Abstract
Nicotine addiction is associated with the development of tolerance and the emergence of withdrawal symptoms upon cessation of chronic nicotine administration. Changes in cognition, including deficits in learning, are one of the most common withdrawal symptoms reported by smokers. However, the neural substrates of tolerance to the effects of nicotine on learning and the substrates of withdrawal deficits in learning are unknown, and in fact it is unclear whether a common mechanism is involved in both. The present study tested the hypothesis that tolerance and withdrawal are separate processes and that nicotinic acetylcholine receptor (nAChR) upregulation underlies changes in learning associated with withdrawal but not tolerance. C57BL/6 male mice were administered a dose of nicotine (3, 6.3, 12, or 24 mg/kg/d) chronically for varying days and tested for the onset of tolerance to the effects of nicotine on learning. Follow up experiments examined the number of days of chronic nicotine treatment required to produce withdrawal deficits in learning and a significant increase in [3H]epibatidine in the hippocampus indicative of receptor upregulation. The results indicate that tolerance onset was influenced by dose of chronic nicotine, that tolerance occurred before withdrawal deficits in learning emerged, and that nAChR upregulation in the dorsal hippocampus was associated with withdrawal but not tolerance. This suggests that for the effects of nicotine on learning, tolerance and withdrawal involve different substrates. These findings are discussed in terms of implications for development of therapeutics that target symptoms of nicotine addiction and for theories of addiction.
Keywords: Addiction, Acetylcholine, Learning, Cognition, Receptor Binding, Withdrawal
1.0 Introduction
Adaptation in behavior and neural substrates occurs with chronic drug use; and for drugs of abuse, these adaptations may contribute to the formation and maintenance of addiction. The DSM-IV lists seven diagnostic criteria for addiction with a minimum of three present needed for a diagnosis of addiction (Association American Psychiatric, 2000). Two of the seven listed are tolerance and withdrawal symptoms. Because of the purported importance of tolerance and withdrawal in addiction, understanding the biological basis of these symptoms could advance treatment of addiction. Models and theories of addiction and drug action have proposed that tolerance and withdrawal are manifestations of the same phenomenon; however, these theories are not universally accepted as some have suggested that tolerance and withdrawal reflect separate processes.
The opponent process theory states that with drug abuse and addiction, a drug will initially produce a positive effect (the A process) but with continued use a countering effect (the B process) is generated to maintain homeostasis (Solomon and Corbit, 1973). As the B process reduces the desired effects of the A process, drug consumption may increase in an attempt to achieve the full A process; this has been suggested to be tolerance. In the absence of the drug and the associated A process, the B process will dominate resulting in a stronger negative effect, which has been proposed to be the mechanism underlying withdrawal (Poulos and Cappell, 1991). Thus, in this model tolerance and withdrawal would reflect the same process.
While the opponent process theory has appeal as it explains how drug-induced stress on homeostatic processes results in addiction phenotypes, there is a long history of work suggesting that tolerance and withdrawal may be separate processes rather than aspects of a single homeostatic process. If this is true, the opponent process theory would need to be amended. For example, Tatum and colleagues (1929) proposed that tolerance reflected a decrease in the depressant effects of morphine whereas withdrawal was related to the stimulant effects of morphine. Furthermore, in a 1985 review of morphine tolerance, Baker and Tiffany concluded that opponent or compensatory processes do not contribute to withdrawal symptoms In both of these cases, tolerance and withdrawal would not reflect the same process.
Nicotine addiction is associated with the development of tolerance and the presence of withdrawal symptoms (Balfour, 1981; Gould and Leach, 2013; Jarvik and Henningfield, 1988; Paolini and De Biasi, 2011), and the effects of nicotine on cognitive processes are sensitive to both tolerance and withdrawal. In both humans and laboratory rodents, acute nicotine enhances learning and cognitive processes (Gould and Higgins, 2003; Gould and Wehner, 1999; Heishman et al., 2010; Kenney and Gould, 2008; Myers et al., 2008). With continued drug administration, tolerance for the cognitive enhancing effects of nicotine develops (Davis et al., 2005; Portugal et al., 2012a), and upon cessation of nicotine treatment, withdrawal-related deficits in learning and other cognitive measures emerge (Ashare et al., 2013; Davis et al., 2005; Heishman, 1999; Hendricks et al., 2006; Hughes et al., 1989; Jacobsen et al., 2005; Raybuck and Gould, 2009). This pattern of an initial enhancement, a decrease in enhancement with continued nicotine administration, and deficits in cognitive processes during abstinence appears to fit well within the opponent process theory of addiction but it remains to be determined if the neural substrates underlying tolerance to the pro-cognitive effects of nicotine are the same substrates underlying nicotine withdrawal disruption of cognition. Understanding the mechanisms that underlie tolerance and withdrawal-related changes in cognition is important because deficits in cognition during abstinence from tobacco are a major withdrawal symptom (Jacobsen et al., 2005; Kleinman et al., 1973; Mendrek et al., 2006; Snyder et al., 1989), and the severity of the cognitive deficits correlates with relapse rates (Patterson et al., 2010).
At the level of the receptor, chronic nicotine is associated with desensitization and upregulation (Hulihan-Giblin et al., 1990; Marks et al., 1983; Schwartz and Kellar, 1983; Sharp and Beyer, 1986). It has been proposed that the onset of desensitization is rapid whereas receptor upregulation occurs over a comparatively longer time period (Bullock et al., 1997; Collins et al., 1994; Ochoa et al., 1989). In addition, both desensitization and receptor upregulation have been proposed to contribute to tolerance for the somatic and locomotor effects of nicotine (Marks et al., 1983; Marks et al., 1985; Robinson et al., 2006; Robinson et al., 2007); though other work suggested that there may be a dissociation of tolerance and receptor upregulation (Collins et al., 1990; McCallum et al., 2000).
In mice, nicotine acts directly in the dorsal hippocampus to enhance hippocampus-dependent learning and to produce withdrawal-related deficits in hippocampus-dependent learning after cessation of chronic administration (Davis and Gould, 2009; Kenney et al., 2012). The withdrawal-related deficits in hippocampus-dependent learning are associated with changes in dorsal hippocampal nicotinic acetylcholinergic receptor (nAChR) upregulation (Gould et al., 2012; Portugal et al., 2012b; Wilkinson et al., 2013) but it is unknown if nAChR upregulation underlies the observed tolerance to the cognitive enhancing effects of nicotine and whether tolerance and withdrawal-associated deficits in learning involve the same process.
The present study investigated whether behavioral tolerance to the effects of nicotine on hippocampus-dependent learning emerged at the same time point as withdrawal deficits in learning emerged and whether a significant change in nAChR upregulation was temporally associated with the development of tolerance and/or withdrawal. It was hypothesized that if tolerance and withdrawal-associated changes in hippocampus-dependent learning involve different neural mechanisms, then tolerance would emerge at a different time than withdrawal deficits and that nAChR upregulation would be temporally related to withdrawal but not tolerance. These experiments also investigated the influence of dose on the onset of tolerance to the cognitive enhancing effects of nicotine.
2.0 Results
2.1 Tolerance
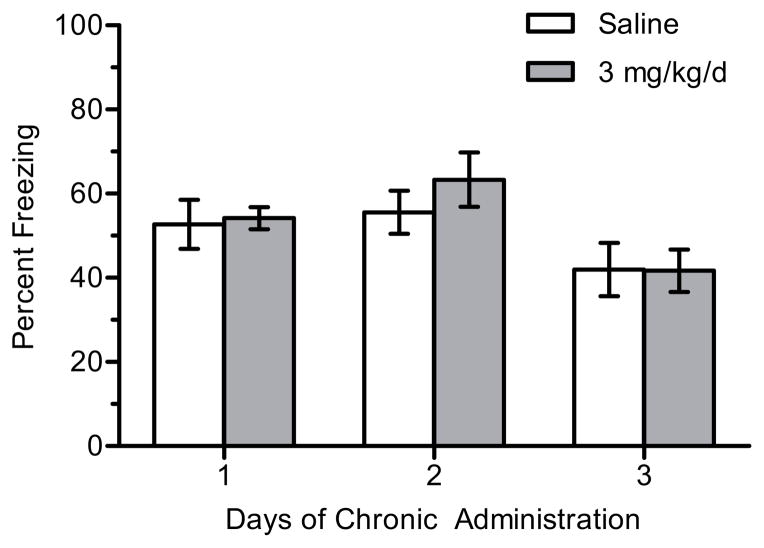
To determine how dose affects onset of tolerance to the effects of nicotine on hippocampus-dependent learning, separate experiments were performed in which mice were implanted with osmotic minipumps that delivered chronic saline or nicotine (3, 6.3, 12, or 24 mg/kg/d) for up to 6 days depending on when tolerance emerged for each dose. There was a significant main effect day for 3 mg/kg/d nicotine (n = 9–12 per group) on contextual freezing, F(2, 61) = 6.049, p < 0.005 (Figure 1). However, this significant omnibus test was due to significant differences between animals that received 2 days of chronic nicotine and 3 days of chronic saline (p < 0.05) and 3 days of chronic nicotine (p < 0.05), comparisons that were not of interest. There were no significant differences between saline- or nicotine-treated animals within each day (ps > 0.05). There were no significant differences in baseline freezing (p > 0.05). Thus, 3 mg/kg/d did not enhance contextual conditioning at any day (1–3) tested.
Figure 1.
The effects of 3 mg/kg/d chronic nicotine on contextual conditioning. There was no significant effect of nicotine on contextual conditioning within each day. Error bars represent ± the standard error of the mean (n = 11 saline day 1; n = 12 nicotine day 1; n = 9 saline day 2; n = 11 nicotine day 2; n = 12 saline day 3; n = 12 nicotine day 3).
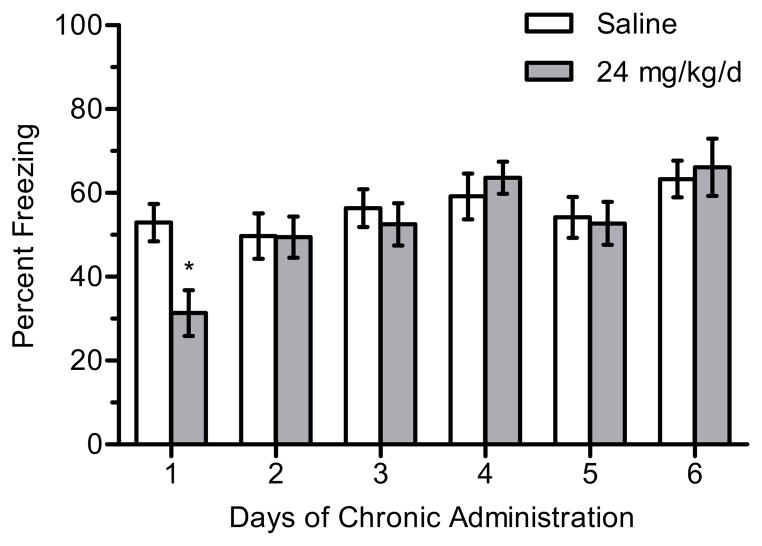
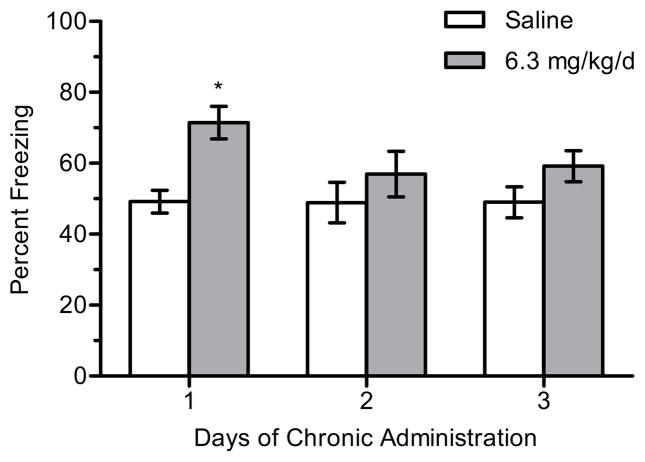
There was a significant main effect of treatment for 6.3 mg/kg/d nicotine (n = 9–14 per group) on contextual freezing, F(1, 62) = 12.700, p < 0.001 (Figure 2). Post-hoc tests revealed that mice treated with 6.3 mg/kg/d nicotine and trained on day 1 of chronic treatment froze more to the context than mice treated with saline (p < 0.05). There were no differences in freezing between chronic saline- and nicotine-treated mice for any other days of chronic treatment (ps > 0.05), suggesting the development of tolerance. In addition, there were no significant differences in baseline freezing (p > 0.05).
Figure 2.
The effects of 6.3 mg/kg/d chronic nicotine on contextual conditioning. 6.3 mg/kg/d chronic nicotine enhanced contextual conditioning for mice trained at day 1 of chronic administration but enhancement was not seen on subsequent days in separate groups of mice. Error bars represent ± the standard error of the mean. (*) indicates p < 0.05 compared to saline treated mice within the same day (n = 12 saline day 1; n = 14 nicotine day 1; n = 9 saline day 2; n = 11 nicotine day 2; n = 10 saline day 3; n = 12 nicotine day 3).
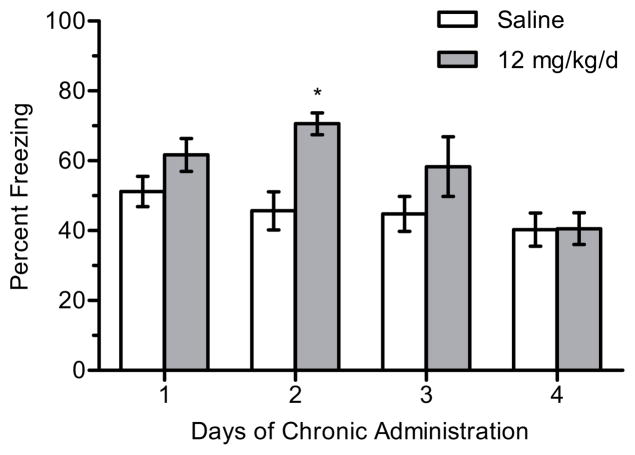
Likewise, for 12 mg/kg/d nicotine (n = 9–12 per group), there were significant main effects of day, F(3, 80) = 4.840, p < 0.005, and treatment, F(1, 80) = 12.506, p < 0.001, on contextual freezing (Figure 3). Post-hoc tests revealed that mice treated with 12 mg/kg/d nicotine and trained on day 2 of treatment froze more to the context than mice treated with saline (p < 0.05). There were no significant differences in freezing between saline- or nicotine-treated mice at any other days of chronic treatment (ps > 0.05). In addition, there were no significant differences in baseline freezing (p > 0.05).
Figure 3.
The effects of 12 mg/kg/d chronic nicotine on contextual conditioning. 12 mg/kg/d chronic nicotine enhanced contextual conditioning for mice trained at day 2 of chronic administration but enhancement was not seen on subsequent days in separate groups of mice. Error bars represent ± the standard error of the mean. (*) indicates p < 0.05 compared to saline treated mice within the same day (n = 10 saline day 1; n = 12 nicotine day 1; n = 10 saline day 2; n = 11 nicotine day 2; n = 9 saline day 3; n = 12 nicotine day 3; n = 12 saline day 4; n = 12 nicotine day 4).
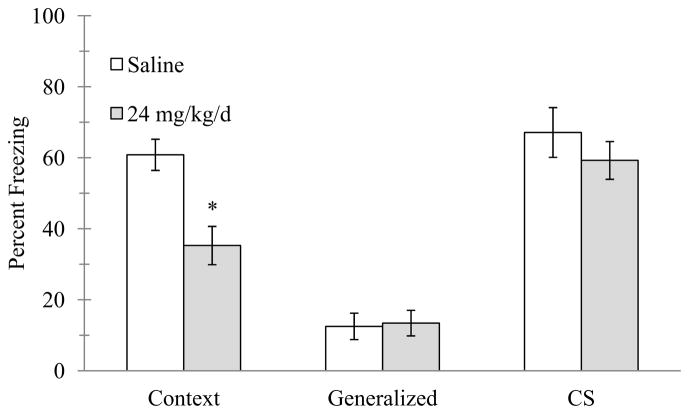
Lastly, there was a significant main effect of day for 24 mg/kg/d nicotine (n = 8–16 per group) on contextual freezing, F(5, 134) = 5.580, p < 0.001 (Figure 4). Post-hoc tests revealed that this was due to mice treated with 24 mg/kg/d and trained on day 1 of treatment freezing significantly less to the context than mice treated with chronic saline (p < 0.05). There were no differences in contextual freezing between saline- and nicotine-treated mice for any other days of chronic treatment (ps > 0.05). There were no significant differences in baseline freezing (p > 0.05)..
Figure 4.
The effects of 24 mg/kg/d chronic nicotine on contextual conditioning. 24 mg/kg/d chronic nicotine produced a deficit in contextual conditioning for mice trained at day 1 of chronic administration; deficits were not seen on subsequent days in separate groups of mice. Error bars represent ± the standard error of the mean. (*) indicates p < 0.05 compared to saline treated mice with the same day (n = 16 saline day 1; n = 15 nicotine day 1; n = 12 saline day 2; n = 12 nicotine day 2; n = 12 saline day 3; n = 12 nicotine day 3; n = 12 saline day 4; n = 12 nicotine day 4; n = 12 saline day 5; n = 11 nicotine day 5; n = 8 saline day 6; n = 12 nicotine day 6).
To determine if the deficit in contextual conditioning with 24 mg/kg/d was specific to contextual conditioning or due to a global deficit in learning or processes that would impact freezing, separate groups of mice (n = 11–12 per group) were trained in cued conditioning (Figure 5). Independent samples t-tests revealed that mice administered 24 mg/kg/d chronic nicotine and trained on day 1 of treatment froze less to the context less than saline-treated mice (t(21) = 3.542, p < 0.05), however, no significant differences existed in freezing between saline- and nicotine-treated mice for pre-CS or CS freezing (p > 0.05), suggesting the reduced freezing was specific to contextual conditioning.
Figure 5.
The effects of 24 mg/kg/d chronic nicotine on cued conditioning. 24 mg/kg/d chronic nicotine produced a deficit in contextual conditioning for mice trained at day 1 of chronic administration but no deficits were seen in cued conditioning. Error bars represent ± the standard error of the mean. (*) indicates p < 0.05 compared to saline treated mice (n = 12 saline; n = 11 nicotine).
2.2 Withdrawal
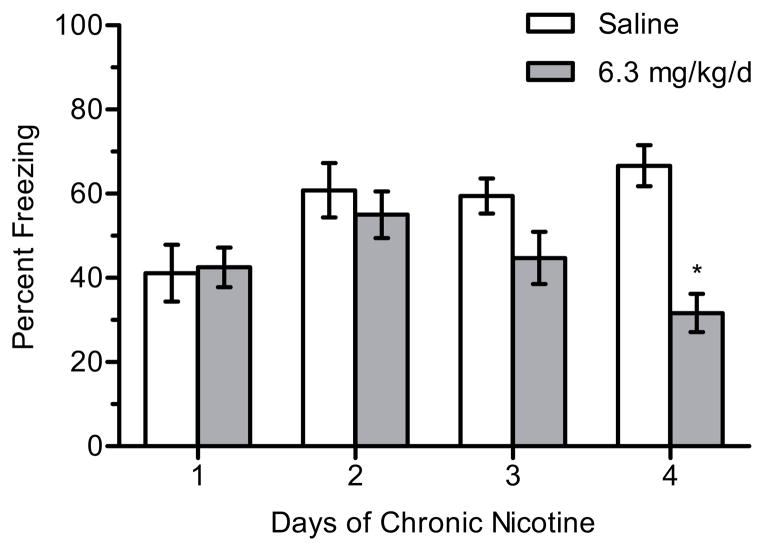
In order to determine the threshold of chronic nicotine treatment required to produce withdrawal-related deficits in contextual conditioning upon cessation of treatment, mice were administered chronic saline or 6.3 mg/kg/d nicotine for 1–4 days (n = 8–12 per group) then withdrawn from chronic treatment for 24 hours prior to training in contextual conditioning (Figure 6). A significant interaction for the effects of day and treatment on withdrawal changes in contextual conditioning was seen, F(3, 71) = 4.766, p < 0.005. Post-hoc tests revealed that mice withdrawn from 4 days of chronic nicotine froze significantly less to the context than mice withdrawn from 4 days of chronic saline (p < 0.05); withdrawal deficits in learning were not seen after cessation of 1–3 days of chronic nicotine treatment. There were no significant differences in baseline freezing (p > 0.05).
Figure 6.
The effects of the number of days of chronic nicotine administration on the emergence of withdrawal deficits in contextual conditioning. 6.3 mg/kg/d chronic nicotine produced a deficit in contextual conditioning following withdrawal from 4 days of chronic nicotine administration. Error bars represent ± the standard error of the mean. (*) indicates p < 0.05 compared to saline treated mice within the same day (n = 11 saline day 1; n = 12 nicotine day 1; n = 8 saline day 2; n = 8 nicotine day 2; n = 12 saline day 3; n = 12 nicotine day 3; n = 8 saline day 4; n = 8 nicotine day 4).
2.3 Binding
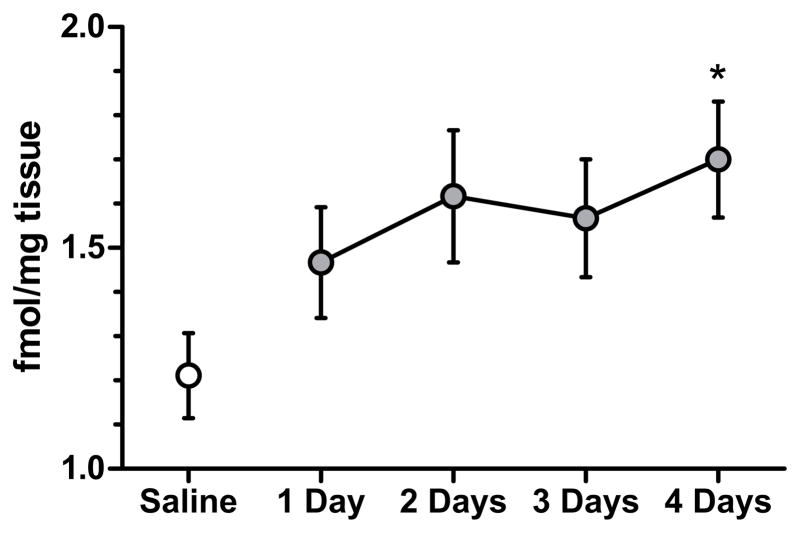
To test if changes in nAChR upregulation corresponded to behavioral changes associated with chronic nicotine and/or withdrawal, [3H]epibatidine binding was assessed in mice treated with 6.3 mg/kg/d chronic nicotine for 1, 2, 3, or 4 days. As shown in Figure 7, 6.3 mg/kg/d chronic nicotine resulted in a time-dependent increase in [3H]epibatidine binding in the dorsal hippocampus of mice, F(4, 28) = 2.685, p = 0.05. Post-hoc tests reveal that binding was significantly greater in mice treated with 4 days of 6.3 mg/kg/day chronic nicotine than in chronic saline-treated mice (p < 0.05).
Figure 7.
The effects of the number of days of chronic nicotine administration on [3H]epibatidine binding in the dorsal hippocampus. Chronic nicotine significantly increased [3H]epibatidine binding in the dorsal hippocampus only after 4 days of administration. Error bars represent ± S.E.M. (*) indicates p < 0.05 compared to saline treated mice (n=9 saline; n= 6 each nicotine group).
3.0 Discussion
This study found that tolerance for the cognitive effects of nicotine emerged earlier in dosing than the cognitive effects of nicotine withdrawal. Because a temporal delay exists between the onset of tolerance and withdrawal-associated deficits, the underlying biological changes responsible for tolerance and withdrawal related changes in learning are most likely different. After one day of chronic nicotine treatment (6.3 mg/kg/day), enhancement of contextual conditioning was no longer seen. In contrast, withdrawal deficits in contextual conditioning were not observed until cessation of four days of chronic nicotine (6.3 mg/kg/day) treatment.
Prior studies also suggest that for nicotine, tolerance and withdrawal may be mediated by separate mechanisms. For instance, Salas et al. (2007) found that α7 KO mice displayed reduced somatic withdrawal symptoms compared to wild-type littermates but both genotypes developed tolerance to nicotine-induced hypolocomotion. In addition, strains of mice that develop tolerance to the effects of acute nicotine on learning do not always display withdrawal deficits in learning (Portugal et al., 2012a). For example, C57BL/6 mice displayed both tolerance to nicotine enhancement of learning and withdrawal deficits in learning; however, 129/SvEv mice showed tolerance to nicotine enhancement of learning without exhibiting associated withdrawal deficits. A disconnect between tolerance and withdrawal is also seen in a subset of smokers who do not meet the criteria for tobacco dependence. Nondependent smokers developed tolerance to the effects of nicotine but did not experience withdrawal effects following cessation of tobacco use (Perkins, 2002; Perkins et al., 2001). Together, these studies and the current data indicate that tolerance can be dissociated from withdrawal, which suggests that separate processes may underlie tolerance and withdrawal and also suggests that theories that propose that withdrawal is a manifestation of tolerance symptoms in the absence of drug may need to be reconsidered.
Whereas the underlying neural changes responsible for withdrawal-associated deficits have not been fully elucidated, increasing evidence suggests that upregulation of high-affinity nAChRs in the dorsal hippocampus is an important contributing factor. In the present study, four days of chronic nicotine treatment were required for withdrawal from nicotine treatment to disrupt learning and a significant increase in nAChR upregulation in the dorsal hippocampus was not seen until the fourth day of chronic nicotine treatment. While these data do not demonstrate causation, they add to a growing literature that suggests nAChR upregulation is necessary for these withdrawal deficits to emerge. Prior work demonstrated that the duration of upregulation of high-affinity hippocampal nAChRs after cessation of nicotine administration paralleled the duration of nicotine withdrawal-associated deficits in learning (Gould et al., 2012). In addition, strains of mice that exhibited nicotine withdrawal-related deficits in contextual learning displayed upregulation of high-affinity nAChRs in the dorsal, but not ventral, hippocampus whereas upregulation was absent in the dorsal hippocampus of strains that did not display nicotine withdrawal deficits in learning (Wilkinson et al., 2013). Finally, early adolescent mice did not display upregulation of high-affinity hippocampal nAChRs or nicotine withdrawal-deficits in learning but both were present in adult mice (Portugal et al., 2012b).
While earlier studies suggested that nAChR upregulation, but not changes in metabolism, may underlie tolerance (Marks et al., 1983; Marks et al., 1985), later studies and the current data suggest nAChR upregulation is not the primary factor underlying tolerance. In the current study, significant nAChR upregulation occurred after the development of tolerance to the effects of nicotine on learning. This finding parallels prior studies examining the relationship between nAChR upregulation and tolerance to the effects of nicotine on activity, respiration, and body temperature. In an examination of genetic differences in tolerance and nAChR upregulation, a relationship between upregulation and tolerance did not hold across strains of mice (Marks et al., 1986); for example C3H mice showed nAChR upregulation but not tolerance (Marks et al., 1986). In another study examining the duration of tolerance to the effects of nicotine on body temperature and activity and the duration of nAChR upregulation, tolerance was short lasting and nAChR upregulation outlasted tolerance (Collins et al., 1990). Together, these findings suggest a mechanism other than nAChR upregulation must underlie tolerance.
If nAChR upregulation does not underlie tolerance, an attractive alternative mechanism is nAChR desensitization. In a 1996 review, Collins and Marks proposed that functional changes in nAChRs, such as densitization, underlie tolerance (Collins and Marks, 1996). In support of nAChR desensitization contributing to tolerance, the development of tolerance in a nicotine discrimination task significantly correlated with nAChR desensitization in the thalamus (Robinson et al., 2006; Robinson et al., 2007). While receptor desensitization was not measured in the current study, we found a dose-dependent shift in the onset of chronic nicotine-associated enhancement of learning; this change could be explained by tolerance. Specifically, 6.3 mg/kg/d of chronic nicotine enhanced contextual learning when mice were trained at day 1 of chronic treatment but failed to alter contextual learning when mice were trained at day 2 of chronic treatment and beyond, indicating the development of tolerance. In comparison, 12 mg/kg/d of chronic nicotine had no effect at 1 day, enhanced contextual learning when mice were trained at day 2 of chronic treatment but failed to alter contextual learning when mice were trained at day 3 of treatment and beyond, demonstrating tolerance. This finding of a delay in cognitive enhancement was seen before for a spatial memory task (Levin et al., 1990). The dose-response curve for nicotine enhancement of learning is a narrow inverted U-shaped curve (Gould and Higgins, 2003; Gould and Wehner, 1999). Therefore, the 12 mg/kg/d dose of chronic nicotine may not enhance learning on the first day of treatment because dose is on the right side of the dose response curve but as nAChR desensitization occurs, the same dose may be less effective and in a sense shifting the effectiveness of the dose towards that center of the dose-response curve and producing enhancement. With continued chronic nicotine administration, further desensitization decreases the effectiveness of the dose, resulting in a response equivalent to responses seen on the far left side of the dose response curve. This, of course, requires further testing. The highest dose of chronic nicotine, 24 mg/kg/d, produced a deficit in contextual, but not cued, conditioning. This deficit, specific to hippocampus-dependent learning, was most likely due to the dose being on the far right tail of the dose response curve. With continued administration, the deficit disappeared suggesting tolerance to this effect of nicotine.
The data presented here support a model of nicotine addiction where desensitization and nAChR upregulation contribute to tolerance and withdrawal symptoms. In this model, chronic nicotine treatment would desensitize nAChRs and then upregulate nAChRs resulting in an increased pool of nAChRs but with a proportion of those receptors being nonfunctional. During periods of abstinence, the increased number of nAChRs may recover from desensitization leading to a hyperexcitable nAChR system (Dani and Heinemann, 1996; Gould and Leach, 2013; Gould et al., 2012). This type of change may contribute to the withdrawal associated deficits in hippocampus-dependent learning. In support, nicotine withdrawal produced an increase in hippocampal CA1 pyramidal cell excitability that persisted up to 9 months (Penton et al., 2011). Furthermore, cognitive deficits during periods of abstinence were associated with greater frontal cortical activity (Jacobsen et al., 2007). Finally, a recent study found that withdrawal from chronic nicotine increased sensitivity to the effects of acute nicotine on learning (Wilkinson and Gould, 2013). These results suggest that withdrawal deficits (at least for cognitive deficits) may be the result of a hypersensitive nAChR system (Wilkinson and Gould, 2013).
If withdrawal is related to an increase in active (or hyperactive) nAChRs, effective therapeutics may be drugs that blunt or desensitize nAChR activation. Varenicline, a partial agonist for α4β2 nAChRs (Mihalak et al., 2006), is the most effective treatment for nicotine addiction (Hudmon et al., 2010). In both mice and smokers, varenicline ameliorated cognitive withdrawal symptoms (Loughead et al., 2010; Patterson et al., 2009; Raybuck et al., 2008; Rhodes et al., 2012). α4β2 nAChRs are critically involved in nicotine withdrawal deficits in learning (Davis and Gould, 2009; Gould et al., 2012; Portugal et al., 2008; Raybuck and Gould, 2009) and as a partial agonist, varenicline may bind to α4β2 nAChR without fully activating them and prevent endogenous and exogenous ligands from activating the receptors (Papke et al., 2011). This blunting of nAChR activation could ameliorate the withdrawal deficits if the nAChR system is in a hypersensitive state. Sazetidine-A is a newly developed compound with high affinity for α4β2 nAChRs that desensitizes but does not upregulate the receptors (Hussmann et al., 2012; Turner et al., 2010; Xiao et al., 2006; Zwart et al., 2008). If the proposed model of nAChR hypersensitivity during withdrawal is accurate, sazetidine-A may be a particularly effective therapeutic. In support, it reduced nicotine self-administration in rats (Levin et al., 2010) and ventral hippocampal infusion of sazetidine-A reduced nicotine withdrawal-associated changes in anxiety (Turner et al., 2013).
4.0 Conclusions
The present study found a delay between the onset of tolerance to the effects of nicotine on learning and the emergence of nicotine withdrawal deficits in learning. This disconnect suggests that the underlying substrates of tolerance and withdrawal are different, at least for nicotine-associated changes in learning. In addition, increased high-affinity nAChR upregulation in the dorsal hippocampus was associated with the withdrawal deficits but not the observed tolerance. It is possible that changes in nAChR function in brain regions other than the dorsal hippocampus also contributed to the behavioral changes; however, prior studies reported that changes in dorsal, but not ventral, hippocampus nAChR binding corresponded with withdrawal changes in cognition (Wilkinson et al., 2013), that the time course of nAChR upregulation in the dorsal hippocampus matched the duration on cognitive withdrawal deficits (Gould et al., 2012), and that chronic nicotine infusion into the dorsal hippocampus was both necessary and sufficient for cognitive withdrawal symptoms to emerge (Davis and Gould, 2009). Overall, the results support a model where nAChR desensitization may contribute to tolerance and nAChR upregulation may primarily contribute to withdrawal symptoms. This suggests that drugs that desensitize without significantly upregulating nAChRs may be effective treatments for nicotine addiction. However, it should be pointed out that it is possible that tolerance and withdrawal could still be related to similar processes that emerge at different times, in which case it would be interesting to identify the process responsible for the delay in onset. Finally, it will be important for future studies to examine if similar mechanisms are involved in all nicotine withdrawal symptoms.
5.0 Methods and Materials
5.1 Subjects
C57BL/6J male mice (Jackson Laboratory, Bar Harbor, ME) aged 8–12 weeks at the beginning of pump implantation were housed 1–4 per cage with ad libitum access to food and water. A 12-hour light/dark cycle was maintained from 7:00 AM to 7:00 PM with all experiments conducted during the light cycle. The Temple University Institutional Animal Care and Use Committee approved all experimental procedures.
5.2 Surgery
Mice were implanted with osmotic minipumps (Alzet, Model 1002, Durect Co, Cupertino, CA) that delivered chronic saline or nicotine for up to 6 days. Osmotic minipumps were surgically inserted subcutaneously via an incision in the lower back of the mouse. Surgery was performed under sterile conditions with 5% isoflurane as the anesthetic. For studies examining nicotine withdrawal, a second similar surgery was performed to remove pumps and induce spontaneous nicotine withdrawal after 1, 2, 3, or 4 days of chronic nicotine.
5.3 Drugs and Duration of Treatment
Nicotine hydrogen tartrate salt (Sigma-Aldrich, St. Louis, MO) was dissolved in 0.9% saline. Osmotic minipumps were filled with 100 μl of a solution that contained saline, 3, 6.3, 12, or 24 mg/kg/d nicotine. Only saline and 6.3 mg/kg/d nicotine was used for nicotine withdrawal studies. All doses are reported as the freebase weight of nicotine and based off of a previous report (Portugal et al., 2012a).
5.4 Apparatus
Mice were trained and tested for contextual conditioning in four identical clear Plexiglas chambers (26.5 × 20.4 × 20.8 cm) housed in sound attenuating boxes (Med-Associates, St. Albans, VT), as previously described (Kenney et al., 2010). The floor of each chamber was made of metal bars (0.20 cm diameter) spaced 1.0 cm apart and connected to a shock generator and scrambler (Med Associates, Model ENV-414). Ventilation fans were mounted on the sides of each box to provide background noise. A 4 W light mounted above each chamber provided illumination. Stimulus administration was controlled by a PC running LabView software.
Cued conditioning testing occurred in four altered context chambers (20.3 × 22.9 × 17.8 cm) housed in sound attenuating boxes (Med-Associates, St. Albans, VT) in a different room from the training room. The floor of each chamber was made of white, opaque plastic. Speakers mounted on the left wall of each chamber delivered the auditory CS. Vanilla extract was added to the tray beneath the floors to further distinguish the altered chambers from the training context.
5.5 Behavioral Procedure
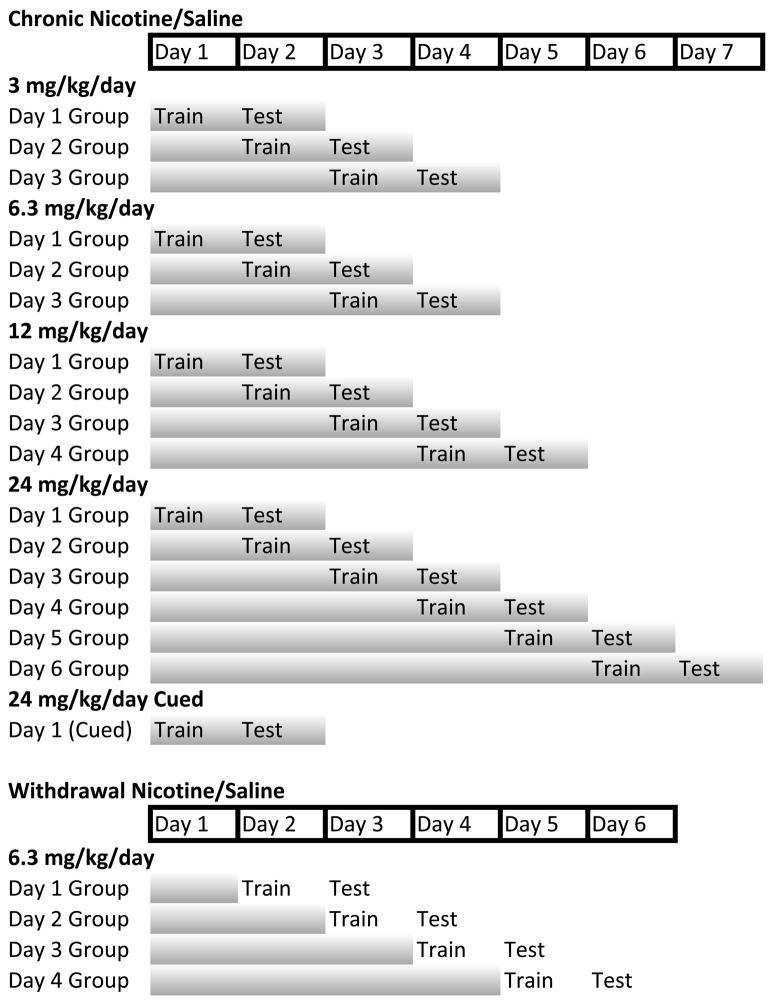
To determine the impact of dose on the development of tolerance to the cognitive enhancing effects of nicotine, separate groups of mice were implanted before training with osmotic minipumps that delivered chronic saline or nicotine (3, 6.3, 12, or 24 mg/kg/d; see Figure 8 for schematic). Within each dose condition, separate groups of mice were trained and tested at different days after initiation of chronic nicotine treatment; because mice cannot be trained and tested multiple times separate mice were examined for each day. Prior work found that withdrawal from chronic nicotine treatment disrupted training (i.e., learning) but not testing (i.e., recall) (Portugal and Gould, 2009). Thus, for all chronic nicotine experiments, mice received chronic nicotine treatment for both training and testing but treatment day designation for chronic nicotine studies’ results and figures refers to the day of chronic nicotine treatment that training occurred on. Each dose was treated as a separate experiment and had a corresponding saline control group. Training and testing of contextual conditioning was performed as previously described (André et al., 2008). Freezing, defined as the absence of all movement except respiration, was sampled for 1 s every 10 s and served as a measure of learning. During training, mice were placed into one of four conditioning chambers for 5.5 min. Baseline freezing behavior was recorded during the first 120 s of the session. At 148 s, mice were presented with a 2 s 0.57 mA foot shock US. At 298 s, an additional 2 s foot shock US was presented. The mice remained in the chambers for 30 s after the second US presentation. Approximately 24 hours later, testing of contextual conditioning occurred via placement of the mouse into the training context and freezing was scored for 5 min.
Figure 8.
Schematic of experimental design. Shaded bars indicate chronic nicotine/saline administration. For the Chronic Nicotine experiments, Day # Group designation indicates days of chronic nicotine treatment at the time of training. For the Withdrawal experiments, Day # Group designation indicates the number of days of chronic nicotine treatment before withdrawal of nicotine treatment; mice were trained 24 hours after withdrawal of nicotine treatment.
One dose of nicotine, 24 mg/kg/d, was found to produce deficits in contextual conditioning after one day of treatment. Therefore, an experiment was performed in a separate group of mice to determine if this deficit was specific to contextual conditioning or due to a global deficit in learning and memory by testing mice in cued fear conditioning, which unlike contextual fear conditioning does not involve the hippocampus (Kim et al., 1993; Logue et al., 1997). The behavioral procedure was performed as previously described (Gould and Higgins, 2003). Mice were placed into the training context and after a 120 s baseline period a 30 s auditory CS (85 dB white noise) was sounded that co-terminated with a 2 s US footshock (0.57 mA). After a 120 s ITI, another CS-US pairing was presented. Mice remained in the chambers for an additional 30 s after the second CS-US pairing. Approximately 24 hours later mice were placed back into the original training context without the CS for 5.5 min and freezing to the context freezing was scored for 5 min. Approximately 1 h later, mice were placed into the altered context for a total of 6 min. Pre-CS freezing was scored for the first 3 min in the absence of the CS. The CS was then turned on and CS freezing was scored for 3 min.
To test if the same number of days of chronic nicotine treatment required for tolerance to emerge were also required for withdrawal deficits in contextual conditioning to emerge, separate groups of mice were implanted with osmotic minipumps that delivered saline or nicotine (6.3 mg/kg/d) for 1–4 days and withdrawal deficits in contextual conditioning were assessed 24 hours after cessation of 1, 2, 3, or 4 days of chronic treatment. The osmotic minipumps were removed to induce withdrawal. Each time was treated as a separate condition with appropriate nicotine and saline groups.
5.6 Receptor Binding
To test if the temporal onset of significant changes in nAChR upregulation corresponded to development of tolerance and/or withdrawal symptoms, binding was performed as previously described (Wilkinson et al., 2013). Separate groups of mice received 6.3 mg/kg/d chronic nicotine or saline for 1–4 days and then and their dorsal hippocampi were dissected on ice (n = 6–9 per group). The dorsal hippocampus was targeted because previous work found that changes in dorsal, but not ventral, hippocampus nAChR binding corresponded to withdrawal deficits in contextual learning (Wilkinson et al., 2013), and that chronic nicotine infusion into the dorsal hippocampus was both necessary and sufficient for cognitive withdrawal symptoms to emerge (Davis and Gould, 2009). Initial analyses found no difference in binding between animals that received chronic saline for 1–4 days. Therefore, data from these animals were combined into one group. The samples were homogenized in 50 mM Tris–HCl (Sigma-Aldrich) buffer, pH 7.4 at 24°C, and centrifuged twice at 35,000 × g for 10 min in fresh buffer. The membrane pellets were resuspended in fresh buffer and added to tubes containing a saturating concentration (2 nM) of [3H]epibatidine (PerkinElmer, Boston, MA). Epibatidine was used because prior research showed that changes in high affinity nAChR binding were associated with nicotine withdrawal deficits in learning (Portugal et al., 2008; Wilkinson et al., 2013). Incubations were performed in Tris buffer at pH 7.4 for 2 hr at 24°C with [3H]epibatidine. Bound receptors were separated from free ligand by vacuum filtration over GF/C glass fiber filters (Brandel, Gaithersburg, MD) that were pre-treated with 0.5% polyethyleneimine (Sigma-Aldrich). The filters were then counted in a liquid scintillation counter. Nonspecific binding was determined in the presence of 300 μM nicotine, and specific binding was defined as the difference between total binding and nonspecific binding. Binding data were expressed as fmol/mg tissue (Turner et al., 2010, 2011).
5.7 Statistical Analyses
For studies examining the effects of chronic nicotine and withdrawal from chronic nicotine on contextual conditioning, freezing data were analyzed using two-way analysis of variance (ANOVA). Significant omnibus tests were followed by Tukey’s post-tests. Games-Howell post-hoc tests were used when the homogeneity of variance assumption was not satisfied. For the experiment using cued conditioning, independent samples t-tests were used to compare freezing levels within each condition. Binding data were analyzed using oneway ANOVA followed by Dunnett’s post-hoc tests. One animal that was 2.5 standard deviations from the mean was considered an outlier and excluded from data analysis.
Chronic nicotine treatment produces tolerance to the effects of nicotine on learning
Onset of tolerance is dose dependent
Nicotine withdrawal disrupts learning
The onset of tolerance occurs before the onset of withdrawal
Acknowledgments
We would like to thank Nicole Yohn for providing training in the binding assay. This work was supported by grants from the NIH: DA017949 (TJG), DA024787 (TJG), and CA143187 (Caryn Lerman).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- André JM, Gulick D, Portugal GS, Gould TJ. Nicotine withdrawal disrupts both foreground and background contextual fear conditioning but not pre-pulse inhibition of the acoustic startle response in C57BL/6 mice. Behav Brain Res. 2008;190:174–181. doi: 10.1016/j.bbr.2008.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashare RL, Falcone M, Lerman C. Cognitive function during nicotine withdrawal: Implications for nicotine dependence treatment. Neuropharmacology. 2013 doi: 10.1016/j.neuropharm.2013.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Association American Psychiatric. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: 2000. text rev. [Google Scholar]
- Baker TB, Tiffany ST. Morphine tolerance as habituation. Psychol Rev. 1985;92:78–108. [PubMed] [Google Scholar]
- Balfour DJ. The pharmacology of nicotine dependence: a working hypothesis. Pharmacol Ther. 1981;15:239–250. doi: 10.1016/0163-7258(81)90044-9. [DOI] [PubMed] [Google Scholar]
- Bullock AE, Clark AL, Grady SR, Robinson SF, Slobe BS, Marks MJ, Collins AC. Neurosteroids modulate nicotinic receptor function in mouse striatal and thalamic synaptosomes. J Neurochem. 1997;68:2412–2423. doi: 10.1046/j.1471-4159.1997.68062412.x. [DOI] [PubMed] [Google Scholar]
- Collins AC, Luo Y, Selvaag S, Marks MJ. Sensitivity to nicotine and brain nicotinic receptors are altered by chronic nicotine and mecamylamine infusion. J Pharmacol Exp Ther. 1994;271:125–133. [PubMed] [Google Scholar]
- Collins AC, Marks MJ. Are nicotinic receptors activated or inhibited following chronic nicotine treatment? Drug Dev Res. 1996;38:231–242. [Google Scholar]
- Collins AC, Romm E, Wehner JM. Dissociation of the apparent relationship between nicotine tolerance and up-regulation of nicotinic receptors. Brain Res Bull. 1990;25:373–379. doi: 10.1016/0361-9230(90)90222-l. [DOI] [PubMed] [Google Scholar]
- Dani JA, Heinemann S. Molecular and cellular aspects of nicotine abuse. Neuron. 1996;16:905–908. doi: 10.1016/s0896-6273(00)80112-9. [DOI] [PubMed] [Google Scholar]
- Davis JA, Gould TJ. Hippocampal nAChRs mediate nicotine withdrawal-related learning deficits. Eur Neuropsychopharmacol. 2009;19:551–561. doi: 10.1016/j.euroneuro.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JA, James JR, Siegel SJ, Gould TJ. Withdrawal from chronic nicotine administration impairs contextual fear conditioning in C57BL/6 mice. J Neurosci. 2005;25:8708–8713. doi: 10.1523/JNEUROSCI.2853-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould TJ, Higgins JS. Nicotine enhances contextual fear conditioning in C57BL/6J mice at 1 and 7 days post-training. Neurobiol Learn Mem. 2003;80:147–157. doi: 10.1016/s1074-7427(03)00057-1. [DOI] [PubMed] [Google Scholar]
- Gould TJ, Leach PT. Cellular, molecular, and genetic substrates underlying the impact of nicotine on learning. Neurobiol Learn Mem. 2013 doi: 10.1016/j.nlm.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould TJ, Portugal GS, André JM, Tadman MP, Marks MJ, Kenney JW, Yildirim E, Adoff M. The duration of nicotine withdrawal-associated deficits in contextual fear conditioning parallels changes in hippocampal high affinity nicotinic acetylcholine receptor upregulation. Neuropharmacology. 2012;62:2118–2125. doi: 10.1016/j.neuropharm.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould TJ, Wehner JM. Nicotine enhancement of contextual fear conditioning. Behav Brain Res. 1999;102:31–39. doi: 10.1016/s0166-4328(98)00157-0. [DOI] [PubMed] [Google Scholar]
- Heishman SJ. Behavioral and cognitive effects of smoking: relationship to nicotine addiction. Nicotine Tob Res. 1999;1(Suppl 2):S143–147. doi: 10.1080/14622299050011971. discussion S165–146. [DOI] [PubMed] [Google Scholar]
- Heishman SJ, Kleykamp BA, Singleton EG. Meta-analysis of the acute effects of nicotine and smoking on human performance. Psychopharmacology (Berl) 2010;210:453–469. doi: 10.1007/s00213-010-1848-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks PS, Ditre JW, Drobes DJ, Brandon TH. The early time course of smoking withdrawal effects. Psychopharmacology (Berl) 2006;187:385–396. doi: 10.1007/s00213-006-0429-9. [DOI] [PubMed] [Google Scholar]
- Hudmon KS, Corelli RL, Prokhorov AV. Current approaches to pharmacotherapy for smoking cessation. Ther Adv Respir Dis. 2010;4:35–47. doi: 10.1177/1753465809353768. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Keenan RM, Yellin A. Effect of tobacco withdrawal on sustained attention. Addict Behav. 1989;14:577–580. doi: 10.1016/0306-4603(89)90079-8. [DOI] [PubMed] [Google Scholar]
- Hulihan-Giblin BA, Lumpkin MD, Kellar KJ. Effects of chronic administration of nicotine on prolactin release in the rat: inactivation of prolactin response by repeated injections of nicotine. J Pharmacol Exp Ther. 1990;252:21–25. [PubMed] [Google Scholar]
- Hussmann GP, Turner JR, Lomazzo E, Venkatesh R, Cousins V, Xiao Y, Yasuda RP, Wolfe BB, Perry DC, Rezvani AH, Levin ED, Blendy JA, Kellar KJ. Chronic sazetidine-A at behaviorally active doses does not increase nicotinic cholinergic receptors in rodent brain. J Pharmacol Exp Ther. 2012;343:441–450. doi: 10.1124/jpet.112.198085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen LK, Krystal JH, Mencl WE, Westerveld M, Frost SJ, Pugh KR. Effects of smoking and smoking abstinence on cognition in adolescent tobacco smokers. Biol Psychiatry. 2005;57:56–66. doi: 10.1016/j.biopsych.2004.10.022. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Mencl WE, Constable RT, Westerveld M, Pugh KR. Impact of smoking abstinence on working memory neurocircuitry in adolescent daily tobacco smokers. Psychopharmacology (Berl) 2007;193:557–566. doi: 10.1007/s00213-007-0797-9. [DOI] [PubMed] [Google Scholar]
- Jarvik ME, Henningfield JE. Pharmacological treatment of tobacco dependence. Pharmacol Biochem Behav. 1988;30:279–294. doi: 10.1016/0091-3057(88)90456-x. [DOI] [PubMed] [Google Scholar]
- Kenney JW, Florian C, Portugal GS, Abel T, Gould TJ. Involvement of hippocampal jun-N terminal kinase pathway in the enhancement of learning and memory by nicotine. Neuropsychopharmacology. 2010;35:483–492. doi: 10.1038/npp.2009.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney JW, Gould TJ. Modulation of hippocampus-dependent learning and synaptic plasticity by nicotine. Mol Neurobiol. 2008;38:101–121. doi: 10.1007/s12035-008-8037-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney JW, Raybuck JD, Gould TJ. Nicotinic receptors in the dorsal and ventral hippocampus differentially modulate contextual fear conditioning. Hippocampus. 2012;22:1681–1690. doi: 10.1002/hipo.22003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Rison RA, Fanselow MS. Effects of amygdala, hippocampus, and periaqueductal gray lesions on short- and long-term contextual fear. Behav Neurosci. 1993;107:1093–1098. doi: 10.1037//0735-7044.107.6.1093. [DOI] [PubMed] [Google Scholar]
- Kleinman KM, Vaughn RL, Christ TS. Effects of cigarette smoking and smoking deprivation on paired-associate learning of high and low meaningful nonsense syllables. Psychol Rep. 1973;32:963–966. doi: 10.2466/pr0.1973.32.3.963. [DOI] [PubMed] [Google Scholar]
- Levin ED, Lee C, Rose JE, Reyes A, Ellison G, Jarvik M, Gritz E. Chronic nicotine and withdrawal effects on radial-arm maze performance in rats. Behav Neural Biol. 1990;53:269–276. doi: 10.1016/0163-1047(90)90509-5. [DOI] [PubMed] [Google Scholar]
- Levin ED, Rezvani AH, Xiao Y, Slade S, Cauley M, Wells C, Hampton D, Petro A, Rose JE, Brown ML, Paige MA, McDowell BE, Kellar KJ. Sazetidine-A, a selective alpha4beta2 nicotinic receptor desensitizing agent and partial agonist, reduces nicotine self-administration in rats. J Pharmacol Exp Ther. 2010;332:933–939. doi: 10.1124/jpet.109.162073. [DOI] [PubMed] [Google Scholar]
- Logue SF, Paylor R, Wehner JM. Hippocampal lesions cause learning deficits in inbred mice in the Morris water maze and conditioned-fear task. Behav Neurosci. 1997;111:104–113. doi: 10.1037//0735-7044.111.1.104. [DOI] [PubMed] [Google Scholar]
- Loughead J, Ray R, Wileyto EP, Ruparel K, Sanborn P, Siegel S, Gur RC, Lerman C. Effects of the alpha4beta2 partial agonist varenicline on brain activity and working memory in abstinent smokers. Biol Psychiatry. 2010;67:715–721. doi: 10.1016/j.biopsych.2010.01.016. [DOI] [PubMed] [Google Scholar]
- Marks MJ, Burch JB, Collins AC. Effects of chronic nicotine infusion on tolerance development and nicotinic receptors. J Pharmacol Exp Ther. 1983;226:817–825. [PubMed] [Google Scholar]
- Marks MJ, Romm E, Gaffney DK, Collins AC. Nicotine-induced tolerance and receptor changes in four mouse strains. J Pharmacol Exp Ther. 1986;237:809–819. [PubMed] [Google Scholar]
- Marks MJ, Stitzel JA, Collins AC. Time course study of the effects of chronic nicotine infusion on drug response and brain receptors. J Pharmacol Exp Ther. 1985;235:619–628. [PubMed] [Google Scholar]
- McCallum SE, Caggiula AR, Booth S, Breese CR, Lee MJ, Donny EC, Leonard S, Sved AF. Mecamylamine prevents tolerance but enhances whole brain [3H]epibatidine binding in response to repeated nicotine administration in rats. Psychopharmacology (Berl) 2000;150:1–8. doi: 10.1007/s002130000401. [DOI] [PubMed] [Google Scholar]
- Mendrek A, Monterosso J, Simon SL, Jarvik M, Brody A, Olmstead R, Domier CP, Cohen MS, Ernst M, London ED. Working memory in cigarette smokers: comparison to non-smokers and effects of abstinence. Addict Behav. 2006;31:833–844. doi: 10.1016/j.addbeh.2005.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihalak KB, Carroll FI, Luetje CW. Varenicline is a partial agonist at alpha4beta2 and a full agonist at alpha7 neuronal nicotinic receptors. Mol Pharmacol. 2006;70:801–805. doi: 10.1124/mol.106.025130. [DOI] [PubMed] [Google Scholar]
- Myers CS, Taylor RC, Moolchan ET, Heishman SJ. Dose-related enhancement of mood and cognition in smokers administered nicotine nasal spray. Neuropsychopharmacology. 2008;33:588–598. doi: 10.1038/sj.npp.1301425. [DOI] [PubMed] [Google Scholar]
- Ochoa EL, Chattopadhyay A, McNamee MG. Desensitization of the nicotinic acetylcholine receptor: molecular mechanisms and effect of modulators. Cell Mol Neurobiol. 1989;9:141–178. doi: 10.1007/BF00713026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolini M, De Biasi M. Mechanistic insights into nicotine withdrawal. Biochem Pharmacol. 2011:1–12. doi: 10.1016/j.bcp.2011.07.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke RL, Trocmé-Thibierge C, Guendisch D, Al Rubaiy SAA, Bloom SA. Electrophysiological perspectives on the therapeutic use of nicotinic acetylcholine receptor partial agonists. J Pharmacol Exp Ther. 2011;337:367–379. doi: 10.1124/jpet.110.177485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson F, Jepson C, Loughead J, Perkins K, Strasser AA, Siegel S, Frey J, Gur R, Lerman C. Working memory deficits predict short-term smoking resumption following brief abstinence. Drug Alcohol Depend. 2010;106:61–64. doi: 10.1016/j.drugalcdep.2009.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson F, Jepson C, Strasser AA, Loughead J, Perkins KA, Gur RC, Frey JM, Siegel S, Lerman C. Varenicline improves mood and cognition during smoking abstinence. Biol Psychiatry. 2009;65:144–149. doi: 10.1016/j.biopsych.2008.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penton RE, Quick MW, Lester RAJ. Short- and long-lasting consequences of in vivo nicotine treatment on hippocampal excitability. J Neurosci. 2011;31:2584–2594. doi: 10.1523/JNEUROSCI.4362-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA. Chronic tolerance to nicotine in humans and its relationship to tobacco dependence. Nicotine Tob Res. 2002;4:405–422. doi: 10.1080/1462220021000018425. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Gerlach D, Broge M, Grobe JE, Sanders M, Fonte C, Vender J, Cherry C, Wilson A. Dissociation of nicotine tolerance from tobacco dependence in humans. J Pharmacol Exp Ther. 2001;296:849–856. [PubMed] [Google Scholar]
- Portugal GS, Gould TJ. Nicotine withdrawal disrupts new contextual learning. Pharmacology Biochemistry And Behavior. 2009;92:117–123. doi: 10.1016/j.pbb.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portugal GS, Kenney JW, Gould TJ. Beta2 subunit containing acetylcholine receptors mediate nicotine withdrawal deficits in the acquisition of contextual fear conditioning. Neurobiol Learn Mem. 2008;89:106–113. doi: 10.1016/j.nlm.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portugal GS, Wilkinson DS, Kenney JW, Sullivan C, Gould TJ. Strain-dependent Effects of Acute, Chronic, and Withdrawal from Chronic Nicotine on Fear Conditioning. Behav Genet. 2012a;42:133–150. doi: 10.1007/s10519-011-9489-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portugal GS, Wilkinson DS, Turner JR, Blendy JA, Gould TJ. Developmental effects of acute, chronic, and withdrawal from chronic nicotine on fear conditioning. Neurobiol Learn Mem. 2012b;97:482–494. doi: 10.1016/j.nlm.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulos CX, Cappell H. Homeostatic theory of drug tolerance: a general model of physiological adaptation. Psychol Rev. 1991;98:390–408. doi: 10.1037/0033-295x.98.3.390. [DOI] [PubMed] [Google Scholar]
- Raybuck JD, Gould TJ. Nicotine withdrawal-induced deficits in trace fear conditioning in C57BL/6 mice--a role for high-affinity beta2 subunit-containing nicotinic acetylcholine receptors. The European Journal of Neuroscience. 2009;29:377–387. doi: 10.1111/j.1460-9568.2008.06580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raybuck JD, Portugal GS, Lerman C, Gould TJ. Varenicline ameliorates nicotine withdrawal-induced learning deficits in C57BL/6 mice. Behav Neurosci. 2008;122:1166–1171. doi: 10.1037/a0012601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes JD, Hawk LW, Ashare RL, Schlienz NJ, Mahoney MC. The effects of varenicline on attention and inhibitory control among treatment-seeking smokers. Psychopharmacology (Berl) 2012;223:131–138. doi: 10.1007/s00213-012-2700-6. [DOI] [PubMed] [Google Scholar]
- Robinson SE, James JR, Lapp LN, Vann RE, Gross DF, Philibin SD, Rosecrans JA. Evidence of cellular nicotinic receptor desensitization in rats exhibiting nicotine-induced acute tolerance. Psychopharmacology (Berl) 2006;184:306–313. doi: 10.1007/s00213-005-0049-9. [DOI] [PubMed] [Google Scholar]
- Robinson SE, Vann RE, Britton AF, O’Connell MM, James JR, Rosecrans JA. Cellular nicotinic receptor desensitization correlates with nicotine-induced acute behavioral tolerance in rats. Psychopharmacology (Berl) 2007;192:71–78. doi: 10.1007/s00213-006-0687-6. [DOI] [PubMed] [Google Scholar]
- Salas R, Main A, Gangitano D, De Biasi M. Decreased withdrawal symptoms but normal tolerance to nicotine in mice null for the alpha7 nicotinic acetylcholine receptor subunit. Neuropharmacology. 2007;53:863–869. doi: 10.1016/j.neuropharm.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz RD, Kellar KJ. Nicotinic cholinergic receptor binding sites in the brain: regulation in vivo. Science. 1983;220:214–216. doi: 10.1126/science.6828889. [DOI] [PubMed] [Google Scholar]
- Sharp BM, Beyer HS. Rapid desensitization of the acute stimulatory effects of nicotine on rat plasma adrenocorticotropin and prolactin. J Pharmacol Exp Ther. 1986;238:486–491. [PubMed] [Google Scholar]
- Snyder FR, Davis FC, Henningfield JE. The tobacco withdrawal syndrome: performance decrements assessed on a computerized test battery. Drug Alcohol Depend. 1989;23:259–266. doi: 10.1016/0376-8716(89)90090-2. [DOI] [PubMed] [Google Scholar]
- Solomon RL, Corbit JD. An opponent-process theory of motivation. II Cigarette addiction. J Abnorm Psychol. 1973;81:158–171. doi: 10.1037/h0034534. [DOI] [PubMed] [Google Scholar]
- Tatum A, Seevers M, Collins K. Morphine addiction and its physiological interpretation based on experimental evidences. J Pharmacol Exp Ther. 1929;36:401–410. [Google Scholar]
- Turner JR, Castellano LM, Blendy JA. Nicotinic partial agonists varenicline and sazetidine-A have differential effects on affective behavior. J Pharmacol Exp Ther. 2010;334:665–672. doi: 10.1124/jpet.110.166280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JR, Castellano LM, Blendy JA. Parallel anxiolytic-like effects and upregulation of neuronal nicotinic acetylcholine receptors following chronic nicotine and varenicline. Nicotine Tob Res. 2011;13:41–46. doi: 10.1093/ntr/ntq206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JR, Wilkinson DS, Poole RL, Gould TJ, Carlson GC, Blendy JA. Divergent functional effects of sazetidine-a and varenicline during nicotine withdrawal. Neuropsychopharmacology. 2013;38:2035–2047. doi: 10.1038/npp.2013.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson DS, Gould TJ. Withdrawal from chronic nicotine and subsequent sensitivity to nicotine challenge on contextual learning. Behav Brain Res. 2013;250:58–61. doi: 10.1016/j.bbr.2013.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson DS, Turner JR, Blendy JA, Gould TJ. Genetic background influences the effects of withdrawal from chronic nicotine on learning and high-affinity nicotinic acetylcholine receptor binding in the dorsal and ventral hippocampus. Psychopharmacology (Berl) 2013;225:201–208. doi: 10.1007/s00213-012-2808-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y, Fan H, Musachio JL, Wei Z-l, Chellappan SK, Kozikowski AP, Kellar KJ. Sazetidine-A, a novel ligand that desensitizes alpha4beta2 nicotinic acetylcholine receptors without activating them. Mol Pharmacol. 2006;70:1454–1460. doi: 10.1124/mol.106.027318. [DOI] [PubMed] [Google Scholar]
- Zwart R, Carbone AL, Moroni M, Bermudez I, Mogg AJ, Folly EA, Broad LM, Williams AC, Zhang D, Ding C, Heinz BA, Sher E. Sazetidine-A is a potent and selective agonist at native and recombinant alpha 4 beta 2 nicotinic acetylcholine receptors. Mol Pharmacol. 2008;73:1838–1843. doi: 10.1124/mol.108.045104. [DOI] [PubMed] [Google Scholar]