Abstract
Background
Emerging data suggest that ovarian cancers differ by tumor grade. However, the reliability of microscopic grade as assigned in the general medical community and reflected in cancer registries is unknown.
Methods
We examined grade agreement between two gynecologic pathologists and the Surveillance Epidemiology and End Results (SEER) program. Grade agreement was assessed with percent observer agreement and kappa coefficients for 664 invasive ovarian carcinomas from SEER’s Residual Tissue Repository. We used three-tier and two-tier grading schemes. A random subset of ovarian carcinomas was selected to compare intra- and inter-pathologist agreement.
Results
Five hundred and eighty-six of SEER’s 664 tumors were confirmed invasive. Percent agreement was 49% with fair kappa coefficient = 0.25 (95% CI: 0.20 to 0.30) for the 664 tumors. Agreement improved slightly when restricted to the 586 confirmed invasive cancers; was better for high than low grade tumors, two-tier than three-tier grading systems, and within (66%) than between study pathologists (43%). Tumor grade was not a robust independent predictor of ovarian cancer-specific survival.
Conclusions
Grade agreement was fair irrespective of grading system between SEER and study pathologists. Recorded grade in SEER should be used with caution and is probably not a reliable metric for ovarian cancer epidemiology.
Keywords: epidemiology, ovarian cancer, tumor grade, SEER, kappa coefficient
Introduction
The molecular classification of ovarian epithelial carcinomas as low grade (type I) or high grade (type II) identifies two sets of cancers with contrasting incidence, molecular characteristics, and clinical outcomes (1–7). The importance of ovarian cancer grade also transcends relevance for individuals with implications for cancer epidemiology and surveillance. It, therefore, would be important to establish the reliability of ovarian cancer grading in a population-based cancer resource such as the National Cancer Institute’s SEER database. We, therefore, examined the agreement between recorded ovarian carcinoma grade in SEER’s Residual Tissue Repository (RTR) and two independent gynecologic pathologists using three different grading schemes; 1) the International Federation of Gynecology and Obstetrics grading system (FIGO) (8, 9), 2) Shimizu and Silverberg system (9–11), and 3) Malpica et al scheme (12–15).
Community-based pathologists commonly use the FIGO system; a three-tier grading scheme (low, intermediate, or high grade) that is modeled after the system for endometrial (uterine) carcinoma, which reflects the level of cellular organization into differentiated structures such as glands and papillae as opposed to solid sheets of tumor cells. Shimizu and Silverberg also devised a three-tier grading system (low, intermediate, or high grade; herein referred to SS) that is similar to grading for breast carcinoma, incorporating architecture, nuclear cytology, and mitotic index. Malpica and colleagues at the M.D. Anderson Cancer Center proposed a two-tier system (low or high grade; herein referred to MDACC) for serous ovarian carcinomas (12–15), which is based upon a dualistic conceptual framework where low and high grade carcinomas proceed along two separate cancer pathways (1–7).
Material and Methods
The National Cancer Institute’s SEER program established its Residual Tissue Repository (RTR) in 2003 to facilitate population-based cancer research using archival biospecimens (16, 17). SEER’s RTR included Tumor Registries in Hawaii, Iowa, and Los Angeles County. The Los Angeles County Tumor Registry did not participate in this study. We retrieved the available formalin-fixed and paraffin-embedded tissue blocks for primary invasive ovarian carcinomas in the Hawaii and Iowa Tumor Registries, excluding tubal and peritoneal tumors. There were 664 ovarian tumors; 516 from the Hawaii Tumor Registry that were diagnosed from 1983 through 2004, which represented 38% of all ovarian tumors in the Hawaii catchment area during that time period. The remaining 148 ovarian cases were derived from the Iowa Tumor Registry diagnosed from 1987 through 2003, representing 4% of all ovarian tumors in the Iowa catchment area during that time period. Because SEER’s RTR data were anonymized, the National Institutes of Health’s Office of Human Subjects Research designated the project as exempt from IRB approval; nonetheless, IRB approvals were provided at the Universities of Hawaii and Iowa.
Demographic data included age at diagnosis, year of diagnosis, and race (White, Asian or Pacific Islander [API], and other/unknown). Tumor characteristics were the American Joint Committee on Cancer (AJCC) TNM stage (18); and histological type, behavior, and grade according to the International Classification of Diseases for Oncology 3rd edition [ICD-O-3] (19). AJCC ovarian cancer stages were stage I (tumors limited to one or both ovaries), stage II (involvement of one or both ovaries with pelvic extension and/or implants), stage III (involvement of one or both ovaries with microscopically-confirmed peritoneal metastasis), and stage IV (distant metastasis, excluding peritoneal metastasis). AJCC guidelines also specify 5 histologic codes for the microscopic assessment of grade (G) that are independent of TNM stage: GX = unknown, G1 = well differentiated, G2 = moderately differentiated, G3 = poorly differentiated, and G4 = undifferentiated. ICD-O-3 codes have six digits; the 1st four digits are for histologic type, the fifth is for behavior (benign or malignant), and the sixth for tumor grade. Ovarian carcinoma histological codes were serous (8441, 8460, and 8461), mucinous (8470, 8471, 8480, and 8481), endometrioid (8380, 8560, 8570, and 8381), clear cell (8310), and other (8010–8580, 9000, 9014). SEER abstracted tumor grade from the 6th ICD-O-3 digit as grade 1 (well differentiated), grade 2 (moderately differentiated, moderately well differentiated, or intermediate differentiation), grade 3 (poorly differentiated), and grade 4 (undifferentiated or anaplastic).
Pathology review
The primary study pathologist (MES) reviewed approximately three H&E stained slides per case (all designated as invasive carcinoma in SEER) to independently re-assess behavior (benign, borderline, or malignant), histological type, and grade for all 664 cases retrieved from the RTR. A set of 19% of the tumors (128 of 664) was selected for repeat pathology panel review. Specifically, this set was constructed by taking a random sample of cancers stratified by histological type, with oversampling of rarer types. Sampling fractions for each histological type were serous (10%, 30 of 298), mucinous (40%, 30 of 75), endometrioid (20%, 20 of 97), clear cell (45%, 28 of 62), and other carcinomas (15%, 20 of 132). The selected ovarian cancers were reexamined by MES to evaluate intra-pathologist agreement and reviewed by the second pathologist (OBI) to assess inter-pathologist agreement between MES and OBI. The pathologists had access to the gross pathologic descriptions but were masked to SEER’s recorded behavior and AJCC stage given that grade is meant to provide a microscopic assessment of ovarian cancer prognosis that is independent of stage (18).
For a complete description of the three ovarian cancer grading systems see supplemental table 1 for: 1) the International Federation of Gynecology and Obstetrics (FIGO) system (8, 9), 2) the Shimizu and Silverberg (SS) (9–11) system, and 3) the MD Anderson (MDACC) system (14). In brief, the FIGO/SS grading schemes are three-tier systems that assign all histological types to ‘low’, ‘intermediate’, or ‘high’ grade. The MDACC grading system is a two-tier system that assigns serous types to ‘low’ or ‘high’ grade.
Statistical analysis
We assessed the representativeness of the SEER RTR ovarian tumors with chi-square tests for heterogeneity, comparing demographic and tumor characteristics for the recovered ovarian carcinomas in SEER’s RTR with the ovarian carcinomas in the corresponding Hawaii and Iowa Tumor Registries. To compare the three-tier FIGO/SS grades to SEER grades 1 to 4 (table 1), we reclassified SEER grade 1 as low, SEER grade 2 as intermediate, and SEER grades 3–4 as high. To compare the two-tier MDACC low and high grades with SEER grades 1 to 4, we dichotomized SEER grade 1 as low and SEER grades 2–4 as high. Finally, the three-tier FIGO/SS schemes were further collapsed to low and high (intermediate + high) grades for survival analyses.
Table 1.
Graphical representation between the reclassified categories of SEER’s 4-tier grading system to the 3-tier and 2-tier grading schemes.
| 4 to 3 tier grade comparison
|
4 to 2 tier grade comparison
|
|||||||
|---|---|---|---|---|---|---|---|---|
| SEER - 4 tier | FIGO - 3 tier | SS - 3 tier | SEER - 4 tier | MDAH - 2 tier | FIGO - 2 tier | SS - 2 tier | ||
|
|
|
|||||||
| Grade 1 | Low | Low | Grade 1 | Low | Low | Low | ||
|
|
|
|||||||
| Grade 2 | Intermediate | Intermediate | Grade 2 |
|
High | High | High | |
| Grade 3 |
|
High | High | Grade 3 | ||||
| Grade 4 | Grade 4 | |||||||
| Unknown | Unknown | Unknown | Unknown | Unknown | Unknown | Unknown | ||
SEER grade 1 = well differentiated; SEER grade 2 = moderately differentiated; SEER grade 3 = poorly differentiated; SEER grade 4 = undifferentiated; FIGO, the International Federation of Gynecology and Obstetrics; SS, Shimizu and Silverberg; M. D. Anderson Cancer Center
Agreement was assessed as percent observer agreement (po) and Cohen’s standard kappa coefficients (κ) (20). Kappa coefficients ranged from 0.00 to 1.00; and were interpreted descriptively as poor κ< 0.20, fair κ = 0.20–0.40, moderate k = 0.40–0.60, good κ = 0.60–0.80, and very good κ = 0.80–1.00. The Kaplan-Meier estimator (21) was used to calculate ovarian cancer-specific survival by low or high grade for all AJCC stages combined, and then by early stage (AJCC I + II) or late stage (AJCC III + IV) stage. The log-rank test was used to assess survival differences by low and high grade (22).
Results
Descriptive statistics
The 664 ovarian carcinomas in SEER’s RTR are shown in Table 2. Approximately three-quarters of the tumors were contributed by the Hawaii RTR (77%, 516 of 664). Mean age at diagnosis was 59.6 years. Serous carcinomas accounted for 45% of the ovarian tumors (298 of 664), 59% were late stage and 40% were high grade. Clear cell carcinomas were more common among APIs (13%, 51 of 379) than among Whites (4%, 11 of 282), p < 0.01. Women with serous carcinomas were diagnosed at older age, later stage, and higher grade than women with other histological types (p < 0.05). Compared to the 664 tumors in the RTRs, the ovarian cancers (5347) reported to the Hawaii and Iowa Tumor Registries demonstrated a higher percentage of White women, slightly older ages at diagnosis, a lower proportion of serous tumors, and lower stage at diagnosis.
Table 2.
Distribution of demographic and tumor characteristics for all 664 ovarian tumors in the Hawaii (1983–2004) and Iowa (1987–2003) Surveillance, Epidemiology and End Results Residual Tissue Repository (SEER RTR)
| Histological type* | All cases | Serous | Mucinous | Endometrioid | Clear cell | Other | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | %** | n | % | n | % | n | % | n | % | n | % | |
| Overall | 664 | 100.0 | 298 | 44.9 | 75 | 11.3 | 97 | 14.6 | 62 | 9.3 | 132 | 19.9 |
| SEER RTR | . | |||||||||||
| Hawaii | 516 | 77.7 | 230 | 77.2 | 58 | 77.3 | 80 | 82.5 | 60 | 96.8 | 88 | 66.7 |
| Iowa | 148 | 22.3 | 68 | 22.8 | 17 | 22.7 | 17 | 17.5 | 2 | 3.2 | 44 | 33.3 |
| Year of diagnosis | ||||||||||||
| 1983–1987 | 33 | 5.0 | 10 | 3.4 | 7 | 9.3 | 2 | 2.1 | 4 | 6.5 | 10 | 7.6 |
| 1988–2000 | 487 | 73.3 | 232 | 77.9 | 54 | 72.0 | 70 | 72.2 | 43 | 69.4 | 88 | 66.7 |
| 2001–2004 | 144 | 21.7 | 56 | 18.8 | 14 | 18.7 | 25 | 25.8 | 15 | 24.2 | 34 | 25.8 |
| Age at diagnosis | ||||||||||||
| Mean age (SE) | 59.6 (0.5) | 61.4 (0.7) | 57.4 (1.6) | 55.6 (1.4) | 55.2 (1.6) | 62.1 (1.3) | ||||||
| <40 years | 46 | 6.9 | 14 | 4.7 | 7 | 9.3 | 10 | 10.3 | 5 | 8.1 | 10 | 7.6 |
| 40–49 years | 122 | 18.4 | 41 | 13.8 | 17 | 22.7 | 31 | 32.0 | 14 | 22.6 | 19 | 14.4 |
| 50–59 years | 157 | 23.6 | 71 | 23.8 | 17 | 22.7 | 18 | 18.6 | 26 | 41.9 | 25 | 18.9 |
| 60–69 years | 170 | 25.6 | 90 | 30.2 | 18 | 24.0 | 20 | 20.6 | 7 | 11.3 | 35 | 26.5 |
| 70+ years | 169 | 25.5 | 82 | 27.5 | 16 | 21.3 | 18 | 18.6 | 10 | 16.1 | 43 | 32.6 |
| Race | ||||||||||||
| White | 282 | 42.5 | 137 | 46.0 | 27 | 36.0 | 31 | 32.0 | 11 | 17.7 | 76 | 57.6 |
| Asian or Pacific Islander | 379 | 57.1 | 160 | 53.7 | 47 | 62.7 | 66 | 68.0 | 51 | 82.3 | 55 | 41.7 |
| Other/unknown | 3 | 0.5 | 1 | 0.3 | 1 | 1.3 | 0 | 0.0 | 0 | 0.0 | 1 | 0.8 |
| AJCC stage | ||||||||||||
| Early (I–II) | 238 | 35.8 | 57 | 19.1 | 51 | 68.0 | 58 | 59.8 | 40 | 64.5 | 32 | 24.2 |
| Late (III–IV) | 391 | 58.9 | 231 | 77.5 | 17 | 22.7 | 37 | 38.1 | 18 | 29.0 | 88 | 66.7 |
| Other/Unknown | 35 | 5.3 | 10 | 3.4 | 7 | 9.3 | 2 | 2.1 | 4 | 6.5 | 12 | 9.1 |
| SEER grade | ||||||||||||
| Grade 1 | 50 | 7.5 | 8 | 2.7 | 23 | 30.7 | 18 | 18.6 | 0 | 0.0 | 1 | 0.8 |
| Grade 2 | 165 | 24.8 | 56 | 18.8 | 32 | 42.7 | 38 | 39.2 | 11 | 17.7 | 28 | 21.2 |
| Grade 3 | 265 | 39.9 | 145 | 48.7 | 5 | 6.7 | 30 | 30.9 | 15 | 24.2 | 70 | 53.0 |
| Grade 4 | 97 | 14.6 | 56 | 18.8 | 2 | 2.7 | 5 | 5.2 | 17 | 27.4 | 17 | 12.9 |
| Other/Unknown | 87 | 13.1 | 33 | 11.1 | 13 | 17.3 | 6 | 6.2 | 19 | 30.6 | 16 | 12.1 |
Key:
ICD-O-3 codes for histological type: serous (8441, 8460, and 8461), mucinous (8470, 8471, 8480, and 8481), endometrioid (8380, 8560, 8570, and 8381), clear cell (8310), and other (8010–8580, 9000, 9014);
%, percentage of all cases
Pathology review
MES classified SEER’s 664 invasive ovarian tumors as primary invasive ovarian carcinoma (n=586), benign (n=3), borderline (n=45), and other (n=30). The other category included ovarian cancers diagnosed at distant metastatic sites (i.e., primary carcinoma in the ovary was unavailable for microscopic examination), non-epithelial ovarian cancers, and non-ovarian carcinomas that were metastatic to the ovary. Grade agreement between the pathologist and SEER was similar for the FIGO (Table 3) and the SS systems (Table 4). Percent agreement with FIGO ranged from 24% (po = 0.24) for clear cell carcinoma to 57% for serous carcinoma with poor to fair kappa coefficients ranging from 0.00 to 0.29 (Table 3A).
Table 3.
Percent agreement and standard Kappa coefficients for MES versus SEER using FIGO three-tier grading schemes for: 3A) all 664 ovarian tumors and 3B) restricted to tumors with assigned grade (low, intermediate, or high) and also classified as invasive by the study pathologist
| 3A: FIGO, all SEER tumors | 3B: FIGO, restricted | |||||
|---|---|---|---|---|---|---|
| low/intermediate/high | low/intermediate/high | |||||
| n | p0 | κ (95% CI) | n | p0 | κ (95% CI) | |
| Overall | 664 | 0.49 | 0.25 (0.20, 0.30) | 477 | 0.62 | 0.32 (0.26, 0.39) |
| Registry | ||||||
| Hawaii | 516 | 0.49 | 0.24 (0.18, 0.30) | 382 | 0.61 | 0.31 (0.23, 0.38) |
| Iowa | 148 | 0.51 | 0.29 (0.18, 0.39) | 95 | 0.65 | 0.41 (0.28, 0.55) |
| P | 0.43 | 0.18 | ||||
| AJCC stage | ||||||
| Early (I–II) | 238 | 0.45 | 0.26 (0.18, 0.34) | 171 | 0.56 | 0.34 (0.23, 0.44) |
| Late (III–IV) | 391 | 0.53 | 0.17 (0.10, 0.24) | 292 | 0.65 | 0.21 (0.11, 0.30) |
| Other/unknown | 35 | 0.37 | 0.15 (−0.07, 0.37) | 14 | 0.71 | 0.53 (0.14, 0.91) |
| P | 0.25 | 0.09 | ||||
| Histologic type | ||||||
| Serous | 298 | 0.57 | 0.21 (0.13, 0.30) | 235 | 0.66 | 0.19 (0.08, 0.31) |
| Mucinous | 75 | 0.44 | 0.26 (0.12, 0.40) | 37 | 0.59 | 0.37 (0.13, 0.61) |
| Endometrioid | 97 | 0.46 | 0.26 (0.14, 0.39) | 82 | 0.55 | 0.34 (0.20, 0.49) |
| Clear cell | 62 | 0.24 | 0.00 (−0.11, 0.12) | 39 | 0.36 | 0.04 (−0.12, 0.20) |
| Other | 132 | 0.49 | 0.16 (0.05, 0.27) | 84 | 0.71 | 0.31 (0.13 0.48) |
| P | 0.01 | 0.04 | ||||
Key: MES, Mark E. Sherman; SEER, Surveillance, Epidemiology, and End Results; FIGO, International Federation of Gynecology and Obstetrics grading scheme; n, number; p0, observed agreement; κ, standard Kappa coefficient; P, two-sided P-value for χ2 test of heterogeneity across strata; AJCC, American Joint Committee on Cancer
Table 4.
Percent agreement and standard Kappa coefficients for MES versus SEER using SS three-tier grading schemes for: 4A) all 664 ovarian tumors and 4B) restricted to tumors with assigned grade (low, intermediate, or high) and also classified as invasive by the study pathologist
| 4A: SS, all SEER tumors | 4B: SS, restricted | |||||
|---|---|---|---|---|---|---|
| low/intermediate/high | low/intermediate/high | |||||
| n | p0 | κ (95% CI) | n | p0 | κ (95% CI) | |
| Overall | 664 | 0.43 | 0.20 (0.15, 0.24) | 447 | 0.57 | 0.27 (0.21, 0.34) |
| Registry | ||||||
| Hawaii | 516 | 0.42 | 0.18 (0.13, 0.24) | 356 | 0.56 | 0.25 (0.17, 0.32) |
| Iowa | 148 | 0.46 | 0.24 (0.14, 0.34) | 91 | 0.62 | 0.37 (0.23, 0.52) |
| P | 0.32 | 0.12 | ||||
| AJCC stage | ||||||
| Early (I–II) | 238 | 0.37 | 0.17 (0.09, 0.24) | 159 | 0.48 | 0.25 (0.15, 0.35) |
| Late (III–IV) | 391 | 0.48 | 0.15 (0.09, 0.22) | 274 | 0.62 | 0.19 (0.10, 0.28) |
| Other/unknown | 35 | 0.37 | 0.15 (−0.07, 0.36) | 14 | 0.64 | 0.39 (−0.04, 0.81) |
| P | 0.96 | 0.50 | ||||
| Histologic type | ||||||
| Serous | 298 | 0.53 | 0.20 (0.12, 0.28) | 224 | 0.63 | 0.18 (0.08, 0.29) |
| Mucinous | 75 | 0.39 | 0.19 (0.05, 0.32) | 34 | 0.56 | 0.29 (0.02, 0.56) |
| Endometrioid | 97 | 0.41 | 0.21 (0.09, 0.33) | 77 | 0.52 | 0.31 (0.16, 0.46) |
| Clear cell | 62 | 0.11 | −0.04 (−0.12, 0.04) | 37 | 0.16 | 0.00 (−0.08, 0.08) |
| Other | 132 | 0.41 | 0.11 (0.01, 0.20) | 75 | 0.65 | 0.26 (0.10, 0.42) |
| P | <0.001 | <0.001 | ||||
Key: MES, Mark E. Sherman; SEER, Surveillance, Epidemiology, and End Results; SS, Shimizu and Silverberg grading scheme; n, number; p0, observed agreement; κ, standard Kappa coefficient; P, two-sided P-value for χ2 test of heterogeneity across strata; AJCC, American Joint Committee on Cancer
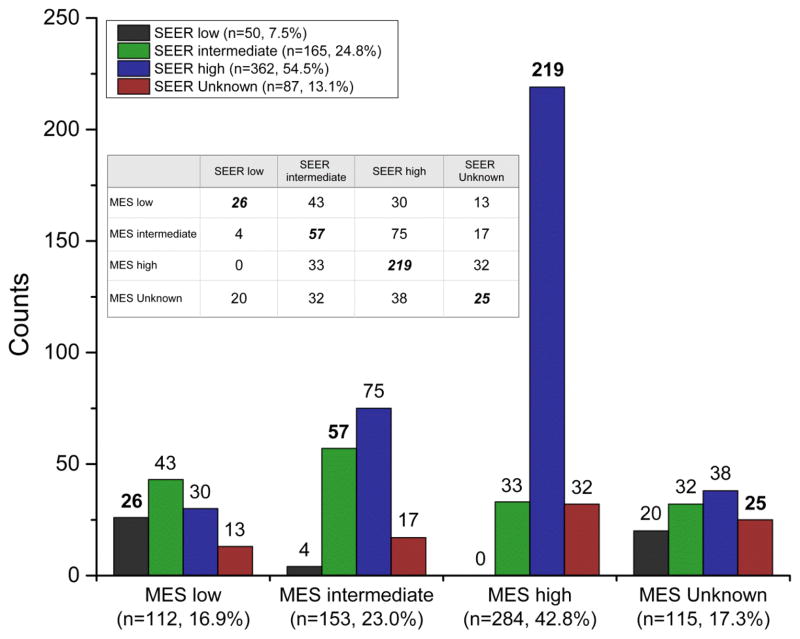
Bar graphs along with an inserted contingency table are used in Figure 1 to supplement FIGO grade agreement between MES and SEER (Table 3A). Percent observer agreement was po = 49% between MES and SEER with 327 of 664 tumors in the diagonal of the contingency table (Figure 1), respectively. MES tended to grade lower than SEER. For example, MES moved 8% of SEER-assigned high grade to low grade cancers (30 of 362) but did not move any SEER-assigned low grade to high grade tumors (0 of 362), Figure 1. Consequently, po rose from low to high grade; e.g., po = 23% for low grade (26 for SEER and 112 for MES), 37% for intermediate grade (57 for SEER and 153 for MES), and 77% for high grade (219 for SEER and 284 for MES). Tumor grade was unknown for 177 of the ovarian carcinomas either because grade was not recorded by SEER or MES could not classify the tumor grade because of insufficient microscopic tissue (87 for SEER and 115 for MES with 25 unknown for both SEER and MES). Grade agreement improved when restricted to those tumors with known grade (low, intermediate, or high) and also labeled as invasive by the study pathologist (Table 3B), i.e., po = 62% and fair kappa coefficient = 0.32 (95% CI: 0.26 to 0.39). Similar improvement was observed for SS grade (Table 4A compared to Table 4B). Grade agreements for the three-tier FIGO/SS systems did not improve substantively even when there was histological type agreement (serous, mucinous, endometrioid, or clear cell) between MES and SEER.
Figure 1.
Contingency table for grade agreement between the study pathologist (MES) and SEER for all 664 ovarian tumors in the Hawaii and Iowa RTRs. The crosstab or contingency table (insert) shows percent observer agreement between MES and SEER in the diagonals with disagreements in the off diagonals. Bold fonts in the bar graph show greater percent agreement between MES and SEER for high grade (77%, 219 of 284) than low grade (23%, 26 of 112) or intermediate grade (37%, 57 of 153). Grading was unknown and/or missing for 177 ovarian tumors.
Percent agreement but not the kappa coefficient was generally better with the two-tier MDACC than three-tier FIGO system (Table 5). For example, overall agreement between the study pathologist and SEER grade with the MDACC system was po = 64% with a poor kappa coefficient = 0.10 (95% CI: 0.01 to 0.19), and improved when restricted to cases that were classified as invasive by the study pathologist (po = 95%).
Table 5.
Percent agreement and standard Kappa coefficients for MES versus SEER using MDACC two-tier grading schemes 5A) all 298 ovarian serous carcinomas recorded in SEER and 5B) restricted to serous tumors with assigned grade (low or high) and also classified as invasive by the primary study pathologist
| 5A: MDACC, all serous tumors | 5B: MDACC, restricted | |||||
|---|---|---|---|---|---|---|
| low/high | low/high | |||||
| n | p0 | κ (95% CI) | n | p0 | κ (95% CI)* | |
| Overall | 298 | 0.64 | 0.10 (0.01, 0.19) | 182 | 0.95 | 0.00 (~) |
| Registry | ||||||
| Hawaii | 230 | 0.69 | 0.13 (0.02, 0.24) | 156 | 0.96 | 0.00 (~) |
| Iowa | 68 | 0.47 | −0.01 (−0.17, 0.14) | 26 | 0.88 | 0.00 (~) |
| P | 0.18 | ~ | ||||
| AJCC stage | ||||||
| Early (I–II) | 57 | 0.56 | 0.17 (0.00, 0.35) | 28 | 0.93 | 0.00 (~) |
| Late (III–IV) | 231 | 0.65 | 0.08 (−0.02, 0.18) | 146 | 0.95 | 0.00 (~) |
| Other/unknown | 10 | 0.80 | 0.00 (0.00, 0.00) | 8 | 1.00 | 0.00 (~) |
| P | 0.35 | ~ | ||||
Key: MES, Mark E. Sherman; SEER, Surveillance, Epidemiology, and End Results; MDACC, MD Anderson Cancer Center grading scheme; n, number; p0, observed agreement; κ, standard Kappa coefficient;
all cases were designated as high grade by SEER;
P, two-sided P-value for χ2 test of heterogeneity across strata; AJCC, American Joint Committee on Cancer
The randomly selected ovarian carcinomas (19%, 128 of 664) were reviewed a second time by MES for intra-pathologist agreement and reviewed by OBI for inter-pathologist agreement. Inter-pathologist agreement between MES and OBI was similar to the agreement between MES and SEER, po = 43% and fair kappa = 0.25 (95% CI: 0.13 to 0.35). Intra-observer agreement for the 1st and 2nd review by MES yielded po = 66% and moderate kappa = 0.52 (95% CI: 0.41 to 0.64).
Ovarian cancer-specific survival
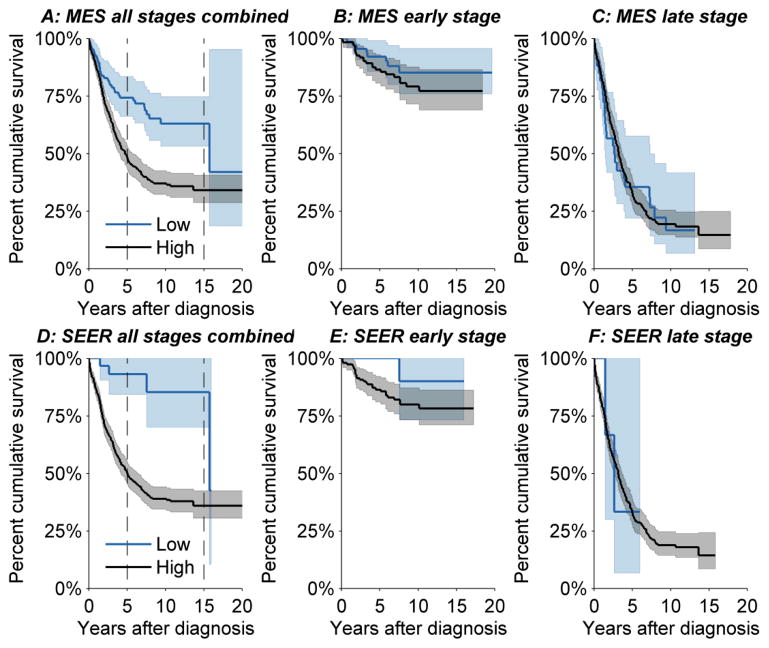
With 20 years of follow-up (Figure 2), ovarian cancer-specific survival for the 586 confirmed invasive ovarian carcinomas was better among low than high tumor grade tumors for MES (Figure 2A, log rank test p<0.001) and for SEER (Figure 2D, log rank test p<0.001). Long term ovarian cancer survival was worse for MES low grade than SEER low grade, e.g., cumulative cancer-specific survival after 15 years of follow-up for MES low grade was 64% (95% CI: 55 to 75%) and for SEER low grade was 90% (95% CI: 80% to 100%). On the other hand, short term cancer survival was similar for MES high grade and SEER high grade, e.g., cancer-specific survival after 5 years of follow-up for MES high grade was 48% (95% CI: 44% to 54%) and for SEER high grade was 51% (95% CI: 47% to 55%). Re-categorizing SEER low grade to include SEER grade 1 + grade 2 and SEER high grade to include SEER grade 3 + grade 4 did not substantively alter the survival analysis (graph available upon request).
Figure 2.
Ovarian cancer-specific survival with 95% confidence limits by low and high grades for the study pathologist (MES) and SEER: all AJCC stages combined (panels A and D), early AJCC stages (panels B and E), and late AJCC stages (panels C and F).
When stratified by AJCC early and late stage, cancer-specific survival was no longer significantly different between low and high grade (Figure 2B, 2C, and 2E), except for SEER late stage (Figure 2F, p = 0.04). For those tumors that were designated as benign by MES, i.e., benign (n=3) or borderline (n=45), ovarian cancer-specific survival was 90% with only 5 of 48 recorded ovarian cancer deaths during follow-up.
Discussion
Our study demonstrated several interesting findings regarding ovarian carcinoma grade between SEER and two independent gynecological pathologists. First, similar to other clinical studies (23), grade agreement was only fair irrespective of grading system and histologic subtype. For example, Gilks et al reported inter-observer kappa coefficients of 0.26 and 0.40 for FIGO and SS grading systems (23), respectively. Second, agreement improved when restricted to tumors with known grade (low, intermediate, or high) and also classified as invasive by the study pathologist (i.e., excluding benign and borderline tumors). Third, agreement was better for high grade than low grade tumors, better for two- than three-tier grading systems, and better for intra- than inter-pathologist comparison. Finally, tumor grade was not a strong independent prognostic factor apart from stage.
Several factors may have affected the generalizability of our results but not the agreement for grade. The 664 ovarian tumors from SEER’s RTR represented only 38% and 4% of the ovarian tumors in the Hawaii and Iowa Tumor Registries, respectively. More than 75% of the data were contributed by the Hawaii Tumor Registry, enriching the study with APIs and clear cell carcinomas, a histological type that is more common among Japanese than White women (24, 25). There were differences between the patient characteristics in the review and those for whom tissue was not retrieved, but grade agreement did not differ by any of these factors.
Percent observer po agreement was generally higher than kappa coefficients, reflecting two limitations of the kappa statistic (26, 27). First, though the kappa statistic attempts to measure the amount of nonrandom agreement (28), one limitation occurs when the categories for a given variable are not equally distributed (27). Given that high grade is proportionately more dominant than low or intermediate grade, high grade ovarian tumors would be more likely by chance alone. The second limitation arises with imbalance of the row and column totals of a contingency table (e.g., figure 1) (27). As shown in Figure 1, there is imbalance in the totals for MES low, intermediate, high, and unknown grade of 16.9%, 23.0%, 42.8%, and 17.3% compared to the corresponding totals for SEER grade of 7.5%, 24.8%, 54.5%, and 13.1%.
Of note, po increased from low to high grade, possibly reflecting the fact that SEER grade comes from community-based pathologists with more clinical information than was available to the study pathologists; e.g., AJCC stage. Even when conditioned upon early and late stage, we observed better agreement between MES and SEER for high than low grade (Table 3A). More specifically, for early stage cancers, po was 29% for low grade and 71% for high grade. For late stage cancers, po was 11% for low grade and 75% for high grade. The knowledge of stage along with a heightened awareness of poor outcomes for advanced stage ovarian carcinomas may have influenced SEER’s pathologists to avoid classifying late stage tumors as low grade. If true, this would tend to yield more conservative low grade carcinomas (because of their association with early stage disease). Figure 2 supports this conjecture since cancer-specific survival was better for SEER early stage than for MES early stage, whereas survival was similar for SEER and MES late stage. Indeed, prior reports from individual pathology laboratories have found lower survival for low grade tumors than reported in SEER (29, 30). Admixing benign and borderline tumors with low grade carcinomas (31) also would tend to improve prognosis for low grade. In fact, 90% cancer-specific survival for the reclassified benign (n = 3) and borderline (n = 45) tumors is clearly better than otherwise would be expected for typical invasive ovarian cancers.
Percent observer po agreement was generally better for the two-tier MDACC than the three-tier staging schemes. Though we cannot exclude better agreement by chance alone, improvement with the two-tier scheme might possibly reflect the dualistic nature of ovarian cancer. Contemporary clinicopathologic and molecular models implicate two main carcinogenic pathways by type I (low grade) or type II (high grade) (1–7). Type I low grade cancers are believed to arise through a stepwise sequence from adenoma to borderline tumor to invasive cancer and are associated with oncogenic mutations that impact cell proliferation (KRAS and/or BRAF) (3, 32). Type II high grade tumors constitute the majority of invasive ovarian cancers in the general population, and typically show molecular changes that are associated with genetic instability (10, 11). As adjuvant chemotherapy regimens continue to evolve, the associations of tumor type, grade, and response to treatment will likely change with greater usage of molecularly targeted drugs such as the anti-VEGF antibody bevacizumab and PARP inhibitors (33).
In sum, grade agreement was fair to moderate between SEER and two independent gynecological pathologists. Agreement improved with higher grade, a two-tier grading scheme for serous tumors, and when restricted to tumors that were classified as invasive by the study pathologist. In this study, grade also was not an independent predictor of ovarian cancer-specific and/or overall survival. Finally, though molecular studies and individual clinical outcomes differ by grade, recorded grade in SEER should be used with caution and may not be a robust metric for population-based cancer epidemiology. Nonetheless, given the compelling molecular evidence for type I and II ovarian cancers, the results of this study suggest that epidemiologists may need to supplement histologic (or microscopic) grade for ovarian cancer with additional biological information such as protein and/or gene expression profiles similar to ‘genomic grade’ for breast cancer. (34, 35).
Footnotes
Disclaimer: None of the co-authors has a financial conflict of interest that would have affected this research. This research was supported by the National Institutes of Health, National Cancer Institute Intramural Research Program, R01CA58598, N01CN67001, N01-PC-35137, and N01-PC-35143. The authors had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the analysis.
References
- 1.Singer G, Kurman RJ, Chang HW, Cho SK, Shih Ie M. Diverse tumorigenic pathways in ovarian serous carcinoma. Am J Pathol. 2002;160(4):1223–8. doi: 10.1016/s0002-9440(10)62549-7. Epub 2002/04/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shih Ie M, Kurman RJ. Ovarian tumorigenesis: a proposed model based on morphological and molecular genetic analysis. Am J Pathol. 2004;164(5):1511–8. doi: 10.1016/s0002-9440(10)63708-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kurman RJ, Shih IM. Pathogenesis of Ovarian Cancer: Lessons From Morphology and Molecular Biology and Their Clinical Implications. Int J Gynecol Pathol. 2008;27:151–60. doi: 10.1097/PGP.0b013e318161e4f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Landen CN, Jr, Birrer MJ, Sood AK. Early events in the pathogenesis of epithelial ovarian cancer. J Clin Oncol. 2008;26(6):995–1005. doi: 10.1200/JCO.2006.07.9970. [DOI] [PubMed] [Google Scholar]
- 5.Levanon K, Crum C, Drapkin R. New insights into the pathogenesis of serous ovarian cancer and its clinical impact. J Clin Oncol. 2008;26(32):5284–93. doi: 10.1200/JCO.2008.18.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuo KT, Guan B, Feng Y, Mao TL, Chen X, Jinawath N, et al. Analysis of DNA copy number alterations in ovarian serous tumors identifies new molecular genetic changes in low-grade and high-grade carcinomas. Cancer Res. 2009;69(9):4036–42. doi: 10.1158/0008-5472.CAN-08-3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grimley PM, Matsuno RK, Rosenberg PS, Henson DE, Schwartz AM, Anderson WF. Qualitative age interactions between low and high grade serous ovarian carcinomas. Cancer Epidemiol Biomarkers Prev. 2009;18(8):2256–61. doi: 10.1158/1055-9965.EPI-09-0240. [DOI] [PubMed] [Google Scholar]
- 8.Classification and staging of malignant tumours in the female pelvis. Acta Obstet Gynecol Scand. 1971;50(1):1–7. doi: 10.3109/00016347109157278. [DOI] [PubMed] [Google Scholar]
- 9.Silverberg SG. Histopathologic grading of ovarian carcinoma: a review and proposal. Int J Gynecol Pathol. 2000;19(1):7–15. doi: 10.1097/00004347-200001000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Shimizu Y, Kamoi S, Amada S, Akiyama F, Silverberg SG. Toward the development of a universal grading system for ovarian epithelial carcinoma: testing of a proposed system in a series of 461 patients with uniform treatment and follow-up. Cancer. 1998;82(5):893–901. doi: 10.1002/(sici)1097-0142(19980301)82:5<893::aid-cncr14>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 11.Shimizu Y, Kamoi S, Amada S, Hasumi K, Akiyama F, Silverberg SG. Toward the development of a universal grading system for ovarian epithelial carcinoma. I. Prognostic significance of histopathologic features--problems involved in the architectural grading system. Gynecol Oncol. 1998;70(1):2–12. doi: 10.1006/gyno.1998.5051. [DOI] [PubMed] [Google Scholar]
- 12.Malpica A. Grading of Ovarian Cancer: A Histotype-Specific Approach. Int J Gynecol Pathol. 2008;27:175–81. doi: 10.1097/PGP.0b013e31816085e0. [DOI] [PubMed] [Google Scholar]
- 13.Malpica A, Deavers MT, Tornos C, Kurman RJ, Soslow R, Seidman JD, et al. Interobserver and intraobserver variability of a two-tier system for grading ovarian serous carcinoma. Am J Surg Pathol. 2007;31(8):1168–74. doi: 10.1097/PAS.0b013e31803199b0. [DOI] [PubMed] [Google Scholar]
- 14.Malpica A, Deavers MT, Lu K, Bodurka DC, Atkinson EN, Gershenson DM, et al. Grading ovarian serous carcinoma using a two-tier system. Am J Surg Pathol. 2004;28(4):496–504. doi: 10.1097/00000478-200404000-00009. [DOI] [PubMed] [Google Scholar]
- 15.McCluggage WG. Morphological subtypes of ovarian carcinoma: a review with emphasis on new developments and pathogenesis. Pathology (Phila) 2011;43(5):420–32. doi: 10.1097/PAT.0b013e328348a6e7. Epub 2011/07/01. [DOI] [PubMed] [Google Scholar]
- 16.SEER. Surveillance Epidemiology and End Results. 2012 [cited 2012 February 06]; Available from: http://seer.cancer.gov/
- 17.Goodman MT, Hernandez BY, Hewitt S, Lynch CF, Cote TR, Frierson HF, Jr, et al. Tissues from population-based cancer registries: a novel approach to increasing research potential. Hum Pathol. 2005;36(7):812–20. doi: 10.1016/j.humpath.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 18.AJCC. Ovary and Primary Peritoneal Carcinoma. In: Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC Cancer Staging Manual. 7. New York: Springer; 2011. pp. 419–28. [Google Scholar]
- 19.Fritz A, Jack A, Shanmugaratnam K, Sobin L, Parkin DM, Whelan S, editors. International Classification of Diseases for Oncology. US Interim Version 2000. 3. Geneva: World Health Organization; 2000. [Google Scholar]
- 20.Cohen CJ. A coefficient of agreement for nominal scales. Educational and Psychological Measurement. 1960;20:37–46. [Google Scholar]
- 21.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. Journal of American Statistical Association. 1958;53:457–81. [Google Scholar]
- 22.Peto R, Peto J. Asymptomatically efficient rank invariant test procedures. Journal of the Royal Statistical Society, A. 1972;135(A):185–98. [Google Scholar]
- 23.Gilks CB, Ionescu DN, Kalloger SE, Kobel M, Irving J, Clarke B, et al. Tumor cell type can be reproducibly diagnosed and is of independent prognostic significance in patients with maximally debulked ovarian carcinoma. Hum Pathol. 2008;39(8):1239–51. doi: 10.1016/j.humpath.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 24.Sugiyama T, Kamura T, Kigawa J, Terakawa N, Kikuchi Y, Kita T, et al. Clinical characteristics of clear cell carcinoma of the ovary: a distinct histologic type with poor prognosis and resistance to platinum-based chemotherapy. Cancer. 2000;88(11):2584–9. Epub 2000/06/22. [PubMed] [Google Scholar]
- 25.Soslow RA. Histologic Subtypes of Ovarian Carcinoma: An Overview. Int J Gynecol Pathol. 2008;27:161–74. doi: 10.1097/PGP.0b013e31815ea812. [DOI] [PubMed] [Google Scholar]
- 26.Cicchetti DV, Feinstein AR. High agreement but low kappa: II. Resolving the paradoxes. J Clin Epidemiol. 1990;43(6):551–8. doi: 10.1016/0895-4356(90)90159-m. Epub 1990/01/01. [DOI] [PubMed] [Google Scholar]
- 27.Feinstein AR, Cicchetti DV. High agreement but low kappa: I. The problems of two paradoxes. J Clin Epidemiol. 1990;43(6):543–9. doi: 10.1016/0895-4356(90)90158-l. Epub 1990/01/01. [DOI] [PubMed] [Google Scholar]
- 28.Last JM. A Dictionary of Epidemiology. 3. Oxford: Oxford University Press; 1995. [Google Scholar]
- 29.Seidman JD, Yemelyanova A, Cosin JA, Smith A, Kurman RJ. Survival rates for international federation of gynecology and obstetrics stage III ovarian carcinoma by cell type: a study of 262 unselected patients with uniform pathologic review. Int J Gynecol Cancer. 2012;22(3):367–71. doi: 10.1097/IGC.0b013e31823c6f80. Epub 2012/01/13. [DOI] [PubMed] [Google Scholar]
- 30.Kobel M, Kalloger SE, Boyd N, McKinney S, Mehl E, Palmer C, et al. Ovarian carcinoma subtypes are different diseases: implications for biomarker studies. PLoS medicine. 2008;5(12):e232. doi: 10.1371/journal.pmed.0050232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leitao MM, Jr, Boyd J, Hummer A, Olvera N, Arroyo CD, Venkatraman E, et al. Clinicopathologic analysis of early-stage sporadic ovarian carcinoma. Am J Surg Pathol. 2004;28(2):147–59. doi: 10.1097/00000478-200402000-00001. [DOI] [PubMed] [Google Scholar]
- 32.Singer G, Shih Ie M, Truskinovsky A, Umudum H, Kurman RJ. Mutational analysis of K-ras segregates ovarian serous carcinomas into two types: invasive MPSC (low-grade tumor) and conventional serous carcinoma (high-grade tumor) Int J Gynecol Pathol. 2003;22(1):37–41. doi: 10.1097/00004347-200301000-00009. [DOI] [PubMed] [Google Scholar]
- 33.Kim A, Ueda Y, Naka T, Enomoto T. Therapeutic strategies in epithelial ovarian cancer. J Exp Clin Cancer Res. 2012;31:14. doi: 10.1186/1756-9966-31-14. Epub 2012/02/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sotiriou C, Pusztai L. Gene-expression signatures in breast cancer. N Engl J Med. 2009;360(8):790–800. doi: 10.1056/NEJMra0801289. [DOI] [PubMed] [Google Scholar]
- 35.Sotiriou C, Wirapati P, Loi S, Harris A, Fox S, Smeds J, et al. Gene expression profiling in breast cancer: understanding the molecular basis of histologic grade to improve prognosis. J Natl Cancer Inst. 2006;98(4):262–72. doi: 10.1093/jnci/djj052. [DOI] [PubMed] [Google Scholar]