HUVEC and MDA-MB-231 cell lines [136]. It was reported that the smaller particles have a greater extent of interaction with cells and, therefore, increased cytotoxicity [136].
QDs
Quantum dots are composed in pairs of semiconductor elements (i.e., ZnS, CdS, CdSe, InP, CdTe, PbS and PbTe). For example, CdSe QDs can be synthesized by adding cadmium oxide to tetradecylphosphonic acid and trioctylphosphine oxide at 300°C under Ar flow to dissolve the Cd. At 270°C, a Se solution of tri-N-octylphosphine is injected. The QDs can be grown at 250°C for different lengths of time to control the size. This solution can then be injected into chloroform and the CdSe will then precipitate out of solution [109]. For biological applications, QDs are sometimes synthesized in a core-shell fashion. The shell is chosen such that the band gap is wider than the core. This improves fluorescence properties, passivates the core, and prevents leaching (i.e., of toxic Cd ions) [110]. Heavy metal ions (i.e., Cd2+) are toxic at low concentrations (0.65 μM); however, when using silica shells and silane-PEGylation (methoxy[polyethyleneoxy] propyltrimethoxysilane [Gelest P/N SIM6492.7; MW = 450–600]), there was no toxicity observed at QD concentrations up to 30 μM [137].
Quantum dots' photoluminescence spectra are resistant to photobleaching [111] and emission is narrow, symmetrical and tunable as a function of core size [110]. Figure 3G shows the color dependence of different radii of CdSe core QDs [104]. The intensity of the fluorescence typically has a half-life of 27 h, which is many-fold greater than other fluorescence agents (i.e., Alexaflour, R-phycoerythrin and FITC) [110]. QDs can be carboxy lated to conjugate peptidyl amine residues or aminated by 2-aminoethane thiol hydrochloride for maleimide derivatization. Thiolated and polyhistidine-conjugated biologicals can also directly interact and self-assemble on the surface of QDs, respectively [110]. QDs have been encapsulated in chitosan nanoparticles to track and monitor siRNA delivery and transfection of SKBR3 breast cancer cells [114]. QDs have been used to quantify and monitor changes in transgene expression of two similar prostate cancer cell lines (PC3 and PC3-PSMA) due to changes in microtubule dynamics [113]. QDs have been used for multiplex fluorescence imaging, tumor cell extravasation tracking and real-time in vivo imaging [111].
SPIONs
Superparamagnetism relates to the stochastic magnetization changes of nanoparticles. In the absence of a magnetic field, the nanoparticles have an average magnetic state of zero. However, under an external magnetic field, magnetism is induced and their magnetic susceptibility is stronger to that of paramagnets [138]. SPION are commonly synthesized by coprecipitation in water [118]. In one example, SPIONs can be synthesized by precipitation in a reverse water-in-oil microemulsion system of water/SDS and 1-butanol/cyclohexane [119]. They can also be synthesized in an aqueous solution by coprecipitation of ferric and ferrous chlorides in alkaline medium [115]. SPIONs constitute a hydrophobic crystalline iron-oxide core that can be coated with relatively hydrophilic and/or biocompatible materials (i.e., dextran, starch, polyol derivatives, phospholipids, silica or amphiliphilic polymers) [116,117]. SPIONs can be conjugated with targeting moieties and gene-delivery carriers and Figure 3H shows doxorubicin-loaded SPIONs.
SPIONS are rapidly cleared by the reticuloendothelial system (RES; also known as the mononuclear phagocyte system). Many are primarily cleared in the liver by Kupffer cells in a nanoparticle size-dependent manner [139]. In one example (AMI-25), there is 80% uptake of the initial dose by Kupffer cells with a half-life of 10 min [140]. SPIONs can be metabolized in the hepato-renal system and are capable of entering the endogenous iron reserves by means of hematopoiesis [141]. SPIONs can be used for delivery systems via magnetofection, contrast enhancers for magnetic resonance imaging for T2 and T2*-weighted imaging [116,142], tissue repair, immunoassays, detoxification and anticancer magnetic hyperthermia [119,120]. Iron oxide nanoparticles associated with Metafectene® and dioleoylphosphatidyl-ethanolamine delivering anti-eGFP and firefly luciferase have been known to accomplish at least 90% knockdown at 48 h post-transfection with reasonable cell viability using the HeLa cell line [143].
LDHNPs
Layered double hydroxide nanoparticles are anionic clay materials that can be generally written as [MIInMIII(OH)2+2n]+ (Am-)1/m × H2O (n = 2–4) (MII/III = di/trivalent metal cation, Am- = anion) [121]. They can be synthesized by dissolving 3:1 mols of Mg(NO3)2 and Al(NO3)3 in deionized water and quickly adding NaOH [121]. Inorganic crystals of controlled sizes can be synthesized in this manner. LDHNPs can be used in cellular drug and gene delivery, are relatively biocompatible, have large cargo capacities and can be tailored to have pH-controlled release of their cargo. In one example of siRNA delivery, there was a significant knockdown to the protein target, HEK293T, and viability was maintained at 94% [121].
Nucleic acid delivery via coprecipitating mineral solutions
Macromolecules such as DNA are capable of codepositing with inorganic minerals (biomineralization) to form bioactive nanocomposites. When in close proximity to cells, these nanocomposites can promote DNA uptake via controllable surface-mediated release [144]. In one example, an inorganic mineral solution constituting of CaCl2, KH2PO4, NaCl, KCl, MgSO4, MgCl2, and NaHCO3 was used to effectively coprecipitate and deliver β-galactosidase DNA (Figure 4) [144].
Figure 4. β-galactosidase delivered to MG-63 cells with acceptable viability (>90%) and greater transfection efficacy than Transfast™ (1 \g=m\g/ml) using inorganic nanocomposites formed from mineral solutions containing various amounts of CaCl2, KH2PO4, NaCl, KCl, MgSO4, MgCl2 and NaHCO3.
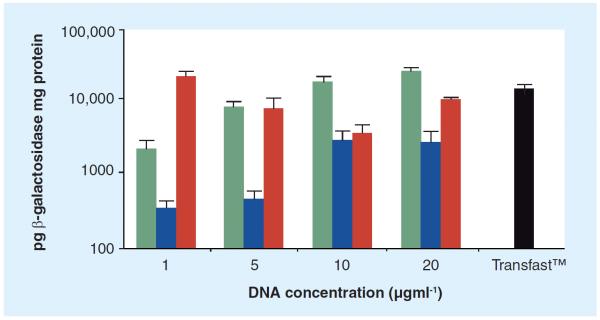
Adapted with permission from [144].
Physical requirements
When designing gene therapy vectors it is important to note that physical properties such as size, aspect ratio, molecular weight, surface area, shape, polydispersity and zeta potential can have an impact on cytotoxicity and delivery [124]. To meet certain barrier requirements for gene delivery, surface modifications can be used to modify the physical properties of the delivery system to improve circulation time and solubility (i.e., PEGylation) [145], localization (i.e., folic acid and RGD) [146], biostability (i.e., zeta potential: amine or carboxylic groups) [145], cyto toxicity (addition of carboxyl or hydroxyl groups) [126,131], internalization and inhibition of RES clearance [93,94].
Shape/surface morphology
Recently, manipulation of particle shape has come into focus as a new method for modulating drug delivery [147]. Local shape of the particle where it makes contact with the cell and not necessarily the overall shape dictate whether or not the particle is internalized by macrophages [148]. Elongated particles have been shown to circulate longer and avoid phagocytosis more effectively than spherical particles [149]; however, spherical particles are much more efficiently internalized into target tissues as compared with elongated particles [150].
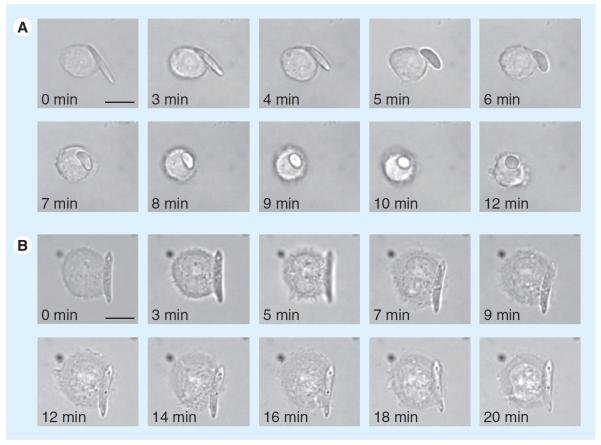
Seeking to take advantage of this property, Yoo et al. has recently constructed PLGA-based, shape-shifting particles (one way, from ellipsoid to spherical) in response to temperature, pH or a chemical signal and demonstrated efficient uptake of the spheres as compared with the ellipsoids (Figure 5) [151]. It is also possible to complex anisotropic faces of AuNPs with DNA oligonucleotides to form sticky patches, which allows for complicated self assembly [152].
Figure 5. Time-lapse video microscopy stills of shape-dependent phagocytosis by macrophage.
(A) Shape-switching poly(lactide-co-glycolide)- ester elliptical disk allows macrophage internalization. (B) Poly(lactide-coglycolide)-ester elliptical disk that does not switch shape prevents internalization. Scale bar: 10 μm.
Reproduced with permission from [151].
Other nanoparticle morphologies may prove worthwhile to investigate tuning cytotoxicity and nucleic acid delivery of potential vectors. Spherical silver nanoparticles can be induced via light to transform into triangular prisms with efficiency nearing 100%. This is accomplished by irradiating a solution of silver nanoparticles with a halogen lamp at 150 W for 5 h (bandpass filter at 550 nm) [153]. DeSimone and coworkers have elegantly used soft lithography using polydimethylsiloxane and perfluoropolyether to make molds enabling nanoparticle replication in a nonwetting template. Using this, nano particles with diverse shapes can be fabricated (i.e., 200 nm trapezoidal particles, 200 × 800 nm bar particles, 3 μm arrow particles and 2.5 × 1 μm2 hexnut particles with 1 μm holes) [154].
Nanoparticle surface morphology has recently been shown to be another important aspect of controlling nanoparticle delivery [155]. Verma et al. demonstrated that AuNPs with surface `ribbon-like' domains of alternating hydrophobic and hydrophilic composition were able to enter the cell without the membrane disruption associated with cationic nanoparticle systems; control particles with random surface organization were unable to penetrate cells at all [156]. Cell-penetrating peptides appear to have similar functionality [156,157]. This property should reduce the toxicity usually associated with membrane disruption [156].
Size
Polymer nanoparticles have been developed with a wide variety of sizes for different purposes. Nanoparticles of approximately 100 nm show prolonged blood circulation and a relatively low rate of mononuclear-phagocyte system uptake [158]. Particles with a 1–5 μm diameter are likely to be trapped in the liver and phagocytosed by Kupffer cells [151]. Particles larger than 5 μm in diameter are likely to be trapped in capillary beds [151]. When NPs are greater than 200 nm they are likely to be filtered in the spleen, whereas the NPs less than 100 nm are likely to leave the blood vessels through fenestrations in the endothelial lining [151]. NPs that are approximately 50–200 nm diameter have been known to accumulate in tumors by the enhanced permeability and retention (EPR) effect (as a result of leaky vasculature and the absence of a draining lymphatic system) [117,159]. It has been suggested that particles must not exceed 300 nm to take advantage of the EPR effect [101]. Nanoparticles smaller than 50 nm are more likely to enter most cells, and those with sizes smaller than 20 nm can get out of the bloodstream and into tissues [142,159]. The glomerular apparatus' capillary wall has fenestrations of approximately 4–5 nm and it has been reported that nanoparticles >8 nm cannot be filtered through the glomerular filtration system [124], as a result this would increase circulation half-life [160].
Charge
To best avoid nonspecific electrostatic interactions and escape the RES, nanoparticles should be designed to have neutral or slightly negative zeta potentials [86]. On the other hand, a positive zeta potential enhances nanoparticle–cell contact and promotes uptake and internalization through stronger affinity for anionic proteoglycans on the cell surface [161]. For example, the zeta potentials of lysine-, arginine- and histidine-modified nanoemulsions were reported to be 50, 43 and 7 mV, with transfection efficiency decreasing with neutralization of the zeta potential [162]. Some nanoparticles may be more or less cytotoxic depending on their charge (i.e., AuNPs are less cytotoxic when anionic) [163]. It is important to consider zeta potentials when complexing nucleic acids via ionic interactions. The interaction must be strong enough to condense the nucleic acid to protect against restriction enzymes. It is important to note that zeta potentials of nanoparticles can switch signs when in the presence of serum and this should be considered in the design and testing process [51,164].
Biocompatibility
Biocompatibility is crucial for maintaining an appropriate host response during gene therapy. In-depth assessments and characterizations are required to elucidate the physicochemical differences responsible for low cytotoxicity and acceptable viability. PEI lacks degradable linkages and is too toxic for therapeutic applications, inducing both apoptosis and necrosis in an endothelial cell model [165]. As a result, a number of investigators have synthesized an array of degradable PEIs consisting of low molecular weight PEIs and degradable crosslinkers, in the hopes of achieving higher efficacy with the reduced cytotoxicity of low molecular weight PEIs [166,167]. Other groups have focused on developing new biodegradable polymers for nonviral gene delivery, which we will review here by method of degradation. Biodegradable polymers should be able to both reduce the cytotoxicity associated with the transfection reagent as well as potentially improve dissociation of the vector from its cargo to allow the cargo to be utilized intracellularly.
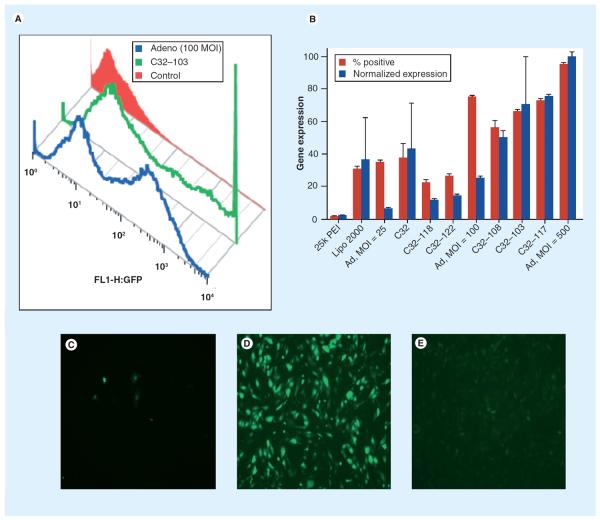
Multiple strategies have been formulated that use ester bonds to allow hydrolytic cleavage of the polymer. Amine-containing hydrolyzable polymers have been utilized, which are effective gene-delivery agents with significantly decreased cytotoxicity as compared with nondegradable polymers such as PEI [38] and PLL [28]. These structures include PLGA [168], hyperbranched poly(amino ester)s [169], poly(lactic acid) (PLA) [47] and linear poly(β-amino ester)s (PBAEs) [49] among others. Libraries of PBAEs have been developed for gene-vector screening [52,54]. Studies have shown that amine-terminated PBAEs are more effective at pDNA transfection than acrylate-terminated versions. Modification of the polymer ends with different amines can lead to virus-like efficacy in human primary cells in vitro (Figure 6) [170]. Tuning of polymer end group leads to significant differences in transfection efficacy, and the optimal end-group for each cell type appears to be cell-type specific [171,172]. PBAEs also have been shown to be nontoxic to human primary cells in vitro [173] and in mice in vivo [174,175].
Figure 6. Gene expression of poly(β-amino ester)s compared with adenovirus.
(A) Gene expression histogram comparing adenovirus, PBAE and negative control. (B) Comparison of various poly(β-amino ester) formulations with adenovirus with respect to % positive cells and normalized expression. Images of GFP+ cells 24 h post-transfection with (C) PEI, (D) C32–103 and (E) 500 MOI adenovirus.
GFP: Green fluorescent protein; MOI: Multiplicity of infection; PEI: Poly(ethylenimine).
Reproduced with permission from [170].
`Stealth' properties
The binding of serum proteins to nanoparticle surfaces after intravenous injection causes the nanoparticles to be internalized by macrophages and removed from the blood [176]. Addition of hydrophilic moieties, such as PEG, poly(N-(2-hydroxypropyl-methacrylamide) (pHPMA) and various oligosaccharides, have been shown to increase solubility, prolong circulation time, neutralize zeta potential and reduce interactions with the environment within the bloodstream due to a higher tolerance against incubation with serum proteins [177,178]. One disadvantage of this approach is that while it may stabilize the polyplex in serum and reduce cytotoxicity, it may also interfere with complexation and reduce transfection efficiencies depending on the extent of addition [179]. Modification of the surface of preformed particles with PEG/pHPMA that can bind to exposed surface amino groups has been shown to alleviate this problem [177,180]. Recently, Yuan et al. demonstrated that adding PEG to PAMAM dendrimers via bis-aryl hydrazone bond linkages into the vector significantly enhanced the buffering capacity of the vector even with a high degree of PEGylation [181]. PEG can be added to a variety of nanoparticles and can be further modified to provide targeting [72,182–184], or can be attached by degradable bonds (such as matrix metaloproteinases [MMPs]) that can be cleaved to expose underlying functionalities [185–189]. Electrostatic coatings can also be used to improve the delivery properties of a charged particle without significantly altering the core particle [190,191].
Nucleic acid complexation
Many polymers for nucleic acid delivery rely on electrostatic interaction between a cationic polymer and the anionic phosphate backbone of nucleic acid substrate. For polymer–DNA complexes, requirements include condensation of the plasmid to an appropriate scale for internalization, neutralization of the negatively charged phosphate backbone of the DNA and protection of DNA from degradation both intra- and extra-cellularly [192,193]. Sufficient cationic charge is crucial to condense DNA, but it is also correlated with increased cytotoxicity, and higher DNA-binding affinity may lead to decreased DNA release and reduced transport through the cytoplasm [194]. AuNPs can also use electrostatic methods for complexation with nucleic acids. AuNP rods conjugated with cationic cetyltrimethylammonium bromide were electrostatically complexed with siRNA (anti-DARPP-32 gene in dopaminergic neuronal cells) with 98% cell viability and 67% expression knockdown at 120 h post-transfection [86]. Alternatively, hydrolytically degradable nanoparticles can be formed through encapsulation of DNA by noncationic polymers such as PLGA. These particles degrade to release their nucleic acid cargo and the size of the particle can be controlled in the nanometer to micrometer range, depending on the method of particle formation used. Methods have been developed to protect the cargo from destruction during these processes [168], but are still limited by low encapsulation efficiency and potential DNA degradation in the hydrolyzing polymer core [195].
Nonviral vectors can encapsulate nucleic acids through other mechanisms as well. Chitosan can form nanoparticles through ionotropic gelation with polyanions such as sodium tripolyphosphate [196]. Chitosan has been optimized to allow for the encapsulation of both hydrophilic and hydrophobic drugs and has been utilized in nucleic acid delivery [78,197], most successfully as hybrid copolymers with various polycations [198–201]. Inorganic nanoparticles can be complexed with nucleic acids via ionic complexation or a covalent bond. For example, AuNPs (4.1 nm) can be covalently attached to cationic N-dodecyl PEI (2 kDa) and complexed with β-gal pDNA. When this conjugated complex was delivered to COS-7 cells, there was 67% cell viability and 50% transfection efficacy, which compared favorably with regular PEI and PEI25 that had 4 and 8% transfection, respectively (~93% cell viability) [202]. Another nucleic acid complexation technique can involve 11-mercaptoundecanoic acid, which can be deposited on Au to bind oppositely charged polyelectrolyte solutions. In one example, deposited Au combined with PEI (23 kDa MW) and double-stranded 21-mer anti-enhanced GFP (EGFP) siRNA was delivered to CHO-K1 cells resulting in ~95% cell viability and ~72% knockdown of EGFP expression [203].
Cellular targeting
By utilizing a targeting moiety, smaller dosages can elicit comparable therapeutic responses while minimizing side effects and reducing the cost of therapy [204]. There are two types of targeting; passive and active. Passive targeting utilizes natural processes such as the EPR, in which the leaky tumor vasculature and lack of efficient lymphatic drainage in a solid tumor leads to passive accumulation of drugs or particles at the tumor site, given sufficient circulation time [205]. Active targeting consists of an additional ligand to assist in localization or internalization such as antibodies or their fragments (i.e., J591 against prostate-specific membrane antigens [206], anti-HER2 [trastu-zumab] [207]) [208], folic acid [209], sugars (i.e., galactose, mannose and lactose) [210], peptides (RGD) [211,212], transferrin [213] and nucleic acid aptamers [214]. Large targeting moieties, however, may hamper internalization and gene unpacking and having triggered removal of the target moiety at the cell surface may be worthwhile [204]. Targeting moieties are typically attached chemically but can be physically adsorbed to the delivery system as well [191]. Interestingly, it has been shown that biodistribution of cargo at the accumulation site can be independent of the presence of targeting ligands [159]. The reason for improved functionality when targeting ligands are used appears to be owing to an increase in cell internalization and specificity of the nanoparticles rather than tissue localization. Passively targeted nanoparticles have a propensity to end up in the extracellular space of tumors and in tumor-associated macrophages [159].
Cationic polymers have been modified with targeting ligands for various applications. For example, the addition of lung surfactant to ternary nanoparticles for aerosol-based gene therapy enhances gene delivery to the lung, resulting in 12-fold higher transfection compared with pure nanoparticles and 30-fold higher compared with polyethylenimine [215]. Insulin adsorption significantly increased gene expression of PEI–pDNA nanoparticles up to 16-fold on alveolar epithelial cells, but not on bronchial epithelial cells [216].
Gold nanoparticles can be complexed with PEG-NH2 and folic acid via noncovalent interactions and can be taken up by cancer cells (OV167, OV202, OVCAR-5) proportional to the degree of folic acid receptors expressed on them. However, unintended delivery to the liver and kidneys can also occur due to overexpression of folic acid receptors there as well [217,218]. AuNPs and the mAb CD11b have been targeted to RAW264.7 macrophages and resulted in 81% cell death post-30 J/cm2 exposure as opposed to 0.9% cell death with nonlabeled cells [94].
Enhancing internalization
Gold nanoparticles can be internalized to a greater degree via electroporation, which causes membranes to become permeabilized by pulsed electric fields (several kV/cm amplitude and submicrosecond duration). Membrane pores occur momentarily as a result, allowing for easier passage of gene therapy systems. However, electroporation can also cause osmotic lysis of the cells. Kawano et al. has delivered AuNP-SS-mPEG-pDNA in vivo in combination with electrical pulses to the mouse liver and observed greater stability in circulation and a gene expression increase by tenfold in comparison to naked DNA [219].
Multiwalled CNTs combined with irradiation of microwaves for 8 s can aid gene delivery by creating transient nanochannels in the cell while maintaining cell viability at 100% [220]. Hexagonal LDHNPs are most likely taken up by clathrin-mediated endocytosis and localize to the perinuclear area of the cytoplasm (where siRNA/mRNA complexes can degrade). By contrast, rod-like LDHNPs concentrate in the nucleus [121].
Gene-associated magnetic nanoparticles (i.e., SPIONs) can be guided toward a particular region of the body via external magnetic fields. Application of external magnetic fields to aid traversing membrane barriers and enhancing cell contact is known as magnetofection [141]. CNTs with ferromagnetic nickel tips have been known to be able to align in an external magnetic field to spear cell membranes. This increases the shuttling efficiency of cargo by 107-fold and was demonstrated to increase transfection rates to approximately 100% to mammalian cells in vitro [221]. Ultrasound is noninvasive and safe at a broad range of frequencies and intensities, and can be used to enhance gene delivery. The main mechanism responsible for increased gene delivery is cavitation, where reversible nano-pores are formed (up to 100 nm with a half-life of a few seconds) by microbubble expansion and collapse [222]. Stride et al. endeavored to combine ultrasound-mediated gene delivery and magneto fection and showed significantly improved transfection over magnetofection and ultrasound alone [223].
Gene guns are biological ballistic hand-held delivery systems that physically propel nucleic acid-complexed nanoparticles (i.e., AuNPs) into cell cytoplasm and nuclei with a low pressured propellant (i.e., helium). Chitosan and poly-gglutamic acid (150–250 nm) nanoparticles have been used to encapsulate reporter genes and transfect liver cells via the gene-gun method, which increases delivery by 2-log orders of magnitude in terms of luciferase RLU/mg protein compared with naked DNA [224].
Endosomal escape
In early experiments with nonviral gene delivery, nondegradable polycations, including PLL and PEI were used. Compared with PLL, a major advantage of PEI is the `proton sponge' effect due to PEI's extensive buffering capacity. When PEI–DNA complexes gain entry to the endosome, the secondary and tertiary amines in PEI function to buffer acidification of the endosome. This causes an influx of negatively charged chloride ions into the endosome to maintain electro-neutrality as protons are continually pumped into the endosome. Eventually, this leads to osmotic swelling, rupture of the endosomes and release of the vectors and cargo into the cytoplasm [225]. This mechanism has been widely explored for gene and siRNA delivery [38,226]. This concept has also been extended and heavily used in the design of next-generation biodegradable vectors that also have this buffering capacity. A widely used buffering moiety is the imidazole ring of histidine. It is a weak base (~6 pKa) capable of buffering the endosome. For example, a poly(phosphazene)-based polymer has been histidylated, and the resulting polymer showed improved transfection and reduced cytotoxicity when compared with the histidine-free polymer and branched PEI [227].
Newer methods for endosomal escape involve functionalizing a polymer with peptides that enhance endosomal release. Melittin enhances endosomal escape and nuclear transport due to the cationic C-terminal sequence lysine–arginine–lysine–arginine [228]. Modifying melittin either by reversible acetylation of a lysine residue in melittin [229] or replacement of two glutamines with glutamic acids, which get neutralized at acidic pH [230], takes advantage of the acidification of the endosome to induce membrane lysis only in the endosomal compartment and reduces the cytotoxicity associated with use. Functionalizing polylysine with PEG- and a pH-responsive melittin peptide was shown to be an efficient siRNA delivery agent [231]. In an alternate fusogenic mechanism, protonation of glutamate-containing peptides causes endosomal escape via spontaneous formation of a membrane-disprupting α-helical structure of the peptide [232–234]. Adding these peptides to polymeric vehicles was shown to enhance the endosomal escape rate constant by two orders of magnitude [235].
Release of cargo/degradation
Nucleic acids must be released from the vector to have an effect. This can be done by taking advantage of the redox potential gradient [236], acidic environment of the endosome [237], MMPs [138,238], photocatalysis [239] and hydrolytic degradation of the carrier [49,240,241]. It has been shown that plasmid unpacking can be a limiting step with regard to gene expression for sufficiently large polymer constructs [242].
Bioreducible polymers
Using bioreducible polymers via incorporation of disulfide linkages takes advantage of the relative reducing environment of the intracellular space. Intracellular reduction of the disulfide bond occurs via the glutathione (GSH) pathway. GSH is regenerated from its oxidized form by GSH reductase, is an important component in many cellular pathways and plays a major role in cellular defense against oxidative stress. Disulfide bonds are stable extra cellularly, preventing particle breakdown before the nano complex reaches the cell surface, whereas the reducing environment of the intracellular space allows for enhanced polymer breakdown and nucleic acid release [243–247]. Disulfide bonds have been shown to degrade intracellularly within 3 h [248]. When cell lines with different intrinsic GSH levels have been compared, increased cellular GSH levels give mixed delivery results. In some cases, there is improved delivery, while in other cases, no clear trend is observed with GSH levels; in these cases, the cell line that demonstrated the best DNA transfection was the fastest dividing cell line [249].
Enhancing release of the pDNA cargo can lead to dramatic gains in transfection efficiency. Chen et al. synthesized a series of reducible hyperbranched PMAMs and found that reducible polymers were able to achieve nearly 200-fold higher transfection as compared with control polymers [250]. Combining hydrolyzable and bioreducible functional groups as a single polymer might also help further tune the release profile [251,252]. Reducible polymers have also been used to deliver siRNA. Histidine-containing reducible polycations based on CH6K3H6C monomers (His6 RPCs) were examined for their utility in delivering siRNA. Co-delivery of EGFP siRNA with EGFP DNA reduced reporter gene expression by 85%. Interestingly, as with most polymer systems, while larger polymer size correlated with increased DNA transfection efficiency, effective delivery of siRNA was only possible with smaller polymers (36–80 kDa) [253].
Low molecular weight PEI has also been cross-linked via disulfide linkages to show reduced cytotoxicity and equivalent DNA transfection efficacy to higher molecular weight PEI [254]. In one study, reducible poly(amido ethylenimine) was synthesized by addition copolymerization of triethylenetetramine and cystamine bis-acrylamide (poly[TETA/CBA]) and used as a carrier for siRNA. Under normal conditions there was significantly higher suppression of VEGF with poly(TETA/CBA) than with linear PEI. The addition of dl-buthionine sulfoxamine, which reduces intracellular levels of reduced glutathione, reduced the RNAi activity level of poly(TETA/CBA) formulation to that of linear PEI, showing that reduction of the polymer was crucial to gene knockdown [255]. Jere et al. used a reducible polyspermine carrier composed of multiple spermine units with disulfide linkages and demonstrated improved efficacy in gene delivery and gene knockdown compared with 25K PEI. Reductable polyspermine delivered anti-Akt1 sh/si/ssiRNA and altered the cancer-cell survival, proliferation and metastasis to different extents depending on the nature of siRNA treatment [256].
Acid-labile linkages
Acid-labile linkages linkages would also be useful for endosomal escape and for enhanced cargo release into the cytoplasm, as they take advantage of the acidification of the endosome to allow for release of the cargo. Acid-labile acetal and ketal bond-bearing polycations were recently developed for this purpose. Oligo-ethylenimines (OEI) linked by either acid-degradable ketal or acetal linkers in a copolymer with 5 kDa PEG formed complexes with half-lives of 3 min at pH 5.0, and 5 h (OEI-MK) or 3.5 h (OEI-BAA) at physiological pH 7.4 [187]. Using acylhydrazides or pyridylhydrazines to link a PEG shield to the polymer backbone enhanced transfection by two (in vitro) or one (in vivo) order(s) of magnitude compared with complexes whose PEG shield was not acid-hydrolyzable [185].
Irradiation release
Irradiation can be used for controlled release. In one example, an Nd:YAG laser was used to release DNA from a 44 nm spherical AuNP complex conjugated to PEG-orthopyridyl-disulfide. The Nd:YAG laser irradiation was applied at 80 mJ/pulse (~10 ns, 6 mm diameter). DNA was released without any degradation seen [239]. When EGFP DNA-SS-AuNR was delivered to HeLa cells and was controlled remotely using femtosecond near-infra red (NIR), a shape change from rod to sphere was observed. It was proposed that this transformation induces DNA release from its conjugate [257]. Also, using NIR, AuNR-EGFP-DNA conjugates were delivered to HeLA cells and there was expression detected at the irradiated spots of NIR exposure (79 uJ/pulse for 1 min). There was 80% cell viability observed and the NIR irradiation induced plasmid release without structural degradation [258].
Gold nanoparticles of different aspect ratios can be melted selectively at their unique longitudinal plasmon resonance by morphing to a sphere to release DNA oligonucleotides. Aspect ratios at 4.0 and 5.4 will have a longitudinal plasmon resonance at 800 and 1100 nm, respectively. By irradiating a combined sample of the aspect ratios at one wavelength, 50–60% of the intended oligonucleotides can be released and are still functional whereas the unintended oligonucleotides from the other aspect ratio released <10% of its cargo [259].
Nuclear translocation
Diffusion of DNA longer than 250 base pairs in length is significantly reduced in the cytoplasm compared with water due to the involvement of the cytoskeleton [260]. The NPC forms a selective permeability barrier, allowing free diffusion of molecules (e.g., ions, small proteins and metabolites) with a mass/size less than ~40 kDa/10 nm [261]. Macromolecules greater than ~40 kDa are transported actively across the nuclear envelope through the NPCs using soluble transport factors or carrier molecules (β-karyopherins) that cycle between the cytoplasm and nucleus [262]. In the classical case, NLS are recognized by importin-α, which then binds to importin-β, and this complex is allowed through the NPC (Figure 7). Once inside the nucleus, the importin-β-binding domain is released by binding to RanGTP and the cargo is released [262]. By utilizing electron microscopy and AuNPs complexed to NLSs, Panté and Kann were able to show that the largest rigid particle to achieve nuclear entry through NPCs was ~39 nm, in diameter including NLSs [263].
Figure 7. Nuclear import through the nuclear pore complex.
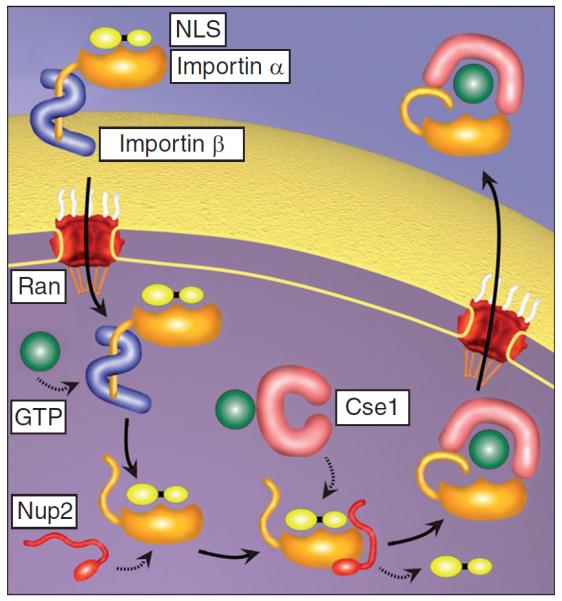
Adapted with permission from [262].
Strategies for obtaining access to the nucleus include diffusion of DNA through the cytoplasm, nuclear breakdown during mitosis and use of NLS. Numerous groups have complexed synthetic or naturally occurring NLS peptides to DNA, with variable efficacy, and transfection enhancement may be due to the NLS peptides inducing improved nanoparticle complexation rather than improved nuclear import [25]. A single NLS has been shown to be sufficient to carry the DNA through the nucleus [264]. However, the addition of many NLS sequences to a plasmid can lead to no nuclear localization of the plasmid at all, perhaps as multiple NLS sites might lead to cellular machinery attempting to pull a single plasmid in multiple directions at the same time [25,264].
Multifunctional nucleic acid carriers
Multifunctionalized constructs are typically a hybrid of materials that are intended to accomplish different objectives simultaneously, such as gene therapy and diagnostics (i.e., imaging), commonly referred to as theranostics. Multifunctional hybrid vectors could also incorporate components to overcome the multiple entry barriers discussed above: nucleic acid complexation, physical requirements (i.e., charge, biocompatibility), targeting, internalization, endosomal escape, cargo release, biodegradation and nuclear translocation.
Hybrid AuNPs/siRNA/PBAE system
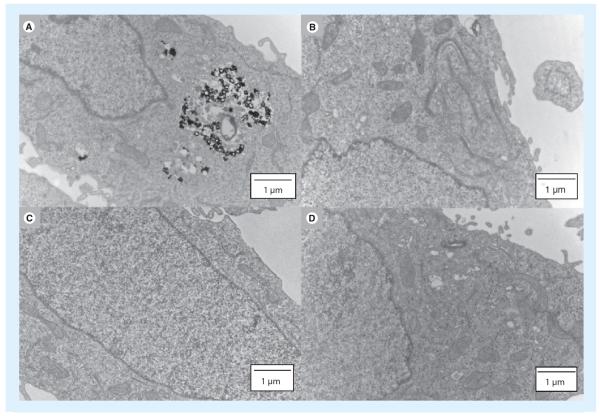
Thiol-modified siRNA can be combined with PBAEs and complexed with thiol-modified AuNPs by disulfide linkages for multiple functionality (AuNP for sensing, siRNA for silencing and reduction-triggered release of cargo) (Figure 8) [93]. PEG can be used as a spacer between the disulfide bond and the AuNP surface as the Au can induce release of the cargo. This system has high stability and low aggregation of the ~100 nm particles [93]. There was no significant cytotoxicity reported and the system resulted in ~95% gene knockdown of luciferase expression in HeLa cells [93].
Figure 8. Transmission electron microscopy images of HeLa cells.
(A) PBAE-siRNA-AuNPs; (B) siRNA-AuNPs without PBAE; (C) unmodified AuNPs; (D) no nanoparticles (control).
AuNPs: Gold nanoparticles; PBAE: Poly(b-amino ester).
Adapted with permission from [93].
Multifunctional QDs
ZnO QDs have been reported to have dual functionality (pDNA delivery and cell labeling) when capped with poly(2-(dimethylamino)ethyl methacrylate). This system was capable of condensing pDNA into nanoplexes and delivering DNA to COS-7 cells with real-time imaging of gene transfection under UV [265].
Mesoporous silicon
Mesoporous silica nanoparticles have been tri-functionalized (imaging, targeting and therapy). In one example, optical agent ATTO 647N, cRGDyK peptides to target αvβ3 integrins over-expressed in tumor metastatic and endothelial cells, and an oxygen-sensing porphyrin-based photosensitizer were used to create such a system [266]. In vitro experiments using MCF-7 human breast cancer cells and U87-MG human glioblastoma cells demonstrated that there was excellent specificity, minimal collateral damage, and potent photodynamic effects [266]. Mesoporous silica can also be used in other ways for combination/theranostic use [267].
Nonviral gene therapy clinical trials
As of June 2010, according to the Journal of Gene Medicine website [301], there have been a total of 1644 gene therapy clinical trials approved worldwide. The leading diseases being treated in these clinical trials are cancer (64.5%), cardiovascular diseases (8.7%) and monogenic diseases (8.2%). The majority (60.5%) of the clinical trials are in Phase I study. The most common gene types used have been antigens (19.8%), cytokines (18.4%), tumor suppressors (10.5%) and growth factors (7.7%). Table 2 is a summary of nonviral gene therapy clinical trials detailing the type, the current or last clinical phase, the nucleic acid being delivered and the disease target. These data highlight that the majority of clinical trial thus far have been with viral vectors (~75%), and the leading nonviral approaches in clinical trials are `naked' free DNA (18%) followed by lipofection (7%). Polymeric and inorganic vectors for gene delivery, although promising for the future, are, for the most part, still in preclinical stages of development. However, one recent approach in polymeric gene delivery to reach clinical trials is Mark Davis' and Calando Pharmaceutical's work with CD-based polymers [70,71]. In this work it has been demonstrated that biocompatible polymeric nanoparticles can reach solid tumors following systemic administration in humans and within these tumors the particles can cause siRNA-specific knockdown of a target gene.
Table 2.
Summary of clinical trials (some of which are ongoing) including naked plasmids, the gene gun, lipofection, siRNA, asDNA, gold nanoparticles and BD Accuspray™.
| Type | Clinical Phase | Nucleic acid | Disease target | Ref. |
|---|---|---|---|---|
| Naked plasmid/DNA (intramyocardial) | II/III | VEGF | Angina | [277,304] |
| Naked plasmid/DNA (lung injection) | II | heNOS | Hypertension | [305] |
| Naked plasmid/DNA (intramuscular) | II | FGF | PAOD | [306] |
| Gene gun | I/II | IL-7,12; GM-CSF | Malignant melanoma | [302] |
| Lipofection for direct gene transfer using Leuvectin™ (intratumoral) | II | IL-2 | Renal cell cancer | [307] |
| Gene gun (intradermal) | I | GM-CSF | Malignant melanoma, sarcoma | [303] |
| Lipofection for direct gene transfer using Allovectin-7 (intratumoral) | II | HLA-B7/β 2 μg globulin | Malignant melanoma | [308] |
| Lipofection (E1A lipid complex); for cancer without overexpression of HER-2/neu (intraperitoneal) | II | E1A | Ovarian cancer | [309] |
| Lipofection using SGT-53 (intravenous) | I | P53 | Solid tumors | [310] |
| Lipofection as a tumor cell vaccine (intradermal) | II | B7.1 (CD80), HLA-A1/2 | Nonsmall cell lung cancer | [311] |
| siRNA (intravenous) | I | I5NP | Acute renal failure | [278,312] |
| Naked DNA (corpus cavernosum injection) | I | hSlo DNA | Erectile dysfunction | [279] |
| siRNA (intravitreal) | III | Bevasiranib (anti-VEGF) | AMD | [280] |
| siRNA (inhalation) | IIa/b | Anti-RSV nucleocapsid gene | RSV | [281] |
| siRNA (systemic, lipid–ionic complexation) | I | Plasmid DNA ≥ 4 RNAi, inhibiting all viral genotypes | Hepatitis B virus | [313] |
| siRNA (topical) | II | Bevasiranib/Cand5 anti-VEGF | Diabetic macular edema | [314] |
| siRNA (injected into callus on foot) | I | TD101 | Pachyonychia congenita | [315] |
| siRNA (intravitreal) | II | AGN211745 | AMD/CNV | [316] |
| cDNA encoding two growth factor isoforms (intramuscular) | II | VM202 (HGF-723/728) | Critical limb ischemia | [317] |
| Anti-sense DNA (intratumoral), DC-Chol liposomes | I | EGF receptor | Head/neck cancer | [318] |
| Anti-sense DNA (intratumoral injection) | I/II | EGF receptor | Head/neck squamous cell carcinoma | [319] |
| siRNA via cyclodextrin-based polymer (intravenous) | I | Anti-M2 subunit of ribonucleotide reductase (R2) | Solid tumor | [320] |
| PEGylated AuNPs | I | rhTNF; not nucleic acid | Advanced-stage cancer patients | [268] |
| siRNA delivered with BD Accuspray™ | II | ALN-RSV01 siRNA | Respiratory syncytial virus | [282] |
| siRNA delivered with stable nucleic acid–lipid particles | I | ALN-TTR01 siRNA | Transthyretin-mediated amyloidosis | [321] |
| Two siRNAs delivered via lipid nanoparticle formulation | I | Contains both kinesin spindle protein VEGF siRNAs (ALN-VSP02) | Liver cancer | [322] |
AMD: Age-related macular degeneration; CNV: Choroidal neovascularization; DC-Chol: 3B[N-(iV',W-dimethylaminoet hane)-carbamoyl] cholesterol; heNOS: Human endothelial nitric oxide synthase; PAOD: Peripheral artery occlusive disease; rhTNF: Recombinant human TNF; RSV: Respiratory syncytial virus.
Inorganic gene delivery systems used in clinical trials to date have so far been limited to the gene gun [302–303]. However, several promising technologies are on the horizon and may prove successful in the near-coming years. For example, AuNPs (27 nm) were recently conjugated with recombinant human TNF (rhTNF) and thiolated PEG (CYT-6091) in a Phase I clinical trial [268]. The results showed that previously toxic concentrations of rhTNF were no longer toxic by systemic administration and that CYT-6091 may target tumors [269].
Conclusion
Polymeric and inorganic-based vectors for nucleic acid delivery need to overcome many crucial barriers in the delivery process, and a variety of novel approaches have been investigated to overcome these challenges. A wide array of materials have been investigated for their potential in this area, including degradable and nondegradable cationic polymers, oligo- and polysaccharides, fullerenes, CNTs, QDs, gold, silver, silica, layered-double hydroxide and iron-oxide nanoparticles. Each has unique properties and potential advantages.
For effective delivery, the vector first must be stably complexed to the nucleic acid cargo and needs to stay compacted until cellular entry. The size, shape, surface charge and surface functionality of the gene delivery particles are critical to efficient delivery, increased circulation time and specific cellular entry. Size is a crucial parameter in determining the passive biodistribution of a nanoparticle delivery system and charge shielding/PEGylation has been shown to improve circulation time and increase accumulation at tumor sites as a result of the EPR effect. Particles can be fabricated in a variety of different shapes and shape-shifting particles whose shape change can be triggered by pH, heat and light are also possible.
A majority of delivery systems achieve cellular entry via endocytosis. The desired delivery compartment within the cell is dependent on the type of nucleic acid being delivered. For delivery of isRNA, interaction with TLR7 in the endosome is the end-goal, so particles should be designed to target and then remain in the endo-some. For siRNA and all DNA-based systems, there needs to be a mechanism for endosomal escape. Mechanisms employed by nonviral vectors for endosomal escape include the proton-sponge effect, endosomolytic peptide-based lysis, and acid-triggered hydrophobic residue exposure. Hydrolysis, bioreduction and photolysis have been utilized to reduce toxicity and promote unpacking of nucleic acid cargo intracellularly. Finally, for cargo such as DNA that needs to localize to the nucleus, particles and nucleic acids can make use of endogenous cell machinery and NLS sequences to allow nuclear import via the NPC.
Future perspective
While nonviral nucleic acid delivery remains less efficient than viral delivery, recent advances offer the promise that soon there will be significant clinical effect from these approaches. CD-based polymers have found early clinical successes and additional biocompatible polymers are likely to soon follow suit. Incorporation of inorganic materials into such particles can also enable multimodality and theranostic applications. Several new directions are evolving, which offer approaches to achieve the goal of targeted, efficient, nonviral nucleic acid delivery.
Mesenchymal stem cells (MSCs) and neural stem/progenitor cells (NSPCs) are capable of migration toward pathological sites such as tumors and associated metastases. MSCs can be used to carry cargo while evading the immune system, as they are hypoimmunogenic and can then engraft into the stroma after arrival [117,270]. For these reasons, MSCs are a very promising avenue for nonviral targeted gene delivery. Recently, it was shown that virally transduced NSPCs could be implanted intracranially as an anticancer therapy. The NSPCs were transduced to stably express an enzyme that activates a 5-fluorocytosine prodrug, and following systemic 5-fluorocytosine treatment there was a significant (71%) reduction in tumor burden [271]. Another group has modified human neural stem cells to secrete anti-HER2 immunoglobulin molecules as a tool to target and attack metastatic breast cancer in the brain. Researchers were able to show that anti-HER2-secreting NSCs exhibit preferential tropism to tumor cells and can deliver antibodies to human breast cancer xenografts in mice [269]. Potential safety complications with viral transduction of these cells could be alleviated with a nonviral approach. Remaining issues with nonviral delivery include timing of gene expression, differentiation of NSPCs/MSCs, and the possibility that these cells become tumorigenic. It is critical that all potential safety concerns with this approach are thoroughly investigated in nonhuman primates before clinical trials commence.
Translocation of pDNA to the nucleus and nuclear import remain critical barriers for gene delivery. This is because in many ways, nonviral gene delivery research has focused on transporting pDNA safely and effectively into the cell, but has not focused as directly on its sub cellular location. Moreover, many biomaterials are designed to release naked DNA to the cytoplasm even though nuclear import is known to be inefficient. Enhancing nuclear import by other modalities in addition to NLS sequences and simple diffusion would be of great interest. It is known that dynein enables transport along microtubules in the direction of the nucleus [272] and that viruses, such as HIV, are able to exploit the cytoskeleton for directed movement towards the nucleus [273]. With further characterization of microtubule-associated transport, synthetic particles could similarly exploit endogenous cell machinery to enhance active transport to the nucleus and nuclear uptake.
Finally, addition of targeting moieties is a widely used and important technique in the field. In addition to targeting ligands to cell surface receptors, a complementary approach is targeting specific enzymes located at a specific microenvironment such as MMPs. MMPs are upregulated during tumor growth (i.e., MMP-3, -7 and -13) and play a role in cell growth, death, malignant conversion and tumor-associated angiogenesis [138]. siRNA has been used to downregulate MMP-9 and was shown to aid the inhibition of invasion and migration of prostate cancer cells, leading to apoptosis both in vitro and in vivo [274]. In one approach, QDs have been conjugated to folic acid, which is sterically shielded from the environment by MMP-7 cleavable PEG (exhaustively cleaved at 5 nM) [275]. This work combines the passive targeting of the EPR effect with MMP-sensitive release of cargo to take a twofold approach for the targeted delivery of the nanoparticles. The cancer type and stage are important in determining which MMP should be used to cleave cargo or be a target itself [138]. Compared with conventional cancer chemotherapies, gene therapy can enable a much wider therapeutic window due to increased specificity. Nanoparticles can be passively targeted by the EPR effect, targeted to a microenvironment through enzyme activity, targeted to a cell receptor through a ligand interaction, and targeted to a cell-type through biomaterial optimization [172]. Once DNA is delivered, it can then be transcriptionally targeted to the cell type of interest and the gene product itself could also be specific to that cell type. Thus, many layers of targeting can be enabled in a nonviral gene-delivery system and particles that use multiple methods of targeting will likely become more widespread in the future. The directions sketched herein and other innovations in biology, bioengineering, materials science and nanotechnology will continue to guide the field of nonviral gene delivery.
Executive summary
-
■
Key barriers to nucleic acid delivery include stable vector–cargo complexation, protection from extracellular degradation, transport to the cell of interest, cellular internalization, endo–lysosomal escape, cargo release, intracellular transport and vector degradation.
-
■
A wide array of materials have been investigated for their potential for delivery, including degradable and nondegradable cationic polymers, oligo- and polysaccharides, fullerenes, carbon nanotubes, quantum dots, gold, silver, silica, layered-double hydroxide and iron-oxide nanoparticles.
-
■
The desired delivery compartment within the cell is dependent on the type of nucleic acid being delivered.
-
■
Size is a crucial parameter in determining the passive biodistribution of a nanoparticle delivery system and charge shielding/PEGylation has been shown to improve circulation time and increase accumulation at tumor sites as a result of the enhanced permeability and retention effect. Particles can be fabricated in a variety of different shapes and shape-shifting particles whose shape-change can be triggered by pH, heat and light are also possible.
-
■
Electroporation, magnetofection, irradiation and ultrasound can be combined with nanoparticle delivery systems in ways that enhance delivery and the therapeutic effect at target cells.
-
■
Mechanisms employed by nonviral vectors for endosomal escape include the proton-sponge effect, endosomolytic peptide-based lysis, and acid-triggered hydrophobic residue exposure.
-
■
Approaches to reduce toxicity and promote intracellular unpacking include hydrolysis, bioreduction and photolysis.
-
■
Multifunctional constructs have been designed that accomplish different objectives simultaneously, such as gene therapy and diagnostics.
-
■
Most current clinical trials for gene therapy are primarily accomplished via viral vectors. Most nonviral vectors currently used for clinical trials involve lipofection and naked nucleic acids, but more polymeric and inorganic methods are expected to be used for clinical trials in the near-coming years.
Acknowledgments
The authors thank the TEDCO MSCR F (2009-MSCRFE-0098-00) for support.
Key Terms
- siRNA
Short, 20–25 base pair dsRNA that directs the target of RNAi. Active siRNA can be produced by the cleavage of longer dsRNA by the enzyme Dicer or siRNA can be used directly for therapeutic application.
- RNAi
The process whereby short dsRNA is used for directed cleavage of mRNA leading to decreased expression of the protein of interest.
- isRNA
Short dsRNA has also been shown to have immunostimulatory properties that are unrelated to their effects in the RNAi pathway; these RNAs interact with Toll-like receptors (TLR) 3,7, and 8; Type I interferon induction by synthetic isRNA requires TLR7 and is sequence dependent.
- shRNA
These are single stranded RNA (ssRNA) that form a `hairpin' secondary structure and have been shown to suppress the expression of desired genes. This method allows delivery of a plasmid that produces shRNA and leads to knockdown of gene expression.
- agRNA and saRNA
Newly recognized classes of RNA that target the promoter region of certain genes to induce or inhibit gene expression.
- Transfection
The successful introduction and expression of exogenous nucleic acid in a cell.
Footnotes
Financial & competing interests disclosure The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
■ of interest
■■ of considerable interest
- 1.Pringle IA, Hyde SC, Gill DR. Non-viral vectors in cystic fibrosis gene therapy: recent developments and future prospects. Expert Opin. Biol. Ther. 2009;9(8):991–1003. doi: 10.1517/14712590903055029. [DOI] [PubMed] [Google Scholar]
- 2.Lam BL, Feuer WJ, Abukhalil F, Porciatti V, Hauswirth WW, Guy J. Leber hereditary optic neuropathy gene therapy clinical trial recruitment: year 1. Arch. Ophthalmol. 2010;128(9):1129–1135. doi: 10.1001/archophthalmol.2010.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sadelain M, Riviere I, Wang X, et al. Strategy for a multicenter Phase I clinical trial to evaluate globin gene transfer in β-thalassemia. Ann. NY Acad. Sci. 2010;1202:52–58. doi: 10.1111/j.1749-6632.2010.05597.x. [DOI] [PubMed] [Google Scholar]
- 4.Perumbeti A, Malik P. Therapy for β-globinopathies: a brief review and determinants for successful and safe correction. Ann. NY Acad. Sci. 2010;1202:36–44. doi: 10.1111/j.1749-6632.2010.05584.x. [DOI] [PubMed] [Google Scholar]
- 5.Viiala NO, Larsen SR, Rasko JE. Gene therapy for hemophilia: clinical trials and technical tribulations. Semin. Thromb. Hemost. 2009;35(1):81–92. doi: 10.1055/s-0029-1214151. [DOI] [PubMed] [Google Scholar]
- 6.Phalon C, Rao DD, Nemunaitis J. Potential use of RNA interference in cancer therapy. Expert Rev. Mol. Med. 2010;12:E26. doi: 10.1017/S1462399410001584. [DOI] [PubMed] [Google Scholar]
- 7.Sangro B, Mazzolini G, Ruiz M, et al. A Phase I clinical trial of thymidine kinase-based gene therapy in advanced hepatocellular carcinoma. Cancer Gene Ther. 2010;17:837–843. doi: 10.1038/cgt.2010.40. [DOI] [PubMed] [Google Scholar]
- 8.Karvinen H, Yla-Herttuala S. New aspects in vascular gene therapy. Curr. Opin. Pharmacol. 2010;10(2):208–211. doi: 10.1016/j.coph.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 9.Nguyen DN, Green JJ, Chan JM, Langer R, Anderson DG. Polymeric materials for gene delivery and DNA vaccination. Adv. Mater. 2009;21(8):847–867. doi: 10.1002/adma.200801478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu J, Vodyanik MA, Smuga-Otto, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318(5858):1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]; ■ Engineering of induced pluripotent stem cells using virally transduced genes.
- 11.Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 12.Green JJ, Zhou BY, Mitalipova MM, et al. Nanoparticles for gene transfer to human embryonic stem cell colonies. Nano Lett. 2008;8(10):3126–3130. doi: 10.1021/nl8012665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Putnam D. Polymers for gene delivery across length scales. Nat. Mater. 2006;5(6):439–451. doi: 10.1038/nmat1645. [DOI] [PubMed] [Google Scholar]
- 14.Check E. Gene therapy put on hold as third child develops cancer. Nature. 2005;433(7026):561. doi: 10.1038/433561a. [DOI] [PubMed] [Google Scholar]
- 15.Viola JR, El-Andaloussi S, Oprea II, Smith CI. Non-viral nanovectors for gene delivery: factors that govern successful therapeutics. Expert Opin. Drug Deliv. 2010;7(6):721–735. doi: 10.1517/17425241003716810. [DOI] [PubMed] [Google Scholar]
- 16.Zamore PD, Tuschl T, Sharp PA, Bartel DP. RNAi: double-stranded RNA directs the ATP-dependent cleavage of mRNA at 21 to 23 nucleotide intervals. Cell. 2000;101(1):25–33. doi: 10.1016/S0092-8674(00)80620-0. [DOI] [PubMed] [Google Scholar]
- 17.Hamilton AJ, Baulcombe DC. A species of small antisense RNA in posttranscriptional gene silencing in plants. Science. 1999;286(5441):950–952. doi: 10.1126/science.286.5441.950. [DOI] [PubMed] [Google Scholar]
- 18.Guo JF, Fisher KA, Darcy R, Cryan JF, O'Driscoll C. Therapeutic targeting in the silent era: advances in non-viral siRNA delivery. Mol. Biosys. 2010;6(7):1143–1161. doi: 10.1039/c001050m. [DOI] [PubMed] [Google Scholar]
- 19.Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391(6669):806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]; ■ Seminal paper on the discovery of RNAi.
- 20.Schlee M, Hornung V, Hartmann G. siRNA and isRNA: two edges of one sword. Mol. Ther. 2006;14(4):463–470. doi: 10.1016/j.ymthe.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 21.Paddison PJ, Caudy AA, Bernstein E, Hannon GJ, Conklin DS. Short hairpin RNAs (shRNAs) induce sequence-specific silencing in mammalian cells. Genes Dev. 2002;16(8):948–958. doi: 10.1101/gad.981002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li LC, Okino ST, Zhao H, et al. Small dsRNAs induce transcriptional activation in human cells. Proc. Natl Acad. Sci. USA. 2006;103(46):17337–17342. doi: 10.1073/pnas.0607015103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Janowski BA, Younger ST, Hardy DB, Ram R, Huffman KE, Corey DR. Activating gene expression in mammalian cells with promoter-targeted duplex RNAs. Nat. Chem. Biol. 2007;3(3):166–173. doi: 10.1038/nchembio860. [DOI] [PubMed] [Google Scholar]
- 24.Miller AM, Dean DA. Tissue-specific and transcription factor-mediated nuclear entry of DNA. Adv. Drug Deliv. Rev. 2009;61(7–8):603–613. doi: 10.1016/j.addr.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 25.Lam AP, Dean DA. Progress and prospects: nuclear import of nonviral vectors. Gene Ther. 2010;17(4):439–447. doi: 10.1038/gt.2010.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schroeder A, Levins CG, Cortez C, Langer R, Anderson DG. Lipid-based nanotherapeutics for siRNA delivery. J. Intern. Med. 2010;267(1):9–21. doi: 10.1111/j.1365-2796.2009.02189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tros De Ilarduya C, Sun Y, Duzgunes N. Gene delivery by lipoplexes and polyplexes. Eur. J. Pharm. Sci. 2010;40(3):159–170. doi: 10.1016/j.ejps.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 28.Laemmli UK. Characterization of DNA condensates induced by poly(ethylene oxide) and polylysine. Proc. Natl Acad. Sci. 1975;72(11):4288–4292. doi: 10.1073/pnas.72.11.4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sela M, Arnon R, Jacobson I. Synthesis of poly-l-lysine and poly-l-lysyl albumin via sigma-N-trifluoroacetyl-A,N-carboxy-l-lysine anhydride. Biopolymers. 1963;1(6):517–525. [Google Scholar]
- 30.Midoux P, Monsigny M. Efficient gene transfer by histidylated polylysine pDNA complexes. Bioconj. Chem. 1999;10(3):406–411. doi: 10.1021/bc9801070. [DOI] [PubMed] [Google Scholar]
- 31.Choi YH, Liu F, Kim JS, Choi YK, Park JS, Kim SW. Polyethylene glycol-grafted poly-l-lysine as polymeric gene carrier. J. Control. Release. 1998;54(1):39–48. doi: 10.1016/s0168-3659(97)00174-0. [DOI] [PubMed] [Google Scholar]
- 32.Wu GY, Wu CH. Receptor-mediated in vitro gene transformation by a soluble DNA carrier system. J. Biol. Chem. 1987;262(10):4429–4432. [PubMed] [Google Scholar]
- 33.Wagner E, Zenke M, Cotten M, Beug H, Birnstiel ML. Transferrin–polycation conjugates as carriers for DNA uptake into cells. Proc. Natl Acad. Sci. USA. 1990;87(9):3410–3414. doi: 10.1073/pnas.87.9.3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nishikawa M, Takemura S, Takakura Y, Hashida M. Targeted delivery of plasmid DNA to hepatocytes in vivo: optimization of the pharmacokinetics of plasmid DNA galactosylated poly(l-lysine) complexes by controlling their physicochemical properties. J. Pharmacol. Exp. Therapeutics. 1998;287(1):408–415. [PubMed] [Google Scholar]
- 35.Hashida M, Takemura S, Nishikawa M, Takakura Y. Targeted delivery of plasmid DNA complexed with galactosylated poly(l-lysine) J. Control. Release. 1998;53(1–3):301–310. doi: 10.1016/s0168-3659(97)00263-0. [DOI] [PubMed] [Google Scholar]
- 36.Midoux P, Mendes C, Legrand A, et al. Specific gene-transfer mediated by lactosylated poly-l-lysine into hepatoma-cells. Nucleic Acids Res. 1993;21(4):871–878. doi: 10.1093/nar/21.4.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mislick KA, Baldeschwieler JD, Kayyem JF, Meade TJ. Transfection of folate-polylysine DNA complexes – evidence for lysosomal delivery. Bioconj. Chem. 1995;6(5):512–515. doi: 10.1021/bc00035a002. [DOI] [PubMed] [Google Scholar]
- 38.Boussif O, Lezoualc'h F, Zanta MA, et al. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc. Natl Acad. Sci. USA. 1995;92(16):7297–7301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]; ■ Original report on the use of poly(ethylenimine) for nucleic acid delivery in vitro and in vivo.
- 39.Brissault B, Kichler A, Guis C, Leborgne C, Danos O, Cheradame H. Synthesis of linear polyethylenimine derivatives for DNA transfection. Bioconj. Chem. 2003;14(3):581–587. doi: 10.1021/bc0200529. [DOI] [PubMed] [Google Scholar]
- 40.Fischer D, Bieber T, Li YX, Elsasser HP, Kissel T. A novel non-viral vector for DNA delivery based on low molecular weight, branched polyethylenimine: effect of molecular weight on transfection efficiency and cytotoxicity. Pharm. Res. 1999;16(8):1273–1279. doi: 10.1023/a:1014861900478. [DOI] [PubMed] [Google Scholar]
- 41.Moghimi SM, Symonds P, Murray JC, Hunter AC, Debska G, Szewczyk A. A two-stage poly(ethylenimine)-mediated cytotoxicity: implications for gene transfer/therapy. Mol. Ther. 2005;11(6):990–995. doi: 10.1016/j.ymthe.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 42.Wightman L, Kircheis R, Rossler V, et al. Different behavior of branched and linear polyethylenimine for gene delivery in vitro and in vivo. J. Gene Med. 2001;3(4):362–372. doi: 10.1002/jgm.187. [DOI] [PubMed] [Google Scholar]
- 43.Bettinger T, Carlisle RC, Read ML, Ogris M, Seymour LW. Peptide-mediated RNA delivery: a novel approach for enhanced transfection of primary and post-mitotic cells. Nucleic Acids Res. 2001;29(18):3882–3891. doi: 10.1093/nar/29.18.3882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thomas M, Lu JJ, Ge Q, Zhang C, Chen J, Klibanov AM. Full deacylation of polyethylenimine dramatically boosts its gene delivery efficiency and specificity to mouse lung. Proc. Natl Acad. Sci. USA. 2005;102(16):5679–5684. doi: 10.1073/pnas.0502067102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Astete CE, Sabliov CM. Synthesis and characterization of PLGA nanoparticles. J. Biomaterials Sci. Polymer Ed. 2006;17(3):247–289. doi: 10.1163/156856206775997322. [DOI] [PubMed] [Google Scholar]
- 46.Visscher GE, Robison RL, Maulding HV, Fong JW, Pearson JE, Argentieri GJ. Biodegradation of and tissue reaction to 50–50 Poly(dl-lactide-co-glycolide) microcapsules. J. Biomed. Mater. Res. 1985;19(3):349–365. doi: 10.1002/jbm.820190315. [DOI] [PubMed] [Google Scholar]
- 47.Shive MS, Anderson JM. Biodegradation and biocompatibility of PLA and PLGA microspheres. Adv. Drug Deliv. Rev. 1997;28(1):5–24. doi: 10.1016/s0169-409x(97)00048-3. [DOI] [PubMed] [Google Scholar]
- 48.Woodrow KA, Cu Y, Booth CJ, Saucier-Sawyer JK, Wood MJ, Saltzman WM. Intravaginal gene silencing using biodegradable polymer nanoparticles densely loaded with small-interfering RNA. Nat. Mat. 2009;8(6):526–533. doi: 10.1038/nmat2444. [DOI] [PMC free article] [PubMed] [Google Scholar]; ■ Precomplexation of siRNA with spermidine allows dense loading of poly(lactide-co-glycolide) nanoparticles with RNA.
- 49.Lynn DM, Langer R. Degradable poly(β-amino esters): synthesis, characterization, and self-assembly with plasmid DNA. J. Am. Chem. Soc. 2000;122(44):10761–10768. [Google Scholar]; ■ Paper detailing the advantages of a combinatorial approach to synthesizing a wide range of poly(β-amino ester)s
- 50.Anderson DG, Akinc A, Hossain N, Langer R. Structure/property studies of polymeric gene delivery using a library of poly(β-amino esters) Mol. Ther. 2005;11(3):426–434. doi: 10.1016/j.ymthe.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 51.Green JJ, Langer R, Anderson DG. A combinatorial polymer library approach yields insight into nonviral gene delivery. Acc. Chem. Res. 2008;41(6):749–759. doi: 10.1021/ar7002336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Akinc A, Lynn DM, Anderson DG, Langer R. Parallel synthesis and biophysical characterization of a degradable polymer library for gene delivery. J. Am. Chem. Soc. 2003;125(18):5316–5323. doi: 10.1021/ja034429c. [DOI] [PubMed] [Google Scholar]
- 53.Anderson DG, Lynn DM, Langer R. Semi-automated synthesis and screening of a large library of degradable cationic polymers for gene delivery. Angew. Chem. Int. Ed. 2003;42(27):3153–3158. doi: 10.1002/anie.200351244. [DOI] [PubMed] [Google Scholar]
- 54.Lynn DM, Anderson DG, Putnam D, Langer R. Accelerated discovery of synthetic transfection vectors: parallel synthesis and screening of a degradable polymer library. J. Am. Chem. Soc. 2001;123(33):8155–8156. doi: 10.1021/ja016288p. [DOI] [PubMed] [Google Scholar]
- 55.Tomalia DA, Baker H, Dewald J, et al. A new class of polymers – starburst-dendritic macromolecules. Polymer J. 1985;17(1):117–132. [Google Scholar]
- 56.Haensler J, Szoka FC. Polyamidoamine cascade polymers mediate efficient transfection of cells in culture. Bioconj. Chem. 1993;4(5):372–379. doi: 10.1021/bc00023a012. [DOI] [PubMed] [Google Scholar]
- 57.Navarro G, De Ilarduya CT. Activated and non-activated PAMAM dendrimers for gene delivery in vitro and in vivo. Nanomed. Nanotechnol. Biol. Med. 2009;5(3):287–297. doi: 10.1016/j.nano.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 58.Bielinska A, Kukowskalatallo JF, Johnson J, Tomalia DA, Baker JR. Regulation of in vitro gene expression using antisense oligonucleotides or antisense expression plasmids transfected using starburst PAMAM dendrimers. Nucleic Acids Research. 1996;24(11):2176–2182. doi: 10.1093/nar/24.11.2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Delong R, Stephenson K, Loftus T, et al. Characterization of complexes of oligonucleotides with polyamidoamine starburst dendrimers and effects on intracellular delivery. J. Pharm. Sci. 1997;86(6):762–764. doi: 10.1021/js960409f. [DOI] [PubMed] [Google Scholar]; ■ Classic paper using dendrimers for intracellular delivery.
- 60.Axel DI, Spyridopoulos I, Riessen R, Runge H, Viebahn R, Karsch KR. Toxicity, uptake kinetics and efficacy of new transfection reagents: Increase of oligonucleotide uptake. J. Vasc. Res. 2000;37(4):221–234. doi: 10.1159/000025737. [DOI] [PubMed] [Google Scholar]
- 61.Ravina M, Paolicelli P, Seijo B, Sanchez A. Knocking down gene expression with dendritic vectors. Mini-Rev. Med. Chem. 2010;10(1):73–86. doi: 10.2174/138955710791112569. [DOI] [PubMed] [Google Scholar]
- 62.Tang MX, Redemann CT, Szoka FC. In vitro gene delivery by degraded polyamidoamine dendrimers. Bioconj. Chem. 1996;7(6):703–714. doi: 10.1021/bc9600630. [DOI] [PubMed] [Google Scholar]
- 63.Wood KC, Little SR, Langer R, Hammond PT. A family of hierarchically self-assembling linear-dendritic hybrid polymers for highly efficient targeted gene delivery. Angew. Chem. Int. Ed. Engl. 2005;44(41):6704–6708. doi: 10.1002/anie.200502152. [DOI] [PubMed] [Google Scholar]
- 64.Morgan WT, Watkins WM. Unravelling the biochemical basis of blood group ABO and Lewis antigenic specificity. Glycoconj. J. 2000;17(7–9):501–530. doi: 10.1023/a:1011014307683. [DOI] [PubMed] [Google Scholar]
- 65.Li H, Sethuraman N, Stadheim TA, et al. Optimization of humanized IgGs in glycoengineered Pichia pastoris. Nat. Biotechnol. 2006;24(2):210–215. doi: 10.1038/nbt1178. [DOI] [PubMed] [Google Scholar]
- 66.Sinclair AM, Elliott S. Glycoengineering: the effect of glycosylation on the properties of therapeutic proteins. J. Pharm. Sci. 2005;94(8):1626–1635. doi: 10.1002/jps.20319. [DOI] [PubMed] [Google Scholar]
- 67.Pun SH, Bellocq NC, Liu AJ, et al. Cyclodextrin-modified polyethylenimine polymers for gene delivery. Bioconj. Chem. 2004;15(4):831–840. doi: 10.1021/bc049891g. [DOI] [PubMed] [Google Scholar]
- 68.Cryan SA, Holohan A, Donohue R, Darcy R, O'Driscoll CM. Cell transfection with polycationic cyclodextrin vectors. Eur. J. Pharm. Sci. 2004;21(5):625–633. doi: 10.1016/j.ejps.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 69.Arima H, Kihara F, Hirayama F, Uekama K. Enhancement of gene expression by polyamidoamine dendrimer conjugates with α-, β-, and γ-cyclodextrins. Bioconj. Chem. 2001;12(4):476–484. doi: 10.1021/bc000111n. [DOI] [PubMed] [Google Scholar]
- 70.Davis ME. The first targeted delivery of siRNA in humans via a self-assembling, cyclodextrin polymer-based nanoparticle: from concept to clinic. Mol. Pharm. 2009;6(3):659–668. doi: 10.1021/mp900015y. [DOI] [PubMed] [Google Scholar]
- 71.Davis ME, Zuckerman JE, Choi CH, et al. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature. 2010;464(7291):1067–1070. doi: 10.1038/nature08956. [DOI] [PMC free article] [PubMed] [Google Scholar]; ■■ Systemic application of siRNA nanoparticles in humans.
- 72.Davis ME. The first targeted delivery of siRNA in humans via a self-assembling, cyclodextrin polymer-based nanoparticle: from concept to clinic. Mol. Pharm. 2009;6(3):659–668. doi: 10.1021/mp900015y. [DOI] [PubMed] [Google Scholar]
- 73.Bellocq NC, Davis ME, Engler H, et al. Transferrin-targeted, cyclodextrin polycation-based gene vector for systemic delivery. Mol. Ther. 2003;7(5):S290–S290. [Google Scholar]
- 74.Pun SH, Tack F, Bellocq NC, et al. Targeted delivery of RNA-cleaving DNA enzyme (DNAzyme) to tumor tissue by transferrin-modified, cyclodextrin-based particles. Cancer Biol. Ther. 2004;3(7):641–650. doi: 10.4161/cbt.3.7.918. [DOI] [PubMed] [Google Scholar]
- 75.Prego C, Garcia M, Torres D, Alonso MJ. Transmucosal macromolecular drug delivery. J. Control. Release. 2005;101(1–3):151–162. doi: 10.1016/j.jconrel.2004.07.030. [DOI] [PubMed] [Google Scholar]
- 76.Roy K, Mao HQ, Huang SK, Leong KW. Oral gene delivery with chitosan – DNA nanoparticles generates immunologic protection in a murine model of peanut allergy. Nat. Med. 1999;5(4):387–391. doi: 10.1038/7385. [DOI] [PubMed] [Google Scholar]
- 77.Liu WG, Zhang X, Sun SJ, et al. N-alkylated chitosan as a potential nonviral vector for gene transfection. Bioconj. Chem. 2003;14(4):782–789. doi: 10.1021/bc020051g. [DOI] [PubMed] [Google Scholar]
- 78.Zhao X, Yin LC, Ding JY, et al. Thiolated trimethyl chitosan nanocomplexes as gene carriers with high in vitro and in vivo transfection efficiency. J. Control. Release. 2010;144(1)):46–54. doi: 10.1016/j.jconrel.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 79.Ghosn B, Singh A, Li M, et al. Efficient gene silencing in lungs and liver using imidazole-modified chitosan as a nanocarrier for small interfering RNA. Oligonucleotides. 2010;20(3):163–172. doi: 10.1089/oli.2010.0235. [DOI] [PubMed] [Google Scholar]
- 80.Opanasopit P, Techaarpornkul S, Rojanarata T, Ngawhirunpat T, Ruktanonchai U. Nucleic acid delivery with chitosan hydroxybenzotriazole. Oligonucleotides. 2010;20(3):127–136. doi: 10.1089/oli.2009.0227. [DOI] [PubMed] [Google Scholar]
- 81.Zhang FX, Srinivasan MP. Multilayered gold-nanoparticle/polyimide composite thin film through layer-by-layer assembly. Langmuir. 2007;23(20)):10102–10108. doi: 10.1021/la0635045. [DOI] [PubMed] [Google Scholar]
- 82.Huang XH, El-Sayed IH, Qian W, El-Sayed MA. Cancer cell imaging and photothermal therapy in the near-infrared region by using gold nanorods. J. Am. Chem. Soc. 2006;128(6):2115–2120. doi: 10.1021/ja057254a. [DOI] [PubMed] [Google Scholar]
- 83.Hartling T, Uhlig T, Seidenstucker A, et al. Fabrication of two-dimensional Au@FePt core-shell nanoparticle arrays by photochemical metal deposition. Appl. Phys. Lett. 2010;96:183111. [Google Scholar]
- 84.Mukherjee P, Bhattacharya R, Wang P, et al. Antiangiogenic properties of gold nanoparticles. Clin. Cancer Res. 2005;11(9)):3530–3534. doi: 10.1158/1078-0432.CCR-04-2482. [DOI] [PubMed] [Google Scholar]
- 85.Qiu HJ, Sun YL, Huang XR, Qu YB. A sensitive nanoporous gold-based electrochemical aptasensor for thrombin detection. Colloids Surfaces B Biointerfaces. 2010;79(1):304–308. doi: 10.1016/j.colsurfb.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 86.Bonoiu AC, Mahajan SD, Ding H, et al. Nanotechnology approach for drug addiction therapy: gene silencing using delivery of gold nanorod-siRNA nanoplex in dopaminergic neurons. Proc. Natl Acad. Sci. 2009;106(14):5546–5550. doi: 10.1073/pnas.0901715106. [DOI] [PMC free article] [PubMed] [Google Scholar]; ■ Gene therapy applied to treating addiction.
- 87.Nikoobakht B, El-Sayed MA. Preparation and growth mechanism of gold nanorods (NRs) using seed-mediated growth method. Chem. Mater. 2003;15(10):1957–1962. [Google Scholar]
- 88.Kayal S, Ramanujan RV. Anti-cancer drug loaded iron-gold core-shell nanoparticles (Fe@Au) for magnetic drug targeting. J. Nanosci. Nanotechnol. 2010;10(9):5527–5539. doi: 10.1166/jnn.2010.2461. [DOI] [PubMed] [Google Scholar]
- 89.Loo C, Lowery A, Halas NJ, West J, Drezek R. Immunotargeted nanoshells for integrated cancer imaging and therapy. Nano Lett. 2005;5(4):709–711. doi: 10.1021/nl050127s. [DOI] [PubMed] [Google Scholar]
- 90.Chang YH, Bau DT, Lee YS, et al. Fabrication of SiO2@Au core-shell multi-functional nanoparticles (MFNPs) for imaging and thermotherapy. Adv. Mater. Res. 2009:79–82. 565–568. [Google Scholar]
- 91.Chao SM, Meen TH, Chen WR, et al. Synthesis of Fe-core/Au-shell nanoparticles under ambient pressure. Key Engineering Mater. 2010:434–435. 799–802. [Google Scholar]
- 92.Daniel MC, Astruc D. Gold nanoparticles: assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem. Rev. 2004;104(1):293–346. doi: 10.1021/cr030698+. [DOI] [PubMed] [Google Scholar]
- 93.Lee JS, Green JJ, Love KT, Sunshine J, Langer R, Anderson DG. Gold, poly(β-amino ester) nanoparticles for small interfering RNA delivery. Nano Lett. 2009;9(6):2402–2406. doi: 10.1021/nl9009793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pissuwan D, Niidome T, Cortie MB. The forthcoming applications of gold nanoparticles in drug and gene delivery systems. J. Control. Release. 2009;149(1):65–71. doi: 10.1016/j.jconrel.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 95.El-Sayed IH, Huang XH, El-Sayed MA. Surface plasmon resonance scattering and absorption of anti-EGFR antibody conjugated gold nanoparticles in cancer diagnostics: applications in oral cancer. Nano Lett. 2005;5(5):829–834. doi: 10.1021/nl050074e. [DOI] [PubMed] [Google Scholar]
- 96.Huang HC, Rege K, Heys JJ. Spatiotemporal temperature distribution and cancer cell death in response to extracellular hyperthermia induced by gold nanorods. ACS Nano. 2010;4(5)):2892–2900. doi: 10.1021/nn901884d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Suwalski A, Dabboue H, Delalande A, et al. Accelerated achilles tendon healing by PDGF gene delivery with mesoporous silica nanoparticles. Biomaterials. 2010;31(19):5237–5245. doi: 10.1016/j.biomaterials.2010.02.077. [DOI] [PubMed] [Google Scholar]
- 98.Branca C, Frusteri F, Magazu V, Mangione A. Characterization of carbon nanotubes by TEM and infrared spectroscopy. J. Phys. Chem. B. 2004;108(11):3469–3473. [Google Scholar]
- 99.Lim SH, Luo Z, Shen Z, Lin J. Plasma-assisted synthesis of carbon nanotubes. Nanoscale Res. Lett. 2010;5(9):1377–1386. doi: 10.1007/s11671-010-9710-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Weber J, Singhal R, Zekri S, Kumar A. One-dimensional nanostructures: fabrication, characterisation and applications. Int. Mater. Rev. 2008;53(4)):235–255. [Google Scholar]
- 101.Reilly RM. Carbon nanotubes: potential benefits and risks of nanotechnology in nuclear medicine. J. Nuclear Med. 2007;48(7)):1039–1042. doi: 10.2967/jnumed.107.041723. [DOI] [PubMed] [Google Scholar]
- 102.Maeda-Mamiya R, Noiri E, Isobe H, et al. In vivo gene delivery by cationic tetraamino fullerene. Proc. Natl Acad. Sci. USA. 2010;107(12)):5339–5344. doi: 10.1073/pnas.0909223107. [DOI] [PMC free article] [PubMed] [Google Scholar]; ■ In vivo gene therapy using a fullerene.
- 103.Sitharaman B, Zakharian TY, Saraf A, et al. Water-soluble fullerene (C-60) derivatives as nonviral gene-delivery vectors. Mol. Pharm. 2008;5(4):567–578. doi: 10.1021/mp700106w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Arachchige IU, Brock SL. Highly luminescent quantum-dot monoliths. J. Am. Chem. Soc. 2007;129(7):1840–1841. doi: 10.1021/ja066749c. [DOI] [PubMed] [Google Scholar]
- 105.Hom C, Lu J, Liong M, et al. Mesoporous Silica nanoparticles facilitate delivery of siRNA to shutdown signaling pathways in mammalian cells. Small. 2010;6(11)):1185–1190. doi: 10.1002/smll.200901966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Liu YY, Miyoshi H. Preparation and characterization of novel drug delivery system of light-sensitive silica nanocapsules with thin shells. J. Biomed. Nanotechnol. 2008;4(1):25–32. [Google Scholar]
- 107.Qian J, Li X, Wei M, Gao XW, Xu ZP, He SL. Bio-molecule-conjugated fluorescent organically modified silica nanoparticles as optical probes for cancer cell imaging. Optics Express. 2008;16(24):19568–19578. doi: 10.1364/oe.16.019568. [DOI] [PubMed] [Google Scholar]
- 108.Moudgil S, Ying JY. Calcium-doped organosilicate nanoparticles for gene delivery vehicles for bone cells. Adv. Mater. 2007;19(20):3130–3135. [Google Scholar]
- 109.Yang XT, Zhang Y. Encapsulation of quantum nanodots in polystyrene and silica micro-/nanoparticles. Langmuir. 2004;20(14)):6071–6073. doi: 10.1021/la049610t. [DOI] [PubMed] [Google Scholar]
- 110.Delehanty JB, Boeneman K, Bradburne CE, Robertson K, Medintz IL. Quantum dots: a powerful tool for understanding the intricacies of nanoparticle-mediated drug delivery. Expert Opin. Drug Deliv. 2009;6(10):1091–1112. doi: 10.1517/17425240903167934. [DOI] [PubMed] [Google Scholar]
- 111.Barat B, Sirk SJ, Mccabe KE, et al. Cysdiabody quantum dot conjugates (ImmunoQdots) for cancer marker detection. Bioconj. Chem. 2009;20(8):1474–1481. doi: 10.1021/bc800421f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Portney NG, Ozkan M. Nano-oncology: drug delivery, imaging, and sensing. Anal. Bioanal. Chem. 2006;384(3):620–630. doi: 10.1007/s00216-005-0247-7. [DOI] [PubMed] [Google Scholar]
- 113.Barua S, Rege K. The influence of mediators of intracellular trafficking on transgene expression efficacy of polymer–plasmid DNA complexes. Biomaterials. 2010;31(22):5894–5902. doi: 10.1016/j.biomaterials.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 114.Tan WB, Jiang S, Zhang Y. Quantum-dot based nanoparticles for targeted silencing of HER2/neu gene via RNA interference. Biomaterials. 2007;28(8):1565–1571. doi: 10.1016/j.biomaterials.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 115.Munnier E, Cohen-Jonathan S, Linassier C, et al. Novel method of doxorubicin-SPION reversible association for magnetic drug targeting. Int. J. Pharm. 2008;363(1–2):170–176. doi: 10.1016/j.ijpharm.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 116.Liu Z, Kiessling F, Gatjens J. Advanced nanomaterials in multimodal imaging: design, functionalization, and biomedical applications. J. Nanomater. DOI:10.1155/2010/894303 (2010) (Epub ahead of print) [Google Scholar]
- 117.Tang C, Russell PJ, Martiniello-Wilks R, Rasko JE, Khatri A. Nanoparticles and cellular carriers - allies in cancer imaging and cellular gene therapy? Stem Cells. 2010;28(9):1686–1702. doi: 10.1002/stem.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Schopf B, Neuberger T, Schulze K, et al. Methodology description for detection of cellular uptake of PVA coated superparamagnetic iron oxide nanoparticles (SPION) in synovial cells of sheep. J. Magn. Magn. Mater. 2005;293(1):411–418. [Google Scholar]
- 119.Prijic S, Scancar J, Romih R, et al. Increased cellular uptake of biocompatible superparamagnetic iron oxide nanoparticles into malignant cells by an external magnetic field. J. Membrane Biol. 2010;236(1):167–179. doi: 10.1007/s00232-010-9271-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gupta AK, Gupta M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials. 2005;26(18):3995–4021. doi: 10.1016/j.biomaterials.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 121.Ladewig K, Niebert M, Xu ZP, Gray PP, Lu GQM. Efficient siRNA delivery to mammalian cells using layered double hydroxide nanoparticles. Biomaterials. 2010;31(7):1821–1829. doi: 10.1016/j.biomaterials.2009.10.058. [DOI] [PubMed] [Google Scholar]
- 122.Bhattacharya R, Patra CR, Earl A, et al. Attaching folic acid on gold nanoparticles using noncovalent interaction via different polyethylene glycol backbones and targeting of cancer cells. Nanomed. Nanotechnol. Biol. Med. 2007;3(3):224–238. [Google Scholar]
- 123.Xiao Y, Gao XG, Taratula O, et al. Anti-HER2 IgY antibody-functionalized single-walled carbon nanotubes for detection and selective destruction of breast cancer cells. BMC Cancer. 2009;9:351. doi: 10.1186/1471-2407-9-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gormley AJ, Ghandehari H. Evaluation of toxicity of nanostructures in biological systems. In: Sahu SC, Casciano DA, editors. Nanotechnology. John Wiley & Sons, Ltd; NJ, USA: 2009. [Google Scholar]
- 125.Kane AB, Hurt RH. Nanotoxicology: the asbestos analogy revisited. Nat. Nanotechnol. 2008;3(7):378–379. doi: 10.1038/nnano.2008.182. [DOI] [PubMed] [Google Scholar]
- 126.Zhang BL, Chen QO, Tang H, et al. Characterization of and biomolecule immobilization on the biocompatible multi-walled carbon nanotubes generatedby functionalization with polyamidoamine dendrimers. Colloids Surfaces B Biointerfaces. 2010;80(1):18–25. doi: 10.1016/j.colsurfb.2010.05.023. [DOI] [PubMed] [Google Scholar]
- 127.Wu P, Chen X, Hu N, et al. Biocompatible carbon nanotubes generated by functionalization with glycodendrimers. Angew. Chem. Int. Ed. Engl. 2008;47(27):5022–5025. doi: 10.1002/anie.200705363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Cheung W, Pontoriero F, Taratula O, Chen AM, He H. DNA and carbon nanotubes as medicine. Adv. Drug Deliv. Rev. 2010;62(6):633–649. doi: 10.1016/j.addr.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 129.Smart SK, Cassady AI, Lu GQ, Martin DJ. The biocompatibility of carbon nanotubes. Carbon. 2006;44(6):1034–1047. [Google Scholar]
- 130.Wick P, Manser P, Limbach LK, et al. The degree and kind of agglomeration affect carbon nanotube cytotoxicity. Toxicol. Lett. 2007;168(2):121–131. doi: 10.1016/j.toxlet.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 131.Sayes CM, Liang F, Hudson JL, et al. Functionalization density dependence of single-walled carbon nanotubes cytotoxicity in vitro. Toxicol. Lett. 2006;161(2):135–142. doi: 10.1016/j.toxlet.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 132.Dong L, Joseph KL, Witkowski CM, Craig MM. Cytotoxicity of single-walled carbon nanotubes suspended in various surfactants. Nanotechnology. 2008;19(25):255702. doi: 10.1088/0957-4484/19/25/255702. [DOI] [PubMed] [Google Scholar]
- 133.Bakry R, Vallant RM, Najam-Ul-Haq M, et al. Medicinal applications of fullerenes. Int. J. Nanomed. 2007;2(4):639–649. [PMC free article] [PubMed] [Google Scholar]
- 134.Lelong G, Bhattacharyya S, Kline S, Cacciaguerra T, Gonzalez MA, Saboungi ML. Effect of surfactant concentration on the morphology and texture of MCM-41 materials. J. Phys. Chem. C. 2008;112(29):10674–10680. [Google Scholar]
- 135.Park EJ, Park K. Oxidative stress and pro-inflammatory responses induced by silica nanoparticles in vivo and in vitro. Toxicol. Lett. 2009;184(1):18–25. doi: 10.1016/j.toxlet.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 136.Nan AJ, Bai X, Son SJ, Lee SB, Ghandehari H. Cellular uptake and cytotoxicity of silica nanotubes. Nano Lett. 2008;8(8):2150–2154. doi: 10.1021/nl0802741. [DOI] [PubMed] [Google Scholar]
- 137.Kirchner C, Liedl T, Kudera S, et al. Cytotoxicity of colloidal CdSe and CdSe/ZnS nanoparticles. Nano Lett. 2005;5(2):331–338. doi: 10.1021/nl047996m. [DOI] [PubMed] [Google Scholar]
- 138.Coussens LM, Fingleton B, Matrisian LM. Cancer therapy – matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science. 2002;295(5564):2387–2392. doi: 10.1126/science.1067100. [DOI] [PubMed] [Google Scholar]
- 139.Raynal I, Prigent P, Peyramaure S, Najid A, Rebuzzi C, Corot C. Macrophage endocytosis of superparamagnetic iron oxide nanoparticles – mechanisms and comparison of ferumoxides and ferumoxtran-10. Invest. Radiol. 2004;39(1):56–63. doi: 10.1097/01.rli.0000101027.57021.28. [DOI] [PubMed] [Google Scholar]
- 140.Ferrucci JT, Stark DD. Iron-oxide enhanced mr-imaging of the liver and spleen – review of the 1st-5 years. Am. J. Roentgenol. 1990;155(5):943–950. doi: 10.2214/ajr.155.5.2120963. [DOI] [PubMed] [Google Scholar]
- 141.Tang M, Russell PJ, Khatri A. Magnetic nanoparticles: prospects in cancer imaging and therapy. Discov. Med. 2007;7(38):68–74. [PubMed] [Google Scholar]
- 142.Sekhon BS, Kamboj SR. Inorganic nanomedicines – part 1. Nanomedicine. 2010;6(4):516–522. doi: 10.1016/j.nano.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 143.Del Pino P, Munoz-Javier A, Vlaskou D, Gil PR, Plank C, Parak WJ. Gene silencing mediated by magnetic lipospheres tagged with small interfering rna. Nano Lett. 2010;10(10):3914–3921. doi: 10.1021/nl102485v. [DOI] [PubMed] [Google Scholar]
- 144.Shen H, Tan J, Saltzman WM. Surface-mediated gene transfer from nanocomposites of controlled texture. Nat. Mater. 2004;3(8):569–574. doi: 10.1038/nmat1179. [DOI] [PubMed] [Google Scholar]; ■ Coprecipitation of DNA with inorganic minerals onto cell-culture surfaces.
- 145.Van Vlerken LE, Vyas TK, Amiji MM. Poly(ethylene glycol)-modified nanocarriers for tumor-targeted and intracellular delivery. Pharm. Res. 2007;24(8):1405–1414. doi: 10.1007/s11095-007-9284-6. [DOI] [PubMed] [Google Scholar]
- 146.Wang JQ, Tao XY, Zhang YF, Wei DZ, Ren YH. Reversion of multidrug resistance by tumor targeted delivery of antisense oligodeoxynucleotides in hydroxypropylchitosan nanoparticles. Biomaterials. 2010;31(15):4426–4433. doi: 10.1016/j.biomaterials.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 147.Champion JA, Katare YK, Mitragotri S. Particle shape: a new design parameter for micro- and nanoscale drug delivery carriers. J. Control. Release. 2007;121(1–2):3–9. doi: 10.1016/j.jconrel.2007.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Champion JA, Mitragotri S. Role of target geometry in phagocytosis. Proc. Natl Acad. Sci. USA. 2006;103(13):4930–4934. doi: 10.1073/pnas.0600997103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Geng Y, Dalhaimer P, Cai SS, et al. Shape effects of filaments versus spherical particles in flow and drug delivery. Nat. Nanotechnol. 2007;2(4):249–255. doi: 10.1038/nnano.2007.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Muro S, Garnacho C, Champion JA, et al. Control of endothelial targeting and intracellular delivery of therapeutic enzymes by modulating the size and shape of ICAM-1-targeted carriers. Mol. Ther. 2008;16(8):1450–1458. doi: 10.1038/mt.2008.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Yoo JW, Mitragotri S. Polymer particles that switch shape in response to a stimulus. Proc. Natl Acad. Sci. USA. 2010;107(25):11205–11210. doi: 10.1073/pnas.1000346107. [DOI] [PMC free article] [PubMed] [Google Scholar]; ■ Innovative shape-switching polymer particles.
- 152.Glotzer SC, Anderson JA. Nanoparticle Assembly Made to Order. Nat. Mater. 2010;9(11):885–887. doi: 10.1038/nmat2892. [DOI] [PubMed] [Google Scholar]
- 153.Xue C, Metraux GS, Millstone JE, Mirkin CA. Mechanistic study of photomediated triangular silver nanoprism growth. J. Am. Chem. Soc. 2008;130(26):8337–8344. doi: 10.1021/ja8005258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Jeong W, Napier ME, DeSimone JM. Challenging nature's monopoly on the creation of well-defined nanoparticles. Nanomed. 2010;5(4):633–639. doi: 10.2217/nnm.10.34. [DOI] [PubMed] [Google Scholar]
- 155.Verma A, Stellacci F. Effect of surface properties on nanoparticle–cell interactions. Small. 2010;6(1):12–21. doi: 10.1002/smll.200901158. [DOI] [PubMed] [Google Scholar]
- 156.Verma A, Uzun O, Hu Y, et al. Surface-structure-regulated cell-membrane penetration by monolayer-protected nanoparticles. Nat. Mater. 2008;7(7):588–595. doi: 10.1038/nmat2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Herbig ME, Assi F, Textor M, Merkle HP. The cell penetrating peptides pVEC and W2-pVEC induce transformation of gel phase domains in phospholipid bilayers without affecting their integrity. Biochemistry. 2006;45(11):3598–3609. doi: 10.1021/bi050923c. [DOI] [PubMed] [Google Scholar]
- 158.Li SD, Huang L. Pharmacokinetics and biodistribution of nanoparticles. Mol. Pharm. 2008;5(4):496–504. doi: 10.1021/mp800049w. [DOI] [PubMed] [Google Scholar]
- 159.Phillips MA, Gran ML, Peppas NA. Targeted nanodelivery of drugs and diagnostics. Nano Today. 2010;5(2):143–159. doi: 10.1016/j.nantod.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]; ■ Excellent review of targeted nanoparticle delivery.
- 160.Tong R, Cheng JJ. Anticancer polymeric nanomedicines. Polymer Rev. 2007;47(3):345–381. [Google Scholar]
- 161.Ortiz Mellet C, Benito JM, Garcia Fernandez JM. Preorganized, macromolecular, gene-delivery systems. Chemistry. 2010;16(23):6728–6742. doi: 10.1002/chem.201000076. [DOI] [PubMed] [Google Scholar]
- 162.Liu CH, Yu SY. Cationic nanoemulsions as non-viral vectors for plasmid DNA delivery. Colloids Surf. B Biointerfaces. 2010;79(2):509–515. doi: 10.1016/j.colsurfb.2010.05.026. [DOI] [PubMed] [Google Scholar]
- 163.Goodman CM, Mccusker CD, Yilmaz T, Rotello VM. Toxicity of gold nanoparticles functionalized with cationic and anionic side chains. Bioconj. Chem. 2004;15(4):897–900. doi: 10.1021/bc049951i. [DOI] [PubMed] [Google Scholar]
- 164.Arsianti M, Lim M, Marquis CP, Amal R. Assembly of polyethylenimine-based magnetic iron oxide vectors: insights into gene delivery. Langmuir. 2010;26(10):7314–7326. doi: 10.1021/la9041919. [DOI] [PubMed] [Google Scholar]
- 165.Godbey WT, Wu KK, Mikos AG. Poly(ethylenimine)-mediated gene delivery affects endothelial cell function and viability. Biomaterials. 2001;22(5):471–480. doi: 10.1016/s0142-9612(00)00203-9. [DOI] [PubMed] [Google Scholar]
- 166.Kloeckner J, Wagner E, Ogris M. Degradable gene carriers based on oligomerized polyamines. Eur. J. Pharm. Sci. 2006;29(5):414–425. doi: 10.1016/j.ejps.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 167.Jere D, Jiang HL, Arote R, et al. Degradable polyethylenimines as DNA and small interfering RNA carriers. Expert Opin. Drug Deliv. 2009;6(8):827–834. doi: 10.1517/17425240903029183. [DOI] [PubMed] [Google Scholar]
- 168.Ando S, Putnam D, Pack DW, Langer R. PLGA microspheres containing plasmid DNA: Preservation of supercoiled DNA via cryopreparation and carbohydrate stabilization. J. Pharm. Sci. 1999;88(1):126–130. doi: 10.1021/js9801687. [DOI] [PubMed] [Google Scholar]
- 169.Lim Y, Kim SM, Lee Y, et al. Cationic hyperbranched poly(amino ester): a novel class of DNA condensing molecule with cationic surface, biodegradable three-dimensional structure, and tertiary amine groups in the interior. J. Am. Chem. Soc. 2001;123(10):2460–2461. doi: 10.1021/ja005715g. [DOI] [PubMed] [Google Scholar]
- 170.Green JJ, Zugates GT, Tedford NC, et al. Combinatorial modification of degradable polymers enables transfection of human cells comparable to adenovirus. Adv. Mater. 2007;19(19):2836–2842. [Google Scholar]
- 171.Zugates GT, Tedford NC, Zumbuehl A, et al. Gene delivery properties of end-modified poly(β-amino ester)s. Bioconj. Chem. 2007;18(6):1887–1896. doi: 10.1021/bc7002082. [DOI] [PubMed] [Google Scholar]
- 172.Sunshine J, Green JJ, Mahon K, et al. Small molecule end group of linear polymer determine cell-type gene delivery efficacy. Adv. Mater. 2009;21(48):4947–4951. doi: 10.1002/adma.200901718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Green JJ, Shi J, Chiu E, Leshchiner ES, Langer R, Anderson DG. Biodegradable polymeric vectors for gene delivery to human endothelial cells. Bioconj. Chem. 2006;17:1162–1169. doi: 10.1021/bc0600968. [DOI] [PubMed] [Google Scholar]; ■ Polymer rivals adenovirus for gene delivery to human primary endothelial cells in vitro.
- 174.Anderson DG, Peng W, Akinc A, et al. A polymer library approach to suicide gene therapy for cancer. Proc. Natl Acad. Sci. USA. 2004;101(45):16028–16033. doi: 10.1073/pnas.0407218101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Huang YH, Zugates GT, Peng W, et al. Nanoparticle-delivered suicide gene therapy effectively reduces ovarian tumor burden in mice. Cancer Res. 2009;69(15):6184–6191. doi: 10.1158/0008-5472.CAN-09-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Opanasopit P, Nishikawa M, Hashida M. Factors affecting drug and gene delivery: effects of interaction with blood components. Crit. Rev. Ther. Drug Carrier Syst. 2002;19(3):191–233. doi: 10.1615/critrevtherdrugcarriersyst.v19.i3.10. [DOI] [PubMed] [Google Scholar]
- 177.Ogris M, Brunner S, Schuller S, Kircheis R, Wagner E. PEGylated DNA/transferrin-PEI complexes: reduced interaction with blood components, extended circulation in blood and potential for systemic gene delivery. Gene Ther. 1999;6(4):595–605. doi: 10.1038/sj.gt.3300900. [DOI] [PubMed] [Google Scholar]
- 178.Harada A, Kimura Y, Kojima C, Kono K. Effective tolerance to serum proteins of head-tail type polycation vectors by PEGylation at the periphery of the head block. Biomacromolecules. 2010;11(4):1036–1042. doi: 10.1021/bm1000108. [DOI] [PubMed] [Google Scholar]
- 179.Petersen H, Fechner PM, Martin AL, et al. Polyethylenimine-graft-poly(ethylene glycol) copolymers: influence of copolymer block structure on DNA complexation and biological activities as gene delivery system. Bioconj. Chem. 2002;13(4):845–854. doi: 10.1021/bc025529v. [DOI] [PubMed] [Google Scholar]
- 180.Subr V, Konak C, Laga R. Ulbrich K: Coating of DNA/poly(l-lysine) complexes by covalent attachment of poly[N-(2-hydroxypropyl)methacrylamide] Biomacromolecules. 2006;7(1):122–130. doi: 10.1021/bm050524x. [DOI] [PubMed] [Google Scholar]
- 181.Yuan Q, Yeudall WA, Yang H. PEGylated polyamidoamine dendrimers with bis-aryl hydrazone linkages for enhanced gene delivery. Biomacromolecules. 2010;11(8):1940–1947. doi: 10.1021/bm100589g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Liu Y, Li J, Shao K, et al. A leptin derived 30-amino-acid peptide modified pegylated poly-l-lysine dendrigraft for brain targeted gene delivery. Biomaterials. 2010;31(19):5246–5257. doi: 10.1016/j.biomaterials.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 183.Chen Y, Wu JJ, Huang L. Nanoparticles targeted with NGR motif deliver c-myc siRNA and doxorubicin for anticancer therapy. Mol. Ther. 2010;18(4):828–834. doi: 10.1038/mt.2009.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Liu Z, Tabakman SM, Chen Z, Dai H. Preparation of carbon nanotube bioconjugates for biomedical applications. Nat. Protoc. 2009;4(9):1372–1382. doi: 10.1038/nprot.2009.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Walker GF, Fella C, Pelisek J, et al. Toward synthetic viruses: endosomal pH-triggered deshielding of targeted polyplexes greatly enhances gene transfer in vitro and in vivo. Mol. Ther. 2005;11(3):418–425. doi: 10.1016/j.ymthe.2004.11.006. [DOI] [PubMed] [Google Scholar]; ■ Novel approach to endosomal escape.
- 186.Knorr V, Ogris M, Wagner E. An acid sensitive ketal-based polyethylene glycol-oligoethylenimine copolymer mediates improved transfection efficiency at reduced toxicity. Pharm. Res. 2008;25(12):2937–2945. doi: 10.1007/s11095-008-9700-6. [DOI] [PubMed] [Google Scholar]
- 187.Knorr V, Russ V, Allmendinger L, Ogris M, Wagner E. Acetal linked oligoethylenimines for use as pH-sensitive gene carriers. Bioconjug. Chem. 2008;19(8):1625–1634. doi: 10.1021/bc8001858. [DOI] [PubMed] [Google Scholar]
- 188.Carlisle RC, Etrych T, Briggs SS, Preece JA, Ulbrich K, Seymour LW. Polymer-coated polyethylenimine/DNA complexes designed for triggered activation by intracellular reduction. J. Gene Med. 2004;6(3):337–344. doi: 10.1002/jgm.525. [DOI] [PubMed] [Google Scholar]
- 189.Murthy N, Campbell J, Fausto N, Hoffman AS, Stayton PS. Design and synthesis of pH-responsive polymeric carriers that target uptake and enhance the intracellular delivery of oligonucleotides. J. Control. Release. 2003;89(3):365–374. doi: 10.1016/s0168-3659(03)00099-3. [DOI] [PubMed] [Google Scholar]
- 190.Harris TJ, Green JJ, Fung PW, Langer R, Anderson DG, Bhatia SN. Tissue-specific gene delivery via nanoparticle coating. Biomaterials. 2010;31(5):998–1006. doi: 10.1016/j.biomaterials.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Shmueli RB, Anderson DG, Green JJ. Electrostatic surface modifications to improve gene delivery. Expert Opin. Drug Deliv. 2010;7(4):535–550. doi: 10.1517/17425241003603653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Abdelhady HG, Allen S, Davies MC, Roberts CJ, Tendler SJB, Williams PM. Direct real-time molecular scale visualisation of the degradation of condensed DNA complexes exposed to DNase I. Nucleic Acids Res. 2003;31(14):4001–4005. doi: 10.1093/nar/gkg462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Lechardeur D, Sohn KJ, Haardt M, et al. Metabolic instability of plasmid DNA in the cytosol: a potential barrier to gene transfer. Gene Ther. 1999;6(4):482–497. doi: 10.1038/sj.gt.3300867. [DOI] [PubMed] [Google Scholar]
- 194.Schaffer DV, Fidelman NA, Dan N, Lauffenburger DA. Vector unpacking as a potential barrier for receptor-mediated polyplex gene delivery. Biotechnol. Bioeng. 2000;67(5):598–606. doi: 10.1002/(sici)1097-0290(20000305)67:5<598::aid-bit10>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]; ■ Vector unpacking as a gene-delivery barrier.
- 195.Walter E, Moelling K, Pavlovic J, Merkle HP. Microencapsulation of DNA using poly(dl-lactide-co-glycolide): stability issues and release characteristics. J. Control. Release. 1999;61(3):361–374. doi: 10.1016/s0168-3659(99)00151-0. [DOI] [PubMed] [Google Scholar]
- 196.Shu XZ, Zhu KJ. Chitosan/gelatin microspheres prepared by modified emulsification and ionotropic gelation. J. Microencapsul. 2001;18(2):237–245. doi: 10.1080/02652040010000415. [DOI] [PubMed] [Google Scholar]
- 197.Weecharangsan W, Opanasopit P, Ngawhirunpat T, Rojanarata T, Apirakaramwong A. Chitosan lactate as a nonviral gene delivery vector in COS-1 cells. AAPS PharmSciTech. 2006;7(3):66. doi: 10.1208/pt070366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Lou YL, Peng YS, Chen BH, Wang LF, Leong KW. Poly(ethylene imine)-g-chitosan using EX-810 as a spacer for nonviral gene delivery vectors. J. Biomed. Mater. Res. A. 2009;88A(4):1058–1068. doi: 10.1002/jbm.a.31961. [DOI] [PubMed] [Google Scholar]
- 199.Rethore G, Mathew A, Naik H, Pandit A. Preparation of chitosan/polyglutamic acid spheres based on the use of polystyrene template as a nonviral gene carrier. Tissue Eng. C – Methods. 2009;15(4):605–613. doi: 10.1089/ten.TEC.2008.0581. [DOI] [PubMed] [Google Scholar]
- 200.Yuan XD, Shah BA, Kotadia NK, Li JA, Gu H, Wu ZQ. The Development and mechanism studies of cationic chitosan-modified biodegradable PLGA nanoparticles for efficient siRNA drug delivery. Pharm. Res. 2010;27(7):1285–1295. doi: 10.1007/s11095-010-0103-0. [DOI] [PubMed] [Google Scholar]
- 201.Mao HQ, Roy K, Troung-Le VL, et al. Chitosan-DNA nanoparticles as gene carriers: synthesis, characterization and transfection efficiency. J. Control. Release. 2001;70(3):399–421. doi: 10.1016/s0168-3659(00)00361-8. [DOI] [PubMed] [Google Scholar]
- 202.Thomas M, Klibanov AM. Conjugation to gold nanoparticles enhances polyethylenimine's transfer of plasmid DNA into mammalian cells. Proc. Natl Acad. Sci. USA. 2003;100(16):9138–9143. doi: 10.1073/pnas.1233634100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Elbakry A, Zaky A, Liebl R, Rachel R, Goepferich A, Breunig M. Layer-by-layer assembled gold nanoparticles for siRNA delivery. Nano Lett. 2009;9(5):2059–2064. doi: 10.1021/nl9003865. [DOI] [PubMed] [Google Scholar]
- 204.Bauhuber S, Hozsa C, Breunig M, Göpferich A. Delivery of nucleic acids via disulfide-based carrier systems. Adv. Mater. 2009;21(32–33):3286–3306. doi: 10.1002/adma.200802453. [DOI] [PubMed] [Google Scholar]
- 205.Greish K. Enhanced permeability and retention of macromolecular drugs in solid tumors: a royal gate for targeted anticancer nanomedicines. J. Drug Target. 2007;15(7–8):457–464. doi: 10.1080/10611860701539584. [DOI] [PubMed] [Google Scholar]
- 206.Moffatt S, Cristiano RJ. PEGylated J591 mAb loaded in PLGA-PEG-PLGA tri-block copolymer for targeted delivery: in vitro evaluation in human prostate cancer cells. Int. J. Pharm. 2006;317(1):10–13. doi: 10.1016/j.ijpharm.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 207.Liu Y, Li K, Liu B, Feng SS. A strategy for precision engineering of nanoparticles of biodegradable copolymers for quantitative control of targeted drug delivery. Biomaterials. 2010;31(35):9145–9155. doi: 10.1016/j.biomaterials.2010.08.053. [DOI] [PubMed] [Google Scholar]
- 208.Pissuwan D, Valenzuela SM, Killingsworth MC, Xu XD, Cortie MB. Targeted destruction of murine macrophage cells with bioconjugated gold nanorods. J. Nanoparticle Res. 2007;9(6):1109–1124. [Google Scholar]
- 209.Dauty E, Remy JS, Zuber G, Behr JP. Intracellular delivery of nanometric DNA particles via the folate receptor. Bioconj. Chem. 2002;13(4):831–839. doi: 10.1021/bc0255182. [DOI] [PubMed] [Google Scholar]
- 210.Hashida M, Nishikawa M, Yamashita F, Takakura Y. Cell-specific delivery of genes with glycosylated carriers. Adv. Drug Deliv. Rev. 2001;52(3):187–196. doi: 10.1016/s0169-409x(01)00209-5. [DOI] [PubMed] [Google Scholar]
- 211.Kunath K, Merdan T, Hegener O, Haberlein H, Kissel T. Integrin targeting using RGD-PEI conjugates for in vitro gene transfer. J. Gene Med. 2003;5:588–599. doi: 10.1002/jgm.382. [DOI] [PubMed] [Google Scholar]
- 212.Green JJ, Chiu E, Leshchiner ES, Shi J, Langer R, Anderson DG. Electrostatic ligand coatings of nanoparticles enable ligand-specific gene delivery to human primary cells. Nano Lett. 2007;7(4):874–879. doi: 10.1021/nl062395b. [DOI] [PubMed] [Google Scholar]
- 213.Ogris M, Walker G, Blessing T, Kircheis R, Wolschek M, Wagner E. Tumor-targeted gene therapy: strategies for the preparation of ligand–polyethylene glycol-polyethylenimine–DNA complexes. J. Control. Release. 2003;91(1–2):173–181. doi: 10.1016/s0168-3659(03)00230-x. [DOI] [PubMed] [Google Scholar]
- 214.Levy-Nissenbaum E, Radovic-Moreno AF, Wang AZ, Langer R, Farokhzad OC. Nanotechnology and aptamers: applications in drug delivery. Trends Biotechnol. 2008;26(8):442–449. doi: 10.1016/j.tibtech.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 215.Nguyen J, Reul R, Betz T, et al. Nanocomposites of lung surfactant and biodegradable cationic nanoparticles improve transfection efficiency to lung cells. J. Control. Release. 2009;140(1):47–54. doi: 10.1016/j.jconrel.2009.07.017. [DOI] [PubMed] [Google Scholar]
- 216.Elfinger M, Pfeifer C, Uezguen S, et al. Self-assembly of ternary insulin-polyethylenimine (PEI)-DNA nanoparticles for enhanced gene delivery and expression in alveolar epithelial cells. Biomacromolecules. 2009;10(10):2912–2920. doi: 10.1021/bm900707j. [DOI] [PubMed] [Google Scholar]
- 217.Alexis F, Rhee JW, Richie JP, Radovic-Moreno AF, Langer R, Farokhzad OC. New frontiers in nanotechnology for cancer treatment. Urol. Oncol. 2008;26(1):74–85. doi: 10.1016/j.urolonc.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 218.Liao H, Hafner JH. Gold nanorod bioconjugates. Chem. Mater. 2005;17(18):4636–4641. [Google Scholar]
- 219.Kawano T, Yamagata M, Takahashi H, et al. Stabilizing of plasmid DNA in vivo by PEG-modified cationic gold nanoparticles and the gene expression assisted with electrical pulses. J. Control. Release. 2006;111(3):382–389. doi: 10.1016/j.jconrel.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 220.Rojas-Chapana J, Troszczynska J, Firkowska I, Morsczeck C, Giersig M. Multi-walled carbon nanotubes for plasmid delivery into Escherichia coli cells. Lab. Chip. 2005;5(5):536–539. doi: 10.1039/b500681c. [DOI] [PubMed] [Google Scholar]; ■ Irradiated multiwalled carbon nanotubes deliver genes with high efficiency.
- 221.Cai D, Mataraza JM, Qin ZH, et al. Highly efficient molecular delivery into mammalian cells using carbon nanotube spearing. Nat. Methods. 2005;2(6):449–454. doi: 10.1038/nmeth761. [DOI] [PubMed] [Google Scholar]
- 222.Rahim A, Taylor SL, Bush NL, Ter Haar GR, Bamber JC, Porter CD. Physical parameters affecting ultrasound/microbubble-mediated gene delivery efficiency in vitro. Ultrasound Med. Biol. 2006;32(8):1269–1279. doi: 10.1016/j.ultrasmedbio.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 223.Stride E, Porter C, Prieto AG, Pankhurst Q. Enhancement of microbubble mediated gene delivery by simultaneous exposure to ultrasonic and magnetic fields. Ultrasound Med. Biol. 2009;35(5):861–868. doi: 10.1016/j.ultrasmedbio.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 224.Lee PW, Peng SF, Su CJ, et al. The use of biodegradable polymeric nanoparticles in combination with a low-pressure gene gun for transdermal DNA delivery. Biomaterials. 2008;29(6):742–751. doi: 10.1016/j.biomaterials.2007.10.034. [DOI] [PubMed] [Google Scholar]
- 225.Sonawane ND, Szoka FC, Jr, Verkman AS. Chloride accumulation and swelling in endosomes enhances DNA transfer by polyamine-DNA polyplexes. J. Biol. Chem. 2003;278(45):44826–44831. doi: 10.1074/jbc.M308643200. [DOI] [PubMed] [Google Scholar]; ■ Investigation of the proton-sponge hypothesis for endosomal escape.
- 226.Akinc A, Thomas M, Klibanov AM, Langer R. Exploring polyethylenimine-mediated DNA transfection and the proton sponge hypothesis. J. Gene Med. 2005;7(5):657–663. doi: 10.1002/jgm.696. [DOI] [PubMed] [Google Scholar]
- 227.Yang YX, Xul ZH, Chen SW, et al. Histidylated cationic polyorganophosphazene/DNA self-assembled nanoparticles for gene delivery. Int. J. Pharm. 2008;353(1–2):277–282. doi: 10.1016/j.ijpharm.2007.11.041. [DOI] [PubMed] [Google Scholar]
- 228.Ogris M, Carlisle RC, Bettinger T, Seymour LW. Melittin enables efficient vesicular escape and enhanced nuclear access of nonviral gene delivery vectors. J. Biol. Chem. 2001;276(50):47550–47555. doi: 10.1074/jbc.M108331200. [DOI] [PubMed] [Google Scholar]; ■ Use of endosomolytic peptides for gene delivery.
- 229.Rozema DB, Ekena K, Lewis DL, Loomis AG, Wolff JA. Endosomolysis by masking of a membrane-active agent (EMMA) for cytoplasmic release of macromolecules. Bioconj. Chem. 2003;14(1):51–57. doi: 10.1021/bc0255945. [DOI] [PubMed] [Google Scholar]
- 230.Boeckle S, Fahrmeir J, Roedl W, Ogris M, Wagner E. Melittin analogs with high lytic activity at endosomal pH enhance transfection with purified targeted PEI polyplexes. J. Control. Release. 2006;112(2):240–248. doi: 10.1016/j.jconrel.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 231.Meyer M, Philipp A, Oskuee R, Schmidt C, Wagner E. Breathing life into polycations: functionalization with pH-responsive endosomolytic peptides and polyethylene glycol enables siRNA delivery. J. Am. Chem. Soc. 2008;130(11):3272–3273. doi: 10.1021/ja710344v. [DOI] [PubMed] [Google Scholar]
- 232.Murata M, Kagiwada S, Hishida R, Ishiguro R, Ohnishi S, Takahashi S. Modification of the N-terminus of membrane fusion-active peptides blocks the fusion activity. Biochem. Biophys. Res. Commun. 1991;179(2):1050–1055. doi: 10.1016/0006-291x(91)91925-3. [DOI] [PubMed] [Google Scholar]
- 233.Midoux P, Mayer R, Monsigny M. Membrane permeabilization by a-helical peptides: a flow cytometry study. Biochim. Biophys. Acta. 1995;1239(2):249–256. doi: 10.1016/0005-2736(95)00163-w. [DOI] [PubMed] [Google Scholar]
- 234.Wagner E, Plank C, Zatloukal K, Cotten M, Birnstiel ML. Influenza virus hemagglutinin HA-2 N-terminal fusogenic peptides augment gene transfer by transferrin-polylysine-DNA complexes: toward a synthetic virus-like gene-transfer vehicle. Proc. Natl Acad. Sci. USA. 1992;89(17):7934–7938. doi: 10.1073/pnas.89.17.7934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Moore NM, Sheppard CL, Barbour TR, Sakiyama-Elbert SE. The effect of endosomal escape peptides on in vitro gene delivery of polyethylene glycol-based vehicles. J. Gene Med. 2008;10(10):1134–1149. doi: 10.1002/jgm.1234. [DOI] [PubMed] [Google Scholar]
- 236.Hwang C, Sinskey AJ, Lodish HF. Oxidized redox state of glutathione in the endoplasmic reticulum. Science. 1992;257(5076):1496–1502. doi: 10.1126/science.1523409. [DOI] [PubMed] [Google Scholar]
- 237.Mellman I. Endocytosis and molecular sorting. Annu. Rev. Cell Dev. Biol. 1996;12:575–625. doi: 10.1146/annurev.cellbio.12.1.575. [DOI] [PubMed] [Google Scholar]
- 238.Gross J, Lapiere CM. Collagenolytic activity in amphibian tissues – a tissue culture assay. Proc. Natl Acad. Sci. USA. 1962;48(6):1014–1016. doi: 10.1073/pnas.48.6.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Niidome Y, Niidome T, Yamada S, Horiguchi Y, Takahashi H, Nakashima K. Pulsed-laser induced fragmentation and dissociation of DNA immobilized on gold nanoparticles. Mol. Crystals Liquid Crystals. 2006;445:201–206. [Google Scholar]
- 240.Jon S, Anderson DG, Langer R. Degradable poly(amino alcohol esters) as potential DNA vectors with low cytotoxicity. Biomacromolecules. 2003;4(6):1759–1762. doi: 10.1021/bm034176f. [DOI] [PubMed] [Google Scholar]
- 241.Jewell CM, Zhang JT, Fredin NJ, Wolff MR, Hacker TA, Lynn DM. Release of plasmid DNA from intravascular stents coated with ultrathin multilayered polyelectrolyte films. Biomacromolecules. 2006;7(9):2483–2491. doi: 10.1021/bm0604808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Schaffer DV, Lauffenburger DA. Optimization of cell surface binding enhances efficiency and specificity of molecular conjugate gene delivery. J. Biol. Chem. 1998;273(43):28004–28009. doi: 10.1074/jbc.273.43.28004. [DOI] [PubMed] [Google Scholar]
- 243.Lin C, Engbersen JF. The role of the disulfide group in disulfide-based polymeric gene carriers. Expert Opin. Drug Deliv. 2009;6(4):421–439. doi: 10.1517/17425240902878010. [DOI] [PubMed] [Google Scholar]
- 244.Lin C, Lammens TM, Zhong ZY, et al. Disulfide-containing poly(β-amino ester)s for gene delivery. J. Control. Release. 2006;116(2):E79–E81. doi: 10.1016/j.jconrel.2006.09.060. [DOI] [PubMed] [Google Scholar]
- 245.Lin C, Zhong Z, Lok MC, et al. Linear poly(amido amine)s with secondary and tertiary amino groups and variable amounts of disulfide linkages: synthesis and in vitro gene transfer properties. J. Control. Release. 2006;116(2):130–137. doi: 10.1016/j.jconrel.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 246.Piest M, Lin C, Mateos-Timoneda MA, et al. Novel poly(amido amine)s with bioreducible disulfide linkages in their diamino-units: structure effects and in vitro gene transfer properties. J. Control. Release. 2008;130(1):38–45. doi: 10.1016/j.jconrel.2008.05.023. [DOI] [PubMed] [Google Scholar]
- 247.Saito G, Swanson JA, Lee KD. Drug delivery strategy utilizing conjugation via reversible disulfide linkages: role and site of cellular reducing activities. Adv. Drug Deliv. Rev. 2003;55(2):199–215. doi: 10.1016/s0169-409x(02)00179-5. [DOI] [PubMed] [Google Scholar]
- 248.Lee Y, Mo H, Koo H, et al. Visualization of the degradation of a disulfide polymer, linear poly(ethylenimine sulfide), for gene delivery. Bioconjug. Chem. 2007;18(1):13–18. doi: 10.1021/bc060113t. [DOI] [PubMed] [Google Scholar]
- 249.Manickam DS, Li J, Putt DA, et al. Effect of innate glutathione levels on activity of redox-responsive gene delivery vectors. J. Control. Release. 2010;141(1):77–84. doi: 10.1016/j.jconrel.2009.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Chen J, Wu C, Oupicky D. Bioreducible hyperbranched poly(amido amine)s for gene delivery. Biomacromolecules. 2009;10(10):2921–2927. doi: 10.1021/bm900724c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Sunshine J, Bhise N, Green JJ. Degradable polymers for gene delivery. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2009;2009:2412–2415. doi: 10.1109/IEMBS.2009.5334767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Bhise NS, Gray RS, Sunshine JC, Htet S, Ewald AJ, Green JJ. The relationship between terminal functionalization and molecular weight of a gene delivery polymer and transfection efficacy in mammary epithelial 2-D cultures and 3-D organotypic cultures. Biomaterials. 2010;31(31):8088–8096. doi: 10.1016/j.biomaterials.2010.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Stevenson M, Ramos-Perez V, Singh S, et al. Delivery of siRNA mediated by histidine-containing reducible polycations. J. Control. Release. 2008;130(1):46–56. doi: 10.1016/j.jconrel.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 254.Gosselin MA, Guo WJ, Lee RJ. Efficient gene transfer using reversibly cross-linked low molecular weight polyethylenimine. Bioconj. Chem. 2001;12(6):989–994. doi: 10.1021/bc0100455. [DOI] [PubMed] [Google Scholar]
- 255.Hoon Jeong J, Christensen LV, Yockman JW, et al. Reducible poly(amido ethylenimine) directed to enhance RNA interference. Biomaterials. 2007;28(10):1912–1917. doi: 10.1016/j.biomaterials.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 256.Jere D, Kim JE, Arote R, et al. Akt1 silencing efficiencies in lung cancer cells by sh/si/ssiRNA transfection using a reductable polyspermine carrier. Biomaterials. 2009;30(8):1635–1647. doi: 10.1016/j.biomaterials.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 257.Chen CC, Lin YP, Wang CW, et al. DNA-gold nanorod conjugates for remote control of localized gene expression by near infrared irradiation. J. Am. Chem. Soc. 2006;128(11):3709–3715. doi: 10.1021/ja0570180. [DOI] [PubMed] [Google Scholar]; ■ Remotely controlled DNA release from shape changing gold nanoparticles.
- 258.Takahashi H, Niidome Y, Yamada S. Controlled release of plasmid DNA from gold nanorods induced by pulsed near-infrared light. Chem. Commun. (Camb) 2005;17:2247–2249. doi: 10.1039/b500337g. [DOI] [PubMed] [Google Scholar]
- 259.Wijaya A, Schaffer SB, Pallares IG, Hamad-Schifferli K. Selective release of multiple DNA oligonucleotides from gold nanorods. ACS Nano. 2009;3(1):80–86. doi: 10.1021/nn800702n. [DOI] [PubMed] [Google Scholar]
- 260.Dauty E, Verkman AS. Actin cytoskeleton as the principal determinant of size-dependent DNA mobility in cytoplasm: a new barrier for non-viral gene delivery. J. Biol. Chem. 2005;280(9):7823–7828. doi: 10.1074/jbc.M412374200. [DOI] [PubMed] [Google Scholar]
- 261.Pouton CW, Wagstaff KM, Roth DM, Moseley GW, Jans DA. Targeted delivery to the nucleus. Adv. Drug Deliv. Rev. 2007;59(8):698–717. doi: 10.1016/j.addr.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 262.Lange A, Mills RE, Lange CJ, Stewart M, Devine SE, Corbett AH. Classical nuclear localization signals: definition, function, and interaction with importin a. J. Biol. Chem. 2007;282(8):5101–5105. doi: 10.1074/jbc.R600026200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Pante N, Kann M. Nuclear pore complex is able to transport macromolecules with diameters of similar to 39 nm. Mol. Biol. Cell. 2002;13(2):425–434. doi: 10.1091/mbc.01-06-0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Zanta MA, Belguise-Valladier P, Behr JP. Gene delivery: a single nuclear localization signal peptide is sufficient to carry DNA to the cell nucleus. Proc. Natl Acad. Sci. USA. 1999;96(1):91–96. doi: 10.1073/pnas.96.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Zhang P, Liu WG. ZnO QD@PMAA-co-PDMAEMA nonviral vector for plasmid DNA delivery and bioimaging. Biomaterials. 2010;31(11):3087–3094. doi: 10.1016/j.biomaterials.2010.01.007. [DOI] [PubMed] [Google Scholar]; ■ ZnO quantum dots used for gene therapy and real-time imaging of transfection.
- 266.Cheng S-H, Lee C-H, Chen M-C, et al. Tri-functionalization of mesoporous silica nanoparticles for comprehensive cancer theranostics-the trio of imaging, targeting and therapy. J. Mater. Chem. 2010;20(29):6149–6157. [Google Scholar]; ■ Mesoporous silicon particles show excellent specificity, minimal collateral damage and potent photodynamic effects for imaging, targeting and therapy.
- 267.Tasciotti E, Liu X, Bhavane R, et al. Mesoporous silicon particles as a multistage delivery system for imaging and therapeutic applications. Nat. Nano. 2008;3(3):151–157. doi: 10.1038/nnano.2008.34. [DOI] [PubMed] [Google Scholar]
- 268.Libutti SK, Paciotti GF, Byrnes AA, et al. Phase I and pharmacokinetic studies of CYT-6091, a novel PEGylated colloidal gold-rhTNF nanomedicine. Clin. Cancer Res. 2010;16(24):6139–6149. doi: 10.1158/1078-0432.CCR-10-0978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Frank RT, Edmiston M, Kendall SE, et al. Neural stem cells as a novel platform for tumor-specific delivery of therapeutic antibodies. PLoS One. 2009;4(12):e8314. doi: 10.1371/journal.pone.0008314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Corsten MF, Shah K. Therapeutic stem-cells for cancer treatment: hopes and hurdles in tactical warfare. Lancet Oncol. 2008;9(4):376–384. doi: 10.1016/S1470-2045(08)70099-8. [DOI] [PubMed] [Google Scholar]
- 271.Aboody KS, Najbauer J, Schmidt NO, et al. Targeting of melanoma brain metastases using engineered neural stem/progenitor cells. Neuro. Oncol. 2006;8(2):119–126. doi: 10.1215/15228517-2005-012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Hirokawa N. Kinesin and dynein superfamily proteins and the mechanism of organelle transport. Science. 1998;279(5350):519–526. doi: 10.1126/science.279.5350.519. [DOI] [PubMed] [Google Scholar]
- 273.Arhel N, Genovesio A, Kim KA, et al. Quantitative four-dimensional tracking of cytoplasmic and nuclear HIV-1 complexes. Nat. Methods. 2006;3(10):817–824. doi: 10.1038/nmeth928. [DOI] [PubMed] [Google Scholar]
- 274.Nalla AK, Gorantla B, Gondi CS, Lakka SS, Rao JS. Targeting MMP-9, uPAR, and cathepsin B inhibits invasion, migration and activates apoptosis in prostate cancer cells. Cancer Gene Ther. 2010;17(9):599–613. doi: 10.1038/cgt.2010.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Sewell SL, Giorgio TD. Synthesis and enzymatic cleavage of dual-ligand quantum dots. Mater. Sci. Eng. C – Biomimetic Supramol. Syst. 2009;29(4):1428–1432. [Google Scholar]; ■ Combines passive targeting of the enhanced permeability and retention effect with matrix metaloproteinase-sensitive release of cargo.
- 276.Iijima S. Carbon nanotubes: past, present, and future. Physica B Condensed Matter. 2002;323(1–4):1–5. [Google Scholar]
- 277.Stewart DJ, Kutryk MJ, Fitchett D, et al. VEGF gene therapy fails to improve perfusion of ischemic myocardium in patients with advanced coronary disease: results of the NORTHERN trial. Mol. Ther. 2009;17(6):1109–1115. doi: 10.1038/mt.2009.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Whitehead KA, Langer R, Anderson DG. Knocking down barriers: advances in siRNA delivery. Nat. Rev. Drug Discov. 2010;9(5):412–412. doi: 10.1038/nrd2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Melman A, Rojas L, Christ G. Gene transfer for erectile dysfunction: will this novel therapy be accepted by urologists? Current Opin. Urol. 2009;19(6):595–600. doi: 10.1097/MOU.0b013e3283314985. [DOI] [PubMed] [Google Scholar]
- 280.Singerman L. Combination therapy using the small interfering RNA bevasiranib. Retina J. Retinal Vitreous Dis. 2009;29(6):S49–S50. doi: 10.1097/IAE.0b013e3181ad2341. [DOI] [PubMed] [Google Scholar]
- 281.Vaishnaw A. A status report on RNAi therapeutics. J. Silence. 2010;1(1):14. doi: 10.1186/1758-907X-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Devincenzo J, Lambkin-Williams R, Wilkinson T, et al. A randomized, double-blind, placebo-controlled study of an RNAi-based therapy directed against respiratory syncytial virus. Proc. Natl Acad. Sci. USA. 2010;107(19):8800–8805. doi: 10.1073/pnas.0912186107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Websites
- 301.Gene Therapy Clinical Trials Worldwide. 2011 www.wiley.com/legacy/wileychi/genmed/clinical
- 302.Schadendorf D. Gene therapy in patients with melanoma. Gene Therapy Clinical Trials Worldwide. www.abedia.com/wiley/record_detail. php?ID=149
- 303.Mahvi D. Phase I/IB study of immunization with autologous tumor cells transfected with the GM-CSF gene by particle-mediated transfer in patients with melanoma or sarcoma. Gene Therapy Clinical Trials Worldwide. 1996 doi: 10.1089/hum.1997.8.7-875. www.abedia.com/wiley/record_detail. php?ID=755 [DOI] [PubMed]
- 304.Stewart DJ. Multicentre, randomized, double blind, placebo controlled trial of myocardial angiogenesis using VEGF165, intramyocardial gene delivery in patients with severe angina. Gene Therapy Clinical Trials Worldwide. 2002 www.abedia.com/wiley/record_detail. php?ID=68
- 305.Ward M. The PHACeT Trial: pulmonary hypertension: assessment of cell therapy (Phase II) Gene Therapy Clinical Trials Worldwide. 2006 www.abedia.com/wiley/record_detail. php?ID=70
- 306.Baumgartner Gene therapy in patients with severe peripheral artery occlusive disease (PAOD) Gene Therapy Clinical Trials Worldwide. 2001 www.abedia.com/wiley/record_detail. php?ID=104
- 307.Figlin R. Phase II study of direct gene transfer of IL-2 plasmid DNA/DMRIE/DOPE lipid complex (Leuvectin) as an immunotherapeutic regimen in patients with metastatic renal cell carcinoma. Gene Therapy Clinical Trials Worldwide. 1998 www.abedia.com/wiley/record_detail. php?ID=856
- 308.Dreicer R. Phase II study of direct gene transfer of HLA-B7 plasmid DNA/DMRIE/ DOPE lipid complex (Allovectin-7) as an immunotherapeutic agent in patients with stage III or IV melanoma with no treatment alternatives. Gene Therapy Clinical Trials Worldwide. 1998 www.abedia.com/wiley/record_detail. php?ID=830
- 309.Ueno N. A Phase II Study of intraperitoneal E1A-lipid complex for patients without advanced epithelial ovarian cancer without HER-2/neu overexpression. Sponsor: Targeted Genetics Corporation. Gene Therapy Clinical Trials Worldwide. 2000 www.abedia.com/wiley/record_detail. php?ID=1012
- 310.Marshall J. An open-label safety study of escalating doses of sgt-53 for systemic injection in patients with advanced solid tumor malignancies. Gene Therapy Clinical Trials Worldwide. 2003 www.abedia.com/wiley/record_detail. php?ID=1188
- 311.Raez L. A Phase II clinical trial of adjuvant immunotherapy with an allogeneic B7.1/HLAA transfected tumor cell vaccine in patients with stage I–II non-small cell lung cancer. Gene Therapy Clinical Trials Worldwide. 2004 www.abedia.com/wiley/record_detail. php?ID=1269
- 312.Weinreb G. Quark Pharmaceuticals raises $27m. IVC Weekly Newsletter. 2008 www.ivc-online.com/ivcWeeklyItem. asp?articleID=6841
- 313.Kureczka J. Nucleonics initiates hepatitis B clinical trial with expressed interfering RNA therapeutic. EurekAlert. 2011 www.eurekalert.org/pub_releases/2008–2001/ ka-nih011008.php 2008.
- 314.O'Shaughnessy D. Safety and efficacy study of small interfering RNA molecule (Cand5) to treat diabetic macular edema. Clinical Trials. 2008 http://clinicaltrials.gov/show/NCT00306904.
- 315.Leachman SA. Study of TD101, a Small interfering RNA (siRNA) designed for treatment of pachyonychia congenita. Clinical Trials. 2008 http://clinicaltrials.gov/ct2/show/NCT00716 014?term=Pachyonychia+Congenita+AND+T ransDerm&rank=00716011.
- 316.Allergan I. A study using intravitreal injections of a small interfering RNA in patients with age-related macular degeneration. Clinical Trials. 2006 http://clinicaltrials.gov/ct2/show/NCT00395 057?term=Allergan+AND+siRNA+AND+A MD&rank=00395052.
- 317.Mendelsohn F. Safety and efficacy study using gene therapy for critical limb ischemia. Clinical Trials. 2010 http://clinicaltrials.gov/ct2/show/NCT01064 440?term=dna+gene+therapy&ra nk=01064443.
- 318.Grandis J. Gene therapy in treating patients with advanced head and neck cancer. Clinical Trials. 2001 http://clinicaltrials.gov/ct2/show/NCT0000 9841?term=dna+gene+therapy&ra nk=00009841.
- 319.Argiris A. Safety and efficacy of radiation/ cetuximab plus EGFR antisense DNA for head and neck squamous cell carcinoma. Clinical Trials. 2009 http://clinicaltrials.gov/ct2/show/NCT0090 3461?term=dna+gene+therapy+head+and+ne ck+squamous&rank=00903461.
- 320.Ribas A. Safety study of CALAA-01 to treat solid tumor cancers. Clinical Trials. 2008 http://clinicaltrials.gov/ct2/show/ NCT00689065?term=CALAA-00689001&rank=00689061.
- 321.Gollob J. Trial to evaluate safety and tolerability of ALN-TTR01 in transthyretin (TTR) amyloidosis. Clinical Trials. 2010 http://clinicaltrials.gov/ct2/show/NCT01148 953?term=Alnylam&rank=01148954.
- 322.Vaishnaw A. Dose escalation trial to evaluate the safety, tolerability, pharmacokinetics and pharmacodynamics of intravenous ALN-VSP02 in patients with advanced solid tumors. Clinical Trials. 2009 http://clinicaltrials.gov/ct2/show/NCT00882 180?term=Alnylam&rank=00882183.