Abstract
The amount of the future liver remnant volume is fundamental for hepato-biliary surgery, representing an important potential risk-factor for the development of post-hepatectomy liver failure. Despite this, there is no uniform consensus about the amount of hepatic parenchyma that can be safely resected, nor about the modality that should be chosen for this evaluation. The pre-operative evaluation of hepatic volume, along with a precise identification of vascular and biliar anatomy and variants, are therefore necessary to reduce surgical complications, especially for extensive resections. Some studies have tried to validate imaging methods [ultrasound, computed tomography (CT), magnetic resonance imaging] for the assessment of liver volume, but there is no clear evidence about the most accurate method for this evaluation. Furthermore, this volumetric evaluation seems to have a certain degree of error, tending to overestimate the actual hepatic volume, therefore some conversion factors, which should give a more reliable evaluation of liver volume, have been proposed. It is widespread among non-radiologists the use of independent software for an off-site volumetric analysis, performed on digital imaging and communications in medicine images with their own personal computer, but very few studies have provided a validation of these methods. Moreover, while the pre-transplantation volumetric assessment is fundamental, it remains unclear whether it should be routinely performed in all patients undergoing liver resection. In this editorial the role of imaging in the estimation of liver volume is discussed, providing a review of the most recent literature and a brief personal series of correlations between liver volumes and resection specimens’ weight, in order to assess the precision of the volumetric CT evaluation.
Keywords: Liver, Hepatectomy, Ultrasound, Computed tomography, Magnetic resonance imaging
Core tip: Imaging plays a fundamental role in the pre-operative volumetric evaluation of patients undergoing liver resection or transplantation. It seems that computed tomography and magnetic resonance imaging are reliable and substantially equivalent for this evaluation. Automatic or semi-automatic methods are efficient and less time-consuming than manual tracing methods. Further studies are needed to definitely evaluate the accuracy of commercially available software for liver volumetry.
INTRODUCTION
The increased knowledge of anatomical structures and liver function, the development of new surgical techniques, the improvements in chemotherapy and anesthesiological management made possible during recent years has increased the successful performance of liver resections, with mortality decreased to less than 5%; even more extensive hepatic resections are nowadays routinely successfully performed[1,2]. The pre-operative hepatic volumetry has become fundamental for hepato-biliary surgery[3-5]; it is widely accepted that the future liver remnant volume (FLRV) is an important potential risk-factor for the development of post-hepatectomy liver failure (PHLF), which is associated with an increase of post-operative complications and with a longer hospitalization. PHLF is the standardized term to define the post-surgical acquired deterioration of the synthetics, excretory and detoxifying functions of the liver; this syndrome is characterized by an increase of the international normalized ratio values and of serum bilirubin levels from the fifth post-operative day[6]. This syndrome has a reported incidence of 1.2%-32%[7-9]. As mentioned above, the post-hepatectomy mortality reported in recent years varies between 0% and 5% and the onset of PHLF remains the main cause[10-12]. Factors that contribute to the onset of PHLF can be divided into three groups: patient-related factors (age, diabetes, obesity)[13,14]; parenchyma-related factors (cirrhosis, cholestasis, steatosis, chemotherapy effects)[15-17]; surgery-related factors (bleeding, ischemia-reperfusion damage, sepsis, insufficient FLRV)[18-21]. With hepatic resection an amount of liver parenchyma is lost, and in the remnant hepatocytes arise both regeneration and necrosis. The remnant liver must therefore be able to overcome the necrosis, preserving or recovering an adequate synthetic ability[22]; as a consequence, there must be an adequate and functional FLRV to avoid PHLF[23]. Despite this, there is no uniform consensus among hepatic surgeons on the amount of liver volume that can be safely resected, with a wide range of reported values[24,25]. Guglielmi et al[24] reported that in patients with “healthy” liver (absence of hepatic diffuse disease, normal functionality tests) the limit of FLRV% for a safe resection varies between 20% and 30%, while in patients with underlying hepatic disease (cirrhosis, cholestasis, steatosis) the critical FLRV% value rise up to 30%-40%.
IMAGING: WHICH MODALITY SHOULD BE CHOSEN?
Many studies have tried to validate different imaging techniques for liver volumetry, but there is no clear evidence about the most accurate method for this evaluation. Kitajima et al[26] reported the possibility of performing liver volumetry by means of conventional ultrasound, with good correlation with the volume of the actual specimen; Xu et al[27] reported the usefulness of the three-dimensional ultrasound (3DUS) volumetric evaluation: the measured volumes all significantly correlated with the true volumes, with significant intraobserver and interobserver reproducibility. Despite the relative widespread availability of 3DUS probes and a considerable refinement of this technique in recent years, ultrasound has not universally proven to be successful for 3D evaluation of the liver, because of well-known limits, both physical and related to a variable reproducibility of the exam, which mainly depends on the skill of the examiner[28-30]. The use of more objective methods as computed tomography (CT) or magnetic resonance imaging (MRI) seems therefore fundamental, especially for the planning of more extensive resections, such as liver transplants or major hepatectomies. These two imaging techniques in fact showed a very good accuracy in the estimation of the graft dimensions before transplant[31] and in providing a precise quantification of the pre-operative liver volumes[32-34]. Itoh et al[35] stated that the meticulous preoperative evaluation based on volumetric analysis of 3D CT images, together with improved surgical techniques, were fundamental to achieve “zero mortality” and minimized intraoperative blood loss in this 300 hepatic resections series. Regarding liver transplantation, Ringe et al[36] emphasized the role of CT, reporting that this method of imaging of the liver, in combination with dedicated software, plays a key role in the evaluation of candidates for liver donor transplantation: based on the results of liver CT volumetry, 31% of the candidates of this series were excluded as donors. Lee et al[37] reported the usefulness of semi automated liver MR volumetry using hepatobiliary phase gadoxetic acid-enhanced images with the quadratic MR image division to measure liver volume in potential living liver donors; the average volume measurement error of the semi automated MR volumetry was 2.35% ± 1.22%. Zappa et al[38] applied CT volumetry to the evaluation of total and segmental liver regeneration after hepatectomy: CT was able to identify even segmental regeneration, reporting a 64% increase in liver volume from the future remnant 7 d after hepatectomy. CT imaging can be useful also to evaluate volumetric modifications after the induction of liver hypertrophy prior to surgery: Ulla et al[39] reported that CT volumetry, being able to calculate the mean absolute future-liver-remnant (FLR) and FLR/total liver volume (TLV) ratio before and after surgery, plays a key role in decision-making, monitoring and predicting liver hypertrophy pre- and post-operatively; in particular, if the enlargement of the FLR is as expected 6 d after surgery on CT examination, a second-step surgery can be safely performed. It has been reported by Vienne et al[40] that CT volumetry is also important prior to endoscopic biliary drainage, in order to estimate the volume of liver to drain: the main factor associated with drainage effectiveness was a liver volume drained of more than 50%. Kalkmann et al[41] has proposed the use of CT-based liver volumetry as a parameter to assess therapy response in patients with advanced liver metastasis, reporting that progressive disease led to larger median liver volume variations than partial remission or stable disease. Literature seems to provide a substantial equivalence between CT and MRI for liver volume estimation, but it must be otherwise noted that there is a bias related to a higher amount of published papers regarding CT-based volumetry. For example, Aoyama et al[34] proposed a manual segmentation technique that requires the tracing of 4 images, which showed a high linear correlation with the conventional manual tracing technique (r = 0.98; slope 0.97; P < 0.001), and does not depend on imaging modalities, so both MRI and CT images can be used. Kianmanesh et al[42] described a technique based on CT measurements of liver angles (the so-called angulometry) that can be used to predict liver ratios on both CT and MRI slices. Angulometry was described as simple and accurate (mean ± SD percentages of the TLV in angulometry and volumetry: 25% ± 4% and 20% ± 3%, respectively, with P < 0.05; mean ± SD overestimation of the percentage of the TLV in angulometry: 2.7% ± 7.0%). Numminen et al[43] stated that 3D liver models, which can be reconstructed both from modified discrete cosine transform and MRI data, improve the surgeon’s knowledge of liver anatomy and made even more complicated liver resections safe. Despite the reported accuracy, the volumetric evaluation performed both with CT and MRI seems to have a certain degree of error, tending to overestimate the actual hepatic volume in respect to the intra-operative volumetric evaluation, probably due to intra-operative loss of blood, as proposed by Niehues et al[44]: median liver density in his series was 1.07 g/mL. Regression analysis showed a high correlation between CT volumetry and water displacement (r = 0.985), but CT volumetry was found to be 13% higher than water displacement volumetry (P < 0.0001): the only relevant factor leading to this difference seemed to be blood perfusion. For these reasons, some authors have proposed the use of conversion factors and formulas, which should standardize imaging volumetry, providing a more realistic evaluation of liver volume[45,46]. Tongyoo et al[47], for example, proposed a formula that combined sonographic portal vein diameters measurement and CT liver volumetry, providing a precise donor screening for graft size adequacy. Sakei et al[48] proposed another formula to calculate the standard liver volume of children undergoing liver transplantation (standard liver volume = 689.9 × body surface area - 24.7), using CT images as a reference. Li et al[49] proposed the use of an equation (intraoperative weight = 0.844 × preoperative CT volume + 5.271) that can be useful to predict the actual graft weight (r = 0.885). Ribero et al[50] reported that the use of an estimated TLV, measured on the basis of correlation existing with body surface area (-794.41 + 1267.28 × body surface area), can identify about 11% of patients in whom liver volumetry directly calculated by CT images underestimates the risk of hepatic insufficiency. Chun et al[51] assessed the usefulness of future liver remnant calculation by means of CT standardized to body weight or body surface area, reporting a strong correlation for both measurements (r = 0.98). Vauthey[52] stated that the CT-based calculation of future liver remnant to TLV ratio by using a formula based on body surface area (liver volume = 706 × body surface area + 2.4) can provide a precise assessment of the future remnant before resection, and this is also useful in evaluating response to portal vein embolization. Müller et al[53] tested different measurement algorithms to predict TLV and reported that the analysis of 3D CT volumetry showed good correlation between the actual and the calculated liver volume in all tested algorithms; the Heidelberg algorithm reduced the measuring error with deviations of only 1.2%. Kayashima et al[54] created an age-adjusted formula using regression analysis retrospectively in 167 donors: 70.767 + (0.703 × graft volume estimated with 3D CT volumetry) + (1.298 × donor age). The mean reported error ratio for the age-adjusted formula (9.6%) was significantly lower than that from 3D CT (14%).
IMAGING: HOW TO CALCULATE LIVER VOLUME?
Various methods have been developed to calculate hepatic volume using CT or MR images. The first proposed method was the manual tracing of the entire liver, but despite a relative precision it was a very time-consuming technique[55-57]. More recently, automatic or semiautomatic segmentation techniques, for example using mathematical models based on histogram cluster analysis, were introduced[58]. Suzuki et al[59] developed an automated liver extraction scheme for measuring volumes at CT and compared the automated volumetric assessment based on this scheme with the findings at interactive volumetry performed with commercially available assist software and with manual volumetry, considered as the reference standard. The values obtained with automated and interactive CT liver volumetry agreed with the values obtained with manual volumetry (intra-class correlation coefficient = 0.94 and 0.96); automated volumetry required substantially less user time (less than 1 min/case) than manual volumetry (approximately 40 min/case) and interactive volumetry (approximately 30 min/case). With reference to liver transplantation, Radtke et al[60] described and validated a modus 3D volumetry based on unenhanced CT images, which accurately accounted for intrahepatic vascular volumes and offered a precise virtual model of individualized operative conditions for each potential live liver donor. Nakayama et al[61] proposed an automated method to obtain liver volumetry on CT images with good correlation with in vivo measured volumes (r = 0.792) in patients awaiting living related liver transplantation. Soyer et al[62] reported that there is a significant correlation (r = 0.767, P < 0.001) between hepatic height and hepatic volume, thus suggesting that hepatic height can be used to quickly predict hepatic volume, thus avoiding time-consuming evaluations as the manual segmentation. Kim et al[63] reported that the automated measurement of blood-free volume must be performed at automated CT volumetry in live liver donors; this parameter is more accurate than the ratio between blood-filled volume/1.22 in estimation of hepatic weight. From a technical point of view, it seems fundamental to use thin-section images, as reported by Hori et al[64]: liver volumes calculated from 2.5-mm-thick or thicker images resulted significantly smaller than liver volumes calculated from 3D images. If a maximum error of 5% in the calculated graft volume will not have a significant clinical impact, 5-mm-thick images are acceptable for CT volumetry, but if the impact is significant, 3D images could be essential. Luciani et al[65] reported that both manual and automated multiphase CT-based volume measurements were strongly correlated to liver volume (r = 0.87 and 0.90, respectively), but automated segmentation was significantly more rapid than manual segmentation (mean time: 16 ± 5 and 86 ± 3 s, respectively). Suzuki et al[66] developed a general framework for liver segmentation in both CT and MRI, using an anisotropic diffusion filter to reduce noise, a scale-specific gradient magnitude filter to enhance liver boundaries, a fast-marching algorithm to roughly determine liver boundaries, and a geodesic-active-contour model coupled with a level-set algorithm to refine the initial boundaries. The comparison of this computer volumetry with “gold standard” manual volumetry reported an excellent agreement (intra-class correlation coefficient = 0.94 and 0.98, respectively), with smaller average user time for computer volumetry. The usefulness of geodesic active contour segmentation was already reported[67]: in this series, the computer-estimated liver volumetrics agreed excellently with the gold-standard manual volumetrics (intraclass correlation coefficient = 0.95) with no statistically significant difference. The average accuracy, sensitivity, specificity, and percent volume error were 98.4%, 91.1%, 99.1%, and 7.2%, respectively. Zhou et al[68] tested three semiautomatic algorithms: 2D region growing with knowledge-based constraints, 2D voxel classification with propagational learning and Bayesian rule-based 3D region growing, reporting a promising overall performance of the first two methods. The use of independent software for an off-site volumetric analysis is also widespread, mainly among non-radiologists, performed on digital imaging and communications in medicine images with their own personal computer, but very few studies provided a validation of these evaluation methods, comparing, for example, their results to the volumetric analysis performed by the radiologist who performed imaging[69,70]. For example, Dello et al[69] compared ImageJ (http://rsb.info.nih.gov/ij/download.html) and OsiriX (http://www.osirix-viewer.com) in performing prospective CT volumetric analysis of the liver on a personal computer in patients undergoing major liver resection, reporting a significant correlation between the measured weights of resection specimens and the volumes calculated prospectively with ImageJ and OsiriX (r = 0.89 and r = 0.83, respectively) and a significant correlation between the volumes measured with radiological software and the volumes measured with ImageJ and OsiriX (r = 0.93 and r = 0.95, respectively). van der Vorst et al[70] assessed the accuracy of OsiriX (http://www.osirix-viewer.com) CT volumetry for predicting liver resection volume (RV) and FLVR in patients undergoing partial hepatectomy, and found significant correlations between these data and the weight and volume of the resected specimens (r = 0.95). Lu et al[71] reported that the hepatic volume calculation obtained with graphic software was reliable, despite the significant disadvantage in digitalization of CT films and manually copying pixel values.
PERSONAL EXPERIENCE
Materials and methods
A radiologist (M.D.) with 10 years of experience in abdominal imaging, blinded to the actual type of resection and to the final diagnosis, retrospectively reviewed the preoperative CT of all patients with primary liver malignancy (hepatocellular carcinoma or cholangiocarcinoma) that underwent resection of two or more liver segments in the last two years in our liver surgery unit. After reviewing CT, an on-site volumetric analysis was performed, using the post-processing application of our CT workstation (Liver Analysis; Extended Brilliance Workstation, Philips, Eindhoven, The Netherlands). The volumetric data were compared with those obtained at an off-site analysis by the hepatic surgeon (A.R.), with ten years of experience in liver surgery, with an independent off-site post-processing software (OsiriX, www.osirix-viewer.com) on his personal computer; to assess the reliability of the two methods, the RVs obtained with the two analysis methods were then compared with the actual weights of surgical specimen. The patients that underwent surgical resection for liver metastases, as well as those with benign liver tumors, were excluded from the study, because of the possibility of performing atypical resections, whose margins and whose extent cannot be accurately reproduced. Other exclusion criteria were: a pre-operative CT performed in another hospital, to avoid bias deriving from the use of a different scan protocol; the absence of weight data of the surgical specimens. Using these criteria, 14 patients were excluded from the study, 6/14 for a pre-operative CT scan performed in another hospital, 8/14 for the absence of weight data of the surgical specimens. Twenty-two patients were included in this study, 10/22 (45.5%) with hepatocellular carcinoma and 12/22 (54.5%) with cholangiocarcinoma (8/12 hilar cholangiocarcinoma; 4/12 peripheral cholangiocarcinoma).
All exams were performed with a multidetector CT scanner (Brilliance 64, Philips, Eindhoven, The Netherlands), before and after intravenous administration of a iodine contrast medium at a concentration of 370 mg/L (Ultravist 370, BayerScheringPharma AG, Berlin, Germany) through an antecubital vein of the arm, using an automatic double-syringe injector (Stellant, MedRad, Indianola, PA, United States) at a flow rate of 3-4 mL/s, followed by a bolus of 50 mL of saline at the same flow rate; the contrast medium amount was tailored to the patient’s weight, injecting 15 mL/kg of contrast medium. Positive oral contrast-medium was never administered. In all cases a quadriphasic examination was performed, using a bolus tracking technique: a pre-contrastographic scan of the upper abdomen; an arterial phase scan of the upper abdomen, performed 15 s after the reach of the aortic enhancement threshold (120 HU); a portal/venous phase scan of the entire abdomen, performed 70 s after the administration of the contrast media; and a delayed phase scan of the upper abdomen, performed 180 s after the administration of contrast media; an additional 10-min delayed acquisition was performed in patients with cholangiocarcinoma. The following parameters were used: thickness 2 mm; increment 11 mm; tube voltage 120 kV; collimation 64 × 0.625; pitch 0.891 for the pre-contrastographic, venous and delayed phase scan, 0.5 for the arterial phase scan; rotation time 0.75 s. For the on-site analysis, liver volumes were calculated with Liver Analysis application software, which uses the density differences in portal/venous scan images to obtain a semi-automatic liver segmentation. The gallbladder and the main vessels, such as the retro-hepatic inferior cava vein, were manually excluded from segmentation volume. A volumetric reconstruction of the liver and the quantification of TLV were obtained (Figure 1); the lesion was then manually segmented, obtaining the tumor volume (TV) (Figure 2). Then, a virtual hepatectomy was performed (Figure 3), obtaining RV values and FLRV values. The FLRV value was calculated both as an absolute value expressed in cubic centimeters, both as a percentage (FLRV%): for extra-hepatic lesions (i.e., hilar cholangiocarcinoma; the FLRV% was directly calculated as the ratio between FLRV and TLV), while for intra-hepatic lesions the FLRV% was indirectly calculated, first obtaining the actual TLV (ATLV) value, calculated as the difference between TLV and TV, because intra-hepatic lesions represent a non-functioning hepatic portion, which must not be included in the FLRV% value. The surgical specimen’s weight was used as the reference standard for the RV measurement, assuming that 1 g of parenchyma was equal to 1 cc; despite some authors[72] having reported that CT overestimates hepatic volume in comparison with the immersion of the surgical specimen in water, used for the ex vivo intraoperative volume measurement according to Archimedes’ principle. This approximation is acceptable because we considered it as the same for the two analysis methods. The Bland-Altman method was used to test the difference (delta) between the values obtained with the two methods against their average. The Pearson’s correlation test was used to assess the correlation between the estimated RV and the weight of surgical specimens. The statistical analysis was performed with GraphPad Prism 5.03.
Figure 1.
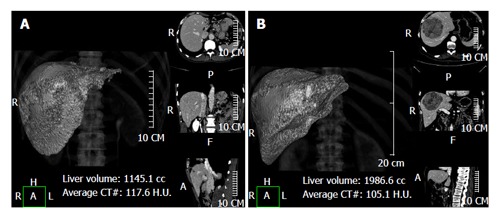
Using a semi-automatic method liver analysis application provides a 3D and a multi-planar reconstruction of the liver. A case of hilar cholangiocarcinoma involving the left hepatic duct, with marked hypotrophy of the left lobe (type IIIb according to the Bismuth-Corlette classification) (A) and a case of hepatocarcinoma in segments 4-5-8 (B) are shown.
Figure 2.
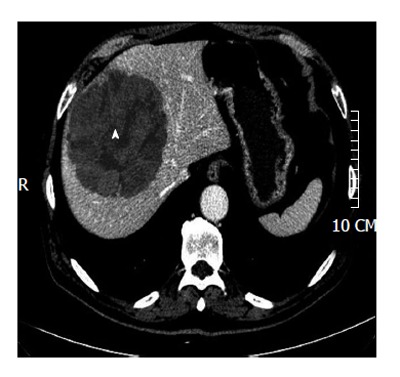
For intra-hepatic masses the manual segmentation of the lesion is needed. A huge hepatocarcinoma (arrowhead) in segments 4-8-5 is shown.
Figure 3.
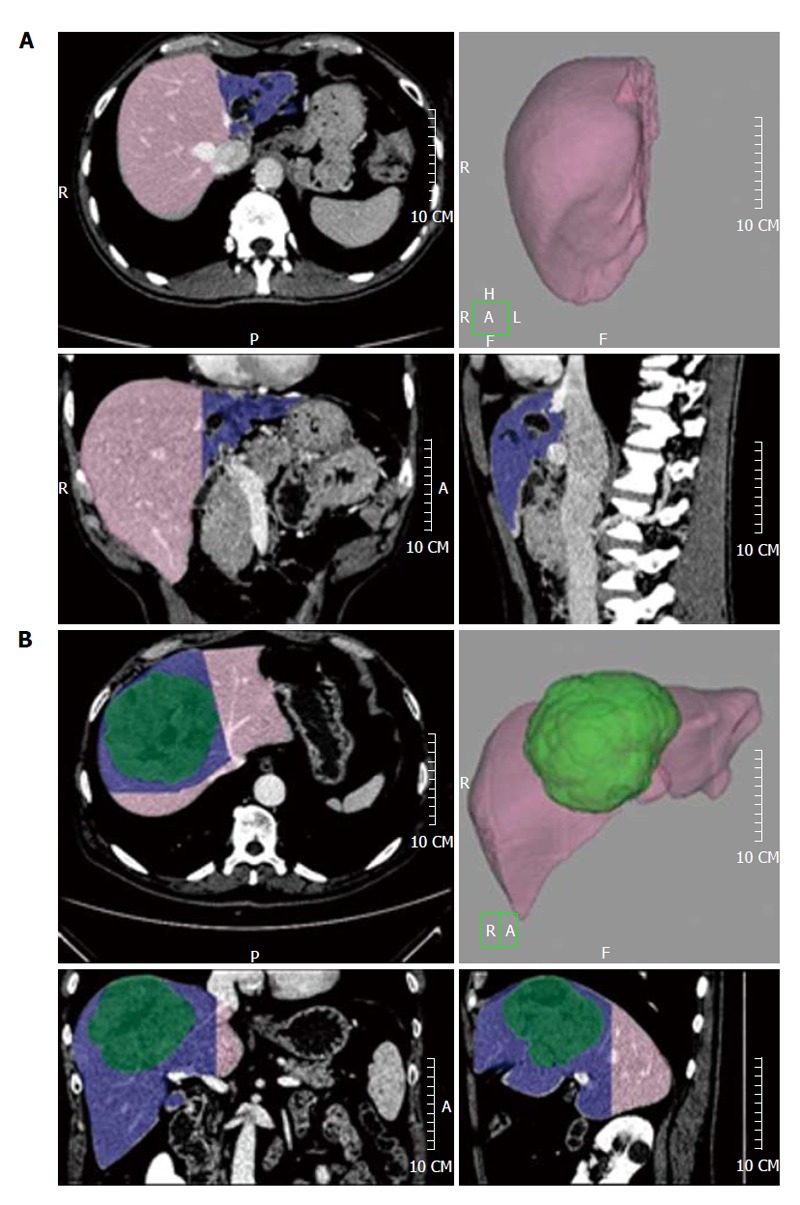
The future liver remnant volume is shown in pink, while the resection volume is shown in blue. A left hepatectomy for a hilar cholangiocarcinoma involving the left hepatic duct (A) and a mesohepatectomy (resection of liver segments 4-8-5) for a hepatocarcinoma (shown in green, B) are shown.
Results
The following resections were performed: left hepatectomy (resection of segments 2, 3 and 4 in 1 hilar cholangiocarcinoma), right hepatectomy (resection of segments 5, 6, 7 and 8 for 7 hepatocellular carcinomas and 2 cholangiocarcinomas); right hepatectomy and resection of caudate lobe (resection of segments 1, 4, 5, 6, 7 and 8 for 5 hilar cholangiocarcinomas); left hepatectomy and resection of caudate lobe (resection of segments 1, 2, 3, 4, 5 and 8 in 1 hilar cholangiocarcinoma); mesohepatectomy (resection of segments 4, 5 and 8 for 1 hepatocellular carcinoma); bisegmentectomy (1 cholangiocarcinoma, 2 hepatocellular carcinomas).
The average and delta values (difference between the values) of TLV, TV, ATLV, RV, and FLRV obtained with the two analysis methods are shown in Table 1.
Table 1.
Mean values of total liver volume, tumor volume, actual total liver volume, resection volume, future liver remnant volume, their mean and difference
| Variable | On-site | Off-site | Mean | Difference |
| TLV | 1787.31 | 1743.58 | 1765.48 | 43.73 |
| TV | 248.67 | 227.68 | 238.18 | 20.99 |
| RV | 1021.23 | 995.48 | 1008.36 | 25.75 |
| ATLV | 1538.65 | 1515.89 | 1527.27 | 22.76 |
| FLRV | 766.08 | 748.10 | 757.09 | 17.98 |
Values in cubic centimeters. TLV: Total liver volume; TV: Tumor volume; RV: Resection volume; ATLV: Actual total liver volume; FLRV: Future liver remnant volume.
Discussion
All patients included in this series had undergone resection of two or more liver segments; in these cases it seems important to perform a pre-operative liver volumetry to assess the FLRV; mean FLRV% obtained with on-site and off-site analysis were equivalent (50% and 49%, respectively). No patient developed PHLF.
The accuracy of the on-site analysis for the prediction of resection type was 100%, because in all cases the virtual resection correctly predicted the actual surgical resection.
The Bland-Altman comparison between the results of the two analysis methods is shown in Figure 4: the overall comparison between the mean values of the volumetric data obtained with on-site analysis and off-site analysis showed good correlation, with bias value of 26.24 (SD = 10.17, 95% limits of agreement from 6.301 to 46.18), thus configuring a substantial equivalence between the two analysis methods. The regression analysis did not show significant differences between the slope of the two regression curves (F = 0.09, P = 0.76, pooled slope = 0.88).
Figure 4.
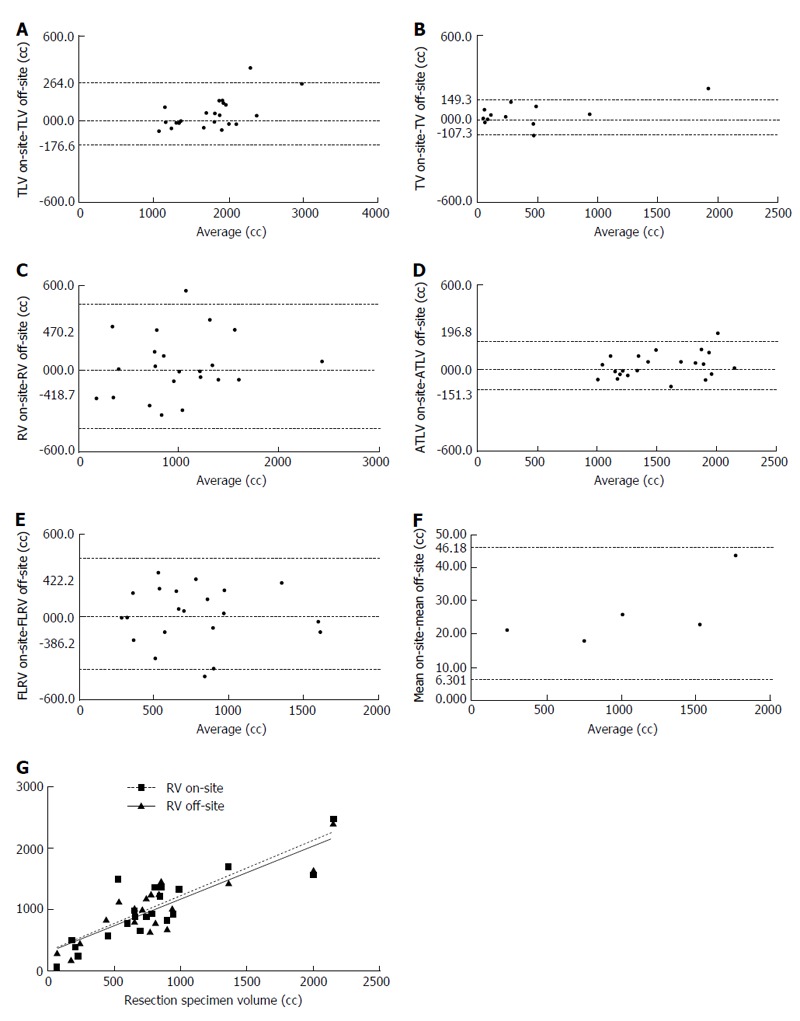
Bland-Altman graphs plotting the mean against the difference for total liver volume (A), tumor volume (B), resection volume (C), actual total liver volume (D), future liver remnant volume (E), and overall mean (F) with the two measurement methods; the correlation lines for the comparison between the resection volume and the actual volume of the resection specimens obtained with Pearson’s correlation test are shown in (G). TLV: Total liver volume; TV: Tumor volume; RV: Resection volume; ATLV: Actual total liver volume; FLRV: Future liver remnant volume.
The RV values obtained with the on-site analysis and the off-site analysis showed high correlation with the actual surgical specimen’s volume, with R values equal to 0.86 (95% confidence interval 0.69 to 0.94, P < 0.0001) and 0.88 for the off-site analysis (95% confidence interval 0.73 to 0.95, P < 0.0001).
Conclusion
The volumetric analysis obtained with on-site and with off-site analysis methods seems comparable and reliable. Further studies including a larger population are required.
CONCLUSION
The role of imaging in the pre-operative volumetric evaluation of patients undergoing liver resection or transplantation is fundamental. It seems that CT and MRI are substantially equivalent in this evaluation. Automatic or semi-automatic volumetry are efficient and less time-consuming than manual segmentation. Further studies are needed to evaluate the accuracy of commercially available software for liver volumetry.
Footnotes
P- Reviewers: Henninger B, Li JJ S- Editor: Wen LL L- Editor: O’Neill M E- Editor: Liu SQ
References
- 1.Scheele J, Stangl R, Altendorf-Hofmann A. Hepatic metastases from colorectal carcinoma: impact of surgical resection on the natural history. Br J Surg. 1990;77:1241–1246. doi: 10.1002/bjs.1800771115. [DOI] [PubMed] [Google Scholar]
- 2.Scheele J. [Vasculature based segmental resection of the liver] Langenbecks Arch Chir. 1990;375:308–317. doi: 10.1007/BF00184174. [DOI] [PubMed] [Google Scholar]
- 3.Okamoto E, Kyo A, Yamanaka N, Tanaka N, Kuwata K. Prediction of the safe limits of hepatectomy by combined volumetric and functional measurements in patients with impaired hepatic function. Surgery. 1984;95:586–592. [PubMed] [Google Scholar]
- 4.Cohnert TU, Rau HG, Buttler E, Hernandez-Richter T, Sauter G, Reuter C, Schildberg FW. Preoperative risk assessment of hepatic resection for malignant disease. World J Surg. 1997;21:396–400; discussion 401. doi: 10.1007/pl00012260. [DOI] [PubMed] [Google Scholar]
- 5.Rau HG, Schauer R, Helmberger T, Holzknecht N, von Rückmann B, Meyer L, Buttler E, Kessler M, Zahlmann G, Schuhmann D, et al. Impact of virtual reality imaging on hepatic liver tumor resection: calculation of risk. Langenbecks Arch Surg. 2000;385:162–170. doi: 10.1007/s004230050260. [DOI] [PubMed] [Google Scholar]
- 6.Rahbari NN, Garden OJ, Padbury R, Brooke-Smith M, Crawford M, Adam R, Koch M, Makuuchi M, Dematteo RP, Christophi C, et al. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS) Surgery. 2011;149:713–724. doi: 10.1016/j.surg.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 7.Farges O, Malassagne B, Flejou JF, Balzan S, Sauvanet A, Belghiti J. Risk of major liver resection in patients with underlying chronic liver disease: a reappraisal. Ann Surg. 1999;229:210–215. doi: 10.1097/00000658-199902000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cucchetti A, Ercolani G, Vivarelli M, Cescon M, Ravaioli M, La Barba G, Zanello M, Grazi GL, Pinna AD. Impact of model for end-stage liver disease (MELD) score on prognosis after hepatectomy for hepatocellular carcinoma on cirrhosis. Liver Transpl. 2006;12:966–971. doi: 10.1002/lt.20761. [DOI] [PubMed] [Google Scholar]
- 9.Mullen JT, Ribero D, Reddy SK, Donadon M, Zorzi D, Gautam S, Abdalla EK, Curley SA, Capussotti L, Clary BM, et al. Hepatic insufficiency and mortality in 1,059 noncirrhotic patients undergoing major hepatectomy. J Am Coll Surg. 2007;204:854–862; discussion 862-864. doi: 10.1016/j.jamcollsurg.2006.12.032. [DOI] [PubMed] [Google Scholar]
- 10.Poon RT, Fan ST, Lo CM, Liu CL, Lam CM, Yuen WK, Yeung C, Wong J. Improving perioperative outcome expands the role of hepatectomy in management of benign and malignant hepatobiliary diseases: analysis of 1222 consecutive patients from a prospective database. Ann Surg. 2004;240:698–708; discussion 708-710. doi: 10.1097/01.sla.0000141195.66155.0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kamiyama T, Nakanishi K, Yokoo H, Kamachi H, Tahara M, Yamashita K, Taniguchi M, Shimamura T, Matsushita M, Todo S. Perioperative management of hepatic resection toward zero mortality and morbidity: analysis of 793 consecutive cases in a single institution. J Am Coll Surg. 2010;211:443–449. doi: 10.1016/j.jamcollsurg.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 12.Simmonds PC, Primrose JN, Colquitt JL, Garden OJ, Poston GJ, Rees M. Surgical resection of hepatic metastases from colorectal cancer: a systematic review of published studies. Br J Cancer. 2006;94:982–999. doi: 10.1038/sj.bjc.6603033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nanashima A, Abo T, Nonaka T, Fukuoka H, Hidaka S, Takeshita H, Ichikawa T, Sawai T, Yasutake T, Nakao K, et al. Prognosis of patients with hepatocellular carcinoma after hepatic resection: are elderly patients suitable for surgery? J Surg Oncol. 2011;104:284–291. doi: 10.1002/jso.21932. [DOI] [PubMed] [Google Scholar]
- 14.Balzan S, Nagarajan G, Farges O, Galleano CZ, Dokmak S, Paugam C, Belghiti J. Safety of liver resections in obese and overweight patients. World J Surg. 2010;34:2960–2968. doi: 10.1007/s00268-010-0756-1. [DOI] [PubMed] [Google Scholar]
- 15.Khuntikeo N, Pugkhem A, Bhudhisawasdi V, Uttaravichien T. Major hepatic resection for hilar cholangiocarcinoma without preoperative biliary drainage. Asian Pac J Cancer Prev. 2008;9:83–85. [PubMed] [Google Scholar]
- 16.Takenaka K, Kanematsu T, Fukuzawa K, Sugimachi K. Can hepatic failure after surgery for hepatocellular carcinoma in cirrhotic patients be prevented? World J Surg. 1990;14:123–127. doi: 10.1007/BF01670561. [DOI] [PubMed] [Google Scholar]
- 17.Selzner M, Clavien PA. Failure of regeneration of the steatotic rat liver: disruption at two different levels in the regeneration pathway. Hepatology. 2000;31:35–42. doi: 10.1002/hep.510310108. [DOI] [PubMed] [Google Scholar]
- 18.Silva MA, Muralidharan V, Mirza DF. The management of coagulopathy and blood loss in liver surgery. Semin Hematol. 2004;41:132–139. doi: 10.1053/j.seminhematol.2003.11.022. [DOI] [PubMed] [Google Scholar]
- 19.Peralta C, Prats N, Xaus C, Gelpí E, Roselló-Catafau J. Protective effect of liver ischemic preconditioning on liver and lung injury induced by hepatic ischemia-reperfusion in the rat. Hepatology. 1999;30:1481–1489. doi: 10.1002/hep.510300622. [DOI] [PubMed] [Google Scholar]
- 20.Hessheimer AJ, Fondevila C, Taurá P, Muñoz J, Sánchez O, Fuster J, Rimola A, García-Valdecasas JC. Decompression of the portal bed and twice-baseline portal inflow are necessary for the functional recovery of a “small-for-size” graft. Ann Surg. 2011;253:1201–1210. doi: 10.1097/SLA.0b013e3181ffb2d7. [DOI] [PubMed] [Google Scholar]
- 21.Gross K, Katz S, Dunn SP, Cikrit D, Rosenthal R, Grosfeld JL. Bacterial clearance in the intact and regenerating liver. J Pediatr Surg. 1985;20:320–323. doi: 10.1016/s0022-3468(85)80211-6. [DOI] [PubMed] [Google Scholar]
- 22.Michalopoulos GK, DeFrances MC. Liver regeneration. Science. 1997;276:60–66. doi: 10.1126/science.276.5309.60. [DOI] [PubMed] [Google Scholar]
- 23.Shoup M, Gonen M, D’Angelica M, Jarnagin WR, DeMatteo RP, Schwartz LH, Tuorto S, Blumgart LH, Fong Y. Volumetric analysis predicts hepatic dysfunction in patients undergoing major liver resection. J Gastrointest Surg. 2003;7:325–330. doi: 10.1016/s1091-255x(02)00370-0. [DOI] [PubMed] [Google Scholar]
- 24.Guglielmi A, Ruzzenente A, Conci S, Valdegamberi A, Iacono C. How much remnant is enough in liver resection? Dig Surg. 2012;29:6–17. doi: 10.1159/000335713. [DOI] [PubMed] [Google Scholar]
- 25.Hiroshige S, Shimada M, Harada N, Shiotani S, Ninomiya M, Minagawa R, Soejima Y, Suehiro T, Honda H, Hashizume M, et al. Accurate preoperative estimation of liver-graft volumetry using three-dimensional computed tomography. Transplantation. 2003;75:1561–1564. doi: 10.1097/01.TP.0000053755.08825.12. [DOI] [PubMed] [Google Scholar]
- 26.Kitajima K, Taboury J, Boleslawski E, Savier E, Vaillant JC, Hannoun L. Sonographic preoperative assessment of liver volume before major liver resection. Gastroenterol Clin Biol. 2008;32:382–389. doi: 10.1016/j.gcb.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 27.Xu HX, Yin XY, Lu MD, Liu GJ, Xu ZF. Estimation of liver tumor volume using a three-dimensional ultrasound volumetric system. Ultrasound Med Biol. 2003;29:839–846. doi: 10.1016/s0301-5629(02)00775-5. [DOI] [PubMed] [Google Scholar]
- 28.Oldhafer KJ, Högemann D, Stamm G, Raab R, Peitgen HO, Galanski M. [3-dimensional (3-D) visualization of the liver for planning extensive liver resections] Chirurg. 1999;70:233–238. doi: 10.1007/s001040050636. [DOI] [PubMed] [Google Scholar]
- 29.Lang H, Wolf GK, Prokop M, Weimann A, Pichlmayr R, Zoller WG. [Volumetry of circumscribed liver changes with 3-D ultrasound in comparison with 3-D computerized tomography] Langenbecks Arch Chir Suppl Kongressbd. 1998;115:1478–1480. [PubMed] [Google Scholar]
- 30.Marescaux J, Clément JM, Tassetti V, Koehl C, Cotin S, Russier Y, Mutter D, Delingette H, Ayache N. Virtual reality applied to hepatic surgery simulation: the next revolution. Ann Surg. 1998;228:627–634. doi: 10.1097/00000658-199811000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lang H, Radtke A, Liu C, Sotiropoulos GC, Hindennach M, Schroeder T, Peitgen HO, Broelsch CE. Improved assessment of functional resectability in repeated hepatectomy by computer-assisted operation planning. Hepatogastroenterology. 2005;52:1645–1648. [PubMed] [Google Scholar]
- 32.Tu R, Xia LP, Yu AL, Wu L. Assessment of hepatic functional reserve by cirrhosis grading and liver volume measurement using CT. World J Gastroenterol. 2007;13:3956–3961. doi: 10.3748/wjg.v13.i29.3956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Torzilli G, Montorsi M, Del Fabbro D, Palmisano A, Donadon M, Makuuchi M. Ultrasonographically guided surgical approach to liver tumours involving the hepatic veins close to the caval confluence. Br J Surg. 2006;93:1238–1246. doi: 10.1002/bjs.5321. [DOI] [PubMed] [Google Scholar]
- 34.Aoyama M, Nakayama Y, Awai K, Inomata Y, Yamashita Y. A simple method for accurate liver volume estimation by use of curve-fitting: a pilot study. Radiol Phys Technol. 2013;6:180–186. doi: 10.1007/s12194-012-0186-x. [DOI] [PubMed] [Google Scholar]
- 35.Itoh S, Shirabe K, Taketomi A, Morita K, Harimoto N, Tsujita E, Sugimachi K, Yamashita Y, Gion T, Maehara Y. Zero mortality in more than 300 hepatic resections: validity of preoperative volumetric analysis. Surg Today. 2012;42:435–440. doi: 10.1007/s00595-011-0108-2. [DOI] [PubMed] [Google Scholar]
- 36.Ringe KI, Ringe BP, von Falck C, Shin HO, Becker T, Pfister ED, Wacker F, Ringe B. Evaluation of living liver donors using contrast enhanced multidetector CT - The radiologists impact on donor selection. BMC Med Imaging. 2012;12:21. doi: 10.1186/1471-2342-12-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee J, Won Kim K, Yeon Kim S, Kim B, Lee SJ, Jung Kim H, Seok Lee J, Gyu Lee M, Song GW, Hwang S, et al. Feasibility of semiautomated MR volumetry using gadoxetic acid-enhanced MRI at hepatobiliary phase for living liver donors. Magn Reson Med. 2013:Epub ahead of print. doi: 10.1002/mrm.24964. [DOI] [PubMed] [Google Scholar]
- 38.Zappa M, Dondero F, Sibert A, Vullierme MP, Belghiti J, Vilgrain V. Liver regeneration at day 7 after right hepatectomy: global and segmental volumetric analysis by using CT. Radiology. 2009;252:426–432. doi: 10.1148/radiol.2523080922. [DOI] [PubMed] [Google Scholar]
- 39.Ulla M, Ardiles V, Levy-Yeyati E, Alvarez FA, Spina JC, Garcia-Mónaco RD, De Santibañes E. New surgical strategy to induce liver hypertrophy: role of MDCT-volumetry to monitor and predict liver growth. Hepatogastroenterology. 2013;60:337–342. doi: 10.5754/hge12717. [DOI] [PubMed] [Google Scholar]
- 40.Vienne A, Hobeika E, Gouya H, Lapidus N, Fritsch J, Choury AD, Chryssostalis A, Gaudric M, Pelletier G, Buffet C, et al. Prediction of drainage effectiveness during endoscopic stenting of malignant hilar strictures: the role of liver volume assessment. Gastrointest Endosc. 2010;72:728–735. doi: 10.1016/j.gie.2010.06.040. [DOI] [PubMed] [Google Scholar]
- 41.Kalkmann J, Forsting M, Stattaus J. Liver volume variations as a parameter to assess therapy response in advanced metastatic liver disease. Onkologie. 2011;34:30–34. doi: 10.1159/000323373. [DOI] [PubMed] [Google Scholar]
- 42.Kianmanesh R, Piardi T, Tamby E, Parvanescu A, Bruno O, Palladino E, Bouché O, Msika S, Sommacale D. Liver angulometry: a simple method to estimate liver volume and ratios. HPB (Oxford) 2013;15:976–984. doi: 10.1111/hpb.12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Numminen K, Sipilä O, Mäkisalo H. Preoperative hepatic 3D models: virtual liver resection using three-dimensional imaging technique. Eur J Radiol. 2005;56:179–184. doi: 10.1016/j.ejrad.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 44.Niehues SM, Unger JK, Malinowski M, Neymeyer J, Hamm B, Stockmann M. Liver volume measurement: reason of the difference between in vivo CT-volumetry and intraoperative ex vivo determination and how to cope it. Eur J Med Res. 2010;15:345–350. doi: 10.1186/2047-783X-15-8-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Karlo C, Reiner CS, Stolzmann P, Breitenstein S, Marincek B, Weishaupt D, Frauenfelder T. CT- and MRI-based volumetry of resected liver specimen: comparison to intraoperative volume and weight measurements and calculation of conversion factors. Eur J Radiol. 2010;75:e107–e111. doi: 10.1016/j.ejrad.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 46.Lemke AJ, Brinkmann MJ, Schott T, Niehues SM, Settmacher U, Neuhaus P, Felix R. Living donor right liver lobes: preoperative CT volumetric measurement for calculation of intraoperative weight and volume. Radiology. 2006;240:736–742. doi: 10.1148/radiol.2403042062. [DOI] [PubMed] [Google Scholar]
- 47.Tongyoo A, Pomfret EA, Pomposelli JJ. Accurate estimation of living donor right hemi-liver volume from portal vein diameter measurement and standard liver volume calculation. Am J Transplant. 2012;12:1229–1239. doi: 10.1111/j.1600-6143.2011.03909.x. [DOI] [PubMed] [Google Scholar]
- 48.Saeki I, Tokunaga S, Matsuura T, Hayashida M, Yanagi Y, Taguchi T. A formula for determining the standard liver volume in children: a special reference for neonates and infants. Pediatr Transplant. 2012;16:244–249. doi: 10.1111/j.1399-3046.2011.01624.x. [DOI] [PubMed] [Google Scholar]
- 49.Li YC, Hu Y, Zhang MM, Jin XQ, Fan X, Pu CL, Guo CB, Kang Q, Dai XK, Deng YH. Usage of 64-detector-row spiral computed tomography volumetry in preoperative volume prediction in living donor liver transplantation in children. Pediatr Surg Int. 2011;27:445–449. doi: 10.1007/s00383-010-2830-z. [DOI] [PubMed] [Google Scholar]
- 50.Ribero D, Amisano M, Bertuzzo F, Langella S, Lo Tesoriere R, Ferrero A, Regge D, Capussotti L. Measured versus estimated total liver volume to preoperatively assess the adequacy of the future liver remnant: which method should we use? Ann Surg. 2013;258:801–806; discussion 806-807. doi: 10.1097/SLA.0000000000000213. [DOI] [PubMed] [Google Scholar]
- 51.Chun YS, Ribero D, Abdalla EK, Madoff DC, Mortenson MM, Wei SH, Vauthey JN. Comparison of two methods of future liver remnant volume measurement. J Gastrointest Surg. 2008;12:123–128. doi: 10.1007/s11605-007-0323-8. [DOI] [PubMed] [Google Scholar]
- 52.Vauthey JN, Chaoui A, Do KA, Bilimoria MM, Fenstermacher MJ, Charnsangavej C, Hicks M, Alsfasser G, Lauwers G, Hawkins IF, et al. Standardized measurement of the future liver remnant prior to extended liver resection: methodology and clinical associations. Surgery. 2000;127:512–519. doi: 10.1067/msy.2000.105294. [DOI] [PubMed] [Google Scholar]
- 53.Müller SA, Bläuer K, Kremer M, Thorn M, Mehrabi A, Meinzer HP, Hinz U, Metzger J, Büchler MW, Schmied BM. Exact CT-based liver volume calculation including nonmetabolic liver tissue in three-dimensional liver reconstruction. J Surg Res. 2010;160:236–243. doi: 10.1016/j.jss.2009.04.050. [DOI] [PubMed] [Google Scholar]
- 54.Kayashima H, Taketomi A, Yonemura Y, Ijichi H, Harada N, Yoshizumi T, Soejima Y, Yoshimitsu K, Maehara Y. Accuracy of an age-adjusted formula in assessing the graft volume in living donor liver transplantation. Liver Transpl. 2008;14:1366–1371. doi: 10.1002/lt.21547. [DOI] [PubMed] [Google Scholar]
- 55.Soyer P, Roche A, Elias D, Levesque M. Hepatic metastases from colorectal cancer: influence of hepatic volumetric analysis on surgical decision making. Radiology. 1992;184:695–697. doi: 10.1148/radiology.184.3.1509051. [DOI] [PubMed] [Google Scholar]
- 56.Henderson JM, Heymsfield SB, Horowitz J, Kutner MH. Measurement of liver and spleen volume by computed tomography. Assessment of reproducibility and changes found following a selective distal splenorenal shunt. Radiology. 1981;141:525–527. doi: 10.1148/radiology.141.2.6974875. [DOI] [PubMed] [Google Scholar]
- 57.Sandrasegaran K, Kwo PW, DiGirolamo D, Stockberger SM, Cummings OW, Kopecky KK. Measurement of liver volume using spiral CT and the curved line and cubic spline algorithms: reproducibility and interobserver variation. Abdom Imaging. 1999;24:61–65. doi: 10.1007/s002619900441. [DOI] [PubMed] [Google Scholar]
- 58.Gao L, Heath DG, Kuszyk BS, Fishman EK. Automatic liver segmentation technique for three-dimensional visualization of CT data. Radiology. 1996;201:359–364. doi: 10.1148/radiology.201.2.8888223. [DOI] [PubMed] [Google Scholar]
- 59.Suzuki K, Epstein ML, Kohlbrenner R, Garg S, Hori M, Oto A, Baron RL. Quantitative radiology: automated CT liver volumetry compared with interactive volumetry and manual volumetry. AJR Am J Roentgenol. 2011;197:W706–W712. doi: 10.2214/AJR.10.5958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Radtke A, Sotiropoulos GC, Nadalin S, Molmenti EP, Schroeder T, Saner FH, Sgourakis G, Cicinnati VR, Valentin-Gamazo C, Broelsch CE, et al. Preoperative volume prediction in adult live donor liver transplantation: 3-D CT volumetry approach to prevent miscalculations. Eur J Med Res. 2008;13:319–326. [PubMed] [Google Scholar]
- 61.Nakayama Y, Li Q, Katsuragawa S, Ikeda R, Hiai Y, Awai K, Kusunoki S, Yamashita Y, Okajima H, Inomata Y, et al. Automated hepatic volumetry for living related liver transplantation at multisection CT. Radiology. 2006;240:743–748. doi: 10.1148/radiol.2403050850. [DOI] [PubMed] [Google Scholar]
- 62.Soyer P, Sirol M, Dohan A, Gayat E, Placé V, Hristova L, Hamzi L, Boudiaf M. Hepatic height on coronal computed tomography images predicts total liver volume in European adults without liver disease. Dig Dis Sci. 2012;57:1692–1697. doi: 10.1007/s10620-012-2077-8. [DOI] [PubMed] [Google Scholar]
- 63.Kim KW, Lee J, Lee H, Jeong WK, Won HJ, Shin YM, Jung DH, Park JI, Song GW, Ha TY, et al. Right lobe estimated blood-free weight for living donor liver transplantation: accuracy of automated blood-free CT volumetry--preliminary results. Radiology. 2010;256:433–440. doi: 10.1148/radiol.10091897. [DOI] [PubMed] [Google Scholar]
- 64.Hori M, Suzuki K, Epstein ML, Baron RL. Computed tomography liver volumetry using 3-dimensional image data in living donor liver transplantation: effects of the slice thickness on the volume calculation. Liver Transpl. 2011;17:1427–1436. doi: 10.1002/lt.22419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Luciani A, Rusko L, Baranes L, Pichon E, Loze B, Deux JF, Laurent A, Tran-Van-Nhieu J, Rahmouni A. Automated liver volumetry in orthotopic liver transplantation using multiphase acquisitions on MDCT. AJR Am J Roentgenol. 2012;198:W568–W574. doi: 10.2214/AJR.11.7468. [DOI] [PubMed] [Google Scholar]
- 66.Suzuki K, Huynh HT, Liu Y, Calabrese D, Zhou K, Oto A, Hori M. Computerized segmentation of liver in hepatic CT and MRI by means of level-set geodesic active contouring. Conf Proc IEEE Eng Med Biol Soc. 2013;2013:2984–2987. doi: 10.1109/EMBC.2013.6610167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Suzuki K, Kohlbrenner R, Epstein ML, Obajuluwa AM, Xu J, Hori M. Computer-aided measurement of liver volumes in CT by means of geodesic active contour segmentation coupled with level-set algorithms. Med Phys. 2010;37:2159–2166. doi: 10.1118/1.3395579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhou JY, Wong DW, Ding F, Venkatesh SK, Tian Q, Qi YY, Xiong W, Liu JJ, Leow WK. Liver tumour segmentation using contrast-enhanced multi-detector CT data: performance benchmarking of three semiautomated methods. Eur Radiol. 2010;20:1738–1748. doi: 10.1007/s00330-010-1712-z. [DOI] [PubMed] [Google Scholar]
- 69.Dello SA, Stoot JH, van Stiphout RS, Bloemen JG, Wigmore SJ, Dejong CH, van Dam RM. Prospective volumetric assessment of the liver on a personal computer by nonradiologists prior to partial hepatectomy. World J Surg. 2011;35:386–392. doi: 10.1007/s00268-010-0877-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.van der Vorst JR, van Dam RM, van Stiphout RS, van den Broek MA, Hollander IH, Kessels AG, Dejong CH. Virtual liver resection and volumetric analysis of the future liver remnant using open source image processing software. World J Surg. 2010;34:2426–2433. doi: 10.1007/s00268-010-0663-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lu Y, Wu Z, Liu C, Wang HH. Hepatic volumetry with PhotoShop in personal computer. Hepatobiliary Pancreat Dis Int. 2004;3:82–85. [PubMed] [Google Scholar]
- 72.Van Thiel DH, Hagler NG, Schade RR, Skolnick ML, Heyl AP, Rosenblum E, Gavaler JS, Penkrot RJ. In vivo hepatic volume determination using sonography and computed tomography. Validation and a comparison of the two techniques. Gastroenterology. 1985;88:1812–1817. doi: 10.1016/0016-5085(85)90005-8. [DOI] [PubMed] [Google Scholar]


