Abstract
Acute gastrointestinal bleeding (GIB) can lead to significant morbidity and mortality without appropriate treatment. There are numerous causes of acute GIB including but not limited to infection, vascular anomalies, inflammatory diseases, trauma, and malignancy. The diagnostic and therapeutic approach of GIB depends on its location, severity, and etiology. The role of interventional radiology becomes vital in patients whose GIB remains resistant to medical and endoscopic treatment. Radiology offers diagnostic imaging studies and endovascular therapeutic interventions that can be performed promptly and effectively with successful outcomes. Computed tomography angiography and nuclear scintigraphy can localize the source of bleeding and provide essential information for the interventional radiologist to guide therapeutic management with endovascular angiography and transcatheter embolization. This review article provides insight into the essential role of Interventional Radiology in the management of acute GIB.
Keywords: Interventional radiology, Angiography, Therapeutic management, Upper gastrointestinal bleeding, Lower gastrointestinal bleeding, Embolization
Core tip: Acute gastrointestinal bleeding can lead to significant morbidity and mortality without appropriate treatment. The role of interventional radiology is crucial in patients that have persistent bleeding despite medical and endoscopic treatment. Computed tomography angiography and nuclear scintigraphy can localize lesions and provide information helpful for the Interventional Radiologist. The source of bleeding can be then be stabilized with endovascular angiography/transcatheter arterial embolization which is safe and effective with minimal complications due to the advances in catheter technology.
INTRODUCTION
Acute gastrointestinal bleeding (GIB) is a common clinical presentation that can lead to significant morbidity and mortality without appropriate treatment. The estimated annual incidence is approximately 40-150 cases per 10000 persons for upper GIB and 20-27 cases per 100000 persons for lower GIB[1,2]. Mortality rate for both upper and lower GIB is estimated to be around 4%-10%[1,2]. GIB can be a sequelae of many different etiologies, such as infection, vascular anomaly, inflammatory diseases, trauma, and malignancy[2-9]. GIB is conventionally categorized by the anatomical location of the bleeding source. A GIB source proximal to the ligament of Treitz, which occurs more frequently, is classified as part of upper gastrointestinal (GI), and a source distal to the ligament of Treitz is considered to be part of lower GI. Diagnostic and treatment approach of GIB depends on its location, severity, and etiology. The role of radiology becomes especially important in patients whose GIB remains resistant to medical and endoscopic treatment. Radiology offers diagnostic imaging studies and endovascular therapeutic interventions that can be performed promptly and effectively.
CLINICAL EVALUATION AND MANAGEMENT OF THE PATIENT
Initial evaluation of patients with GIB begins with a history and physical examination[10,11]. GIB can manifest with various signs, such as tachycardia, orthostatic hypotension, and chronic anemia[11,12]. In patients who are hemodynamically unstable, resuscitation with fluid replacement and blood product administration should occur promptly to maintain intravascular volume and stabilize vital signs[10]. Correction of coagulopathy may also be needed in certain cases[10]. Diagnostic workup should immediately follow assessment and resuscitation, if not occurring simultaneously, to minimize adverse patient outcomes[10,13,14].
Patient history and physical examination can help to determine whether GIB is of upper or lower GI source and guide subsequent workup[11,15]. GIB that manifests as hematemesis or melena are commonly due to an upper GI source[11,13]. Patients with active brisk upper GIB can also present with hematochezia and without any associated hematemesis or melena[11,13,15]. Nasogastric tube lavage is sometimes performed to confirm an upper GI source of bleeding, but a negative result does not necessarily rule it out[11,13,15]. Because of the intermittent nature of GIB and the possibility of a bleeding source distal to the pylorus, gastric lavage test is expected to yield negative results in certain cases. Approximately one quarter of upper GI hemorrhage is due to peptic ulcer disease and often associated with non-steroidal anti-inflammatory drug use and Helicobacter pylori infection[15,16]. Other causes of acute upper GIB include varices, vascular abnormalities, angiodysplasia, gastritis, esophagitis, post Endoscopic Retrograde Cholangiopancreatography-papillotomy, and neoplasms[2,4,10].
Patients with lower GIB commonly presents with hematochezia as most lower GIB sources are located in the colon. Less commonly, patients may present with melena if the source of bleeding is located in the small bowel or right colon. Of note, 10% to 15% of patients with hematochezia are reported to have an upper GI bleeding site[17]. Diverticulosis is the most common cause of hematochezia, with the incidence increasing with ages older than 65. Other causes include inflammatory bowel disease, ischemic colitis, neoplasia, polyps, vascular malformations, post-polypectomy, and angiodysplasia[12,18]. Although most lower GIB resolves spontaneously with conservative management, 10%-15% of cases eventually require endovascular intervention[19].
Endoscopy is the first diagnostic and therapeutic intervention of choice for both upper and lower GIB and thus a consultation with a gastroenterologist should not be delayed when a patient presents with GIB. For patients suspected of having an upper GI source of bleeding, esophagoduodenoscopy (EGD) is performed. Factors that may predict endoscopic treatment failure include patients that present with shock, hemoglobin less than 10, greater than six units of blood transfused, and significant comorbidities.
With regards to upper GI bleeding, larger ulcer size and location of an ulcer on the posterior wall of the duodenal bulb are also associated with increased rates of technical failure[20,21].
Patients that present with hematochezia and suspected of having a lower GI source, colonoscopy is the initial diagnostic test of choice. For active lower GIB that is rapid and heavy, endoscopic view may be limited and yield inconclusive results. If a colonoscopy fails to identify the source of bleeding, then EGD may be performed in addition. Some studies have shown that endoscopy has a 92% sensitivity and near 100% specificity of identifying upper GI lesions and sensitivity of 90% and positive predictive value of 87% for identifying lower GI lesions[22,23]. An unprepared colon limits the colonoscopy study and while blood may be seen within the colon lumen the exact site of bleeding is difficult to identify[23].
If a lesion is identified endoscopically, therapeutic intervention can be done to effectively stabilize bleeding. Endoscopic therapies include epinephrine injection, sclerotherapy, and metal clip placement. Metal clips are especially useful in patients who require transcatheter or surgical intervention later on as clips can be visualized by imaging studies and facilitate lesion localization during angiography or surgery[9,17].
INDICATIONS FOR ANGIOGRAPHY
When a patient has non-diagnostic endoscopic results or remains refractory to medical and endoscopic treatment, radiologic imaging and endovascular intervention are the next intervention of choice. Non-invasive radiologic imaging options include computed tomography angiography (CTA) and nuclear scintigraphy. However, these imaging modalities are only diagnostic and require subsequent endovascular or surgical intervention to stabilize bleeding[24,25].
COMPUTED TOMOGRAPHY
CTA can detect flow rates as low as 0.3 mL/min and has a sensitivity of 50%-86% and specificity of 92%-95% for identifying lesions responsible for GIB[24,26,27]. In addition to identifying the site of bleeding; CTA can often identify the etiology of GIB which may be useful for further management.
At our institution, we use the following protocol for CTA: noncontrast (unenhanced), arterial phase, and portal venous phase with intravenous contrast at 4-5 mL/s. We also recommend the following acquisition parameters: section thickness of 1 mm with reconstruction interval of 0.8 mm, pitch of 0.900, rotation time of 0.5 s, tube voltage of 120 kV, and automatic tube current modulation in the x/y/z axis directions. We do not administer oral contrast as this may mask the bleeding source.
On nonenhanced CT, focal hyperattenuation within the bowel is indicative of recent hemorrhage and may represent a “sentinel clot”. Extravasation of contrast is the hallmark finding used to determine the source of bleeding. Further, a changing appearance of the focus of extravasated contrast with time between phases confirms the presence of active bleeding[25]. Although CTA can only serve as a diagnostic tool; it provides important information about vascular anatomy variance that becomes useful for endovascular intervention or surgical planning.
NUCLEAR SCINTIGRAPHY
The role of nuclear medicine for the detection of acute GI bleeding varies on an institutional basis. Nuclear scintigraphy plays a very important role in the detection of lower GI bleeding and when positive, has the ability to stratify patients that would benefit from intervention versus medical management. Although there is significant variability in reported detection of bleeding site by scintigraphy, the Society of Nuclear Medicine procedure guidelines states that bleeding rates as low as 0.1-0.35 mL/min can be detected[28]. Tc-99m labeled red blood cell studies have an overall sensitivity of 95% and specificity of 93%[29]. Patients with immediate blush on red blood cell scintigraphy are more likely to require urgent angiography and those with delayed blush have low angiographic yield[30]. GI bleeding often is intermittent and nuclear scintigraphy has the advantage of continuous monitoring to localize sites of intermittent bleeding for potential angiography and intervention[31].
ANGIOGRAPHIC EVALUATION OF ACUTE GI HEMORRHAGE
In emergent cases or in hospitals where CTA or nuclear scintigraphy is not available, patients with active GIB who fail medical and endoscopic intervention should undergo endovascular angiographic evaluation. Angiography is able to identify an active bleeding rate of at least 0.5 to 1 mL/min[32,33]. For lower GIB, angiography performed with digital subtraction has a sensitivity of 60%, specificity of 100%, positive and negative predictive values of 100% and 24%, respectively[34].
Access for endovascular angiography is gained via the common femoral artery[35,36]. The aim of endovascular angiography is to identify bleeding vessel(s) and use selective catheterization to prepare for embolization[36,37]. At our institution, based on clinical scenario (patient history, CTA, endoscopic findings) the most suspected bleeding vessel is first studied. For suspected upper GIB, the celiac artery is commonly interrogated first as a majority of upper GIB is caused by gastroduodenal ulcers which are supplied by branches of the celiac artery[35-38] (Figure 1). If angiographically negative, selective left gastric and the gastroduodenal artery evaluation is done. If the source of bleeding is thought to be in the small bowel or if no evidence of bleeding is seen upon interrogation of the celiac artery or its branches, the superior mesenteric artery (SMA) is evaluated next[36,37]. If these angiographic studies are all negative, then evaluation of the inferior mesenteric artery (IMA) is considered. Selective injections are used to confirm any findings that are suspicious on nonselective angiograms[36,37].
Figure 1.
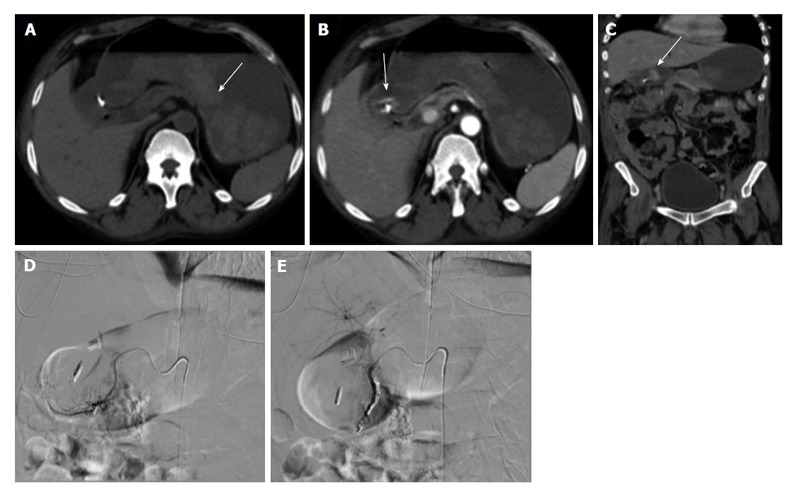
Upper gastrointestinal bleed secondary to gastric/duodenal ulcers. Fifty-four-year-old male with history of gastric ulcers which were treated by clipping through endoscopy. Despite endoscopic intervention, the patient presented with dropping hematocrit requiring transfusion. A: Noncontrast; B and C: contrast enhanced computed tomography imaging demonstrates active extravasation at the level of the gastric antrum with blood product filling the stomach; D and E: Active extravasation was found at the gastroduodenal artery (not shown) which was embolized with coils and gelfoam.
For suspected lower GIB, the SMA and IMA are examined[36]. If bleeding appears to originate the proximal colon, the SMA is initially evaluated. If bleeding appears to originate in the distal colon, the IMA is selected[36,39]. The two most common causes of lower GIB are colonic diverticular disease and angiodysplasia[2,32] (Figures 2 and 3). However, when congenital variant vascular anatomy is suspected, such as in cases where a lower GI bleed simulates an upper GI bleed, all three major arterial supplies should be evaluated[36]. If negative, the internal iliac arteries should be evaluated as the middle and inferior rectal arteries can be a source of hemorrhage[36,39].
Figure 2.
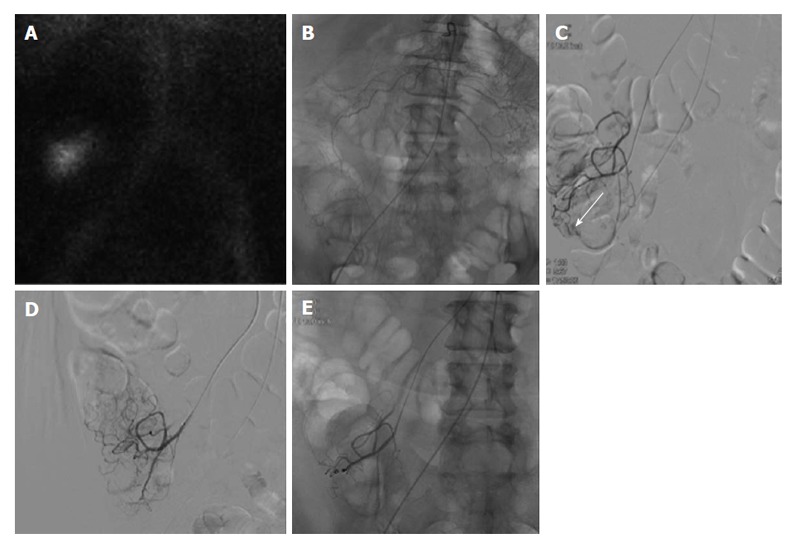
Lower gastrointestinal bleeding secondary to angiodysplasia. Sixty-one-year-old female with multiple bloody stools prior to admission and negative colonoscopy. A: Tc-99m red blood cell study demonstrates active bleeding in the region of the cecum; B and C: Selective catheterization of the distal ileocolic artery demonstrates a small focus of hemorrhage consistent with an area of angiodysplasia; D and E: Coil embolization was performed with two 3 mm coils. Post embolization images demonstrates resolved bleeding.
Figure 3.
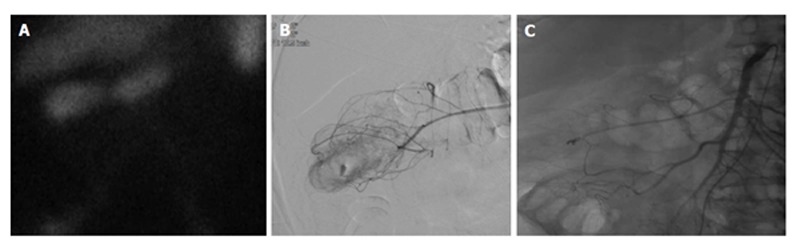
Lower gastrointestinal bleed secondary to diverticulitis. Sixty-eight-year-old male with history of esophageal carcinoma with acute diverticulitis with drifting hematocrit. A: Tc-99m labeled red blood cell study demonstrates bleeding at the hepatic flexure of the colon; B: Selective catheter angiography at the middle colic artery demonstrates active extravasation into a diverticulum present at the hepatic flexure the colon; C: Coil embolization of the right lateral aspect of the middle colic artery, across a small perforating vessel associated with a diverticular hemorrhage.
At our institution, under fluoroscopy we use nonionic contrast is injected at a flow rate of 5-7 mL/s for celiac and super mesenteric arteriography, and 2-3 mL/s for inferior mesenteric arteriography. Digital subtraction angiogram is used to better visualize the vasculature by subtracting pre-contrast image from later images and effectively removing soft tissue and bones from the images. This is limited by peristalsis (consider giving glucagon) or patient breathing (which can be dealt with by studying unsubtracted images). Extravasation of contrast agent is indicative of active bleeding[36]. Positive findings include mucosal blushes with abnormal vessels suggestive of tumor, prolonged contrast spots suggestive of inflammation, and visualization of arteries and veins on the same phase of the study suggestive of an arteriovenous malformation. Other lesions to consider include pseudoaneurysms and arteriovenous fistulas[36,39].
There are several artifacts that mimic extravasation including bowel subtraction artifact, hypervascular mucosa, parts of the renal collecting system, and adrenal gland opacification[39]. With respect to angiography, contrast extravasation may not been seen; however, a pathologic finding may indicate the source of bleeding. For example, visualization of varices at unsuspected sites may indicate the site of pathology. Further, angiodysplasia is often diagnosed by early and persistent filling of a draining vein and by abnormal clusters of vessels within the bowel wall. Possible pitfalls for failing to identify the bleeding focus include bleeding from a venous origin and technically related issues such as failure to inject the correct artery and bleeding outside the field of imaging[39,40].
If patient is not actively bleeding or contrast extravasation is not visualized under fluoroscopy, the interventional radiologist may choose to restudy the same vessels or sub-selectively catheterize vessels likely supplying the suspected site of bleeding identified by prior endoscopic clipping or imaging studies to help increase the diagnostic yield and reduce false negative studies[39,40].
ANGIOGRAPHIC MANAGEMENT OF ACUTE GI HEMORRHAGE
One of the advantages of endovascular angiography is that it can be both a diagnostic and therapeutic tool. Also, endovascular angiography can be performed emergently without any bowel preparation. However, if the patient had prior oral contrast this may limit the diagnostic ability of a mesenteric angiogram thus oral contrast should be avoided in patients who undergo CT imaging prior to angiographic intervention[40-42].
Endovascular angiography serves as an effective and safe alternative to surgical intervention for patients whose GIB is refractory to medical and endoscopic treatment. Hemostasis is achieved by reducing blood flow to the bleeding vessel and thus decreasing perfusion pressure and facilitating clot formation at the site of bleeding[40-43].
TRANSCATHETER ARTERIAL EMBOLIZATION
Transcatheter arterial embolization (TAE) is effective for controlling acute GIB[41]. Some studies have shown that TAE is safer than surgical intervention in the high risk patient population and has a lower 30-d mortality rate[38,44]. TAE is a viable option and temporizing measure in circumstances where endoscopic and/or surgical approach is not ideal.
The goal of TAE is super-selective embolization of bleeding vessels to reduce arterial perfusion pressure while maintaining adequate collateral blood flow to minimize the risk of bowel infarction[43]. A 5 French angiographic catheter is used to access the celiac, superior mesenteric, or inferior mesenteric arteries depending on the suspected location of bleeding and its supplying vasculature. In some cases this catheter can be guided to the site of bleeding; however, if it does not reach the bleeding site, then a smaller coaxial 3 French microcatheter can be advanced through the 4 or 5 Fr catheter.
Smaller guidewires, such as 0.018 in or smaller are used to guide the microcatheters as close as possible to the bleeding site. Caution must be taken to move the guide wire and microcatheter as carefully and steadily as possible to avoid vessel perforation, dissection, and vasospasm while reaching as close as possible to the site of bleeding. When no contrast extravasation is visualized under fluoroscopy, blind embolization of suspected bleeding vessel may be done at the discretion of the interventional radiologist[38,45].
For upper GI bleeding, bleeding visualized in the stomach fundus is treated by left gastric artery embolization and bleeding in the gastric antrum/proximal duodenum by gastroduodenal embolization. When embolizing the gastroduodenal artery, if only the proximal portion is occluded, then bleeding may by the pancreaticoduodenal arcade, also known as bleeding via the “back door”. mpiric embolization has also shown to be effective[40,42] (Figures 4 and 5).
Figure 4.
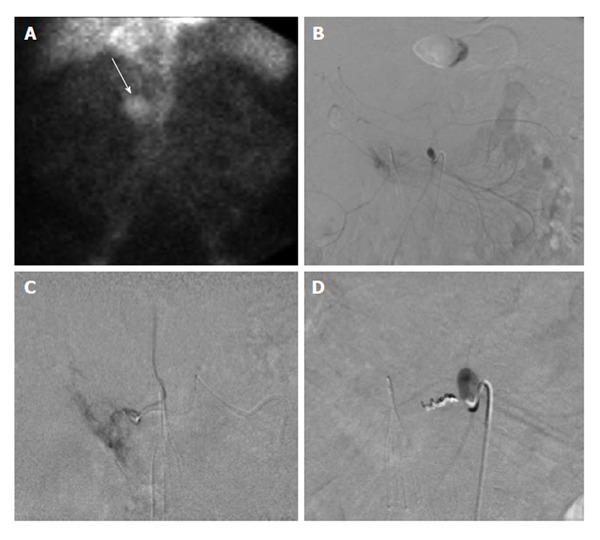
Sixty-six-year-old male with ongoing gastrointestinal hemorrhage requiring multiple transfusions over the last 72 h. EGD performed revealed ulcers in the first and second portion of the duodenum. A: Technetium-99m tagged RBC scan demonstrates brisk hemorrhage arising from the proximal small bowel/duodenum; B and C: Digitally subtracted images reveal active extravasation in the second portion of the duodenum from the inferior pancreaticoduodenal artery corresponding to area of hemorrhage on tagged RBC scan; D: Successful and uncomplicated coil embolization of the inferior pancreaticoduodenal artery with cessation of active hemorrhage. EGD: Esophagoduodenoscopy; RBC: Red blood cell.
Figure 5.
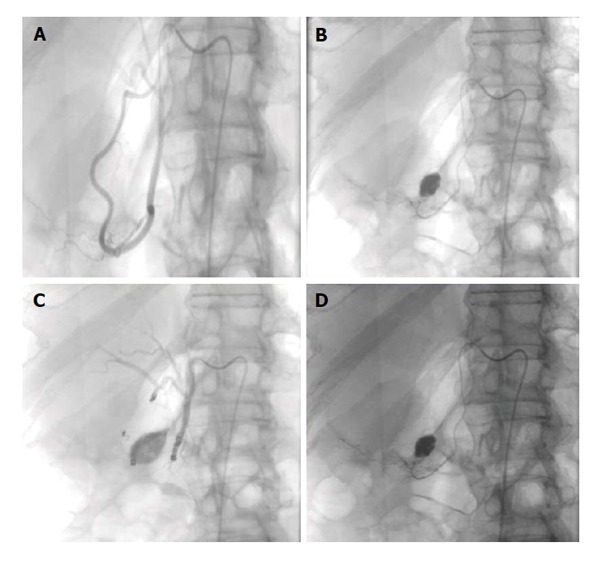
Seventy-two-year-old female with worsening abdominal pain and acute gastrointestinal hemorrhage. Upper gastrointestinal endoscopy reveals multiple large bleeding ulcers in the duodenum. A and B: Selective catheter angiography of the gastroduodenal and pancreaticoduodenal arteries demonstrates active extravasation; C: A combination of gelfoam slurry and coils were used to embolize branches of the pancreaticoduodenal and gastroduodenal artery; D: Representative post embolization image demonstrates no further evidence of active extravasation or bleeding.
For lower GI bleeding the catheter should be positioned as close to the bleeding site as possible. If the source is in the SMA, the catheter advanced to the vasa rectum and if in the IMA the catheter should be placed in the marginal or terminal artery if possible. Embolization should only be performed when the catheter has been advanced to the mesenteric border of the colon. The bowel distal to the ligament of Treitz does not have a dual supply; therefore, the risk of bowel infarction is higher[39,40,42] (Figures 6 and 7).
Figure 6.
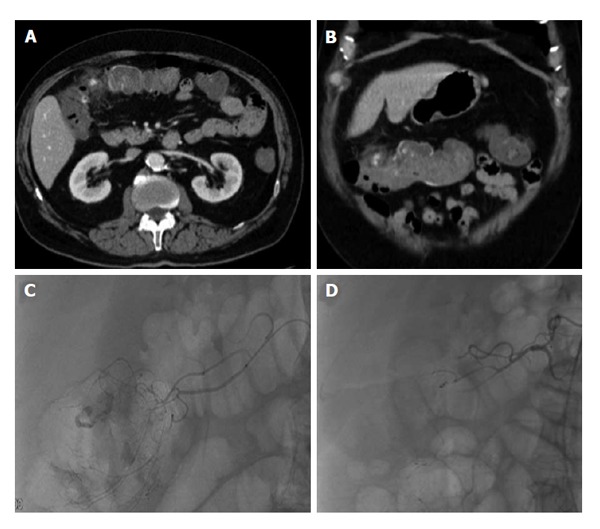
Lower gastrointestinal bleed from acute diverticulitis. Seventy-three-year-old male patient with bloody diarrhea, severely hypotensive (blood pressure 70/40) requiring 10 units of packed red blood cells. A and B: Contrast enhanced computed tomography abdomen demonstrates acute diverticulitis at the hepatic flexure, with active hemorrhage; C: Visceral angiography demonstrates the region of active bleeding in the ascending colon at the hepatic flexure; D: Successful distal Gelfoam and coil embolization of the supplying right colic artery branches.
Figure 7.
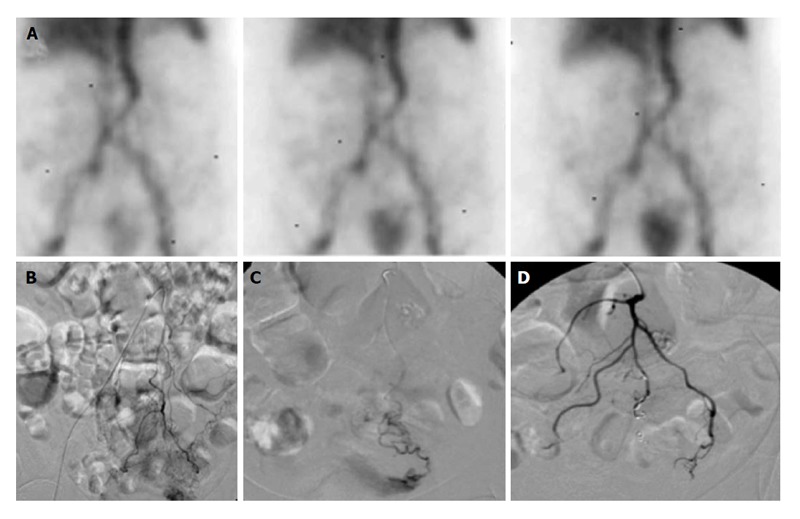
Lower gastrointestinal bleed secondary to supratherapeutic international normalized ratio. Seventy-six-year-old female with supratherapeutic INR (3.5) with painless hematochezia. A: Tc-99m labeled RBC study demonstrates brisk gastrointestinal bleeding localized to the sigmoid colon; B and C: Catheter angiography demonstrates active extravasation from a tertiary branch of the inferior mesenteric artery supplying the distal sigmoid colon which was subsequently embolized; D: With coils and no evidence of continued bleeding. RBC: Red blood cell.
The type of embolic agent used is conventionally dependent on the interventional radiologist’s experience and preference, etiology of bleeding, and availability of the agent. Embolic agents include coils, glue, onyx, Gelfoam, polyvinyl alcohol particles (PVA), and Amplatzer vascular plugs. The most commonly used embolic agents are coils and PVA[46-49].
Coils come in a variety of sizes and shapes, ranging from sub-millimeter to centimeters. Coils are composed of a metal component that acts as a physical occlusion and a fiber component that stimulates the thrombogenic process. Coils can be visualized under fluoroscopy after placement which is an important advantage when compared to Gelfoam or PVA. Newer types of embolization coils have the ability to be removed after deployment if the initial placement is felt to be unsatisfactory[46,50].
Gelfoam (absorbable compressed sponge) is a temporary agent made of subcutaneous porcine adipose tissue that remains effective for weeks to months before re-canalization occurs. For this reason, Gelfoam is not recommended as a single agent. Gelfoam can also be mixed with saline to form a slurry, which helps with delivery. Advantages of gelfoam include: widespread availability, cost-effectiveness, and allows future access to embolized vessels after resorption. Disadvantages include that the preparation of particles can be time consuming and recanalization of vessels is unpredictable[51]. In addition, because Gelfoam is made of small particulates, it is difficult to control its placement and can be deployed more distally than intended, which can result in higher risk of bowel ischemia from embolization of nearby collateral vessels[36,50,51].
Several studies have shown that recurrent bleeding is more likely to occur when PVA particles, Gelfoam, or coils are used alone[46,48,52]. Using coils with Gelfoam or PVA particles on both sides of the bleeding vessel is recommended to avoid “backdoor” bleeding and decrease the risk of recurrent bleeding[48,53]. Some studies have shown that for upper GIB, which is commonly due to gastroduodenal ulcers, successful hemostasis can be achieved by embolizing the gastroduodenal artery or pancreaticoduodenal artery using coils alone[54-56], or using coils and Gelfoam together to embolize distally and proximally in the gastroduodenal arterial trunk[46,48,52]. Clinical success rate of embolization for upper GIB have been cited to be around 44%-100%[41,44,46,57].
For lower GIB, some studies recommend against using Gelfoam, and instead advocate using coils and larger PVA particles[43,58,59]. Small PVA particles, less than 250 μm, and Gelfoam particles may travel distally and occlude vessels at the arteriolar level. This results in occlusion of intramural circulation or submucosal plexus beyond the level of collateralization and increases the risk of bowel infarction[59,60]. More peripheral embolization just proximal to the vasa recta is recommended to minimize the length of bowel at risk for ischemia. In most of the reported series in the literature, the target artery of embolization was the vasa recta and in technically difficult cases the marginal artery or more proximally[57,61,62].
Anecdotally, coil embolization at the marginal artery may result in a higher rebleeding rate. Further, if a secondary intervention is required, this may close the door for future access to the bleeding vessel. Advantages to using microcoils include the ability to visualize under direct fluoroscopy and permitting decreasing perfusion pressure while collateral flow prevents infarction. Early rebleeding (less than 30 d) is reported to range from 10%-30%. Rebleeding may be secondary to a new site of bleed or recanalization of the previously embolized artery. Success rate for embolization of lower GIB has ranged from 88%-93%[43,57,60].
N-butyl 2-cyanoacrylate (NBCA) glue or ethylene-vinyl alcohol copolymer (Onyx®, Micro Therapeutics, Inc., Irvine, CA, United States of America) is a promising newer embolic agent to control GI bleeding. There is a growing body of evidence that supports the use of cyanoacrylates in embolization for lower GI hemorrhage. Advantages of using NBCA include the ability to occlude vessel beyond the most distal site of microcatheter advancement, permanent vessel closure, the option for using ultra-microcatheters not suitable for microcoil delivery, and more efficient obliteration of bleeding pseudoaneurysms with complex anatomy. Further, the rebleeding rate after use of cyanoacrylate is 4%-15%, which appears lower than the rate reported from employing coils or particles 0%-26%[39,63-66].
However, NBCA is considerably more expensive and requires a steeper learning curve. It has been reported in the literature that the time for TAE using NBCA was significantly lower than when using other liquid agents[49,55]. There is a significant risk of glue reflux, bowel infarction, and future bowel stenosis[67]. Also, the glue may polymerize with the catheter tip, which may subsequently get stripped off as the catheter is retracted. This poses the risk of non-target embolization or the catheter becoming adherent to the artery. Prompt catheter removal and aspiration of the guide catheter after microcatheter removal can significantly reduce this risk[52,68].
Another potential agent, Onyx, is a liquid embolic agent composed of ethylene-vinyl alcohol copolymer dissolved in dimethyl sulphoxide (DMSO). In 2010, Lenhart et al[69] reported their experience with the use of Onyx in the setting of acute upper GIB, becoming the first study published on arterial embolotherapy with Onyx as an embolic agent in the gastrointestinal tract. Their reported success rate was 81% and the complication rate minimal. The main advantages of Onyx® are its nonadhesive properties, high radiopacity and long solidification periods which make the embolization procedure more predictable. The DMSO solvent has disadvantages including severe vasospasm, excretion via respiration/perspiration which can cause an odor for days. The most prohibitive and restrictive factor however is its high cost and requirement for DMSO compatible catheters[52,69].
VASOPRESSIN INFUSION
Vasopressin infusion is a less frequently used treatment for acute GIB[58]. It was more commonly used before the advancement and improvement of transcatheter technique. Vasopressin acts by constricting arteries and reducing blood flow to the target site, but can also cause systemic side effects such as cardiac arrhythmia and bowel ischemia. In addition, vasopressin infusion has a high rebleeding rate after infusion is stopped. It requires much longer procedure time including catheter placement for 24-48 h and intensive monitoring during vasopressin infusion. However, when GIB is caused by diffuse lesions or super-selective catheterization is not possible, vasopressin infusion may be the remaining therapeutic option before surgical intervention. Vasopressin infusion is used more often for lower GIB than upper GIB as vessels responsible for lower GIB tend to be smaller in diameter and thus more responsive to the constricting effect of vasopressin[58,70]. Some studies have shown that the cardiac side effects of vasopressin can be alleviated by using intravenous nitroglycerin infusion to increase coronary blood flow and cardiac output[70]. Vasopressin infusion has a success rate of 59%-90% and a high rate of rebleeding rate of up to 36%-43%[70].
COMPLICATIONS
Endovascular embolization and vasopressin infusion can increase the risk of bowel ischemia by reducing blood flow to the segment of bowel supplied by the target vessel[71,72]. Non-target embolization can occur as well[41]. However, the advancement of super-selective catheterization technique has helped to reduce such risk in recent years[42,73]. Other complications with transcatheter intervention include arterial injury such as dissection, perforation, pseudoaneurysm, and vasospasm. For vasopressin infusion, there is a higher risk of catheter associated infection or thrombus formation because of the need for much longer duration of catheter placement[74,75]. Contrast use during fluoroscopy can induce nephropathy, but with adequate hydration and vigilance of contrast use, the risk can be decreased[76,77]. Hematoma is a common minor complication associated with puncture site. Patients may complain of temporary minor abdominal pain after the procedure but this most often resolves spontaneously.
CONCLUSION
Although many cases of acute GIB resolve spontaneously or respond to medical and endoscopic treatment, patients with GIB refractory to such treatment are at higher risk for adverse outcome. Various subspecialties contribute to the care of bleeding patients. Patients with acute GIB should be considered for prompt radiologic imaging studies and endovascular intervention to prevent morbidity and mortality. CTA can localize lesions and provide information helpful for endovascular intervention and surgery. Rapid GIB stabilization can be achieved with endovascular angiography and transcatheter embolization. It is a safe and effective alternative to surgery.
Footnotes
P- Reviewers: Chan WP, Murata S, Metwalli ZA S- Editor: Song XX L- Editor: A E- Editor: Liu SQ
References
- 1.Manning-Dimmitt LL, Dimmitt SG, Wilson GR. Diagnosis of gastrointestinal bleeding in adults. Am Fam Physician. 2005;71:1339–1346. [PubMed] [Google Scholar]
- 2.Longstreth GF. Epidemiology and outcome of patients hospitalized with acute lower gastrointestinal hemorrhage: a population-based study. Am J Gastroenterol. 1997;92:419–424. [PubMed] [Google Scholar]
- 3.Boonpongmanee S, Fleischer DE, Pezzullo JC, Collier K, Mayoral W, Al-Kawas F, Chutkan R, Lewis JH, Tio TL, Benjamin SB. The frequency of peptic ulcer as a cause of upper-GI bleeding is exaggerated. Gastrointest Endosc. 2004;59:788–794. doi: 10.1016/s0016-5107(04)00181-6. [DOI] [PubMed] [Google Scholar]
- 4.Cook DJ, Fuller HD, Guyatt GH, Marshall JC, Leasa D, Hall R, Winton TL, Rutledge F, Todd TJ, Roy P. Risk factors for gastrointestinal bleeding in critically ill patients. Canadian Critical Care Trials Group. N Engl J Med. 1994;330:377–381. doi: 10.1056/NEJM199402103300601. [DOI] [PubMed] [Google Scholar]
- 5.Enestvedt BK, Gralnek IM, Mattek N, Lieberman DA, Eisen G. An evaluation of endoscopic indications and findings related to nonvariceal upper-GI hemorrhage in a large multicenter consortium. Gastrointest Endosc. 2008;67:422–429. doi: 10.1016/j.gie.2007.09.024. [DOI] [PubMed] [Google Scholar]
- 6.Hreinsson JP, Kalaitzakis E, Gudmundsson S, Björnsson ES. Upper gastrointestinal bleeding: incidence, etiology and outcomes in a population-based setting. Scand J Gastroenterol. 2013;48:439–447. doi: 10.3109/00365521.2012.763174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Longstreth GF. Epidemiology of hospitalization for acute upper gastrointestinal hemorrhage: a population-based study. Am J Gastroenterol. 1995;90:206–210. [PubMed] [Google Scholar]
- 8.van Leerdam ME. Epidemiology of acute upper gastrointestinal bleeding. Best Pract Res Clin Gastroenterol. 2008;22:209–224. doi: 10.1016/j.bpg.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 9.Zuccaro G. Epidemiology of lower gastrointestinal bleeding. Best Pract Res Clin Gastroenterol. 2008;22:225–232. doi: 10.1016/j.bpg.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 10.Ahmed A, Stanley AJ. Acute upper gastrointestinal bleeding in the elderly: aetiology, diagnosis and treatment. Drugs Aging. 2012;29:933–940. doi: 10.1007/s40266-012-0020-5. [DOI] [PubMed] [Google Scholar]
- 11.Srygley FD, Gerardo CJ, Tran T, Fisher DA. Does this patient have a severe upper gastrointestinal bleed? JAMA. 2012;307:1072–1079. doi: 10.1001/jama.2012.253. [DOI] [PubMed] [Google Scholar]
- 12.Strate LL, Orav EJ, Syngal S. Early predictors of severity in acute lower intestinal tract bleeding. Arch Intern Med. 2003;163:838–843. doi: 10.1001/archinte.163.7.838. [DOI] [PubMed] [Google Scholar]
- 13.Barkun A, Bardou M, Marshall JK, Nonvariceal Upper GI Bleeding Consensus Conference Group. Consensus recommendations for managing patients with nonvariceal upper gastrointestinal bleeding. Ann Intern Med. 2003;139:843–857. doi: 10.7326/0003-4819-139-10-200311180-00012. [DOI] [PubMed] [Google Scholar]
- 14.Barnert J, Messmann H. Diagnosis and management of lower gastrointestinal bleeding. Nat Rev Gastroenterol Hepatol. 2009;6:637–646. doi: 10.1038/nrgastro.2009.167. [DOI] [PubMed] [Google Scholar]
- 15.Wilkins T, Khan N, Nabh A, Schade RR. Diagnosis and management of upper gastrointestinal bleeding. Am Fam Physician. 2012;85:469–476. [PubMed] [Google Scholar]
- 16.Prasad Kerlin M, Tokar JL. Acute gastrointestinal bleeding. Ann Intern Med. 2013;159:ITC2–IT1, ITC2-IT1, ITC2-IT1, ITC2-IT1, ITC2-IT1, ITC2-IT1, ITC2-IT1, ITC2-IT1, ITC2-IT1, ITC2-IT1, ITC2-IT1, ITC2-IT1, ITC2-IT1, ITC2-IT1, ITC2-IT1, quiz ITC2-IT1. [Google Scholar]
- 17.Zuccaro G. Management of the adult patient with acute lower gastrointestinal bleeding. American College of Gastroenterology. Practice Parameters Committee. Am J Gastroenterol. 1998;93:1202–1208. doi: 10.1111/j.1572-0241.1998.00395.x. [DOI] [PubMed] [Google Scholar]
- 18.Strate LL. Lower GI bleeding: epidemiology and diagnosis. Gastroenterol Clin North Am. 2005;34:643–664. doi: 10.1016/j.gtc.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 19.Billingham RP. The conundrum of lower gastrointestinal bleeding. Surg Clin North Am. 1997;77:241–252. doi: 10.1016/s0039-6109(05)70542-9. [DOI] [PubMed] [Google Scholar]
- 20.Brullet E, Calvet X, Campo R, Rue M, Catot L, Donoso L. Factors predicting failure of endoscopic injection therapy in bleeding duodenal ulcer. Gastrointest Endosc. 1996;43:111–116. doi: 10.1016/s0016-5107(06)80110-0. [DOI] [PubMed] [Google Scholar]
- 21.Siva R, Al Zubaidi G, Masoud AK, Nihar M. Predictive factors for failure of endoscopic management therapy in peptic ulcer bleeding. Saudi J Gastroenterol. 2002;8:17–21. [PubMed] [Google Scholar]
- 22.Smith GA, O’Dwyer PJ. Sensitivity of double contrast barium enema and colonoscopy for the detection of colorectal neoplasms. Surg Endosc. 2001;15:649–652. doi: 10.1007/s004640000372. [DOI] [PubMed] [Google Scholar]
- 23.Zuckerman GR, Prakash C, Askin MP, Lewis BS. AGA technical review on the evaluation and management of occult and obscure gastrointestinal bleeding. Gastroenterology. 2000;118:201–221. doi: 10.1016/s0016-5085(00)70430-6. [DOI] [PubMed] [Google Scholar]
- 24.Chua AE, Ridley LJ. Diagnostic accuracy of CT angiography in acute gastrointestinal bleeding. J Med Imaging Radiat Oncol. 2008;52:333–338. doi: 10.1111/j.1440-1673.2008.01964.x. [DOI] [PubMed] [Google Scholar]
- 25.Artigas JM, Martí M, Soto JA, Esteban H, Pinilla I, Guillén E. Multidetector CT angiography for acute gastrointestinal bleeding: technique and findings. Radiographics. 2013;33:1453–1470. doi: 10.1148/rg.335125072. [DOI] [PubMed] [Google Scholar]
- 26.García-Blázquez V, Vicente-Bártulos A, Olavarria-Delgado A, Plana MN, van der Winden D, Zamora J, EBM-Connect Collaboration. Accuracy of CT angiography in the diagnosis of acute gastrointestinal bleeding: systematic review and meta-analysis. Eur Radiol. 2013;23:1181–1190. doi: 10.1007/s00330-012-2721-x. [DOI] [PubMed] [Google Scholar]
- 27.Rondonotti E, Marmo R, Petracchini M, de Franchis R, Pennazio M. The American Society for Gastrointestinal Endoscopy (ASGE) diagnostic algorithm for obscure gastrointestinal bleeding: eight burning questions from everyday clinical practice. Dig Liver Dis. 2013;45:179–185. doi: 10.1016/j.dld.2012.07.012. [DOI] [PubMed] [Google Scholar]
- 28.Ford PV, Bartold SP, Fink-Bennett DM, Jolles PR, Lull RJ, Maurer AH, Seabold JE. Procedure guideline for gastrointestinal bleeding and Meckel’s diverticulum scintigraphy. Society of Nuclear Medicine. J Nucl Med. 1999;40:1226–1232. [PubMed] [Google Scholar]
- 29.Bunker SR, Lull RJ, Tanasescu DE, Redwine MD, Rigby J, Brown JM, Brachman MB, McAuley RJ, Ramanna L, Landry A. Scintigraphy of gastrointestinal hemorrhage: superiority of 99mTc red blood cells over 99mTc sulfur colloid. AJR Am J Roentgenol. 1984;143:543–548. doi: 10.2214/ajr.143.3.543. [DOI] [PubMed] [Google Scholar]
- 30.Ng DA, Opelka FG, Beck DE, Milburn JM, Witherspoon LR, Hicks TC, Timmcke AE, Gathright JB. Predictive value of technetium Tc 99m-labeled red blood cell scintigraphy for positive angiogram in massive lower gastrointestinal hemorrhage. Dis Colon Rectum. 1997;40:471–477. doi: 10.1007/BF02258395. [DOI] [PubMed] [Google Scholar]
- 31.Allen TW, Tulchinsky M. Nuclear medicine tests for acute gastrointestinal conditions. Semin Nucl Med. 2013;43:88–101. doi: 10.1053/j.semnuclmed.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 32.Zuckerman GR, Prakash C. Acute lower intestinal bleeding. Part II: etiology, therapy, and outcomes. Gastrointest Endosc. 1999;49:228–238. doi: 10.1016/s0016-5107(99)70491-8. [DOI] [PubMed] [Google Scholar]
- 33.Winzelberg GG, Froelich JW, McKusick KA, Waltman AC, Greenfield AJ, Athanasoulis CA, Strauss HW. Radionuclide localization of lower gastrointestinal hemorrhage. Radiology. 1981;139:465–469. doi: 10.1148/radiology.139.2.6971455. [DOI] [PubMed] [Google Scholar]
- 34.Defreyne L, Uder M, Vanlangenhove P, Van Maele G, Kunnen M, Kramann B. Angiography for acute lower gastrointestinal hemorrhage: efficacy of cut film compared with digital subtraction techniques. J Vasc Interv Radiol. 2003;14:313–322. doi: 10.1097/01.rvi.0000058414.01661.77. [DOI] [PubMed] [Google Scholar]
- 35.Navuluri R, Patel J, Kang L. Role of interventional radiology in the emergent management of acute upper gastrointestinal bleeding. Semin Intervent Radiol. 2012;29:169–177. doi: 10.1055/s-0032-1326925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Walker TG, Salazar GM, Waltman AC. Angiographic evaluation and management of acute gastrointestinal hemorrhage. World J Gastroenterol. 2012;18:1191–1201. doi: 10.3748/wjg.v18.i11.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walker TG. Acute gastrointestinal hemorrhage. Tech Vasc Interv Radiol. 2009;12:80–91. doi: 10.1053/j.tvir.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 38.Eriksson LG, Ljungdahl M, Sundbom M, Nyman R. Transcatheter arterial embolization versus surgery in the treatment of upper gastrointestinal bleeding after therapeutic endoscopy failure. J Vasc Interv Radiol. 2008;19:1413–1418. doi: 10.1016/j.jvir.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 39.Valji K. The practice of interventional radiology with online cases and videos. Philadelphia, PA: Elsevier/Saunders; 2012. [Google Scholar]
- 40.Funaki B. On-call treatment of acute gastrointestinal hemorrhage. Semin Intervent Radiol. 2006;23:215–222. doi: 10.1055/s-2006-948758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yap FY, Omene BO, Patel MN, Yohannan T, Minocha J, Knuttinen MG, Owens CA, Bui JT, Gaba RC. Transcatheter embolotherapy for gastrointestinal bleeding: a single center review of safety, efficacy, and clinical outcomes. Dig Dis Sci. 2013;58:1976–1984. doi: 10.1007/s10620-012-2547-z. [DOI] [PubMed] [Google Scholar]
- 42.Funaki B, Kostelic JK, Lorenz J, Ha TV, Yip DL, Rosenblum JD, Leef JA, Straus C, Zaleski GX. Superselective microcoil embolization of colonic hemorrhage. AJR Am J Roentgenol. 2001;177:829–836. doi: 10.2214/ajr.177.4.1770829. [DOI] [PubMed] [Google Scholar]
- 43.Evangelista PT, Hallisey MJ. Transcatheter embolization for acute lower gastrointestinal hemorrhage. J Vasc Interv Radiol. 2000;11:601–606. doi: 10.1016/s1051-0443(07)61612-1. [DOI] [PubMed] [Google Scholar]
- 44.Mirsadraee S, Tirukonda P, Nicholson A, Everett SM, McPherson SJ. Embolization for non-variceal upper gastrointestinal tract haemorrhage: a systematic review. Clin Radiol. 2011;66:500–509. doi: 10.1016/j.crad.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 45.Eriksson LG, Sundbom M, Gustavsson S, Nyman R. Endoscopic marking with a metallic clip facilitates transcatheter arterial embolization in upper peptic ulcer bleeding. J Vasc Interv Radiol. 2006;17:959–964. doi: 10.1097/01.RVI.0000223719.79371.46. [DOI] [PubMed] [Google Scholar]
- 46.Aina R, Oliva VL, Therasse E, Perreault P, Bui BT, Dufresne MP, Soulez G. Arterial embolotherapy for upper gastrointestinal hemorrhage: outcome assessment. J Vasc Interv Radiol. 2001;12:195–200. doi: 10.1016/s1051-0443(07)61825-9. [DOI] [PubMed] [Google Scholar]
- 47.Loffroy R, Guiu B, D’Athis P, Mezzetta L, Gagnaire A, Jouve JL, Ortega-Deballon P, Cheynel N, Cercueil JP, Krausé D. Arterial embolotherapy for endoscopically unmanageable acute gastroduodenal hemorrhage: predictors of early rebleeding. Clin Gastroenterol Hepatol. 2009;7:515–523. doi: 10.1016/j.cgh.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 48.Loffroy R, Guiu B, Mezzetta L, Minello A, Michiels C, Jouve JL, Cheynel N, Rat P, Cercueil JP, Krausé D. Short- and long-term results of transcatheter embolization for massive arterial hemorrhage from gastroduodenal ulcers not controlled by endoscopic hemostasis. Can J Gastroenterol. 2009;23:115–120. doi: 10.1155/2009/795460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Toyoda H, Nakano S, Kumada T, Takeda I, Sugiyama K, Osada T, Kiriyama S. Estimation of usefulness of N-butyl-2-cyanoacrylate-lipiodol mixture in transcatheter arterial embolization for urgent control of life-threatening massive bleeding from gastric or duodenal ulcer. J Gastroenterol Hepatol. 1996;11:252–258. doi: 10.1111/j.1440-1746.1996.tb00071.x. [DOI] [PubMed] [Google Scholar]
- 50.Frisoli JK, Sze DY, Kee S. Transcatheter embolization for the treatment of upper gastrointestinal bleeding. Tech Vasc Interv Radiol. 2004;7:136–142. doi: 10.1053/j.tvir.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 51.Abdel-Aal AK, Bag AK, Saddekni S, Hamed MF, Ahmed FY. Endovascular management of nonvariceal upper gastrointestinal hemorrhage. Eur J Gastroenterol Hepatol. 2013;25:755–763. doi: 10.1097/MEG.0b013e32835fb9a9. [DOI] [PubMed] [Google Scholar]
- 52.Loffroy RF, Abualsaud BA, Lin MD, Rao PP. Recent advances in endovascular techniques for management of acute nonvariceal upper gastrointestinal bleeding. World J Gastrointest Surg. 2011;3:89–100. doi: 10.4240/wjgs.v3.i7.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lubarsky M, Ray CE, Funaki B. Embolization agents-which one should be used when? Part 1: large-vessel embolization. Semin Intervent Radiol. 2009;26:352–357. doi: 10.1055/s-0029-1242206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ledermann HP, Schoch E, Jost R, Decurtins M, Zollikofer CL. Superselective coil embolization in acute gastrointestinal hemorrhage: personal experience in 10 patients and review of the literature. J Vasc Interv Radiol. 1998;9:753–760. doi: 10.1016/s1051-0443(98)70387-2. [DOI] [PubMed] [Google Scholar]
- 55.Toyoda H, Nakano S, Takeda I, Kumada T, Sugiyama K, Osada T, Kiriyama S, Suga T. Transcatheter arterial embolization for massive bleeding from duodenal ulcers not controlled by endoscopic hemostasis. Endoscopy. 1995;27:304–307. doi: 10.1055/s-2007-1005697. [DOI] [PubMed] [Google Scholar]
- 56.van Vugt R, Bosscha K, van Munster IP, de Jager CP, Rutten MJ. Embolization as treatment of choice for bleeding peptic ulcers in high-risk patients. Dig Surg. 2009;26:37–42. doi: 10.1159/000193476. [DOI] [PubMed] [Google Scholar]
- 57.Defreyne L, Vanlangenhove P, De Vos M, Pattyn P, Van Maele G, Decruyenaere J, Troisi R, Kunnen M. Embolization as a first approach with endoscopically unmanageable acute nonvariceal gastrointestinal hemorrhage. Radiology. 2001;218:739–748. doi: 10.1148/radiology.218.3.r01mr05739. [DOI] [PubMed] [Google Scholar]
- 58.Darcy M. Treatment of lower gastrointestinal bleeding: vasopressin infusion versus embolization. J Vasc Interv Radiol. 2003;14:535–543. doi: 10.1097/01.rvi.0000064862.65229.8a. [DOI] [PubMed] [Google Scholar]
- 59.Kusano S, Murata K, Ohuchi H, Motohashi O, Atari H. Low-dose particulate polyvinylalcohol embolization in massive small artery intestinal hemorrhage. Experimental and clinical results. Invest Radiol. 1987;22:388–392. doi: 10.1097/00004424-198705000-00006. [DOI] [PubMed] [Google Scholar]
- 60.Gordon RL, Ahl KL, Kerlan RK, Wilson MW, LaBerge JM, Sandhu JS, Ring EJ, Welton ML. Selective arterial embolization for the control of lower gastrointestinal bleeding. Am J Surg. 1997;174:24–28. doi: 10.1016/S0002-9610(97)00044-5. [DOI] [PubMed] [Google Scholar]
- 61.Nicholson AA, Ettles DF, Hartley JE, Curzon I, Lee PW, Duthie GS, Monson JR. Transcatheter coil embolotherapy: a safe and effective option for major colonic haemorrhage. Gut. 1998;43:79–84. doi: 10.1136/gut.43.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Peck DJ, McLoughlin RF, Hughson MN, Rankin RN. Percutaneous embolotherapy of lower gastrointestinal hemorrhage. J Vasc Interv Radiol. 1998;9:747–751. doi: 10.1016/s1051-0443(98)70386-0. [DOI] [PubMed] [Google Scholar]
- 63.Hur S, Jae HJ, Lee M, Kim HC, Chung JW. Safety and efficacy of transcatheter arterial embolization for lower gastrointestinal bleeding: a single-center experience with 112 patients. J Vasc Interv Radiol. 2014;25:10–19. doi: 10.1016/j.jvir.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 64.Yata S, Ihaya T, Kaminou T, Hashimoto M, Ohuchi Y, Umekita Y, Ogawa T. Transcatheter arterial embolization of acute arterial bleeding in the upper and lower gastrointestinal tract with N-butyl-2-cyanoacrylate. J Vasc Interv Radiol. 2013;24:422–431. doi: 10.1016/j.jvir.2012.11.024. [DOI] [PubMed] [Google Scholar]
- 65.Huang CC, Lee CW, Hsiao JK, Leung PC, Liu KL, Tsang YM, Liu HM. N-butyl cyanoacrylate embolization as the primary treatment of acute hemodynamically unstable lower gastrointestinal hemorrhage. J Vasc Interv Radiol. 2011;22:1594–1599. doi: 10.1016/j.jvir.2011.07.018. [DOI] [PubMed] [Google Scholar]
- 66.Frodsham A, Berkmen T, Ananian C, Fung A. Initial experience using N-butyl cyanoacrylate for embolization of lower gastrointestinal hemorrhage. J Vasc Interv Radiol. 2009;20:1312–1319. doi: 10.1016/j.jvir.2009.06.031. [DOI] [PubMed] [Google Scholar]
- 67.Lang EK. Transcatheter embolization in management of hemorrhage from duodenal ulcer: long-term results and complications. Radiology. 1992;182:703–707. doi: 10.1148/radiology.182.3.1535883. [DOI] [PubMed] [Google Scholar]
- 68.Lee CW, Liu KL, Wang HP, Chen SJ, Tsang YM, Liu HM. Transcatheter arterial embolization of acute upper gastrointestinal tract bleeding with N-butyl-2-cyanoacrylate. J Vasc Interv Radiol. 2007;18:209–216. doi: 10.1016/j.jvir.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 69.Lenhart M, Paetzel C, Sackmann M, Schneider H, Jung EM, Schreyer AG, Feuerbach S, Zorger N. Superselective arterial embolisation with a liquid polyvinyl alcohol copolymer in patients with acute gastrointestinal haemorrhage. Eur Radiol. 2010;20:1994–1999. doi: 10.1007/s00330-010-1762-2. [DOI] [PubMed] [Google Scholar]
- 70.Bush HL, Nabseth DC. Intravenous nitroglycerin to improve coronary blood flow and left ventricular performance during vasopressin therapy. Surg Forum. 1979;30:226–228. [PubMed] [Google Scholar]
- 71.Gomes AS, Lois JF, McCoy RD. Angiographic treatment of gastrointestinal hemorrhage: comparison of vasopressin infusion and embolization. AJR Am J Roentgenol. 1986;146:1031–1037. doi: 10.2214/ajr.146.5.1031. [DOI] [PubMed] [Google Scholar]
- 72.Leitman IM, Paull DE, Shires GT. Evaluation and management of massive lower gastrointestinal hemorrhage. Ann Surg. 1989;209:175–180. doi: 10.1097/00000658-198902000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bandi R, Shetty PC, Sharma RP, Burke TH, Burke MW, Kastan D. Superselective arterial embolization for the treatment of lower gastrointestinal hemorrhage. J Vasc Interv Radiol. 2001;12:1399–1405. doi: 10.1016/s1051-0443(07)61697-2. [DOI] [PubMed] [Google Scholar]
- 74.Mallory A, Schaefer JW, Cohen JR, Holt SA, Norton LW. Selective intra-arterial vasopression in fusion for upper gastrointestinal tract hemorrhage: a controlled trial. Arch Surg. 1980;115:30–32. doi: 10.1001/archsurg.1980.01380010022004. [DOI] [PubMed] [Google Scholar]
- 75.Formanek G, Frech RS, Amplatz K. Arterial thrombus formation during clinical percutaneous catheterization. Circulation. 1970;41:833–839. doi: 10.1161/01.cir.41.5.833. [DOI] [PubMed] [Google Scholar]
- 76.Barrett BJ, Parfrey PS. Clinical practice. Preventing nephropathy induced by contrast medium. N Engl J Med. 2006;354:379–386. doi: 10.1056/NEJMcp050801. [DOI] [PubMed] [Google Scholar]
- 77.Murphy SW, Barrett BJ, Parfrey PS. Contrast nephropathy. J Am Soc Nephrol. 2000;11:177–182. doi: 10.1681/ASN.V111177. [DOI] [PubMed] [Google Scholar]


